Abstract
Modern urologic endoscopy is the result of continuous innovations since early 19th century. White light cystoscopy is the primary strategy for identification, resection, and local staging of bladder cancer. While highly effective, white light cystoscopy has several well-recognized shortcomings. Recent advances in optical imaging technologies and device miniaturization hold the potential to improve bladder cancer diagnosis and resection. Photodynamic diagnosis and narrow band imaging are the first to enter the clinical arena. Confocal laser endomicroscopy, optical coherence tomography, Raman spectroscopy, UV autofluorescence, and others have shown promising clinical and pre-clinical feasibility. We review their mechanisms of action, highlight their respective advantages, and propose future directions.
Keywords: optical imaging, fluorescence imaging, in vivo microscopy, multimodal imaging, cystoscopy, bladder cancer
Introduction
Landmark innovations over the last two centuries have shaped modern urologic endoscopy and particularly endoscopic management of bladder tumors.[1•] Bozzini first conceptualized endoscopy with his lichleiter (‘light conductor’) to illuminate various accessible body cavities including the bladder.[2] Subsequent innovations include the first endoscopic surgery (i.e. extraction of a urethral papilloma) by Desormeaux in 1853, invention of the cystoscope by Nitze in 1894, and the development and refinement of the resectoscope by Stern and McCarthy in the early 1930’s.[3] The introduction of flexible fiberoptic cystoscopes in the 1980’s and more recently the digital videoscopes further decreased the morbidity associated with diagnostic cystoscopy.
Today, white light cystoscopy (WLC) is the cornerstone for office-based hematuria work-up, bladder cancer detection and surveillance, as well as detection of numerous other benign lower urinary tract disorders. Transurethral resection (TUR) under WLC is the standard for excisional biopsy and local staging of bladder cancer. While widely used, WLC has several well-recognized shortcomings. First, nonpapillary bladder cancer such as carcinoma in situ (CIS) may be difficult to visualize or differentiate from inflammation.[4] Second, smaller or satellite tumors may be missed. Third, bladder cancer may be incompletely resected therefore understaged.[5] Lastly, cancer grading and staging requires pathological analysis, which has a significant time delay. Suboptimal endoscopic management of early stage bladder cancer increases the risk of cancer persistence, recurrence, and progression.[6]
To address the shortcomings of WLC, new optical imaging technologies to improve detection and characterization of suspected bladder tumors are being developed. Table 1 summarizes the characteristics and properties of the technologies reviewed. PDD, NBI, CLE and OCT have been recently reviewed elsewhere [7] and their recent progress highlighted here. Several promising new technologies at the initial clinical feasibility (e.g. Raman spectroscopy, UV autofluorescence) or pre-clinical stage (e.g. scanning fiber endoscopy) will be introduced. Taken together, these technologies offer an exciting glimpse into future possibilities to improve optical diagnosis and endoscopic management of bladder cancer.
Table 1.
Characteristics and Properties of Emerging Imaging Modalities
| Name | Mechanism | Contrast Agent | Resolution | Depth | Status | Scope or Probe (diameter) | Image |
|---|---|---|---|---|---|---|---|
| PDD | Fluorescence | HAL | mm-cm | Surface | Clinical | Rigid scope within standard sheath |

|
| NBI | Absorption | None | mm-cm | Surface | Clinical | Flexible video- cystoscope (5.5 mm) or standard rigid cystoscope |

|
| CLE | Fluorescence | Fluorescein | 1–3.5 μm | 120 μm | Investigational (in vivo) | Probe (0.85–2.6 mm) |

|
| OCT | Scattering | None | 10–20 μm | ~2.5 mm | Clinical/ Investigational (in vivo) | Probe (2.7 mm) |

|
| Raman | Scattering | Optional (SERS) | - | 2 mm | Investigational (in vivo) | Probe (2.1 mm) |

|
| UV | Fluorescence | None | mm-cm | Surface | Investigational (in vivo) | Probe (3 mm) |

|
| SFE | Reflectance + Fluorescence | None | mm-cm | Surface | Investigational (ex vivo) | Scope/Probe (1.2 mm) |

|
| MPM | Fluorescence | None | *1–6 μm | *0.25-0.5 mm | Investigational (ex vivo) | Probe (3 mm) |

|
Spatial resolution and imaging depth were calculated from a custom built upright multiphoton microscope.
OCT and UV images reproduced with permission from Elsevier. Raman image reproduced with permission from American Chemical Society. SFE image courtesy of Eric Seibel, University of Washington. MPM image reproduced with permission from National Academy of Sciences.
