Abstract
Objectives: To study the relationship between platelet glycoprotein IIIa gene (GP IIIa) polymorphism (Leu33Pro) and aspirin resistance in a very elderly Chinese population. Methods: Four hundred fifty very elderly Chinese people receiving aspirin therapy were enrolled in the study. Patients who underwent arachnoid acid–induced platelet aggregation were then divided into two groups based on their resistance to aspirin: aspirin-resistant (AR) group (n=236) and aspirin-sensitive (AS) group (n=214). The Leu33Pro polymorphism of the GP IIIa gene was scanned by polymerase chain reaction–restriction fragment-length polymorphism. Results: In the AR group, 224 participants had the A1/A1 genotype and 12 had the A1/A2 genotype. All patients in the AS group had the A1/A1 genotype. Thus, there was significant difference between these two groups in the genotype distribution (p<0.05). Conclusion: The genetic polymorphism of the GP IIIa gene was associated with AR in a very elderly Chinese population.
Introduction
Because of its capacity in inhibiting platelet aggregation, aspirin has been extensively used in the primary and secondary prevention of thromboembolic cardiovascular and cerebrovascular diseases. Its efficacy in preventing myocardial infarction, stroke, and cardiovascular death has been well established. However, recent studies further revealed that in certain cases platelet function was inadequately inhibited, thereby leading to thrombotic events despite therapy with the standard dose of aspirin. This phenomenon was called aspirin resistance (Hanjis et al., 2006; Feher et al., 2011), with an incidence of 9–24% (Cattaneo 2004; Campbell and Steinhubl, 2005). Although the underlying mechanism for aspirin resistance was largely unknown, it was postulated that genetic polymorphism of certain key genes in mediating platelet biofunctions might be an important cause. Platelet glycoprotein IIb/IIIa receptor, a glycoprotein complex playing a key role in platelet functioning, is considered the crucial factor in the final common pathway of platelet activation. The most common polymorphism in the glycoprotein IIIa gene was the substitution of leucine (PLA1) by proline (PLA2); thus, alleles of platelet antigen1 (PLA1) and 2 (PLA2) were determined (Papp et al., 2005). A large body of evidence showed that GP receptor polymorphism was a hereditary risk factor for arterial thrombosis (Meisel et al., 2001; Liu et al., 2006).
China has become an aging society, in which senile citizens older than 80 years of age had made up 11% of the entire elderly population by 2005. Considering the increasing percentage and significance of this specific group in the overall population, our study was designed to detect GP IIIa (Leu33Pro) T/C polymorphism in a very elderly Chinese population and its relation to aspirin resistance, aiming to provide evidence and support for further medical decision making.
Materials and Methods
Patients
A total of 450 very elderly (defined as >80 years of age) Chinese patients treated from June 2009 to December 2011 in the outpatient clinic and hospitalization center of our hospital with long-term aspirin therapy were enrolled in the study. The qualifying long-term aspirin therapy was defined as an aspirin dose of 100 mg daily for >2 months. Hypertension and diabetes mellitus were diagnosed according to 2004 World Health Organization/International Society of Hypertension guideline and 2003 American Diabetes Association criteria, respectively. The average age of enrolled patients (±standard deviation) was 83.78±3.00 years (range, 80–95 years). Exclusion criteria included concomitant antiplatelet therapy other than aspirin or anticoagulation therapy, bleeding, hematologic diseases, and liver or kidney dysfunctions. On the basis of their reactivities to aspirin via platelet aggregation studies, patients were divided into the following groups: aspirin full resistant, aspirin semi-resistant, and aspirin sensitive (AS). The first two groups were also classified as aspirin resistant (AR) (236 patients) and the latter as AS (214 patients).
Demographic data
Demographic factors considered included age, duration of disease, concomitant medical therapies, cardiovascular diseases, risk factors, and other potential factors that might be relevant.
