Abstract
Background
The purpose of our study was to analyze the frequency of focal fatty replacement (FR) of the heart, as well as the distribution and detailed morphology of FR in a large group of patients referred to multi-slice computed tomography with ECG-gating examinations (ECG-MSCT) for various clinical reasons.
Material/Methods
The ECG-MSCT examinations of 1830 consecutive patients were analyzed. The examinations were performed using 8-row (1015 patients) and 64-row (815 patients) MSCT, in pre- and post-contrast scanning.
We analyzed the morphology of FR, the dimensions and densities of changes, as well as the morphology and localization of FR with regard to clinical diagnosis.
Results
204 subjects (11.1%) had FR within the heart (113 men; 91 women; mean age 57.8 years); 66% of fatty foci were seen only in the native scanning. The distribution of the fat was: right ventricle (RV) 31.9%, left ventricle (LV) 21.5%, biventricular 39.7%, interventricular or atrial septum 5.9%, and atria 1%. In the RV, fat was localized mainly in the papillary muscles, while in the LV fat was mainly subendocardial (p<0.001). The morphology of the fat was: linear 61.6%, oval 14.8%, punctuate 10.6%, irregular 10.2%, and bilobular 2.8%.
Fat was primarily located subendocardially in the LV in patients after myocardial infarction. In patients with suspected coronary artery disease, it was mainly observed subpericardially in the RV and in papillary muscles (p<0.001).
Conclusions
The incidental frequency of FR within the heart in patients diagnosed with the ECG-MSCT examinations is about 11%. Pre-contrast scanning is the most valuable for FR assessment.
MeSH Keywords: Arrhythmogenic Right Ventricular Dysplasia, LHIS, Fat, ECG-MSCT, Myocardial Infarction, Myocardium
Background
ECG-gated multi-slice computed tomography (ECG-MSCT) is an important noninvasive diagnostic tool in cardiac imaging. As the number of ECG-MSCT studies continues to grow, radiologists are noting with increasing frequency various pathological changes within cardiac muscle, including the presence of fatty foci. Although a search of available literature did reveal a small number of publications addressing this topic, to date there are no studies that take a comprehensive look at the occurrence and detailed morphology of fatty changes within the heart in ECG-MSCT examinations.
The presence of fat within the heart has been linked to a number of pathologies. Replacement of the ventricular myocardium by fat is a feature of arrhythmogenic right ventricular dysplasia (ARVD) [1,2]. Fat within the ventricular myocardium is also related to prior myocardial infarction [3,4]. Lipomatous hypertrophy of the interatrial septum (LHIS) is a benign disorder characterized by fat accumulation in the interatrial septum [5–8]. Myocardial fat is also seen in patients with cardiac lipomas and tuberous sclerosis complex [9,10]. Fatty infiltration of the right ventricle, however, can also be present in healthy people [11,12].
The purpose of our study was to analyze the frequency of focal fatty replacement of the heart, as well as the distribution and morphology of fatty deposits in a large group of patients referred for ECG-MSCT examinations for various clinical reasons.
Material and Methods
Our study group consisted of 1830 patients (802 women, 1028 men), averaging 53.5 years of age (range 6 to 90 years of age). ECG-MSCT examinations were carried out and analyzed retrospectively regarding the occurrence of fatty deposits within the heart. This study was approved by the Bioethics Committee of the Medical University of Lublin, Poland (number KE-0254/153/2009) and patient consent was waived.
Examination of 1015 patients was performed using an 8-slice scanner (LightSpeed Ultra, GE Medical Systems), and in 815 patients a 64-slice scanner was used (LightSpeed VCT GE Medical Systems, Milwaukee, Wisconsin).
The examinations were performed in layers: 2.5 mm in native scans and 0.625mm (64-row CT) or 1.25 mm (8-row CT) after intravenous (IV) administration of contrast medium: a bolus of 70–100 ml of Ultravist (iopromide) 370 mgI/ml at a rate of 4–5 ml/s, followed by 30 ml of saline at 4–5 ml/s (64-row CT). In all cases retrospective ECG-gating was employed. When necessary, patients received 25–50 mg of oral metoprolol for heart rate reduction administered 30–60 minutes prior to examination. Reconstruction windows were set manually, depending on heart size, FOV 20–27 cm and a few centimeters above the tracheal bifurcation and below the lower heart border. The output data were sent to a diagnostic console after scanning (Advantage Workstation 4.2 or 4.3, GE Medical Systems). Two experienced radiologists analyzed all ECG-MSCT images.