Photodynamic diagnosis (PDD)
Of the new imaging technologies, PDD has the most extensive clinical track record.[8] PDD is based on selective accumulation of heme-precursors, 5-aminolevulinic acid (5-ALA) or its ester derivative hexaminolevulinate (HAL), by cancer cells when introduced intravesically. Under blue fluorescence, bladder cancer appears red in contrast to the surrounding benign urothelium (Figure 1A).[9] PDD requires a specialized rigid cystoscope, light source and camera head, which can be used in conjunction with different size sheaths. Multi-institutional randomized clinical studies have demonstrated that PDD improves detection of both papillary tumors and CIS compared to white light [10, 11] and patients treated with PDD-guided transurethral resection of bladder tumor (TURBT) had longer recurrence-free intervals.[11, 12] Limitations of PDD include false positive fluorescence [10] from inflammatory lesions or previous biopsy sites. The contrast agent, HAL, is currently approved for single use and not for patients who received intravesical immunotherapy or chemotherapy within 90 days. More studies are needed to better define the optimal indications for PDD use and strategies to improve PDD’s diagnostic specificity.
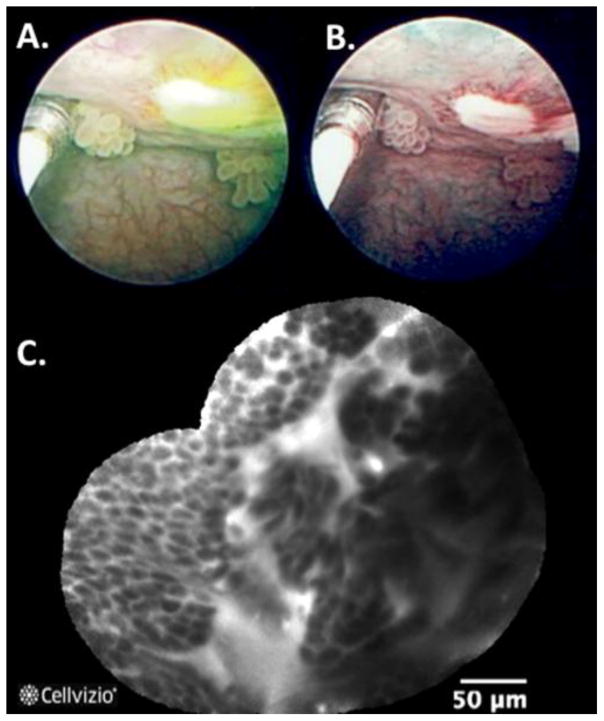
Figure 1. New bladder imaging technologies with corresponding white light cystoscopy (WLC).
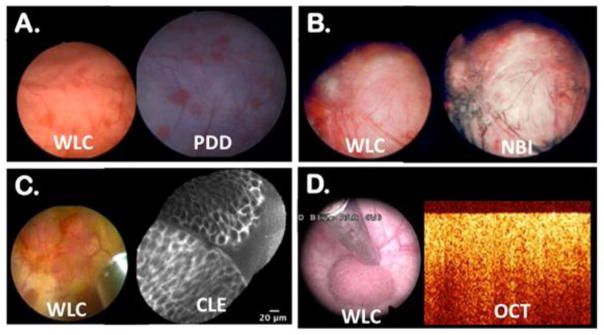
(A) Bladder tumors appeared pink under blue fluorescence of photodynamic diagnosis (PDD); (B) Narrow band imaging (NBI) enhances visualization of aberrant tumor vasculature; (C) Confocal laser endomicroscopy (CLE) enables in vivo microscopy of a papillary urothelial carcinoma; (D) Subsurface imaging of a papillary urothelial cancer using optical coherence tomography (OCT) showing loss of normal urothelial layers. Image reproduced with permission from Elsevier.
Narrow Band Imaging (NBI)
Similar to PDD, NBI is an endoscope-based imaging modality that the user can switch dynamically between NBI and white light during cystoscopy. NBI improves visualization of bladder neoplasia through enhanced visualization of mucosal and submucosal vasculature without the need for exogenous contrast agent. White light is filtered into blue (415 nm) and green (540 nm), which are wavelengths that hemoglobin absorbs (Figure 1B). NBI is approved for clinical use, either in an integrated videocystoscope or through a camera head that can be attached to standard white light cystoscopes. Both flat and papillary tumors are more easily visualized with NBI compared to WLC due to the increased and aberrant vascularity associated with bladder cancer. Single center studies have found a detection rate of 94.7% vs. 79.2% for WLC.[13] Studies have shown the feasibility of NBI-assisted TUR[14] with decreased 1 year recurrence in comparison to standard TUR.[15] The results from an ongoing multi-center international study comparing NBI- and WLC-assisted TUR are currently pending.[16]
Confocal Laser Endomicroscopy (CLE)
Based on the well-established principle of fluorescence confocal microscopy, CLE is an optical biopsy technology that enables in vivo high resolution, subsurface imaging.[17–19] CLE is currently approved for gastrointestinal and pulmonary endoscopic applications and has been under investigational use in the urinary tract since 2009. Fluorescein, an FDA-approved drug with an established clinical safety profile, is required as the exogenous contrast agent.[20] Reusable miniaturized imaging probes ranging from 0.85-mm to 2.6-mm diameter are compatible with working channels of standard cystoscopes.[21] Real time microscopy of normal urothelium, inflammation, CIS, low-grade and high-grade urothelial carcinoma have been demonstrated with images comparable to conventional histopathology (Figure 1C).[18•]. A recent study demonstrated moderate interobserver agreement in image interpretation between novice and experienced CLE urologists with respect to cancer diagnosis.[19] Multi-center studies examining the CLE diagnostic accuracy for real time cancer diagnosis and grading remained to be completed. An advantage of CLE is that it may be coupled with fluorescently labeled peptides or antibodies against cancer-specific surface antigens, as it has been demonstrated in the gastrointestinal tract.[22•, 23] Similar endoscopic molecular imaging strategies may be deployed in the bladder to improve cancer diagnosis with molecular specificity.