Clinical laboratory tests
Three milliliters of fasting blood and 1 mL of 2-hr postprandial blood were drawn via peripheral vein for each patient. Total cholesterol, triglyceride, liver and kidney function, and fasting serum glucose were tested after 12 hours of fasting. The test was carried out by using an automatic biochemical analyzer (CL-7200; Shimadzu, Kyoko, Japan). Total cholesterol, triglycerides, liver and kidney function, and fasting and 2-hr postprandial serum glucose were detected by enzymatic tests (variance<5%).
Platelet aggregation test
Fasting blood samples were drawn and Chrono-Log platelet aggregometry was used (Chrono-Log, Havertown, PA). Platelet aggregation was detected by turbidimetry using the following procedures: 4 mL of a (2 mL×2) blood sample was transferred into Vacutainer tubes containing 0.3 mL of 3.18% sodium citrate and then centrifuged for 2 min at 500 rpm. Platelet-rich plasma was obtained and then centrifuged for 10 min at 3000 rpm. Platelet counts were between 10×109/L and approximately 20×109/L. Platelet-poor plasma was used to adjust platelet-rich plasma to a level of 200×109/L to approximately 300×109/L. Platelet-poor plasma was used as an internal control and arachidonic acid (AA; 0.5 mmol/L) or adenosine diphosphate (ADP; 10 μmol/L) was added to induce platelet aggregation.
Determination of aspirin resistance
For the AFR group, the mean ADP-induced platelet aggregation was ≥70% and the mean AA-induced platelet aggregation was ≥20%. For the ASR group, either of the above-mentioned criteria were met. For the AS group, mean ADP-induced platelet aggregation was <70% and AA-induced platelet aggregation was <20%.
DNA analysis
Total DNA was extracted from peripheral blood with a genome DNA extract kit; Primer 5.0 software (PREMIER Biosoft, Palo Alto, CA) was used for primer designing. The length of the target segment was 239 base pairs.
Primer sequences are listed as follows:
Primer FGPIIIa-1: 5′ GTCGCCATAGCTCTGATTGC-3′.
Primer RGPIIIa-2: 5′ GAGCCGGAGTGCAATCCTCT-3′.
Primers were synthesized and purchased from Sigma-Aldrich (St. Louis, MO).
The polymerase chain reaction (PCR) system (40 μL) involved use of 4.0 μL of 10×reaction buffer, 3.2 μL of magnesium 2+, 3.8 μL of deoxyribonucleotide triphosphate, 0.5 μL of upstream primer, 0.5 μL of downstream primer, 0.3 μL of Taq enzyme, 28.7 μL of double-distilled water, and 2.0 μL of DNA. The PCR conditions were as follows: 94°C, initial denaturation for 3 min, 94°C denaturation for 15 s, 57°C annealing for 30 s, and 72°C extension for 30 s, after 35 cycles, 72°C final extension for 10 min, and 12° conservation. The restriction fragment-length polymorphism method was used to evaluate target polymorphism. The enzymatic system (20 μL) includes 2 μL of NEB4 buffer, 0.5 μL of NciI enzyme, 7.5 μL of purified PCR products, and 10 μL of double-distilled water. PCR products were incubated in a 37°C water bath for 3 hr. Ten microliters of enzyme-digested product was then analyzed by electrophoresis in 1.5% agarose gel.
Statistical analysis
The data were analyzed with SPSS software, version 11.0 (SPSS, Inc., Chicago, IL). All results were expressed as mean±standard deviation. We used t-tests to compare each pair of measurement data. Frequencies of genotypes and alleles were assessed by χ2 test. A p value less than 0.05 was considered to represent a statistically significant difference.
Results
Clinical characteristics
Of the 214 patients in the AS group, 161 were male with an average age of 83.60±3.11 (range, 80–95) years and 53 were female with an average age of 83.30±2.46 (range, 80–89) years. Of the 236 patients in the AR group, there were 181 men with an average age of 84.09±3.03 (range, 80–93) years and 55 women with an average age of 83.72±2.97 (range, 80–92) years. Table 1 lists and compares demographic and clinical characteristics such as sex, age, blood glucose, blood pressure, body mass index, coronary heart diseases, cerebrovascular diseases, peripheral vascular diseases, and hypertension.
