Abstract
Patient: Female, 43
Final Diagnosis: —
Symptoms: Diarrhea • generalized weakness • headache • lightheadedness • nausea • rash • short of breath • vomiting
Medication: —
Clinical Procedure: —
Specialty: Pulmonology
Objective:
Rare diseae
Background:
IFN-alpha-2b in combination with ribavirin is now the standard of care for the treatment of hepatitis C. Sarcoidosis is a chronic multisystem granulomatous disorder characterized by noncaseating granulomas in the involved organs. The pathologic hallmark of sarcoidosis is the presence of noncaseating granulomas in the interstitium that typically involve the lymphatics.
Case Report:
A 43-year-old woman presented to our care with 2-week history of nausea, vomiting, diarrhea, shortness of breath, migraine headache, maculopapular rash, generalized weakness, and lightheadedness. She had been treated for hepatitis C with telaprevir, ribavirin, and interferon-alpha-2b for 6 months. Chest radiograph showed bilateral diffuse prominence of bronchovascular markings. CT of the chest revealed bilateral diffuse centrilobular nodules with associated intralobular septal thickening, thickening of the central peribronchovascular interstitium, nodularity of the major fissures, and mediastinal lymphadenopathy. These findings were suspicious for atypical pulmonary sarcoidosis, possibly interferon-induced. The pathology of the mediastinal lymph node biopsy revealed noncaseating granulomatous inflammation consistent with the diagnosis of pulmonary sarcoidosis. Pathology of the skin punch biopsy showed giant-cell granulomatous inflammation without necrosis. The patient was started on prednisone 40 mg daily with a steroid tapering course for 8 weeks.
Conclusions:
The management of IFN-induced sarcoidosis includes the discontinuation of IFN therapy with or without the administration of systemic corticosteroids. With the increasing prevalence of HCV in the United States, it is likely that more IFN-alpha-induced sarcoidosis will be encountered by clinicians.
MeSH Keywords: Hepatitis C, Interferons - adverse effects, Sarcoidosis - chemically induced
Background
The biologic effects of interferon (IFN) involve stimulation of natural killer cell activity, T cell-mediated immunity, cytokine synthesis, and activation of macrophages. Interferons act by up-regulating the immune system and enhancing the immune response [1]. Interferons are classified into 2 types: Type 1 IFN-alpha and IFN-beta, and Type 2 IFN-gamma. Recombinant interferons are immunomodulators in the treatment of viral infections such as hepatitis B and C, malignancies such as melanoma, renal cell carcinoma, lymphoproliferative disorders, and multiple sclerosis [2].
IFN-alpha in combination with ribavirin is now the standard of care for the treatment of hepatitis C [3]. The prevalence of hepatitis C in the general population is 1–2%. Early treatment of acute hepatitis C with interferon-alpha-2b will prevent chronicity in a majority of patients but treatment-related adverse effects can result in the discontinuation of therapy [4]. Adverse reactions from IFN therapy are non-specific, such as generalized malaise, fever, cough, and arthralgia. A variety of pulmonary complications have been associated with IFN, including bronchial asthma exacerbation, bronchiolitis obliterans, pleural effusion, and interstitial pneumonitis [4].
Sarcoidosis is a chronic multisystem granulomatous disorder characterized by noncaseating granulomas in the involved organs. The prevalence is estimated to be 10–20 per 100 000 population [5]. Sarcoidosis is more common in African American women and most commonly affects people 25–40 years old [6]. The etiology is not known, but an exaggerated immune response to an antigenic stimulus could play a role. Possible antigens could include infectious agents such as mycobacteria, environmental agents, or auto-antigens. The natural history of sarcoidosis is variable, with two-thirds of patients having spontaneous remission [7].
Sarcoidosis can be diagnosed by the clinical presentation, radiologic findings, and histopathologic findings of non-caseating granulomas [7]. Sarcoidosis affects the lungs (>90%), lymphoid system (33%), liver (50–80%), eyes (11–83%), and skin (25%) [8]. Usual findings on plain chest radiography in pulmonary sarcoidosis include hilar lymphadenopathy and/or reticulonodular infiltrates [9]. The pathologic hallmark of sarcoidosis is the presence of noncaseating granulomas in the interstitium that typically involve the lymphatics. We herein present the case of a patient with hepatitis C who developed cutaneous and pulmonary sarcoidosis induced by IFN-alpha.