Optical coherence tomography (OCT)
OCT is another optical biopsy technology that enables high-resolution, subsurface tissue interrogation. OCT is analogous to ultrasound in that cross-sectional images are generated (Figure 1D) using near-infrared light instead of sound waves as the source. Image resolutions of 10–20 μm and depths of penetration of 2 mm can be achieved, thus enabling the possibility of real-time cancer diagnosis and staging (i.e. differentiation between non-muscle and muscle invasive cancer).[24–26] Similar to CLE, there is a learning curve associated with image interpretation, which is based on loss of delineation between tissue layers (e.g. lamina propria). Other recent studies have reported an automated image processing algorithm to detect bladder cancer from OCT images [27] and application in the upper tract.[28]
Multimodal imaging
Given the different imaging characteristics, a combination of macroscopic (e.g. PDD and NBI) and microscopic (e.g. CLE and OCT) imaging modalities used in concert may improve diagnostic accuracy.[7] For example, PDD and NBI could be used to identify suspicious lesions while CLE and OCT provide high-resolution characterization to provide grading or staging information. Feasibility of combining endoscope-based PDD and NBI with probe-based CLE has been reported (Figure 2).[29, 30] A recent study showed that PDD combined with OCT had increased the specificity compared to PDD alone.[31] In another study, PDD in combination with standard OCT did not improve the diagnostic accuracy significantly in detecting non-invasive bladder cancer, but the use of cross-polarization OCT and PDD decreased overall false positive and negative cases.[32]
Figure 2. Multimodal imaging using narrow band imaging and confocal laser endomicroscopy.
(A) Endoscopic view of the confocal imaging probe adjacent to a papillary tumor at the bladder dome. Yellow tinge corresponds with intravesical fluorescein as contrast agent for CLE. (B) Narrow band imaging highlights tissue and tumor vasculature. (C) Concomitant endomicroscopy image shows well-organized papillary border and monomorphic urothelial cells consistent with a low grade papillary urothelial carcinoma.
Raman spectroscopy (RS)
RS is based on the principle of inelastic scattering of photons following interaction with intramolecular bonds. Near infrared light (785–845 nm) is used to illuminate biological tissues and alter the vibrational state of molecules. The photons donate energy to molecular bonds and as a result exit the tissue at a different wavelength from the incident light. The change in energy level of the photons is known as a Raman shift.[33] Detection of multiple Raman peaks from the target tissue is plotted to create a spectrum of peaks, achieving a molecular “fingerprint” of the tissue examined without the need for exogenous contrast agent.[34] In ex vivo settings, RS has been shown to differentiate the normal bladder wall layers, identify low- and high-grade bladder cancer, and assess invasiveness.[35, 36] To evaluate the feasibility of in vivo bladder cancer diagnosis, a prototype fiberoptic Raman probe compatible with the working channels of endoscopes has been developed.[34•, 37, 38] In 38 patients suspected of bladder cancer, differentiation of cancer from normal urothelium was feasible in near real-time during cystoscopy.[34•] Recent research led to development of a non-contact Raman probe that detects signals even with imperfect centering of the probe and nonuniform topology of human tissue surface.[39] Recognized limitations of RS include time to obtain a spectrum (1–5 seconds), weak signals and limited field of view. Surface-enhanced Raman scattering (SERS) nanoparticles have been demonstrated to augment the relatively weak signals from Raman scattering. SERS substrates can be conjugated to cancer-specific antibodies to enable multiplexed targeted imaging during endoscopy.[40]
Ultraviolet (UV) autofluorescence
The basis of UV-induced autofluorescence is to differentiate normal, inflammatory and cancerous urothelium by differences in their intrinsic molecular contents. UV light is used to excite endogenous fluorophores (e.g. NAD and tryptophan) present in the tissue of interest. A pilot clinical study composed of 14 patients was recently published.[41•] A UV light-emitting probe (360 and 450 nm) was inserted via the working channel of a standard cystoscope and advanced in close proximity to the region of interest for tissue interrogation. For image processing, the signals were converted into an intensity ratio between the two wavelengths and color coded to facilitate real time interpretation.[41•] In the pilot study, real time differentiation of bladder cancer from benign/normal urothelium based on autofluorescence spectrum was demonstrated (Figure 3). Further studies are needed to investigate signal intensity across different tumor types (including flat lesions), reproducibility, and potential for UV-induced toxicity.
