Abstract
Purpose: This study evaluated the safety and efficacy of half-dose verteporfin combined with half-fluence photodynamic therapy (half-half photodynamic therapy (PDT) for chronic central serous chorioretinopathy (CSC).
Methods: This was a retrospective case series. Fourteen eyes with chronic CSC receiving half-half PDT were included in group 1. Another 28 eyes receiving half-dose verteporfin combined with standard fluence PDT were included in group 2 as a control group. Main outcome measures were the success rates, major complications, best-corrected visual acuity (BCVA), and central subfield foveal thickness (CFT) on optical coherence tomography (OCT) at 6 months in both groups. Success of treatment was defined as complete resolution of subretinal fluid on OCT after treatment without recurrence.
Results: There was no significant difference between groups in their age, gender, duration of symptoms, baseline BCVA, baseline CFT, PDT spot size, and follow-up duration. The success rate was 64% (9/14 eyes) in group 1 and 93% (26/28 eyes) in group 2 at 6 months (P=0.031). No major complications were found in either group. Mean CFT showed significant reduction at 6 months in both groups (-115 μm and P<0.001 in group 1; −150 μm and P<0.001 in group 2). The mean BCVA in group 2 improved significantly (P<0.001) at 6 months. The mean BCVA in group 1 showed a trend of improvement but was not statistically significant (P=0.25) at 6 months.
Conclusions: Half-half PDT is a feasible treatment for chronic CSC. However, there was a lower success rate at 6 months compared with the control group.
Introduction
Chronic central serous chorioretinopathy (CSC) is characterized by persistent accumulation of subretinal fluid (SRF) and the long-standing detachment of neurosensory retina,1,2 both of them possibly causing irreversible visual impairment due to damage of retinal pigment epithelium (RPE) and photoreceptors.1,3,4 Fundus changes may include diffuse RPE decompensation, subretinal precipitates, descending atrophic tracts, cystoid macular degeneration, secondary choroidal neovascularization (CNV), fibrous scarring, and RPE atrophy.3,5–10
Although standard photodynamic therapy (PDT) had been reported to be effective for resolution of SRF in chronic CSC,1,11–13 side-effects of PDT such as RPE atrophy, secondary CNV, choriocapillaris ischemia, and transient reduction of macular function have also been reported.1,11,14–21 In order to reduce the damage to RPE and normal choroidal vasculature, several methods, such as reducing the dosage of verteporfin or laser fluence, have been attempted.1,2 Half-dose verteporfin22–25 or low-fluence PDT have yielded good results, comparable to those achieved by conventional PDT with reduced adverse effects on RPE and retaining normal choroidal perfusion.26–28 However, the optimal PDT protocol for effective management of chronic CSC with minimal damage to RPE functions remains unclear. As far as we know, reducing the dosage of verteporfin and laser fluence at the same time for chronic CSC has never been reported. The purpose of this study is thus to evaluate the safety and efficacy of half-dose verteporfin combined with half-fluence PDT (half-half PDT) in the treatment of chronic CSC. We also compare its success rate with half-dose verteporfin combined with standard fluence photodynamic therapy (half-dose PDT).
Methods
We retrospectively reviewed medical records and images of patients who received PDT treatment for chronic CSC between December 2007 and January 2012 at Chang Gung Memorial Hospital and Mackay Memorial Hospital. This study was approved by the Institutional Review Boards of both Chang Gung Memorial Hospital and Mackay Memorial Hospital, and followed the tenets of the Declaration of Helsinki. Patients who had received half-half PDT for chronic CSC were included as the study group (group 1). The control group (group 2) consisted of patients who received half-dose PDT. The number of patients in the control group is twice that of the study group, and these patients were matched with the study group in terms of age, gender, and duration of symptoms. Chronic CSC was defined as CSC with persistent visual disturbance, including distortion and metamorphopsia, for more than 6 months as well as the presence of any sign of long-standing SRF accumulation, such as RPE atrophy, descending atrophic tracts, and subretinal precipitates. The exclusion criteria were (1) disease duration of less than 6 months, (2) absence of SRF on the baseline optical coherence tomography (OCT), (3) previous treatment with PDT, (4) previous or concurrent treatment with laser focal photocoagulation or intravitreal injection of antivascular endothelial growth factor drugs such as bevacizumab or ranibizumab, (5) presence of any evidence of CNV or polypoidal choroidal vasculopathy, (6) current treatment with steroid for any other diseases, (7) a follow-up period of less than 6 months after the PDT treatment, and (8) PDT using a nonstandard infusion protocol.