Table 1.
Patients' Characteristics in Aspirin-Sensitive and Aspirin-Resistant Groups
| Project | AS group (n=214) | AR group (n=236) | Statistics | p-Value |
|---|---|---|---|---|
| Men, n (%) | 161/75.23 | 181/76.69 | χ2=0.131 | 0.717 |
| Age (yr) | 83.53±2.96 | 84.00±3.02 | t=0.687 | 0.092 |
| Fasting glucose (mmol/L) | 5.74±1.50 | 5.61±1.24 | t=0.981 | 0.327 |
| 2-hr postprandial glucose (mmol/L) | 8.59±3.16 | 8.24±2.88 | t=1.243 | 0.214 |
| SBP (mmHg) | 127.99±9.46 | 129.45±9.30 | t=1.655 | 0.099 |
| DBP (mmHg) | 73.05±9.40 | 74.44±9.43 | t=1.561 | 0.119 |
| Heart rate (beats/min) | 71.98±13.59 | 74.30±13.62 | t=1.806 | 0.072 |
| BMI (kg/m2) | 23.58±2.13 | 23.46±2.08 | t=0.604 | 0.546 |
| Coronary artery disease, n (%) | 172 (80.37) | 195 (82.63) | χ2=0.379 | 0.538 |
| Cerebral vascular disease, n (%) | 75 (35.05) | 87 (36.86) | χ2=0.161 | 0.688 |
| Peripheral vascular disease, n (%) | 52 (24.30) | 68 (28.81) | χ2=1.170 | 0.279 |
| Hypertension, n (%) | 85 (39.72) | 107 (45.34) | χ2=1.449 | 0.229 |
Values expressed with a plus/minus sign are the mean±standard deviation.
AR, aspirin-resistant; AS, aspirin-sensitive; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index.
Frequency of PLA genotypes
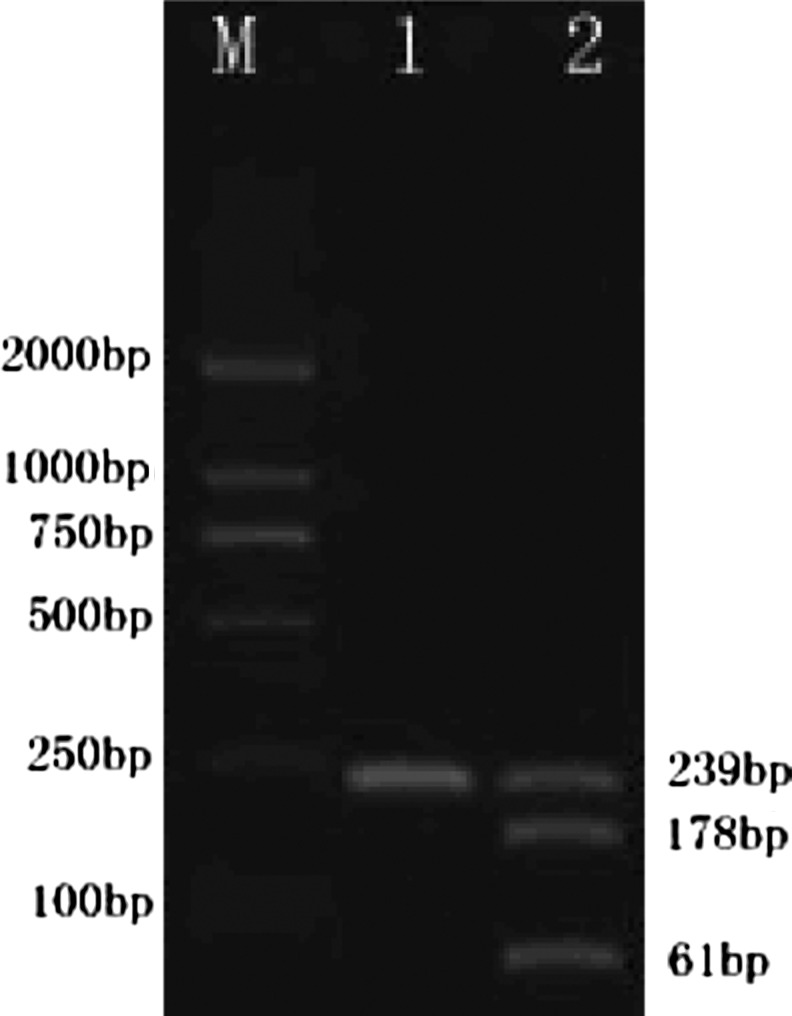
