Abstract
In recent years, Drosophila melanogaster has become an attractive model organism in which to study the structure and development of the cellular immune components. The emergence of immunological markers greatly accelerated the identification of the immune cells (hemocytes), while the creation of genetic reporter constructs allowed unique insight into the structural organization of hematopoietic tissues. However, investigation of the hemocyte compartments by the means of immunological markers requires dissection and fixation, which regularly disrupt the delicate structure and hamper the microanatomical characterization. Moreover, the investigation of transgenic reporters alone can be misleading as their expression often differs from the native expression pattern of their respective genes. We describe here a method that combines the reporter constructs and the immunological tools in live imaging, thereby allowing use of the array of available immunological markers while retaining the structural integrity of the hematopoietic compartments. The procedure allows the reversible immobilization of Drosophila larvae for high-resolution confocal imaging and the time-lapse video analysis of in vivo reporters. When combined with our antibody injection-based in situ immunostaining assay, the resulting double labeling of the hemocyte compartments can provide new information on the microanatomy and functional properties of the hematopoietic tissues in an intact state. Although this method was developed to study the immune system of Drosophila melanogaster, we anticipate that such a combination of genetic and immunological markers could become a versatile technique for in vivo studies in other biological systems too.
Introduction
Fluorescence-based imaging techniques are widely used in studies relating to the development of the hematopoietic system, and in tumor biology and immunity in general. Due to the similarities of the innate immune responses in vertebrates and in insects, its powerful genetic system has led to Drosophila melanogaster becoming a key model organism of innate immunity [1]–[3].
The hemocytes in Drosophila fall into three categories: plasmatocytes, crystal cells and lamellocytes. Plasmatocytes are small round cells that clear microbes by phagocytosis [4], and produce antimicrobial peptides and extracellular matrix components [5], [6]. Crystal cells contain high amounts of the prophenol oxidases required for melanization [7]. Lamellocytes, the large, flat, key effector cells of the encapsulation reaction, appear after immune induction by the eggs of parasitic wasps or in response to tumors [8], [9].
The hemocytes of the Drosophila larva populate three hematopoietic compartments. In the circulation, the cells move freely in the body cavity, pumped by the dorsal vessel. The lymph gland, a compact, multilobular hematopoietic organ attached to the anterior portion of the dorsal vessel [10], [11], comprises plasmatocytes and crystal cells in its cortical zone, and progenitor cells in its medullary zone [12]–[14]. In the sessile hematopoietic tissue, the hemocytes adhere to the subepithelial layer of the body cavity, forming a striped pattern along the longitudinal axis of the larva [15]–[17].
The identification of Drosophila hemocytes initially relied on morphological criteria [18]. However, the advent of hemocyte subset-specific molecular markers allowed a clear definition of morphologically and functionally distinct effector cell types [19]–[21]. All larval hemocytes express a highly glycosylated transmembrane protein, Hemese, a member of the sialophorin protein family [19]. Plasmatocytes express the transmembrane protein NimrodC1, identified as a bacterium-binding phagocytosis receptor [20], [22]. Although these markers have become essential tools for the characterization of hemocytes and hematopoietic tissues in ex vivo samples, the delicate structure of the immobile hemocyte compartments, and especially that of the sessile tissue, is disrupted, which severely hinders their comprehensive structural analysis.
The construction of in vivo reporters and hemocyte-specific GAL4 lines in recent years [15], [23]–[26] allowed a detailed anatomical and functional characterization of the hematopoietic compartments, and in particular the lymph gland [12], [13] and the sessile hematopoietic tissue [16], [17], [27], [28].
We set out to complement the in vivo reporters with immunological markers in live larvae with a view to studying the composition and structure of the hematopoietic tissues in an undisturbed state. This requires a simple and effective immobilization of the larva for the duration of the microscopic analysis. This was earlier achieved by dissection [29], [30], by the use of chloroform [31], through the administration of CO2 or isofluorane to the larva [32]–[34], or by placing the specimen in a specially prepared microfluidic chamber and applying vacuum [35]. Isofluorane was found to be very effective, but it arrests the pulsation of the dorsal vessel [32], [33] thereby interfering with the circulation of the hemolymph and the mobile hemocytes. We present here an effective and simple method with which to paralyze the larva for an extended period by the use of an acetylcholinesterase inhibitor. This method of immobilization, combined with the genetic and immunological tools mentioned above, allows the in situ examination and analysis of the hematopoietic compartments with a so far unprecedented resolution.