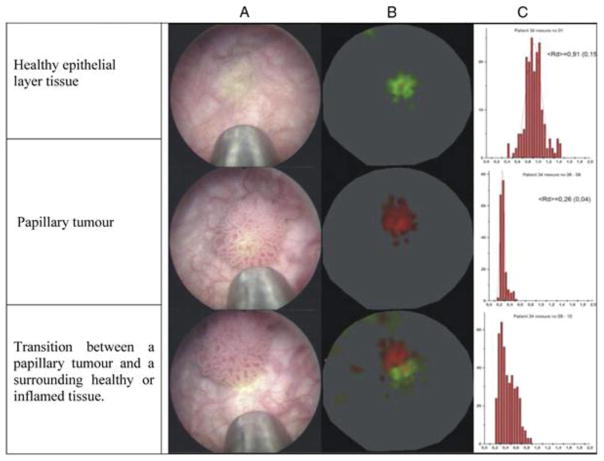
Figure 3. Tumor autofluorescence with an ultraviolet (UV) imaging probe.
(A) Cystoscopic view of the imaging probe adjacent to normal urothelium and tumor; (B) Color-coding is integrated in real-time and facilitates interpretation of autofluorescence measurements and differentiation of normal (green) and malignant (red) tissue; (C) Histogram of the autofluorescent measurements showing the calculated mean diagnostic ratios of healthy tissue (0.91) and the papillary tumor (0.26). Image reproduced with permission from Elsevier.
Multiphoton microscopy (MPM)
In MPM, fluorescence is achieved by nonlinear excitation of molecules after the simultaneous absorption of two or more photons of lesser energy.[42] MPM takes advantages of intrinsic tissue fluorophores (i.e. NADH, FAD and collagen) instead of exogenous contrast agents. In addition to the autofluorescence signals, MPM is also able to visualize non-centrosymmetric structures (e.g. collagen) via second harmonic generation (SHG). The exciting light is produced from a femtosecond pulsed Ti-Sapphire laser tuned to 780 nm while the detected autofluorescence and SHG signals are in the range of 420–530 nm and 355–420 nm respectively.[43] Interpretation of acquired images is simplified by color-coding the detected SHG and autofluorescence signals. Recent in vitro studies using MPM demonstrated high-resolution images from fresh TUR bladder tissues comparable to histopathology and differentiation of malignant and benign features. Similar to CLE, limitations of MPM include limited depth of penetration not suitable for cancer staging and lack of nuclear morphology on the images. Current research is focused on miniaturizing the MPM system into a compact probe suitable for in vivo use.[44–46]
Scanning Fiber Endoscopy (SFE)
SFE uses an ultrathin (1.2 mm tip) flexible endoscope that provides wide angle, full color, high-resolution images.[47] The tip contains a single-mode optical fiber that spirally scans red, green and blue laser light that is focused onto tissue. The backscattered light is collected by multiple optical fibers and an image is generated pixel by pixel.[48] The small diameter decreases the invasiveness and expands the versatility of how SFE may be deployed either as a standalone miniaturized endoscope or as a probe in conjunction with other imaging modalities.[49] Currently, SFE has been demonstrated in the gastrointestinal tract and in ex vivo bladder models.[50, 51•] A provocative application of SFE involves integrating an automated bladder-scanning device and an ‘image-stitching’ algorithm to generate a 2-D panoramic view of the mucosa. Figure 4 shows examples of such image mosaicing in a phantom model and ex vivo pig bladder.[50, 51•] With integration of automated scanning, SFE cystoscopy may be performed in the future by skilled technologists with the urologist reviewing the images in real time or offline. The panoramic view may also provide a systematic way for tumor mapping and survey the bladder longitudinally.
Figure 4. Scanning fiber endoscopy (SFE) of a bladder phantom and excised pig bladder to generate surface mosaics of the urothelium.
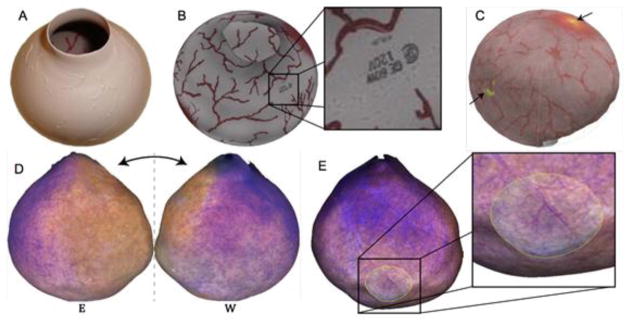
(A) A bladder phantom with painted on vessels was imaged using a conventional endoscope from which a (B) 3D mosaic was constructed. (C) A multi-modal mosaic was generated by SFE imaging of the same phantom with green fluorescent microspheres to mimic hotspots (arrows). Using the SFE, a mosaic (D/E) was constructed from roughly a thousand frames taken within an excised pig bladder. Image courtesy of Eric Seibel, University of Washington.
Telerobotic cystoscopy—cystoscope of the future?