Case Report
A 43-year-old Caucasian woman presented to our care with 2-week history of nausea, vomiting, and watery diarrhea. The nausea and vomiting occurred with about 1–2 episodes per day and was described as non-projectile vomiting with bilious consistency She had 1–2 episodes of watery diarrhea per day but noted no blood in the stool. Due to these symptoms, she had food aversion and therefore lost 6 kilograms in body weight during those 2 weeks. Her other complaints included a migraine headache, lightheadedness, generalized weakness, and shortness of breath. Approximately 6 months prior to this admission, she was being treatment for hepatitis C with telaprevir 750 mg 3 times a day (TID), ribavirin 400 mg daily, and interferon-alpha-2b 3 million unit subcutaneous injections 3 times a week. Within the last 3 months she had developed a diffuse erythematous maculopapular rash on both upper and lower extremities. Her medical history included type 2 diabetes mellitus, hepatitis C, chronic smoker, and intravenous heroin abuse for 15 years (she last used heroin 5 years ago). Interferon-alpha-2b and ribavirin therapy was discontinued 1 month prior because of fatigue and depression symptoms thought to be adverse effects of interferon.
Initial vital signs on admission were not significant. Pertinent physical exam finding were a maculo-papular rash on the entire bilateral upper and lower extremities. There were no other significant physical exam findings. Initial laboratory work-up (Table 1) revealed mild hyponatremia, hyperglycemia, renal insufficiency, hypercalcemia, and elevated alkaline phosphatase. Results of tests for severe hypercalcemia with intact PTH, and PTH-related peptide were normal. The vitamin D 1,25 dehydrogenase, vitamin D 25-hydroxygenase, and TSH levels were normal. Urinalysis was positive for calcium oxalate crystals and random urine calcium of 50 mg/dL. She was initially admitted to the medical intensive care unit (MICU) for correction of the hypercalcemia with intravenous fluid resuscitation and calcitonin 280 units subcutaneously injected twice daily (4 units/kg every 12 hours). The hypercalcemia improved by the 6th hospital day and the renal insufficiency improved by the 10th hospital day.
Table 1.
Initial laboratory work up.
| White blood cell count | 5.63×103 uL |
| Hemoglobin | 15.6 g/dL |
| Hematocrit | 45.5% |
| Platelet count | 329×103/uL |
| Sodium | 132 mmol/L |
| Potassium | 4.5 mmol/L |
| Chloride | 96 mmol/L |
| CO2 | 25 mmol/L |
| Serum glucose | 275 mg/dL |
| BUN | 38 mg/dL |
| Creatinine | 3.01 mg/dL |
| Calcium | 15.6 mmol/L |
| Albumin | 4.0 g/dL |
| Protein | 6.7 g/dL |
| AST | 16 IU/L |
| ALT | 28 IU/L |
| Alk. phosphatase | 141 IU/L |
| Ionized Ca | 1.96 mmol/L |
| Intact PTH | 3.7 pg/mL |
| HIV | Negative |
Chest radiograph (Figure 1) on admission showed bilateral diffuse prominence of bronchovascular markings. Computed tomography (CT) of the chest (Figure 2) revealed bilateral diffuse centrilobular nodules with associated intralobular septal thickening. There was also thickening of the central peribronchovascular interstitium, nodularity of the major fissures, mediastinal lymphadenopathy, and hypercalcemia. These finding were suspicious for atypical pulmonary sarcoidosis, possibly induced by treatment with interferon. Pulmonary function test results (PFT) were normal. A bone survey showed no bony metastasis or lytic lesions. Renal ultrasound showed multiple echogenic foci suggestive of nonobstructive calculi in the left kidney.
Figure 1.
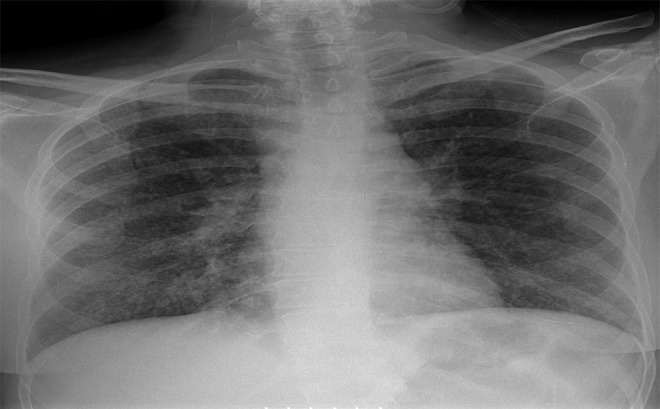
Plain chest radiograph: bilateral diffuse prominence of bronchovascular markings.
Figure 2.
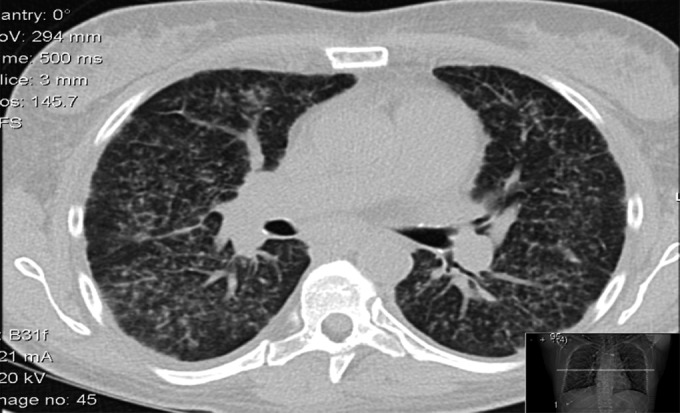
CT chest: Bilateral diffuse centrilobular nodules with associated intralobular septal thickening.