All patients received complete ocular examinations, best-corrected visual acuity (BCVA), OCT [(Stratus OCT; Model 3000 Carl Zeiss Meditec, Inc., Dublin, CA) or (RTVue-100; Optovue Inc., Fremont, CA)], and simultaneous fluorescein angiography (FA) and indocyanine green angiography (ICGA) (Heidelberg Retina Angiography; Heidelberg Engineering, Heidelberg, Germany) at baseline. Informed consent for PDT was obtained after thorough discussion of the potential risks and benefits with the patients.
The PDT procedures are briefly described as follows. All patients in this study received half-dose (3 mg/m2) of verteporfin (Visudyne; Novartis AG, Bülach, Switzerland) by standard infusion protocol.29 The verteporfin was infused for 10 minutes, followed by PDT application 5 minutes later. The laser was 689 nm with a standard light intensity of 600 mW/cm2. In group 1, the irradiation time was shortened to 42 seconds, which was equivalent to the reduction of energy to 25.2 J/cm2. In group 2, patients received a standard irradiation time of 83 seconds. All of the PDT procedures were performed under ICGA guidance. The treatment spot size was the smallest circular area covering choroidal hyperpermeability and dilated vessels on ICGA, which was related to active FA leakage.
After the PDT, patients were followed up at 1 week, 1 month, 3 months, and 6 months. Thereafter, they were recommended to continue the follow-up at 3-month intervals or pro re nata. Both BCVA and OCT were obtained at every visit if possible. In this study, success of treatment was defined as complete resolution of SRF after the PDT and no recurrence of SRF on OCT within 6 months. Treatment failure was defined as nonresponse to the PDT or recurrence of SRF within 6 months after the PDT. Repeated FA and ICGA were performed when recurrence of SRF was suspected or if necessary.
In the statistical analysis, group 1 was compared with group 2 in their demographic data and success rates. Chi-square analysis was applied to categorical data and an independent samples t-test was applied to continuous data. The Fisher's exact test was used when 1 or more of the cells in the χ2 analysis had expected values below 5. In each group, the paired-samples t-test was used for comparing the BCVA and central subfield foveal thickness (CFT) at 3 and 6 months with the baseline. BCVA was measured on the Snellen chart and converted to the logarithm of the minimum angle of resolution (logMAR) for calculation. The Pearson correlation was employed to evaluate the BCVA at baseline and 6 months after treatment. In each patient, only CFT measurements obtained from the same OCT machine were used for comparison. A 2-tailed P value of<0.05 was considered statistically significant. All data were analyzed using IBM SPSS Statistics 21.0 (IBM, Armonk, NY).
Results
Fourteen eyes from 14 patients who received half-half PDT were enrolled in group 1. Twenty-eight eyes from 28 patients who received half-dose PDT were enrolled in group 2. The demographic data of the 2 groups are summarized in Table 1. No significant differences were found in age, gender, right eye or left eye, duration of symptoms, baseline BCVA, baseline CFT, PDT spot size, and follow-up duration.
Table 1.
Comparison of Demographic Data of Patients in the 2 Groups
| Group 1 (n=14) | Group 2 (n=28) | P-value | |
|---|---|---|---|
| Age (mean±SD) | 50.4±7.0 | 49.1±7.3 | 0.59a |
| Gender (male:female) | 11:3 | 22:6 | 1.00b |
| Eye (right:left) | 6:8 | 13:15 | 0.83c |
| Symptoms duration (mean±SD) | 19.9±18.9 months | 13.9±10.9 months | 0.29a |
| Baseline BCVA (mean±SD) | 0.799±0.572 | 0.626±0.398 | 0.26a |
| Baseline CFT (μm) (mean±SD) | 305±75 | 341±90 | 0.21a |
| PDT spot size (μm) (mean±SD) | 2,386±922 | 2,739±913 | 0.25a |
| Follow-up duration (months) (mean±SD) | 14.6±8.0 | 12.8±6.7 | 0.42a |
Comparing between group 1 and group 2, P-values were calculated by independent samples t-test.
Comparing between group 1 and group 2, P-values were calculated by Fisher's exact test.