Fatty changes were defined as areas with negative Hounsfield unit (HU) values in routine or enhanced phase of examination. Fatty changes were divided into: linear, oval, irregular, bilobular, and punctuate based on morphology visible on axial images (Figure 1A–1E). Linear changes were defined as lesions with regular contours for which the longitudinal dimension was twice as large as the transverse dimension. Oval changes were defined as lesions with regular contours for which the longitudinal dimension was equal to or greater than the transverse dimension. Irregular changes were defined as lesions with irregular contours for which the transverse dimension was equal to or greater than the longitudinal dimension. Punctate changes were defined as lesions with regular contours for which the transverse dimension was equal to the longitudinal dimension and with a diameter equal to or less than 2 mm. Bilobular changes were defined as lesions with regular contours for which the longitudinal dimension was greater than the transverse dimension with a visible midline constriction (Figure 2).
Figure 1.
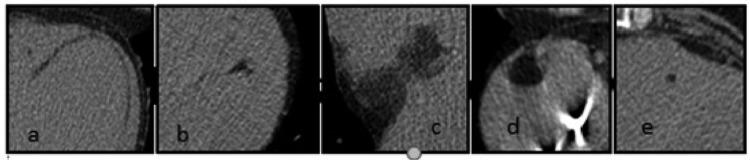
The morphology of the fatty deposits: (A) linear; (B) irregular; (C) bilobular; (D) oval; (E) punctate.
Figure 2.
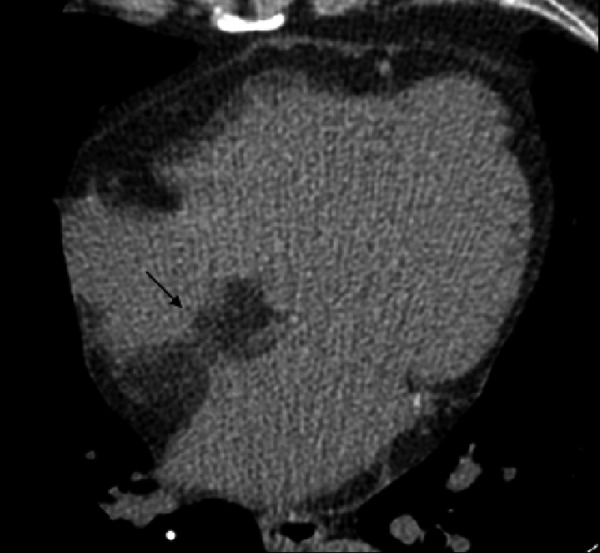
Figure represents the bilobular morphology of the interatrial septum in 69-year-old patient with LHIS.
The following dimensions were determined for each fatty change noted on axial images in both phases of examination:
– The maximum diameter was measured for oval changes.
– The maximal transverse dimension was measured for punctual and irregular changes.
– The greatest length along the curved line was measured for linear changes.
For bilobular changes, the maximum width of the septum was measured within the region of the fossa ovalis. This measurement was made in the native scanning and after contrast administration during the end-diastolic phase on reconstructed images obtained along the long axis of the heart.
The exact location of each fatty change within the heart was noted.
The density of fatty foci was measured on axial images in pre-contrast and post-contrast scanning. Density was measured at a single point for oval and irregular changes measuring up to 5 mm in transverse diameter. For oval and irregular changes measuring greater than 5 mm in transverse diameter, density was measured at 3 points and then estimated mean value of optical density was determined. The mean optical density was measured for changes greater than 10 mm in diameter.
Morphology and localization of fatty changes within the heart were also analyzed with regard to clinical diagnosis. Patients were classified into one of the following groups: after myocardial infarction (MI) (60 patients), suspected coronary artery disease (56 patients) and “other” (88 persons). The number of patients and different clinical diagnoses comprising the group “other” are shown in Table 1.