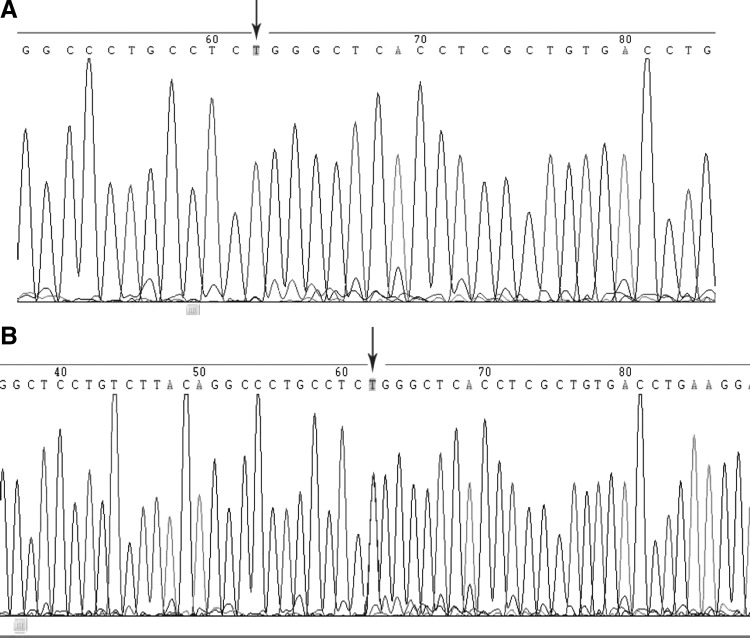
Among the 450 patients total, 438 had the PLA1/A1 homozygous genotype, while 12 had the PLA1/A2 heterozygous genotype (9 men and 3 women). No PLA2/A2 homozygous genotype was found (Table 2). Electrophoresis results of the two genotypes after enzymatic fragmentation are shown in Figure 1. Sequencing of the two genotypes gave the same results as enzymatic fragmentation (Fig. 2). Arrows point to genetic mutation sites. The distribution frequency of the PLA1/A1 genotype was 100% in the AS group and 94.92% in the AR group with a corrected χ2 value of 9.306 and a p value of 0.002, indicating a statistically significant difference between the two groups (p<0.05) (Table 2).
Table 2.
Frequency of PLA Genotypes
| Genotype | AS group (n=214), n (%) | AR group (n=236), n (%) |
|---|---|---|
| A1/A1 | 214 (100) | 224 (94.92) |
| A1/A2 | 0 (0) | 12 (5.08) |
| A2/A2 | 0 (0) | 0 (0) |
FIG. 1.
Genotypes after enzymatic fragmentation. PLA1/A1 (TT): target segment length after enzymatic fragmentation: 239 base pairs; PLA1/A2 (TC): target segment length after enzymatic fragmentation: 239+178+61 base pairs.
FIG. 2.
Sequencing results: (A) PLA1/A1 (TT) sequencing. (B) PLA1/A2 (TC) sequencing.