As new imaging technologies enter into clinical use, new methods to integrate different imaging modalities with endoscopic surgical interventions may be needed. A telerobotic cystoscopy system has recently been developed and tested in ex vivo animal models (Figure 5).[52•] This system contains multiple instrument channels and offers the potential to integrate optical imaging, catheter-based ablative technologies, and new generation of micro-surgical tools with additional degrees of freedom. Such ‘smart’ endoscopic instruments may expand the spectrum of multi-modal endoscopic interventions that can be performed in the urinary tract.
Figure 5. A prototype telerobotic system for the bladder.
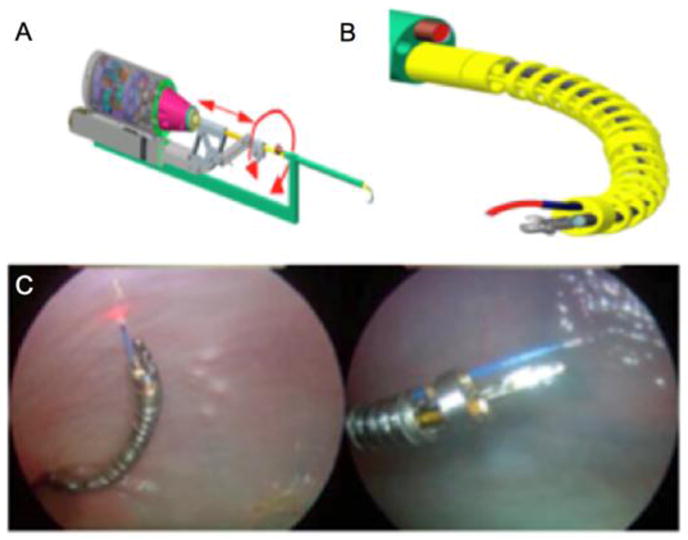
(A) Schematic of the dexterous bladder telerobotic system. The central rigid stem contains an optical scope and irrigation apparatus. (B) The dexterous end effector has multiple channels including its own optics and channels for laser and other tools such as tissue optical interrogation catheters. (C) The dexterous instrumentation manipulated to various areas of an ex vivo bovine bladder. Courtesy of Nabil Simaan and Duke Herrell, Vanderbilt University.
Conclusions
Modern endoscopy and endoscopic surgery of the urinary tract is the culmination of ingenuity and persistence by many innovators in urology, surgery, and engineering over the last two centuries. These innovations introduced the concept of minimally invasive surgery and revolutionized the management of bladder cancer. Recent advances in optical imaging and instrument miniaturization are poised to usher in an era of image-guided surgery, that holds the cancer recurrence and progression, and cost effective utilization of available health care resources.[53]
Acknowledgments
The authors thank Kathleen Mach for critical review of the manuscript. A.L. is supported by the Stanford University School of Medicine MedScholars Research Fellowship. J.C.L. is supported in part by R01 CA160986 from the National Cancer Institute.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Dr. Aristeo Lopez declares no potential conflicts of interest relevant to this article.
Dr. Joseph C. Liao received a research grant from NIH. Dr. Liao received travel support from Mauna Kea Technologies including expenses covered or reimbursed, and payment for the development of educational presentations from Storz.
References
• Of importance
- 1•.Herr H. Early History of Endoscopic Treatment of Bladder Tumors From Grunfeld’s Polypenkneipe to the Stern-McCarthy Resectoscope. J Endourol. 2006;20(2):85–91. doi: 10.1089/end.2006.20.85. This article highlights the most noteworthy innovations that occurred in urologic endoscopy providing an overview of the history of endoscopy and the endoscopic management of bladder tumors. [DOI] [PubMed] [Google Scholar]
- 2.Natalin Ra, Landman J. Where next for the endoscope? Nat Rev Urol. 2009 Nov;6(11):622–8. doi: 10.1038/nrurol.2009.199. [DOI] [PubMed] [Google Scholar]
- 3.Samplaski MK, Jones JS. Two centuries of cystoscopy: the development of imaging, instrumentation and synergistic technologies. BJU Int. 2009 Jan;103(2):154–8. doi: 10.1111/j.1464-410X.2008.08244.x. [DOI] [PubMed] [Google Scholar]
- 4.Witjes Ja. Bladder Carcinoma in Situ in 2003: State of the Art. Eur Urol. 2004 Feb;45(2):142–146. doi: 10.1016/j.eururo.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Neumann RM, Weaver aL, Cheville JC, Leibovich BC, Ramnani DM, Scherer BG, Nehra a, Zincke H, Bostwick DG. Grading and staging of bladder carcinoma in transurethral resection specimens. Correlation with 105 matched cystectomy specimens. Am J Clin Pathol. 2000 Feb;113(2):275–9. doi: 10.1309/94B6-8VFB-MN9J-1NF5. [DOI] [PubMed] [Google Scholar]
- 6.Babjuk M. Transurethral Resection of Non–muscle-invasive Bladder Cancer. Eur Urol Suppl. 2009 Sep;8(7):542–548. [Google Scholar]
- 7.Liu JJ, Droller MJ, Liao JC. New optical imaging technologies for bladder cancer: considerations and perspectives. J Urol. 2012 Aug;188(2):361–8. doi: 10.1016/j.juro.2012.03.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kausch I, Sommerauer M, Montorsi F, Stenzl A, Jacqmin D, Jichlinski P, Jocham D, Ziegler A, Vonthein R. Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol. 2010 Apr;57(4):595–606. doi: 10.1016/j.eururo.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Mark JR, Gelpi-Hammerschmidt F, Trabulsi EJ, Gomella LG. Blue light cystoscopy for detection and treatment of non-muscle invasive bladder cancer. Can J Urol. 2012 Apr;19(2):6227–31. [PubMed] [Google Scholar]