Table 1.
Main clinical diagnosis in the group of patients classified as “other” (n=88).
| Clinical diagnosis | Number of patients |
|---|---|
| Arrhythmias | 14 |
| Cardiomyopathy | 7 |
| Suspicion of LHIS in echocardiography | 2 |
| Tumor within atria diagnosed in echocardiography | 2 |
| Suspicion of pericardial effusion | 2 |
| Aortic aneurysm | 4 |
| Aortic stenosis | 1 |
| Hypertension | 13 |
| Atypical chest pain | 41 |
| Dyspnea | 1 |
| Endocarditis | 1 |
Statistical analysis
Statistical analysis was performed using the Statistica 9.0 program.
Data are expressed as min, max, and mean ± standard deviation. The χ2 test and t test were used to determine the significance between variables. A value of p<0.05 was considered statistically significant.
Results
Presence of fatty foci within the heart was diagnosed in 204 patients (11.1% of the analyzed group).
Patients with fatty deposits within the heart included 113 (11.0% of analyzed group) men and 91 (11.1%) women, averaging 57.8 years of age. Average BMI index in the analyzed group was elevated (28.0 for women, ranging from 23.6 to 42.75; for men 27.9, ranging from 20.3 to 35.4). The average BMI index for patients with LHIS was 28.0 for women and 27.7 for men.
The distribution of fat in 204 patients was as follows: right ventricle (RV) − 65 patients (31.9%), left ventricle (LV) − 44 (21.5%), biventricular − 81 (39.7%), interventricular (IVS) or interatrial septum (IAS) − 12 (5.9%), left atrium (LA) − 1 (0.5%), and right atrium (RA). The exact distribution of fatty foci within the heart is shown in Table 2.
Table 2.
Localization of fatty deposits (n=539).
| Location | Number of fatty foci (n=539) | |
|---|---|---|
| Subendocardially | RV | 39 |
| LV | 95 | |
|
| ||
| Intramyocardially | RV | 10 |
| LV | 10 | |
|
| ||
| Subepicardially | RV | 50 |
| LV | 34 | |
|
| ||
| Entire wall | RV | 23 |
| LV | 12 | |
|
| ||
| Papillary muscles | RV | 139 |
| LV | 50 | |
|
| ||
| Interventricular septum | 60 | |
|
| ||
| Interatrial septum | 15 | |
|
| ||
| Right atrium | 1 | |
|
| ||
| Left atrium | 1 | |
The total number of identified fatty foci was 539. Morphology of fatty deposits was as follows: linear − 332 foci (61.6%), oval − 80 (14.8%), irregular − 55 (10.2%), punctate − 57 (10.6%), bilobular − 15 (2.8%).
The number of fatty foci in particular localization is shown in Table 3.
Table 3.
Location of the various morphological types of fatty lesions within the cardiac wall and papillary muscle n=539.