Discussion
The current definition of AR includes both laboratory resistance and clinical resistance to aspirin. Because different laboratory methods could give out different results, the platelet aggregation rate induced by AA and ADP is the most widely accepted approach to assessing laboratory aspirin resistance. Clinical resistance derived from the clinical observation of long-term prognosis in patients with ischemic vascular diseases, referring to those receiving long-term aspirin therapy but still experiencing thrombotic diseases. AR was commonly observed in both healthy population and patients with cerebrovascular, peripheral vascular, and coronary heart diseases (Cattaneo 2004). Studies had reported an incidence rate of clinical resistance around 8–45% and a still higher rate in elderly patients, women, patients who take other nonsteroidal anti-inflammatory drugs simultaneously, and smokers in the general population. Epidemiologic data showed that the incidence of laboratory aspirin resistance ranges from 5.5% to 61% (Campbell and Steinhubl, 2005; Sanderson et al., 2005). In this study conducted among a very elderly Chinese population, 236 of 450 patients had aspiring resistance, amounting to 52.44% of the total study population. The incidence of aspirin resistance in our study was higher than that of foreign results, suggesting that age itself might be one risk factor for aspirin resistance.
Aspirin resistance is now believed to be associated with smoking, concurrent medication, dose of aspirin, cyclooxyenase-2, gene polymorphism, and platelet function (Goodman et al., 2007; Acikel et al., 2008). Our study focused on the relation between gene polymorphism and aspirin resistance. Gene polymorphism contributed to the different phenotypes. Platelet glycoprotein IIb/IIIa complex, a fibrinogen receptor–mediated platelet aggregation, was considered a key factor involved in the final common pathway for platelet activation. There existed a subgroup of PLA polymorphisms in the platelet glycoprotein IIIa gene. Two alleles had been discovered in platelet glycoprotein IIIa subgroup: PLA1 and PLA2. The most common polymorphism is the substitution of leucine (PLA1) by proline (PLA2), generating two types of platelet antigens, platelet antigen 1 (PLA1) and platelet antigen 2 (PLA2). PLA2/A2 homozygotes were associated with platelet activation and re-stenosis process following stent implantation (Kastrati et al., 2000; Petrovic and Peterlin, 2005). Other studies showed that PLA2 gene carriers were more prone to aspirin resistance (Berent and Sinzinger, 2011; Szczeklik 2011). The single-nucleotide polymorphism might facilitate or be associated with aspirin resistance. Others argued that platelet GP IIIa gene polymorphism was not compatible with changes in platelet function and subsequently denied a relationship between GP IIIa polymorphism and AR.
In this study, all patients in the AS group were PLA1 genotypic homozygotes, whereas 224 in the AR group were PLA1 homozygotes and 12 were PLA1/A2 heterozygotes, including 3 women and 9 men. No PLA2 homozygotes were found in either group. The distribution frequency of PLA2 genotype was 5.08% in the AR group and 2.67% in the whole population. This distribution pattern is significantly different from those in whites but similar to that in Koreans (Lee et al., 2008). Genotype frequency significantly differed between the AR and AS populations; thus, an association between PLA1/A2 heterozygotes and aspirin resistance could be established. In terms of nationalities, PLA1/A2 heterozygosis was associated with incidence of AR in the very elderly Chinese Han population. On the contrary, PLA2 frequency in the Chinese ethnic group was very low, suggesting that PLA genotype frequency varied significantly among different nationalities within the Chinese population. In middle Europe, PLA1/A2 allele was seen in 20–30% of the whole population and 1–3% expressed the PLA2/A2 allele. This was not seen in an Asian population (Chen et al., 2004). Among Koreans, frequency of PLA1 and PLA2 was 100% and 1%, respectively. Thus, among 100 Koreans, only 1 individual was a PLA1/PLA2 heterozygote and none were PLA2 homozygotes. Among black, Japanese, and Indian American populations, systematic expression of PLA antigen was more prone to be in the form of PLA1 phenotype.
Scarce studies are available on the GP IIIa gene in China, which is very rare among very elderly patients. Our study provided data on PLA gene polymorphism and confirms the difference in GP IIIa polymorphism distribution frequencies among different populations. Further studies on the relation between aspirin resistance and GP IIIa gene polymorphism with larger sample sizes and different ages and populations are still required. Our conclusion must be confirmed by future studies. Admittedly, additional studies may also reveal different insights.