Comparing between group 1 and group 2, P-values were calculated by χ2.
BCVA, best-corrected visual acuity, which is expressed in the logarithm of the minimal angle of resolution; CFT, central subfield foveal thickness; n, number of cases; PDT, photodynamic therapy; SD, standard deviation.
In group 1, 13/14 (93%) of the eyes showed complete resolution of SRF after treatment. A typical case is illustrated in Fig. 1. One patient (7%) showed no response to half-half PDT. He received a half-dose PDT at 6 months. Multiple intravitreal injections of bevacizumab were also used as an adjunctive treatment. However, his SRF never resolved completely during the entire follow-up period of 2 years. Four patients (36%) suffered from recurrent SRF within 6 months. One patient had recurrence at 3 months and was successfully treated by PDT with standard verteporfin dosage and standard laser fluence.29 No further recurrence was noted at the 6-month follow-up. One patient had his recurrence at 6 months due to a new leaking area that was successfully managed by focal laser photocoagulation; the SRF was resolved and no further recurrence was noted at 15 months. One patient had recurrence at 6 months but was lost at the 7-month follow-up. One patient had recurrence at 6 months and showed no response to another half-half PDT. The SRF sustained until the last follow-up time of 26 months, at which time the patient refused further treatments. In summary, the success rate in group 1 was 64% (9/14 eyes) at 6 months. In the long-term follow-up, there was 1 more patient who had the recurrence at 9 months, thereby reducing the success rate to 57% (8/14 eyes) with a mean follow-up duration of 14.6 months.
FIG. 1.
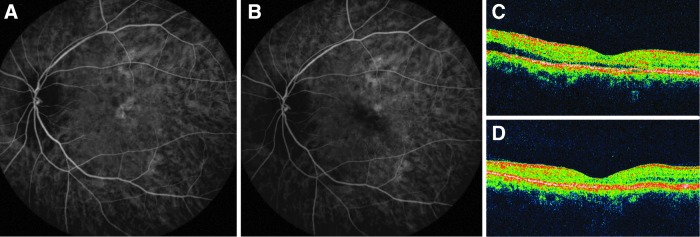
A patient had chronic central serous chorioretinopathy in the left eye for 1 year. The best-corrected visual acuity (BCVA) was 20/100 at baseline. (A) Baseline indocyanine green angiography (ICGA) showed hyperfluorescence over the macula. (B) The hyperfluorescence on ICGA was much decreased at 3 months after half-dose verteporfin combined with half-fluence photodynamic therapy. (C) Baseline optical coherence tomography (OCT) showed shallow subretinal fluid (SRF). (D) OCT at 3 months showed complete resolution of SRF. The BCVA improved to 20/30 at 1 month after treatment and remained stable thereafter.
In group 2, 26/28 (93%) of the eyes showed complete resolution of SRF after half-dose PDT. Two patients (7%) showed no response to half-dose PDT, and SRF persisted to their last follow-up times (12 and 13 months, respectively); the 2 patients refused further treatments. The other 26 patients did not have recurrence either within 6 months or during the entire follow-up period. Therefore, the success rates of the half-dose PDT group were 93% (26/28 eyes) at 6 months, as well as at the mean follow-up duration of 12.6 months. The success rate was significantly higher in group 2 than in group 1 at 6 months (P=0.031) and at the last follow up time (P=0.01).
The CFTs before and after PDT are summarized in Table 2. The CFT significantly decreased at 3 and 6 months in both groups. The BCVA before and after PDT are summarized in Table 3. In group 1, while there was a trend of visual improvement at 3 and 6 months, the statistical analyses indicated nonsignificance. In group 2, the BCVA demonstrated significant improvement at 3 and 6 months (both P<0.001). The BCVA at 6 months was highly correlated with the baseline BCVA in both groups (P=0.002 and P<0.001, respectively). No major complications, such as new RPE atrophy, secondary CNV formation, or significant visual loss were noted in either group.
Table 2.
Central Subfield Foveal Thickness Before and After Photodynamic Therapy
| Group 1 (n=14) | Group 2 (n=28) | |||||
|---|---|---|---|---|---|---|
| CFT (μm) (mean±SD) | Change in CFT (μm) (mean±SD) | P-valuea | CFT (μm) (mean±SD) | Change in CFT (μm) (mean±SD) | P-valuea | |
| Baseline | 305±75 | 341±90 | ||||
| 3 months | 212±97 | −93±125 | 0.015 | 190±44 | −151±103 | <0.001 |
| 6 months | 190±93 | −115±74 | <0.001 | 191±41 | −150±97 | <0.001 |
Compared with baseline, P-values were calculated by paired samples t-test.