Drosophila Stocks and Materials
Drosophila stocks
Flies were kept on cornmeal-yeast food at 25°C. R3-Hml>GFP (R3-w1118; Hml.delta.GAL4, UAS-2xEGFP) [36] was used to visualize plasmatocytes, and atillaminos (Mi{ET1}atillaMB05359) [37] to detect lamellocytes; atillaminos; l(3)mbn1 (Mi{ET1}atillaMB05359; l(3)mbn1/TM6, Tb) [37] is a tumor suppressor mutant with proliferating tumorous hemocytes and GFP-marked lamellocytes in the hematopoietic compartments. The Hml>GFP (w1118; Hml.delta.GAL4, UAS-2xEGFP) line was used as a P1 negative control.
Antibodies
The mouse monoclonal antibody anti-Hemese (1.2) [19] reacts with all hemocytes in the larva, the anti-NimC1 reagent, a mixture of P1a and P1b antibodies, reacts with plasmatocytes [20], and T2/48, a negative control antibody, reacts with the human CD45 molecule [21]. These antibodies were used as tissue culture supernatants, at an immunoglobulin concentration of 15 µg ml−1. The secondary antibody was an anti-mouse Alexa-633 conjugate (Invitrogen) goat polyclonal antibody, or an anti-mouse CF-568 conjugate (Sigma-Aldrich) goat polyclonal antibody.
Methods
Immobilization of the larvae
Larvae were placed in a 25-µl droplet of Drosophila Ringer's solution containing Dichlorvos (Fluka, diluted 1∶1000) for 5 min at 25°C, and then transferred into glass-bottom dishes (Cell E&G). To ensure the stability of the larvae, the coverslip was coated with glue. The glue was dissolved in 80 ml heptane from the surface of a 1-m-long double-sided adhesive tape (3M #415) for 24 h at 25°C, after which the tape was removed. This solution was layered onto the coverslip and dried [38]. To prevent desiccation, larvae were mounted with 10S Voltalef oil (VWR).
Confocal microscopic analysis
Samples were analyzed by means of a Leica TCS SP5 II confocal microscope. The images were merged stacks of 15 slices, combined in ImageJ (maximum intensity stacking). The frames of Video S1 and Video S2 were stacks of 5 slices, combined and sequenced using ImageJ at 5 frames per second (as in [39]).
Preparation of the antibodies for injection
Monoclonal antibodies to Hemese (1.2), to NimrodC1 (a mixture of P1a and P1b), and against human CD45 (T2/48) (negative control) were used in the form of hybridoma-culture supernatants. The secondary antibody was added to the respective supernatant in 1∶1000 final dilution. This mixture was incubated for 10 min at 25°C to generate hemocyte-specific immunocomplexes, prior to injection.
Antibody injection
Third instar larvae were washed in Drosophila Ringer's solution, and placed on a dry paper towel. With a sharpened glass capillary, 1 µl of the mixture of the primary and secondary antibodies was injected into the hemocoel of third instar larvae, near the posterior end, between segments A6 and A7.
Preparation of circulating hemocytes
All procedures described below were performed at room temperature (20°C). Larvae were dissected on 12-spot microscope slides (SM-011, Hendley-Essex) in Shields & Sang medium containing 1-phenyl-2-thiourea (PTU). Hemocytes were left to adhere for 45 min, after which they were fixed in paraformaldehyde (2 per cent, in PBS) for 12 min and washed three times in PBS for 5 min. The samples were then blocked with PBS containing 0.1 per cent bovine serum albumin (PBS-BSA) for 15 min.
Staining of circulating hemocytes with immunocomplexes
The mixture of the respective hybridoma supernatants (anti-Hemese, anti-NimrodC1 or T2/48) and the secondary antibody (anti-mouse CF-568 conjugate, Sigma-Aldrich) in a ratio of 1000∶1 was prepared and incubated for 5 min. The prepared hemocyte samples were treated with the mixture of antibodies for 1 h, and were washed three times with PBS for 5 min. The nuclei were stained with DAPI (Sigma-Aldrich). The samples were mounted with Fluoromount-G (SouthernBiotech) and investigated with a Zeiss Axioskope 2 MOT fluorescent microscope.