Author Disclosure Statement
No competing financial interests exist.
References
- Acikel S, Yildirir A, Aydinalp A, et al. (2008) Incidence of aspirin resistance and its relationship with cardiovascular risk factors and graft function in renal transplant recipients. Transplant Proc 40:3485–3488 [DOI] [PubMed] [Google Scholar]
- Berent R, Sinzinger H. (2011. ) Aspirin—resistance? A few critical considerations on definition, terminology, diagnosis, clinical value, natural course of atherosclerotic disease, and therapeutic consequences.Vasa 40:429–438 [DOI] [PubMed] [Google Scholar]
- Campbell CL, Steinhubl SR. (2005. ) Variability in response to aspirin: do we understand the clinical relevance? J Thromb Haemost 3:665–669 [DOI] [PubMed] [Google Scholar]
- Cattaneo M. (2004) Aspirin and clopidogrel. Efficacy, safety and the issue of drug resistance. Arterioscler Thromb Vasc Biol 24:1980–1987 [DOI] [PubMed] [Google Scholar]
- Chen CH, Lo YK, Ke D, et al. (2004) Platelet glycoprotein Ia C807T, I b C355OT, and IIIa P1 polymorphisms and ischemic stroke in young Taiwanese. J Neurol Sci 227:1–5 [DOI] [PubMed] [Google Scholar]
- Feher A, Pusch G, Harang G, et al. (2011) Aspirin resistance in cerebrovascular patients. Int J Cardiol 152:111–112 [DOI] [PubMed] [Google Scholar]
- Goodman T, Sharma P, Ferro A. (2007) The genetics of aspirin resistance. Int J Clin Pract 61:826–834 [DOI] [PubMed] [Google Scholar]
- Hanjis C, Frishman WH, Lerner RG, et al. (2006) Aspirin resistance: mechanisms and clinical implications. Cardiol Rev 14:18–25 [DOI] [PubMed] [Google Scholar]
- Kastrati A, Koch W, Gawaz M, et al. (2000) PLA polymorphism of glycoprotein IIIa and risk of adverse events after coronary stent placement. J Am Coll Cardiol 36:84–89 [DOI] [PubMed] [Google Scholar]
- Lee YK, Kim HS, Park JY, et al. (2008) Incidence of aspirin resistance in the patient group of a university hospital in Korea. Korean J Lab Med 28:251–257 [DOI] [PubMed] [Google Scholar]
- Liu CZ, Wang YC, Wang JH, et al. (2006) Differential expression of platelet glycoprotein Ia/IIa in Taiwan Chinese corresponds to glycoprotein ia gene polymorphisms. J Pharmacol Sci 101:103–106 [DOI] [PubMed] [Google Scholar]
- Meisel C, Afshar-Kharghan V, Cascorbi I, et al. (2001) Role of Kozak sequence polymorphism of platelet glycoprotein Ibalpha as a risk factor for coronary artery disease and catheter interventions. J Am Coll Cardiol 38:1023–1027 [DOI] [PubMed] [Google Scholar]
- Papp E, Havasi V, Bene J, et al. (2005) Glycoprotein IIIA gene (PlA) polymorphism and aspirin resistance: is there any correlation? Ann Pharmacother 39:1013–1018 [DOI] [PubMed] [Google Scholar]
- Petrovic D, Peterlin B. (2005) Genetic markers of restenosis after coronary angioplasty and after stent implantation. Med Sci Monit 11:127–135 [PubMed] [Google Scholar]
- Sanderson S, Emery J, Baglin T, Kinmonth AL. (2005) Narrative review: aspirin resistance and its clinical implications. Ann Intern Med 142:370–380 [DOI] [PubMed] [Google Scholar]
- Szczeklik A. (2011) Reasons for aspirin resistance. Thromb Haemost 105:1124. [DOI] [PubMed] [Google Scholar]