- 10.Lapini A, Minervini A, Masala A, Schips L, Pycha A, Cindolo L, Giannella R, Martini T, Vittori G, Zani D, Bellomo F, Cosciani Cunico S. A comparison of hexaminolevulinate (Hexvix(®)) fluorescence cystoscopy and white-light cystoscopy for detection of bladder cancer: results of the HeRo observational study. Surg Endosc. 2012 Dec;26(12):3634–41. doi: 10.1007/s00464-012-2387-0. [DOI] [PubMed] [Google Scholar]
- 11.Rink M, Babjuk M, Catto JWF, Jichlinski P, Shariat SF, Stenzl A, Stepp H, Zaak D, Witjes JA. Hexyl Aminolevulinate-Guided Fluorescence Cystoscopy in the Diagnosis and Follow-up of Patients with Non-Muscle-invasive Bladder Cancer: A Critical Review of the Current Literature. Eur Urol. 2013 Oct;64(4):624–38. doi: 10.1016/j.eururo.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Grossman HB, Stenzl A, Fradet Y, Mynderse La, Kriegmair M, Witjes JA, Soloway MS, Karl A, Burger M. Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. J Urol. 2012 Jul;188(1):58–62. doi: 10.1016/j.juro.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauberg ECC, Kloen S, Visser M, de la Rosette JJMCH, Babjuk M, Soukup V, Pesl M, Duskova J, de Reijke TM. Narrow band imaging cystoscopy improves the detection of non-muscle-invasive bladder cancer. Urology. 2010 Sep;76(3):658–63. doi: 10.1016/j.urology.2009.11.075. [DOI] [PubMed] [Google Scholar]
- 14.Naselli A, Introini C, Bertolotto F, Spina B, Puppo P. Feasibility of transurethral resection of bladder lesion performed entirely by means of narrow-band imaging. J Endourol Endourol Soc. 2010 Jul;24(7):1131–4. doi: 10.1089/end.2010.0042. [DOI] [PubMed] [Google Scholar]
- 15.Naselli A, Introini C, Timossi L, Spina B, Fontana V, Pezzi R, Germinale F, Bertolotto F, Puppo P. A randomized prospective trial to assess the impact of transurethral resection in narrow band imaging modality on non-muscle-invasive bladder cancer recurrence. Eur Urol. 2012 May;61(5):908–13. doi: 10.1016/j.eururo.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Naito S, van Rees Vellinga S, de la Rosette J. Global randomized narrow band imaging versus white light study in nonmuscle invasive bladder cancer: accession to the first milestone-enrollment of 600 patients. J Endourol Endourol Soc. 2013 Jan;27(1):1–3. doi: 10.1089/end.2012.1556. [DOI] [PubMed] [Google Scholar]
- 17.Sonn Ga, Jones S-NE, Tarin TV, Du CB, Mach KE, Jensen KC, Liao JC. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J Urol. 2009 Oct;182(4):1299–305. doi: 10.1016/j.juro.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 18•.Wu K, Liu J-J, Adams W, Sonn Ga, Mach KE, Pan Y, Beck AH, Jensen KC, Liao JC. Dynamic real-time microscopy of the urinary tract using confocal laser endomicroscopy. Urology. 2011 Jul;78(1):225–31. doi: 10.1016/j.urology.2011.02.057. This article compiled representative confocal images from the urinary tract into an imaging atlas. The atlas was used to develop the diagnostic imaging criteria for benign and neoplastic conditions of the urinary tract to facilitate the use and enable adaptation of CLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang TC, Liu JJ, Hsiao ST, Pan Y, Mach KE, Leppert JT, McKenney JK, Rouse RV, Liao JC. Interobserver agreement of confocal laser endomicroscopy for bladder cancer. J Endourol Endourol Soc. 2013 May;27(5):598–603. doi: 10.1089/end.2012.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace MB, Meining a, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, Löhr M, Coron E, Filoche B, Giovannini M, Moreau J, Schmidt C, Kiesslich R. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010 Mar;31(5):548–52. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- 21.Adams W, Wu K, Liu JJ, Hsiao STT, Jensen KC, Liao JC. Comparison of 2.6- and 1.4-mm imaging probes for confocal laser endomicroscopy of the urinary tract. J Endourol Endourol Soc. 2011 Jun;25(6):917–21. doi: 10.1089/end.2010.0686. [DOI] [PubMed] [Google Scholar]