Staining of circulating hemocytes with sequential indirect immunofluorescence
The prepared samples were treated with the hybridoma supernatants for 1 h, followed by three washes in PBS for 5 min. The secondary antibody (anti-Mouse CF-568, Sigma-Aldrich) was applied to the sample in a dilution of 1∶1000 in PBS-BSA for 45 min. The nuclei were stained with DAPI. The sample was washed three times with PBS, mounted with Fluoromount-G and inspected with a Zeiss Axioskope 2 MOT fluorescent microscope.
Results
Immobilization of the Drosophila larvae
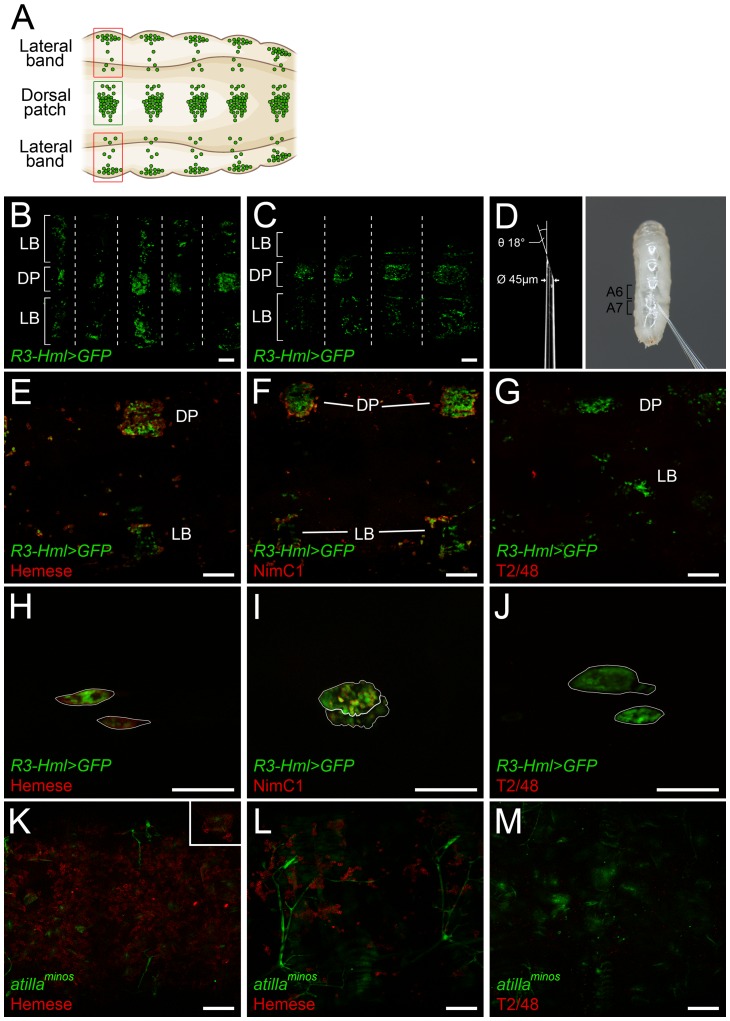
Each larva was physically immobilized in a 25-µl droplet of Dichlorvos (Fluka) solution, diluted in Drosophila Ringer's solution. Dichlorvos (2,2-dichlorovinyl dimethyl phosphate), is an acetylcholinesterase inhibitor. In preliminary experiments (not shown), exposure at a dilution of 1∶1000 in Drosophila Ringer's solution for 5 min at 25°C paralyzed the larvae for over 1 h. For microscopic analysis, larvae were placed on glue-covered glass-bottom dishes, and mounted with Voltalef 10S oil, as shown in Figure 1. The immobilization of the larvae did not affect the characteristic structure (Figure 2A) and the integrity of the sessile hematopoietic compartment (Figure 2B).
Figure 1. Preparation of larvae for in vivo microscopic analysis.