No monoclonal proteins were detected on serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP). Angiotensin-converting enzyme was elevated at 132 U/L (9–67 U/L). On the 6th hospital day, a bronchoscopy was performed, with findings of normal anatomic airways and mucosa. The bronchoalveolar lavage of the right middle lobe did not reveal any malignancy, acid-fast bacilli (AFB), Pneumocystis carinii, or fungal organisms. A transbronchial biopsy of the mediastinal lymph node was subsequently performed on that day.
The pathology of the mediastinal lymph node biopsy revealed noncaseating granulomatous inflammation consistent with the diagnosis of pulmonary sarcoidosis (Figure 3). Special stains for AFB and fungus were negative. A skin punch biopsy (Figure 4) of the maculopapular rash on the right forearm was performed on the 10th hospital day. Pathology findings revealed giant cell granulomatous inflammation without necrosis (Figure 4).
Figure 3.
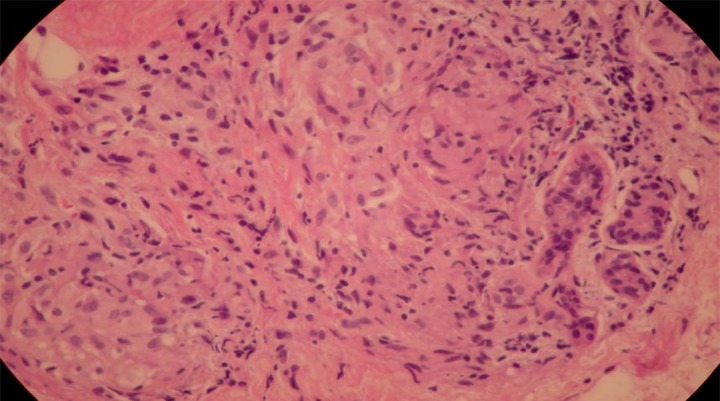
Transbronchial lung biopsy: Noncaseating granulomas.
Figure 4.
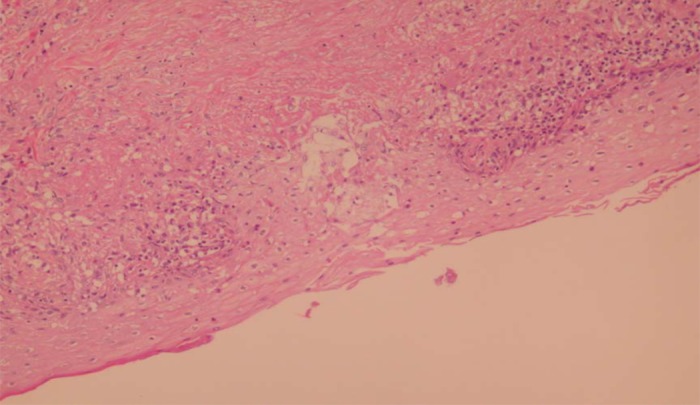
Skin biopsy of right forearm lesion: Giant-cell granulomatous inflammation without necrosis.
The patient was started on prednisone 40 mg daily with a steroid tapering course for 8 weeks. On 1.5-month follow-up at the clinic, she still noted shortness of breath with exertion but was improved since being discharged from the hospital. The maculopapular rash completely resolved. Follow-up PFT’s and chest radiograph were still normal. She was continued on prednisone 20 mg daily, which was tapered to completion of therapy within 2 weeks.
Discussion
The first recognized case of IFN-alpha induced sarcoidosis was reported in 1987 in a woman treated with IFN-alpha for renal cell carcinoma (RCC) [10]. Since then, several cases of IFN-induced sarcoidosis have been reported in the medical literature. Recent data suggest that pulmonary sarcoidosis may be an underreported complication of IFN-a therapy. IFN has been shown to result in pulmonary macrophage activation, a characteristic feature of sarcoidosis. Hepatitis C virus may be a cofactor in the pathogenesis of sarcoidosis in patients receiving IFN. Diffuse nodular parenchymal abnormalities were observed in our patient, but this finding is not commonly seen in sarcoidosis cases. The granulomas are composed of aggregations of epithelioid histiocytes and multinucleated giant cells [11]. One histologic difference between de novo and IFN-associated sarcoidosis is that the granulomas of IFN-associated sarcoidosis are more tightly formed.