- 22•.Hsiung PL, Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, Wang TD. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008 Apr;14(4):454–8. doi: 10.1038/nm1692. This study identified and synthesized a peptide, VRPMPLQ, that bound more strongly to dysplastic colonocytes than to normal cells. By conjugating the peptide to fluorescein the fluorescent signals were detected with CLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturm MB, Joshi BP, Lu S, Piraka C, Khondee S, Elmunzer BJ, Kwon RS, Beer DG, Appelman HD, Turgeon DK, Wang TD. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci Transl Med. 2013 May;5(184):184ra61. doi: 10.1126/scitranslmed.3004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermes B, Spöler F, Naami A, Bornemann J, Först M, Grosse J, Jakse G, Knüchel R. Visualization of the basement membrane zone of the bladder by optical coherence tomography: feasibility of noninvasive evaluation of tumor invasion. Urology. 2008 Sep;72(3):677–81. doi: 10.1016/j.urology.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 25.Goh AC, Tresser NJ, Shen SS, Lerner SP. Optical coherence tomography as an adjunct to white light cystoscopy for intravesical real-time imaging and staging of bladder cancer. Urology. 2008 Jul;72(1):133–7. doi: 10.1016/j.urology.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Willmann E. Optical Coherence Tomography for Bladder Cancer-Ready as a Surrogate for Optical Biopsy? -Results of a Prospective Mono-Centre Study. 2010:131–134. doi: 10.1186/2047-783X-15-3-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingley-Papadopoulos Ca, Loew MH, Manyak MJ, Zara JM. Computer recognition of cancer in the urinary bladder using optical coherence tomography and texture analysis. J Biomed Opt. 2013;13(2):024003. doi: 10.1117/1.2904987. [DOI] [PubMed] [Google Scholar]
- 28.Bus MTJ, Muller BG, de Bruin DM, Faber DJ, Kamphuis GM, van Leeuwen TG, de Reijke TM, de la Rosette JJMCH. Volumetric in vivo visualization of upper urinary tract tumors using optical coherence tomography: a pilot study. J Urol. 2013 Dec;190(6):2236–42. doi: 10.1016/j.juro.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Bonnal JL, Rock A, Gagnat A, Papadopoulos S, Filoche B, Mauroy B. Confocal laser endomicroscopy of bladder tumors associated with photodynamic diagnosis: an ex vivo pilot study. Urology. 2012 Nov;80(5):1162e1–5. doi: 10.1016/j.urology.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 30.Chang TC, Liu J-J, Liao JC. Probe-based Confocal Laser Endomicroscopy of the Urinary Tract: The Technique. J Vis Exp. 2013;71:e4409. doi: 10.3791/4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidbauer J, Remzi M, Klatte T, Waldert M, Mauermann J, Susani M, Marberger M. Fluorescence cystoscopy with high-resolution optical coherence tomography imaging as an adjunct reduces false-positive findings in the diagnosis of urothelial carcinoma of the bladder. Eur Urol. 2009 Dec;56(6):914–9. doi: 10.1016/j.eururo.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 32.Gladkova N, Kiseleva E, Streltsova O, Prodanets N, Snopova L, Karabut M, Gubarkova E, Zagaynova E. Combined use of fluorescence cystoscopy and cross-polarization OCT for diagnosis of bladder cancer and correlation with immunohistochemical markers. J Biophotonics. 2013 Sep;6(9):687–98. doi: 10.1002/jbio.201200105. [DOI] [PubMed] [Google Scholar]
- 33.Rao AR, Hanchanale V, Javle P, Karim O, Motiwala H. Spectroscopic view of life and work of the Nobel Laureate Sir C.V. Raman. J Endourol Endourol Soc. 2007 Jan;21(1):8–11. doi: 10.1089/end.2006.9998. [DOI] [PubMed] [Google Scholar]
- 34•.Draga ROP, Grimbergen MCM, Vijverberg PLM, van Swol CFP, Jonges TGN, Kummer JA, Ruud Bosch JLH. In vivo bladder cancer diagnosis by high-volume Raman spectroscopy. Anal Chem. 2010 Jul;82(14):5993–9. doi: 10.1021/ac100448p. This study showed the feasibility of in vivo RS for diagnosis of bladder cancer using a Raman probe. Bladder cancer and normal urothelium had characteristic Raman spectrum that were used to discriminate healthy and cancerous bladder tissue. [DOI] [PubMed] [Google Scholar]
- 35.de Jong BWD, Bakker Schut TC, Wolffenbuttel KP, Nijman JM, Kok DJ, Puppels GJ. Identification of bladder wall layers by Raman spectroscopy. J Urol. 2002 Oct;168(4 Pt 2):1771–8. doi: 10.1097/01.ju.0000030059.28948.c6. [DOI] [PubMed] [Google Scholar]
- 36.Crow P, Uff JS, Farmer JA, Wright MP, Stone N. The use of Raman spectroscopy to identify and characterize transitional cell carcinoma in vitro. BJU Int. 2004 Jun;93(9):1232–1236. doi: 10.1111/j.1464-410X.2004.04852.x. [DOI] [PubMed] [Google Scholar]
- 37.Grimbergen MCM, van Swol CFP, Draga ROP, van Diest P, Verdaasdonk RM, Stone N, Bosch JHLR. Bladder cancer diagnosis during cystoscopy using Raman spectroscopy. Proc SPIE. 2009 Feb;7161:716114–716114–6. [Google Scholar]