Table 3.
Best-Corrected Visual Acuity Before and After Photodynamic Therapy and Comparison Between 2 Groups
| Group 1 (n=14) | Group 2 (n=28) | |||
|---|---|---|---|---|
| BCVA (mean±SD) | P-value | BCVA (mean±SD) | P-value | |
| Baseline | 0.799±0.572 | — | 0.626±0.398 | — |
| 3 months | 0.728±0.679 | 0.57a | 0.331±0.496 | <0.001a |
| 6 months | 0.669±0.569 | 0.25a | 0.292±0.486 | <0.001a |
Compared with baseline, P-values were calculated by paired samples t-test.
Discussion
Our study was a pilot study on using half-half PDT for chronic CSC. It is believed that chronic CSC is a disease caused by increasing choroidal vascular dilatation with hyperpermeability or poor RPE barrier function.1,30 It has also been postulated that PDT can cause short-term choriocapillaris hypoperfusion and long-term choroidal vascular remodeling, leading to a reduction in choroidal congestion, vascular hyperpermeability, and extravascular leakage.1,14,20,31 The purpose of the treatment, therefore, is to reconstruct the balance between choroidal vasculature permeability and RPE barrier function. However, in those patients whose chronic CSC is caused by compromised RPE barrier function, standard PDT might further damage RPE functions and cause RPE atrophy.1,11,14–21 As a result, attempts to modify the PDT protocol have been made to reduce this adverse effect. The first way is to reduce the fluence (energy) of the radiation. In a previous study, higher fluence laser energy delivery was shown to result in the closure of the deeper choroidal vessels and focal alterations in the RPE.20 A PDT of lower fluence resulted in a higher selectivity to leaking choriocapillaris and caused less damage to weakened RPE cells.20 Several studies also demonstrated that a PDT of lower fluence might be more beneficial in treating chronic CSC with diseased RPE and choriocapillaris.26,27 The second way of modifying the PDT protocol is to reduce the dosage of verteporfin.22–25 In their clinical trials, Chan et al. and Nicolo et al. obtained good results by using half-dose verteporfin to treat chronic CSC. To date, however, the optimal PDT protocol for effective management of chronic CSC with minimal damage to RPE functions remains unclear. The rationale of using half-half PDT in chronic CSC is to try to produce an adequate photodynamic effect with minimized RPE damage.
Previous studies demonstrated that the success rates in standard PDT protocol for chronic CSC were around 90% at 6 months,13 and 79%–100% at 12 months.11,27,28 In studies using standard-dose verteporfin combined with a PDT of reduced fluence, the success rate was around 83% at 6 months and 91%–94% at 12 months.27,28,32 As for half-dose verteporfin combined with PDT of standard fluence, short-term results indicated that SRF resolved in 85% patients at 1 month24 and in 87.5% at 3 months.23 Chan et al. observed the resolution of SRF in 89.6% of 43 patients at 12 months.22 Moreover, Nicolo et al.25 showed that SRF resolved in 92.1% of their patients at the last follow-up visit (mean 14.2 months). In the current study, the success rate in group 2 was 94% at 6 months, and no further recurrence was noted during the mean follow-up duration of 12.6 months. Although direct comparison between studies was not possible due to different baseline conditions and study designs, it seems that our results in the half-dose PDT group were similar to the previous studies published by Chan et al. and Nicolo et al.22,25 In a recent study, Uetani et al. demonstrated the effects of further reducing the verteporfin dose.33 Their success rate in half-dose PDT was 100% at 3 months. However, the success rate in one-third-dose verteporfin combined with standard fluence PDT was markedly decreased to 33% at 3 months. Thus, further reducing the verteporfin below half-dose might compromise the success rate.
To the best of our knowledge, there are no previous reports describing the effect of reducing the verteporfin dosage and laser fluence at the same time for chronic CSC. We found that its success rate of half-half PDT was 64% at 6 months. Theoretically, reducing the verteporfin dosage and laser fluence at the same time can further prevent the damage to RPE. On the other hand, such a reduction may also decrease the ability of closing the choroidal hyperpermeable vessels. For this reason, it is reasonable to expect that group 1 had a significantly lower success rate than that of group 2 (P=0.01). However, it is also worth noting that more than half of our patients could be successfully treated with this extremely low PDT effect.