| Morphology | Localization | Number of fatty foci/(%) |
|---|---|---|
| Linear (n=332) | LV subendocardially | 72/(21.5) |
| LV subepicardially | 15/(4.4) | |
| LV intramyocardially | 4/(1.3) | |
| LV entire wall | 3/(1.1) | |
| LV papillary muscle | 29/(8.7) | |
| RV subendocardially | 28/(8.4) | |
| RV subepicardially | 39/(12.0) | |
| RV intramyocardially | 2/(0.7) | |
| RV entire wall | 15/(4.4) | |
| RV papillary muscle | 73/(21.8) | |
| IV septum | 52/(15.7) | |
| Oval (n=80) | LV subendocardially | 5/(5.9) |
| LV subepicardially | 14/(17.6) | |
| LV entire wall | 5/(5.9) | |
| LV papillary muscle | 8/(10.3) | |
| RV subendocardially | 7/(8.8) | |
| RV subepicardially | 6/(7.3) | |
| RV entire wall | 4/(4.4) | |
| RV papillary muscle | 29/(36.8) | |
| LAwall | 1/(1.5) | |
| RAwall | 1/(1.5) | |
| Punctate (n=57) | LV subendocardially | 6/(9.8) |
| LV subepicardially | 3/(4.9) | |
| LV intramyocardially | 3/(4.9) | |
| LV papillary muscle | 8/(14.6) | |
| RV subendocardially | 3/(4.9) | |
| RV subepicardially | 4/(7.3) | |
| RV intramyocardially | 4/(7.3) | |
| RV papillary muscle | 26/(46.3) | |
| Irregular (n=55) | LV subendocardially | 12/(22.6) |
| LV subepicardially | 2/(3.2) | |
| LV intramyocardially | 3/(4.8) | |
| LV entire wall | 4/(6.5) | |
| LV papillary muscle | 5/(9.7) | |
| RV subendocardially | 1/(1.6) | |
| RV subepicardially | 1/(1.6) | |
| RV intramyocardially | 4/(6.5) | |
| RV entire wall | 4/(8.0) | |
| RV papillary muscle | 11/(21.0) | |
| IV septum | 8/(14.5) | |
| Bilobular | IA septum | 15/(100) |
There were significant differences (p<0.001) in the distribution of fatty foci: in the right ventricle fat was localized mainly in the papillary muscles, while in the left ventricle it was localized mainly subendocardially.
When comparing the localization of fatty deposits within the heart we found that that linear changes localized mainly subendocardially in the left ventricle and in the papillary muscles in the right ventricle. Oval and punctate changes, however, localized primarily in the papillary muscles in the right ventricle. Irregular fatty changes were located mainly subendocardially in the left ventricle. The bilobular fatty changes were localized only within the interatrial septum (Table 4).
Table 4.
Visibility of fatty foci with regard to contrast medium administration.
| Morfological type of fatty foci | Visible only in the pre-contrast scanning n/% | Visible only in the post-contrast scanning n/% | Visible in pre-contrast and post-contrast scanning n/% |
|---|---|---|---|
| Punctate | 57/100.0 | – | – |
| Oval | 48/60.0 | 5/6.6 | 27/33.4 |
| Irregular | 14/25.0 | – | 41/75.0 |
| Linear | 218/65.6 | – | 114/34.4 |
| Bilobular | – | – | 15/100.0 |
| Total number of fatty foci | 337 | 5 | 197 |
The parameters determining the size and optical density of the fatty foci in pre-contrast and post-contrast scanning were analyzed. Effect of administration of contrast medium on the visibility of fatty changes, as well as their size and density in both phases of examination, are presented in Tables 5 and 6.
Table 5.
Morphological parameters of fatty deposits visible in both phases of examination.
| Linear (n=99) | Oval (n=27) | Irregular (n=41) | Bilobular (n=15) | |||
|---|---|---|---|---|---|---|
| Length/diameter (mm) | Pre-contrast phase | Mean | 26.9 | 7.6 | 10.4 | 19.0 |
| Min | 2.5 | 2.7 | 3.3 | 15.0 | ||
| Max | 161.0 | 47.0 | 47.0 | 34.0 | ||
| SD | 29.8 | 8.5 | 9.0 | 4.6 | ||
| Post-contrast phase | Mean | 21.7 | 5.9 | 5.7 | 19.0 | |
| Min | 0.5 | 1.0 | 1.0 | 15.0 | ||
| Max | 166.0 | 39.0 | 19.0 | 34.0 | ||
| SD | 30.2 | 7.3 | 4.1 | 4.6 | ||
| p value | 0.2241 | 0.44 | 0.02 | ? | ||
| Optical density (HU) | Pre-contrast phase | Mean | −38.1 | −44.7 | −43.2 | −73.3 |
| Min | −92.0 | −111.0 | −85.0 | −123.0 | ||
| Max | −2.0 | −2.0 | −13.0 | −6.2 | ||
| SD | 19.9 | 26.4 | 20.8 | 26.5 | ||
| Post-contrast phase | Mean | −30.0 | −30.7 | −31.8 | −25.0 | |
| Min | −93.0 | −81.0 | −91.0 | −107.0 | ||
| Max | 0.0 | −1.0 | −11.0 | 40.5 | ||
| SD | 18.6 | 18.4 | 20.1 | 43.4 | ||
| p value | 0.0035 | 0.0279 | 0.0639 | <0.0009 |
Table 6.