Our patient did not have any clinical findings to suggest preexisting sarcoidosis. After careful evaluation of our patient and other cases in the medical literature, we conclude that it appears that IFN can exacerbate sarcoidosis. Management includes the discontinuation of IFN therapy with or without the administration of systemic corticosteroids [12]. Most patients will have complete resolution of the disease within a few months of discontinuing interferon therapy, without the need for immunosuppressants [13]. The prognosis for IFN-alpha-induced sarcoidosis is very good, with improvement once medication is discontinued [14]. Sarcoidosis should be considered in the differential diagnosis of a patient who develops pulmonary disease, lymphadenopathy, or abnormalities of any organ while undergoing IFN therapy [15]. With the increasing prevalence of HCV in the United States, it is likely that more cases of IFN-alpha-induced sarcoidosis will be encountered by clinicians.
Conclusions
Our case demonstrates that sarcoidosis should be considered in the differential diagnosis of a patient who develops cutaneous or pulmonary manifestations while undergoing IFN therapy.
Footnotes
Disclosures
All participating authors in this study declare no financial, professional, or personal conflicts.
No grant support was received for this case report.
All Authors were involved in manuscript preparation and literature review.
References:
- 1.Ravenel JG, McAdams HP, Plankeel JF, et al. Sarcoidosis induced by interferon therapy. Am J Roentgenol. 2001;177(1):199–201. doi: 10.2214/ajr.177.1.1770199. [DOI] [PubMed] [Google Scholar]
- 2.Petousi N, Thomas EC. Interferon-β-induced pulmonary sarcoidosis in a 30-year-old woman treated for multiple sclerosis: a case report. J Med Case Rep. 2012;6(1):344. doi: 10.1186/1752-1947-6-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle MK, Berggren R, Magnus JH. Interferon-Induced Sarcoidosis. J Clin Rheumatol. 2006;12(5):241–48. doi: 10.1097/01.rhu.0000240035.67652.9d. [DOI] [PubMed] [Google Scholar]
- 4.Baron S, Tyring SK, Fleischmann WR, Jr, et al. The interferons. Mechanisms of action and clinical applications. JAMA. 1991;266:1375–83. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 5.Lelerc S, Myers RP, Moussalli J, et al. Sarcoidosis and interferon therapy: report of five cases and review of the literature. Eur J Inter Med. 2003;14(4):237–43. doi: 10.1016/s0953-6205(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso C, Freire R, Alves A, Oliveira A. Interferon-induced sarcoidosis. BMJ Case Rep, 2011. 2011;pii doi: 10.1136/bcr.03.2011.3929. bcr0320113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YB, Lee JI, Park HJ, et al. Interferon-alpha Induced Sarcoidosis with Cutaneous Involvement along the Lines of Venous Drainage. Ann Dermal. 2011;23(2):239–41. doi: 10.5021/ad.2011.23.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gayet AR, Plaisance P, Bergmann JF, Mouly S. Developmet of sarcoidosis following completion of treatment for hepatitis C with pegylated interferon-alpha 2a and ribavirin: a case report and literature review. Clin Med Res. 2010;8(3–4):163–67. doi: 10.3121/cmr.2010.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes H, Soler P, Valeyre D. Pulmonary sarcoidosis. Allergy. 2005;60(5):565–82. doi: 10.1111/j.1398-9995.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 10.Abdi EA, Nguyen GK, Ludwig RN, Dickout WJ. Pulmonary sarcoidosis following interferon therapy for advanced renal cell carcinoma. Cancer. 1987;59:896–900. doi: 10.1002/1097-0142(19870301)59:5<896::aid-cncr2820590507>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Butnor KJ. Pulmonary sarcoidosis induced by interferon-alpha therapy. Am J Surg Pathol. 2005;29(7):976–79. doi: 10.1097/01.pas.0000160442.23523.0a. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg HJ, Fiedler D, Webb A, et al. Sarcoidosis after treatment with interferon-alpha: a case series and review of the literature. Respir Med. 2006;100(11):2063–68. doi: 10.1016/j.rmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Marzouk K, Saleh S, Kannass M, Sharma OP. Interferon-induced granulomatous lung disease. Curr Opin Pulm Med. 2004;10(5):435–40. doi: 10.1097/01.mcp.0000134400.88832.9c. [DOI] [PubMed] [Google Scholar]
- 14.Alazemi S, Campos MA. Interferon-induced sarcoidosis. Int J Clin Pract. 2006;60(2):201–11. doi: 10.1111/j.1742-1241.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 15.Celik G, Sen E, Ulger AF, et al. Sarcoidosis caused by interferon therapy. Respirology. 2005;10(4):535–40. doi: 10.1111/j.1440-1843.2005.00746.x. [DOI] [PubMed] [Google Scholar]