The changes of CFT in this study were similar to previous studies.22,24,25,27,28,32 In studies using half-dose PDT, Chan et al. showed that the CFT reduced from 320 μm to 175 μm in the first 3 months (P<0.001).22 Then the CFT stabilized at around 175 μm from 3 months to 12 months. Nicola et al. showed that the CFT was significantly reducing from 346 μm to 213 μm at a mean follow-up time of 14.2 months.25 In the current study, we found similar CFT changes in group 2 patients using half-dose PDT. The CFT reduced from 341 μm to 190 μm at 3 months and then remained stable at 6 months. In group 1, despite the low PDT effect, the CFT also showed a significant reduction from 305 μm to 212 μm at 3 months and to 190 μm at 6 months. This showed that both half-half PDT and half-dose PDT were effective in reducing the SRF in CSC.
The BCVA in our group 2 showed significant improvements at both 3 and 6 months, and this result was similar to previous studies.22,24,25,27,28,32 Chan et al. reported that BCVA (logMAR) improved from 0.31 to 0.15 (P<0.001) at 1 year after half-dose PDT.22 Reibaldi et al. reported that BCVA (logMAR) improved from 0.46 to 0.16 at 12 months in the low-fluence PDT group.27 On the other hand, the BCVA in our group 1 showed a trend of improvement at 3 and 6 months, but they were not statistically significant. This may be due to the higher recurrence rate in group 1. The patients with recurrent SRF would have less BCVA improvement. We analyzed the subgroup of patients (8 eyes) whose SRF had permanently disappeared (success subgroup) in group 1. The BCVA was 0.893, 0.662 (P=0.10), and 0.690 (P=0.19) at baseline, 3 months, and 6 months, respectively. There seemed to be a trend of visual improvement at 3 and 6 months, but the statistic was not significant due to the small number of cases.
We also found that the BCVA at 6 months is highly correlated with the baseline BCVA in both half-half PDT and half-dose PDT groups (P=0.002 and P<0.001, respectively). This may be because patients with worse baseline BCVA usually have more severe RPE changes and retina atrophy, possibly leading to a poor visual outcome even after the resolution of SRF. Because of this, in patients with poor baseline BCVA, ophthalmologists should explain to them about the risk of limited visual outcomes in advance. This fact also suggests that early treatment of chronic CSC is desirable before irreversible damages of RPE and retina occur.
Our study is limited by its retrospective nature and small number of cases in group 1. However, this pilot study provides important information on the use of half-half PDT for chronic CSC. Many researchers have been trying to establish the most effective PDT protocol with the least adverse effect. Our study can offer useful indications for designing future randomized prospective studies for this purpose.
In conclusion, half-half PDT is a feasible treatment for chronic CSC. No major complications, such as new RPE atrophy or secondary CNV, were observed. However, it has a lower success rate at 6 months when compared to half-dose PDT (64% versus 93%).
Acknowledgment
We thank Mr. Yu-Jr Lin, who was supported by grants from Biostatistical Center for Clinical Research, Chang Gung Memorial Hospital (CLRPG340599), for statistical consultation.
Author Disclosure Statement
All of the authors declare they have no proprietary/financial interest related to the article nor any conflicts of interest.