- 38.Magee ND, Villaumie JS, Marple ET, Ennis M, Elborn JS, McGarvey JJ. Ex Vivo Diagnosis of Lung Cancer Using a Raman Miniprobe. J Phys Chem B. 2009;113(23):8137–8141. doi: 10.1021/jp900379w. [DOI] [PubMed] [Google Scholar]
- 39.Zavaleta CL, Garai E, Liu JTC, Sensarn S, Mandella MJ, Van de Sompel D, Friedland S, Van Dam J, Contag CH, Gambhir SS. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc Natl Acad Sci U S A. 2013 Jun;110(25):E2288–97. doi: 10.1073/pnas.1211309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vendrell M, Maiti KK, Dhaliwal K, Chang YT. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013 Apr;31(4):249–57. doi: 10.1016/j.tibtech.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 41•.Schäfauer C, Ettori D, Rouprêt M, Phé V, Tualle JM, Tinet E, Avrillier S, Egrot C, Traxer O, Cussenot O. Detection of bladder urothelial carcinoma using in vivo noncontact, ultraviolet excited autofluorescence measurements converted into simple color coded images: a feasibility study. J Urol. 2013 Jul;190(1):271–7. doi: 10.1016/j.juro.2013.01.100. This feasibility study demonstrated that bladder cancer could be detected using ultraviolet laser induced autofluorescence measurements (AM). Converting the AMs into color-coded images, red indicating tumor and green indicating normal tissue, facilitated interpretation. [DOI] [PubMed] [Google Scholar]
- 42.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003 Nov;21(11):1369–77. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 43.Jain M, Robinson BD, Scherr DS, Sterling J, Lee M-M, Wysock J, Rubin Ma, Maxfield FR, Zipfel WR, Webb WW, Mukherjee S. Multiphoton microscopy in the evaluation of human bladder biopsies. Arch Pathol Lab Med. 2012 May;136(5):517–26. doi: 10.5858/arpa.2011-0147-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadav R, Mudalair K, Srivastava A, Rubin MA. Multiphoton microscopy for structure identification in human prostate and periprostatic tissue: implications in prostate cancer surgery. BJU Int. 2012;108(9):1421–1429. doi: 10.1111/j.1464-410X.2011.10169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu C. Multiphoton imaging for deep tissue penetration and clinical endoscopy. Proc SPIE. 2011 Feb;7891:78910H–78910H–4. [Google Scholar]
- 46.Rivera DR, Brown CM, Ouzounov DG, Pavlova I, Kobat D, Webb WW, Xu C. Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue. PNAS. 2011;108(43):17598–17603. doi: 10.1073/pnas.1114746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seibel EJ, Brentnall Ta, Dominitz Ja. New endoscopic and cytologic tools for cancer surveillance in the digestive tract. Gastrointest Endosc Clin N Am. 2009 Apr;19(2):299–307. doi: 10.1016/j.giec.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seibel EJ, Brown CM, Dominitz Ja, Kimmey MB. Scanning single fiber endoscopy: a new platform technology for integrated laser imaging, diagnosis, and future therapies. Gastrointest Endosc Clin N Am. 2008 Jul;18(3):467–78. viii. doi: 10.1016/j.giec.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon WJ, Park S, Reinhall PG, Seibel EJ. Development of an Automated Steering Mechanism for Bladder Urothelium Surveillance. J Med Devices. 2009;3(1):011004. doi: 10.1115/1.3054381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soper TD, Porter MP, Seibel EJ. Surface mosaics of the bladder reconstructed from endoscopic video for automated surveillance. IEEE Trans Biomed Eng. 2012 Jun;59(6):1670–80. doi: 10.1109/TBME.2012.2191783. [DOI] [PubMed] [Google Scholar]
- 51•.Soper TD, Chandler JE, Porter MP, Seibel EJ. Constructing spherical panoramas of a bladder phantom from endoscopic video using bundle adjustment. 2011 Mar 12;7964:796417–796417. This study shows that stitching software is able to create a complete 360° panoramic image of a bladder phantom from video frames obtained with an SFE endoscope. [Google Scholar]
- 52•.Goldman RE, Bajo A, MacLachlan LS, Pickens R, Herrell SD, Simaan N. Design and performance evaluation of a minimally invasive telerobotic platform for transurethral surveillance and intervention. IEEE Trans Biomed Eng. 2013 Apr;60(4):918–25. doi: 10.1109/TBME.2012.2226031. This study demonstrates the design and evaluation of a prototype telerobotic system that provides a dexterous manipulator with access channels for the deployment of multiple tools and is compatible with current resectoscope sheaths. [DOI] [PubMed] [Google Scholar]
- 53.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. PharmacoEconomics. 2003 Jan;21(18):1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]