The differences in distribution and morphology of fatty lesions with regard to clinical diagnosis.
| Clinical diagnosis | MI | Suspected coronary artery disease | Other | ||
|---|---|---|---|---|---|
| Localization | RV | Number of fatty foci | 7 | 8 | 20 |
| LV | 13 | 3 | 7 | ||
| RV+LV | 13 | 6 | 14 | ||
| RV+IAS/IV | 2 | 14 | 13 | ||
| LV+ IAS/IV | 15 | 5 | 2 | ||
| RV+LV+ IAS/IV | 9 | 17 | 22 | ||
| IAS/IV | 0 | 3 | 8 | ||
| LA | 0 | 0 | 1 | ||
| RA | 0 | 0 | 1 | ||
| Morphology | Linear | Number of fatty foci | 32 | 18 | 23 |
| Punctual | 0 | 1 | 5 | ||
| Oval | 0 | 3 | 9 | ||
| Irregular | 1 | 1 | 1 | ||
| Mixed | 27 | 32 | 49 | ||
| Bilobular | 0 | 1 | 1 | ||
| Localization within the heart wall | LV subendocardially | Number of fatty foci | 42 | 11 | 17 |
| LV subpericardially | 6 | 5 | 17 | ||
| LVintramyocardially | 4 | 6 | 6 | ||
| LV entire wall | 4 | 2 | 11 | ||
| RV subendocardially | 7 | 8 | 17 | ||
| RV subpericardially | 7 | 12 | 12 | ||
| RVintramyocardially | 1 | 2 | 2 | ||
| RV entire wall | 5 | 5 | 12 | ||
Fatty foci were visible in pre-contrast scanning in 66% of cases. Almost half as many fatty lesions were seen in both phases of examination. Punctate foci were visible only in the native scans. Oval changes were seen in 60% of cases, linear changes in 65%, and irregular in 25% were visible only in the native scanning. Only the irregular changes in 75% of cases were seen in both phases of examination. All bilobular fatty deposits were seen in both phases of examination.
For fatty deposits that were visible in both phases of examination, significant differences were found in the amount of size change depending on the phase of examination.
The dimensions of all fatty changes evaluated after administration of contrast medium were lower in relation to their size prior to administration of contrast medium. The dimensions of irregular and bilobular fatty changes were significantly lower in post-contrast scanning. Also, the optical density of fatty changes in post-contrast scanning was significantly higher for linear, oval, and bilobular deposits (Table 6).
A significant association was found between the localization of fatty lesions within the heart and clinical diagnosis (p<0.001). In patients after MI, fatty deposits were usually located within the left ventricle and interventricular septum. Among patients with suspected coronary artery disease and diagnosed as “other”, fatty foci were mainly localized simultaneously in both ventricles and septum.
No significant correlation was found between the morphology of fatty changes and clinical diagnosis (p<0.087). In patients with advanced coronary artery disease, only linear, irregular, and mixed changes occurred. In patients with suspected coronary artery disease and a diagnosis of “other”, the most frequent mixed changes were diagnosed (Figure 3). Oval and punctate changes occurred mainly in patients with a diagnosis of “other”.
Figure 3.
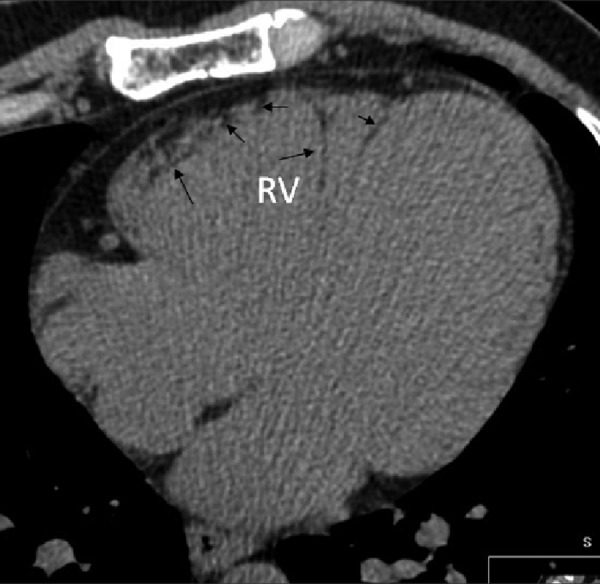
Numerous fatty deposits of various shapes in the right ventricle in 40-year-old patient with arrhythmogenic right ventricular dysplasia.