References
- 1.Gemenetzi M., De Salvo G., and Lotery A.J.Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye 24:1743–1756, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Stewart J.M.Half dose verteporfin PDT for central serous chorioretinopathy. Br. J. Ophthalmol. 90:805–806, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iida T., Yannuzzi L.A., Spaide R.F., et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina 23:1–7; quiz 137–138, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Piccolino F.C., de la Longrais R.R., Ravera G., et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am. J. Ophthalmol. 139:87–99, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ficker L., Vafidis G., While A., and Leaver P.Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br. J. Ophthalmol. 72:829–834, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ie D., Yannuzzi L.A., Spaide R.F., et al. Subretinal exudative deposits in central serous chorioretinopathy. Br. J. Ophthalmol. 77:349–353, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalkh A.E., Jabbour N., Avila M.P., Trempe C.L., and Schepens C.L.Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology 91:1544–1548, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Levine R., Brucker A.J., and Robinson F.Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology 96:854–859, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Loo R.H., Scott I.U., Flynn H.W. Jr., et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 22:19–24, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Yannuzzi L.A., Shakin J.L., Fisher Y.L., and Altomonte M.A.Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology 91:1554–1572, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Cardillo Piccolino F., Eandi C.M., Ventre L., Rigault de la Longrais R.C., and Grignolo F.M.Photodynamic therapy for chronic central serous chorioretinopathy. Retina 23:752–763, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Taban M., Boyer D.S., Thomas E.L., and Taban M.Chronic central serous chorioretinopathy: photodynamic therapy. Am. J. Ophthalmol. 137:1073–1080, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Yannuzzi L.A., Slakter J.S., Gross N.E., et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina 23:288–298, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Chan W.M., Lam D.S., Lai T.Y., et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br. J. Ophthalmol. 87:1453–1458, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isola V., Pece A., and Brancato R.Circulatory changes in the choroidal vasculature after verteporfin-based photodynamic therapy for choroidal neovascularization in age-related macular degeneration. Retina 24:618–620, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Isola V., Pece A., and Parodi M.B.Choroidal ischemia after photodynamic therapy with verteporfin for choroidal neovascularization. Am. J. Ophthalmol. 142:680–683, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Klais C.M., Ober M.D., Freund K.B., et al. Choroidal infarction following photodynamic therapy with verteporfin. Arch. Ophthalmol. 123:1149–1153, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Lee P.Y., Kim K.S., and Lee W.K.Severe choroidal ischemia following photodynamic therapy for pigment epithelial detachment and chronic central serous chorioretinopathy. Jpn. J. Ophthalmol. 53:52–56, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Oner A., Karakucuk S., Mirza E., and Erkilic K.The changes of pattern electroretinography at the early stage of photodynamic therapy. Doc. Ophthalmol. 111:107–112, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Schlotzer-Schrehardt U., Viestenz A., Naumann G.O., et al. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 240:748–757, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Tzekov R., Lin T., Zhang K.M., et al. Ocular changes after photodynamic therapy. Invest. Ophthalmol. Vis. Sci. 47:377–385, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Chan W.M., Lai T.Y., Lai R.Y., Tang E.W., Liu D.T., and Lam D.S.Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina 28:85–93, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Fujita K., Yuzawa M., and Mori R.Retinal sensitivity after photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy: short-term results. Retina 31:772–778, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Lai T.Y., Chan W.M., Li H., et al. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br. J. Ophthalmol. 90:869–874, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolo M., Zoli D., Musolino M., and Traverso C.E.Association between the efficacy of half-dose photodynamic therapy with indocyanine green angiography and optical coherence tomography findings in the treatment of central serous chorioretinopathy. Am. J. Ophthalmol. 153:474–480, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Reibaldi M., Boscia F., Avitabile T., et al. Low-fluence photodynamic therapy in longstanding chronic central serous chorioretinopathy with foveal and gravitational atrophy. Eur. J. Ophthalmol. 19:154–158, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Reibaldi M., Cardascia N., Longo A., et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am. J. Ophthalmol. 149:307–315, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Shin J.Y., Woo S.J., Yu H.G., and Park K.H.Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina 31:119–126, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch. Ophthalmol. 117:1329–1345, 1999 [PubMed] [Google Scholar]
- 30.Ciardella A.P., Guyer D.R., Spitznas M., and Yannuzzi L.A.Central serous chorioretinopathy. In: Ryan S.J., Schachat A.P., and Hengst T.C., eds. Retina, 3rdedn Philadelphia: Mosby; 2001; p. 1153–1181 [Google Scholar]
- 31.Schmidt-Erfurth U., Laqua H., Schlotzer-Schrehard U., Viestenz A., and Naumann G.O.Histopathological changes following photodynamic therapy in human eyes. Arch. Ophthalmol. 120:835–844, 2002 [PubMed] [Google Scholar]
- 32.Inoue R., Sawa M., Tsujikawa M., and Gomi F.Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am. J. Ophthalmol. 149:441–446, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Uetani R., Ito Y., Oiwa K., Ishikawa K., and Terasaki H.Half-dose vs one-third-dose photodynamic therapy for chronic central serous chorioretinopathy. Eye 26:640–649, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