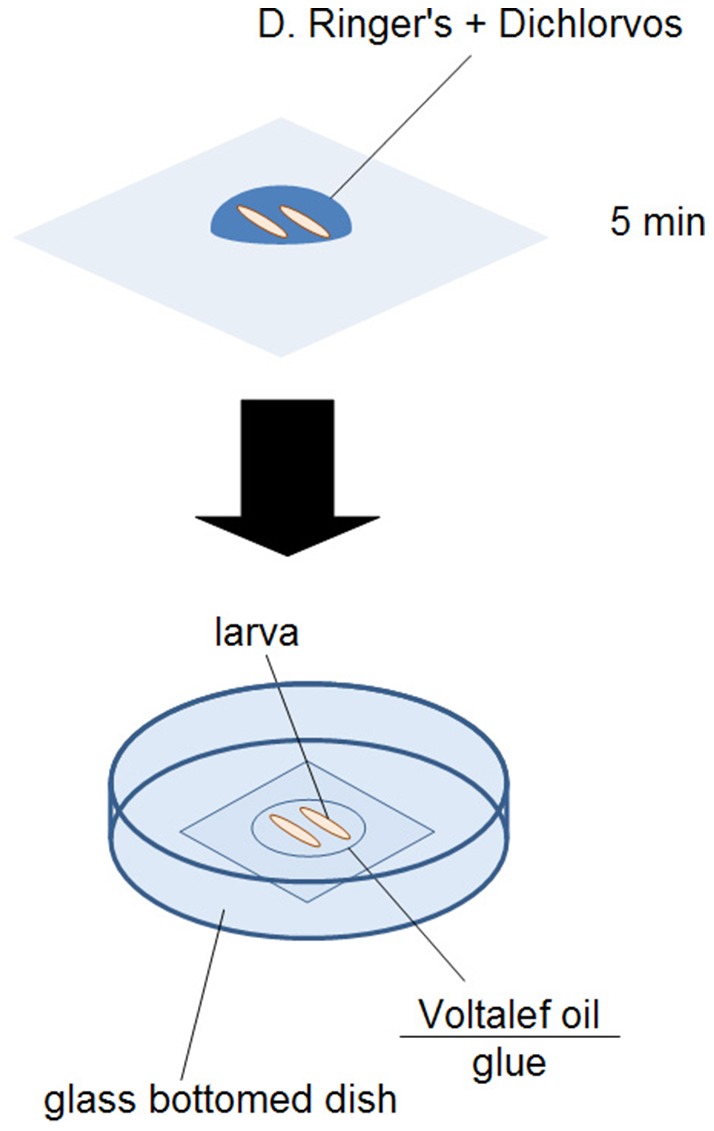
Figure 2. The schematic representation of the larval sessile hematopoietic tissue (A).
The sessile tissue of an immobilized R3-Hml>GFP larva (B), and a mock-injected R3-Hml>GFP larva (C). The dorsal patches are indicated by DP, and the lateral bands are indicated by LB. Segmental borders are marked with dashed lines. The parameters for the sharpened capillaries used for the injection of larvae, and a high-magnification photograph of the injection (D). Staining (red) of the sessile hemocytes (green) in R3-Hml>GFP larva with anti-Hemese (E), anti-NimC1 (F) and T2/48 (G) antibodies. The lymph glands (outlined in white) of R3-Hml>GFP larvae (green) stained in situ with anti-Hemese (H), anti-NimC1 (I) or T2/48 (J) antibodies (red). The sessile hematopoietic tissue of atillaminos; l(3)mbn1 (K) and atillaminos (L) larvae stained for Hemese (red). Negative control staining of atillaminos; l(3)mbn1 larva with T2/48 antibody (M). The lamellocytes (green) of atillaminos; l(3)mbn1 larva in the hematopoietic tissue stained for Hemese (K, insert), indicated by the arrows. All scale bars indicate 50 µm.
The hemocyte compartments of the paralyzed larvae
Paralyzed R3-Hml>GFP larvae were subjected to confocal microscopic time-lapse analysis (Video S1). Examination of the generated video files revealed that the hematopoietic compartments were intact, the pulsation of the dorsal vessel was not influenced and the circulation of the hemocytes in the dorsal vessel and in the hemocoel was normal. In some cases, minor movements of the larva were noticed (Video S1), but these did not affect the video-analysis.