A statistically significant relationship was found between the localization of fatty foci within the heart wall and clinical diagnosis (p<0.001). Fatty lesions were primarily located subendocardially in the left ventricle in patients with MI (Figure 4). In patients with suspected coronary artery disease, fatty foci were mainly observed subpericardially in the right ventricle and papillary muscles. In patients with a diagnosis of “other”, fatty changes were most frequently localized subendocardially and subpericardially in the left ventricle and subendocardially in the right ventricle (Table 6).
Figure 4.
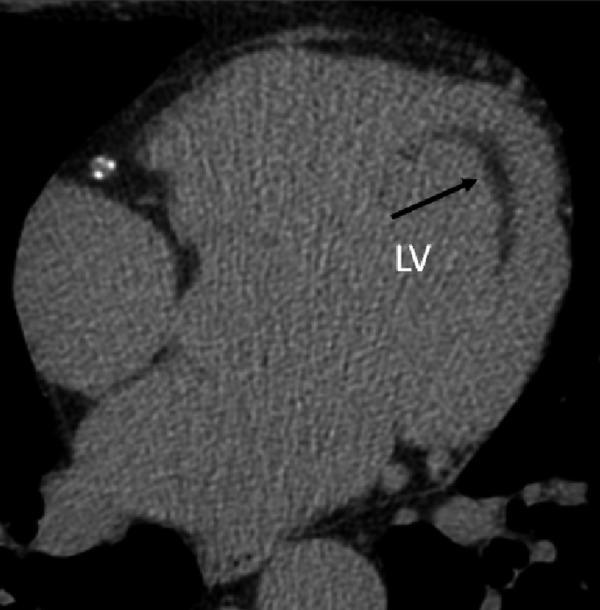
Linear fatty focus localized subendocardially in the left ventricle in a 55-year-old patient after myocardial infarction.
Discussion
Development of new cardiac imaging techniques like ECG-MSCT and magnetic resonance imaging (MRI) enables detailed visualization of the heart as well as the presence of fatty foci within heart muscle. The most frequently reported diseases in which fatty foci are seen are ARVD, LHIS, lipomatous tumor, and post-myocardial infarction [1–12].
ARVD is a disease characterized by fibro-fatty replacement of the ventricular myocardium [1,2]. Dysplastic changes are localized within the right ventricular free wall and are located in the so-called “triangle of dysplasia”, which is an area between the tip, funnel, and right ventricular outflow tract (RVOT). As a result, weakening of the ventricular wall occurs, causing right ventricular enlargement and the formation of aneurysms [13].
The main diagnostic modalities for the detection of ARVD are magnetic resonance imaging, echocardiography, and radionuclide imaging. To date, there are only a few reports in the literature on the diagnosis of ARVD by computed tomography (CT) [14–16].
Lipomatous hypertrophy of the interatrial septum is a rare pathology that leads to mild adipose infiltration of the interatrial septum. The frequency of occurrence for this condition is estimated at 1–8% and, depending on the diagnostic method employed, from 1% in autopsy examination to 2–8% in echocardiography [17].
The etiology of LHIS is unknown, but it is generally thought to be a hamartoma type of change [18]. In clinical research, lipomatous hypertrophy may develop without any symptoms but it can also cause supraventricular cardiac arrhythmias and even sudden death [19]. Imaging diagnostics of lipomatous hypertrophy is primarily based on transthoracic echocardiography (TTE), trans-oesophageal echocardiography (TOE), MRI, and CT.