In vivo immunostaining of hemocytes
With a glass capillary, third instar R3-Hml>GFP larvae were injected with the mixture of antibodies to established hemocyte-specific markers [19]–[21] and far-red-fluorescence-labeled secondary antibodies (Figure 2D), and the hematopoietic tissues were analyzed with the aid of a confocal microscope 15 min after the injection. Although the injury and the volume injected had no effect on the structure of the sessile tissue (Figure 2C) or the other compartments (not shown), elongation of the larva was observed (Figure 2C). Mixtures of anti-Hemese or anti-NimC1 antibodies and secondary antibodies stained membranes of hemocytes in the sessile compartment (Figure 2E, F), while the negative control antibody (T2/48) gave no signal (Figure 2G). Analysis of the samples confirmed that the expressions of the in vivo marker R3-Hml>GFP and the Hemese antigen overlapped. Moreover, the NimC1 marker was expressed in more than 80 per cent of the R3-Hml>GFP-positive sessile hemocytes. In the lymph gland, the anti-Hemese antibody (Figure 2H) reacted with the whole surface of the lymph gland, whereas the anti-NimC1 antibody gave a patchy staining pattern in the cortical zone of the primary lobes (Figure 2I). The negative control antibody, T2/48, did not give a signal (Figure 2J). In order to confirm that the specificity of the antibody was not altered in the immunocomplex, circulating hemocytes from R3-Hml>GFP larvae were immunostained with sequential indirect immunofluorescent staining and with the mixture of primary and secondary antibodies (Figure S1). Even though the staining of the antibody mixture was weaker (Figure S1B) than the staining of the sequential method (Figure S1A), no difference was observed in the staining pattern of the two procedures. Furthermore, to provide a genetic control, a NimC1 negative Hml>GFP sample was also included and was stained against NimC1; no staining was observed in either case (Figure S1).
To generate time-lapse confocal videos from in situ immunostained specimens, larvae were injected with a mixture of anti-Hemese antibody and Alexa-633-conjugated secondary antibody, paralyzed and mounted on glass-bottomed dishes. Twenty minute video recordings showed the specific far-red staining of GFP-expressing hemocytes (Video S2). As no changes in signal intensity were detected during the experiment, we concluded that this method is a viable option for the creation of time-lapse series of in situ immunostained larvae.
Malignant tissue transformations are known to trigger a cellular immune response, and cause blood cell proliferation and differentiation [40]. Since these events may affect the composition and structure of the hematopoiteic compartments, we tested and validated the in situ immunostaining technique in tumorous animals. Third instar l(3)mbn1 homozygous larvae contain approximately 150 times as many circulating hemocytes as in uninduced wild-type controls, with severely swollen and often melanized lymph glands [40]. Since no information was available on the state of the sessile hematopoietic tissue in this tumorous mutant, we investigated the sessile compartment of homozygous atillaminos; l(3)mbn1 larvae, which also carry an in vivo GFP reporter of the Atilla (L1) lamellocyte-specific marker [37]. The larvae were injected with a mixture of anti-Hemese and the far-red-labeled secondary antibody and the sessile hematopoietic tissue was analyzed with confocal microscopy. The structure of the sessile tissue of the mutant larvae was altered (Figure 2K) as compared with that of the non-tumorous atillaminos control (Figure 2L). Double-positive lamellocytes, displaying both GFP expression and staining for the pan-hemocyte Hemese marker, were clearly visible in the sessile tissue (Figure 2K, insert).
Discussion
The localization of cells and the structure of tissues in the organism is generally studied on dissected specimens by light microscopy or a high-resolution confocal microscopic analysis after the use of histochemical or immunofluorescent techniques. These methods have a number of limitations: in consequence of their invasive nature of the preparation and the fixation procedures, the fine structure of the tissues and the cell morphology are often disrupted. Live imaging overcomes most of these limitations. Since the translucent cuticle of the Drosophila melanogaster embryo and the larva makes such investigations much easier, it has become an ideal model organism for in vivo imaging studies.
Analysis of the hematopoietic compartments and the cell-mediated immunity of Drosophila has played an important role in our understanding of the vertebrate hematopoiesis and immune response. Most of our knowledge on Drosophila hemocytes was gained through the labeling of different cell types with cell-type-specific antibodies [19]–[21], and the use of transgenic reporter constructs [13], [15]–[17]. In vivo labeling has been utilized to visualize hemocyte migration in the embryo [25] and also in genetic screens to identify factors that regulate the structure of the sessile hematopoietic tissue [27].
We have presented here a simple approach through which to amalgamate the strengths of these methods. Immobilization of the larvae renders the experimental subjects accessible for confocal microscopic studies, and thereby allows the high-resolution visualization of hemocytes in their normal environment (e.g. in the sessile tissue). As the larvae survive the immobilization, their hematopoietic tissues can be investigated throughout the whole of the larval life. This novel method permits the immunostaining of hemocytes in situ, and this has proved to be very useful when no transgenic reporter is available to label a certain hemocyte subset, or when two distinct hemocyte populations are to be labeled. The present study has confirmed that the expressions of the in vivo marker R3-Hml>GFP and the pan-hemocyte Hemese antigen overlap. Moreover, it has demonstrated that the NimrodC1 markers are present on more than 80 per cent of R3-Hml>GFP-expressing sessile hemocytes. These results indicate that the method is suitable for the investigation of overlapping or differential expressions of in vivo and immunological markers.
Investigation of tumorous atillaminos; l(3)mbn1 larvae revealed that the sessile tissue in these specimens is more massive than in the atillaminos control larvae, and additionally includes fully differentiated lamellocytes.
The combination of immunostaining with transgenic reporters allows the in situ investigation of multilabeled hemocytes, and monitoring of the expression of several independent markers on hemocytes throughout the course of the development or during the immune response. As the technique is easy to perform, such an antibody injection may also facilitate the in situ investigation of other arthropod model species in which there are no transgenic reporter constructs, but where immunological markers are available, such as Manduca sexta [41], Bombyx mori [42] or Anopheles gambiae [43].
Supporting Information
Immunostaining of circulating hemocytes with the sequential indirect immunofluorescent method (A), and the mixture of antibodies (B). The staining is shown in red, and the hemocytes are marked by their GFP expression (green), and DAPI nuclear staining (blue). The scale bar indicates 50 µm.
(TIF)
The sessile hematopoietic tissue (green) of an immobilized R3-Hml>GFP larva. The video was rendered with 5 frames per second, and represents 20 min of recording.
(AVI)
The sessile hematopoietic tissue (green) of an immobilized R3-Hml>GFP larva immunostained in situ with anti-Hemese (red). The video was rendered with 5 frames per second, and represents 20 min of recording.
(AVI)
Acknowledgments
We express our thanks to the unnamed Referee for the constructive suggestions. We are grateful to Olga Kovalcsik, Anita Balázs, Szilvia Tápai and Anikó Képíró for their technical assistance. Our thanks are also due to the Imaging Laboratory of the Biological Research Center of the Hungarian Academy of Sciences.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Funding Statement
This research was financed by grants from the Hungarian Science Foundation, OTKA grant NK 101730 to (IA) (www.otka.hu), and TÁMOP 4.2.2.A-11/1KONV-2012-0035 to (IA) (www.ujszechenyiterv.gov.hu). This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4.A/2-11-1-2012-0001 ‘National Excellence Program’ (for VH and GIBV) (www.nemzetikivalosag.hu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hultmark D (1994) Insect immunology. Ancient relationships. Nature 367: 116–117. [DOI] [PubMed] [Google Scholar]
- 2. Dushay MS, Eldon ED (1998) Drosophila immune responses as models for human immunity. Am J Hum Genet. 62: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans CJ, Hartenstein V, Banerjee U (2003) Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 5: 673–90. [DOI] [PubMed] [Google Scholar]
- 4. Ulvila J, Vanha-Aho LM, Rämet M (2011) Drosophila phagocytosis – still many unknowns under the surface. APMIS. 119: 651–662. [DOI] [PubMed] [Google Scholar]
- 5. Martinek N, Shahab J, Saathoff M, Ringuette M (2008) Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 121: 1671–1680. [DOI] [PubMed] [Google Scholar]
- 6. Samakovlis C, Kimbrell DA, Kylsten P, Engström A, Hultmark D (1990) The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J. 9: 2969–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rizki TM, Rizki RM (1959) Functional significance of the crystal cells in the larva of Drosophila melanogaster. J Biophys Biochem Cytol. 5: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rikzi TM (1957) Alterations in the haemocyte population of Drosophila melanogaster. J Morphology 100: 437–458. [Google Scholar]
- 9. Minakhina S, Steward R (2006) Melanotic mutants in Drosophila: pathways and phenotypes. Genetics 174: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Röhrborn G (1961) Drosophila tumors and the structure of larval lymph glands. Experientia 17: 507–509. [DOI] [PubMed] [Google Scholar]
- 11. Shrestha R, Gateff E (1982) Ultrastructure and Cytochemistry of the Cell-types in the Tumorous Hematopoietic Organs and the Hemolymph of the Mutant Lethal (1) Malignant Blood Neoplasm (l(1)mbn) of Drosophila Melanogaster . Development, Growth & Differentiation 1: 83–98. [DOI] [PubMed] [Google Scholar]
- 12. Sorrentino RP, Carton Y, Govind S (2002) Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 243: 65–80. [DOI] [PubMed] [Google Scholar]
- 13. Jung SH, Evans CJ, Uemura C, Banerjee U (2005) The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132: 2521–2533. [DOI] [PubMed] [Google Scholar]
- 14. Krzemień J, Oyallon J, Crozatier M, Vincent A (2010) Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 346: 310–319. [DOI] [PubMed] [Google Scholar]
- 15. Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz É, et al. (2004) A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 101: 14192–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Márkus R, Laurinyecz B, Kurucz É, Honti V, Bajusz I, et al. (2009) Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A. 106: 4805–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Honti V, Csordás G, Márkus R, Kurucz É, Jankovics F, et al. (2010) Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster. Mol Immunol. 47: 1997–2004. [DOI] [PubMed] [Google Scholar]
- 18. Rizki TM, Rizki RM (1980) Properties of the larval hemocytes of Drosophila melanogaster . Experientia 36: 1223–1226. [Google Scholar]
- 19. Kurucz É, Zettervall CJ, Sinka R, Vilmos P, Pivarcsi A, et al. (2003) Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc Natl Acad Sci U S A. 100: 2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurucz É, Márkus R, Zsámboki J, Folkl-Medzihradszky K, Darula Z, et al. (2007) Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 17: 649–654. [DOI] [PubMed] [Google Scholar]
- 21. Kurucz É, Váczi B, Márkus R, Laurinyecz B, Vilmos P, et al. (2007) Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol Hung. 58: 95–111. [DOI] [PubMed] [Google Scholar]
- 22. Zsámboki J, Csordás G, Honti V, Pintér L, Bajusz I, et al. (2013) Drosophila Nimrod proteins bind bacteria. Cent. Eur. J. Biol. 7: 633–645. [Google Scholar]
- 23. Olofsson B, Page DT (2005) Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 279: 233–243. [DOI] [PubMed] [Google Scholar]
- 24. Sinenko SA, Mathey-Prevot B (2004) Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene. 23: 9120–9128. [DOI] [PubMed] [Google Scholar]
- 25. Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, et al. (2005) Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 168: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tokusumi T, Shoue DA, Tokusumi Y, Stoller JR, Schulz RA (2009) New hemocyte-specific enhancer-reporter transgenes for the analysis of hematopoiesis in Drosophila. Genesis 47: 771–774. [DOI] [PubMed] [Google Scholar]
- 27. Stofanko M, Kwon SY, Badenhorst P (2008) A misexpression screen to identify regulators of Drosophila larval hemocyte development. Genetics. 180: 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K (2011) The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 138: 5379–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pilling AD, Horiuchi D, Lively CM, Saxton WM (2006) Kinesin-1 and Dynein Are the Primary Motors for Fast Transport of Mitochondria in Drosophila Motor Axons. Mol Biol Cell 17: 2057–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gunawardena S, Her LS, Brusch RG, Laymon RA, Niesman IR, et al. (2003) Disruption of Axonal Transport by Loss of Huntingtin or Expression of Pathogenic PolyQ Proteins in Drosophila. Neuron 40: 25–40. [DOI] [PubMed] [Google Scholar]
- 31. Miller KE, DeProto J, Kaufmann N, Patel BN, Duckworth A, et al. (2005) Direct Observation Demonstrates that Liprin-[alpha] Is Required for Trafficking of Synaptic Vesicles. Current Biology 15: 684–689. [DOI] [PubMed] [Google Scholar]
- 32. Fuger P, Behrends LB, Mertel S, Sigrist SJ, Rasse TM (2007) Live imaging of synapse development and measuring protein dynamics using two-color fluorescence recovery after photo-bleaching at Drosophila synapses. Nat Protocols 2: 3285–3298. [DOI] [PubMed] [Google Scholar]
- 33. Schmid A, Hallermann S, Kittel RJ, Khorramshahi O, Frölich AM, et al. (2008) Activity-dependent site-specific changes of glutamate receptor composition in vivo. Nat Neurosci 11: 659–666. [DOI] [PubMed] [Google Scholar]
- 34. Badre NH, Martin ME, Cooper RL (2004) The physiological and behavioral effects of carbon dioxide on Drosophila melanogaster larvae. Comp Biochem Physiol A Mol Integr Physiol 140: 363–376. [DOI] [PubMed] [Google Scholar]
- 35. Ghannad-Rezaie M, Wang X, Mishra B, Collins C, Chronis N (2012) Microfluidic chips for in vivo imaging of cellular responses to neural injury in Drosophila larvae. PLoS One 7: e29869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honti V, Cinege G, Csordás G, Kurucz É, Zsámboki J, et al.. (2013) Variation of NimC1 expression in Drosophila stocks and transgenic strains. Fly (Austin). 7. [DOI] [PMC free article] [PubMed]
- 37. Honti V, Kurucz É, Csordás G, Laurinyecz B, Márkus R, et al. (2009) In vivo detection of lamellocytes in Drosophila melanogaster. Immunol Lett. 126: 83–84. [DOI] [PubMed] [Google Scholar]
- 38. Vilmos P, Jankovics F, Szathmári M, Lukácsovich T, Henn L, et al. (2009) Live imaging reveals that the Drosophila actin-binding ERM protein, moesin, co-localizes with the mitotic spindle. Eur J Cell Biol. 88: 609–619. [DOI] [PubMed] [Google Scholar]
- 39. Jankovics F, Brunner D (2006) Transiently reorganized microtubules are essential for zippering during dorsal closure in Drosophila melanogaster. Dev Cell. 11: 375–385. [DOI] [PubMed] [Google Scholar]
- 40. Dreschers S, Hotz-Wagenblatt A, Gateff E (1994) Tumorous blood cells in Drosophila melanogaster: Experiments concerning the expression of the lethal(3)malignant blood neoplasm, l(3)mbn, tumor suppressor gene. Europ. J. Cell Biol. 63: 82. [Google Scholar]
- 41. Beetz S, Brinkmann M, Trenczek T (2004) Differences between larval and pupal hemocytes of the tobacco hornworm, Manduca sexta, determined by monoclonal antibodies and density centrifugation. J Insect Physiol. 50: 805–819. [DOI] [PubMed] [Google Scholar]
- 42. Nakahara Y, Shimura S, Ueno C, Kanamori Y, Mita K, et al. (2009) Purification and characterization of silkworm hemocytes by flow cytometry. Dev Comp Immunol. 33: 439–448. [DOI] [PubMed] [Google Scholar]
- 43. King JG, Hillyer JF (2013) Spatial and temporal in vivo analysis of circulating and sessile immune cells in mosquitoes: hemocyte mitosis following infection. BMC Biol. 11: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunostaining of circulating hemocytes with the sequential indirect immunofluorescent method (A), and the mixture of antibodies (B). The staining is shown in red, and the hemocytes are marked by their GFP expression (green), and DAPI nuclear staining (blue). The scale bar indicates 50 µm.
(TIF)
The sessile hematopoietic tissue (green) of an immobilized R3-Hml>GFP larva. The video was rendered with 5 frames per second, and represents 20 min of recording.
(AVI)
The sessile hematopoietic tissue (green) of an immobilized R3-Hml>GFP larva immunostained in situ with anti-Hemese (red). The video was rendered with 5 frames per second, and represents 20 min of recording.
(AVI)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.