Fatty tissue may be also present within the scar after a healed myocardial infarction. Patients with peripheral artery disease are at particular risk for the incidence of myocardial infarction [20].
The presence of fat in the post-infarction scar in post-mortem material was described for the first time in 1997 [3]. Histological examination was performed on 247 hearts with fatty metaplasia of the infarction scar in 68% of cases.
The presence of myocardial fatty metaplasia in post-myocardial infarction patients was first diagnosed in vivo in 2004 [21]. The proposed mechanism responsible for this process is probably related to the presence of multipotential cells in the myocardium which, in response to an external stimulus (e.g., the therapeutic process), differentiate into fat cells [4]. The incidence of fatty changes after myocardial infarction correlates with increased age, the male sex, and after placement of a coronary artery bypass graft [22].
Cardiac lipomas are benign tumors composed of mature adipose tissue cells [23]. They are visible on CT as homogenous fatty masses within the heart chambers without contrast enhancement. The presence of fatty deposits was also described in patients with tuberous sclerosis complex [9].
Fatty deposits within the heart muscle may also be present in healthy elderly people [11,12,24]. Physiologic fat is located in the anterolateral right ventricular free wall, RVOT, and sometimes in the right ventricular trabeculae and the apex of the heart. In these cases the myocardial wall is either normal or thick [12].
To our knowledge there are no reports in the literature of a comprehensive and detailed approach to the morphology and localization of fatty foci within the heart. Available articles only partly address this issue. The majority of articles selectively present data on fatty foci in the right ventricle in a selected group of patients, as well as the presence of fatty foci in the left ventricle in a group of patients after myocardial infarction.
In our study, fatty foci were diagnosed in 204 patients (11.1% of the analyzed group). It is difficult to compare this result with data in the literature because of differences in data analysis and different criteria for selecting groups of patients. In our study, a large group of consecutive patients (1830) referred for ECG-MSCT examinations for different clinical reasons were analyzed. There is also a lack of systematic analysis of all localizations of fatty deposits within the heart by other authors. Jacobi et al. [25] detected fatty metaplasia of the heart in 1.4% of diagnosed patients compared to 11.1% in our study. This discrepancy between our 2 studies is most likely due to differences in data analysis and different criteria for selecting the patients to the analysis. Jacobi et al. analyzed non-contrast chest CT studies conducted without ECG gating in a group of patients sent for CT studies with the suspicion of respiratory diseases. Imada et al. [26] detected fatty metaplasia of heart muscle in 42.8% of diagnosed patients, but these authors analyzed fatty foci localized only in the right ventricle. They also examined a smaller group consisting of only 49 patients referred for ECG-MSCT examinations for the evaluation of the degree of atherosclerosis of coronary arteries or the presence of an aortic aneurysm. Such large differences in frequency of the incidence of fatty changes within the heart are most likely the result of different criteria of patient selection for analysis. Kim et al. [11] detected fatty metaplasia of the heart muscle in 17% of examined patients who underwent ECG-MSCT for the measurement of coronary calcium scores, but they too only analyzed the fatty deposits localized in the right ventricle. Hori et al. [24] detected fatty replacement in the right ventricle in 31 (25.8%) atherosclerotic patients.
Linear fatty changes were observed most often in our study (61.5% of fatty foci). Prior to our investigation, Kim et al. [11] was the only group to attempt to evaluate the morphology of fatty changes located in right ventricular muscle. The authors found that linear changes occurred in 53% of cases, with the band-like type occurring in 24% of cases and the punctate type in 23% of patients. The degree of right ventricle infiltration was also determined in their study. In 70% of cases, fat cells accumulated within one-third of the right ventricular wall. In 26% of the patients, fat occupied more than one-third, but less than two-thirds, of the right ventricular wall. Only 4% of fatty infiltration included the full thickness of the ventricular wall.
In our study, fatty foci most often localized simultaneously biventricularly and in the interventricular septum (23.5% of cases). In 17.1% of cases, fatty deposits were localized in the right ventricle and biventricularly in 16.1% of cases. To our knowledge only Jacobi et al. [25] and Raney et al. [27] analyzed the fatty foci localized in both ventricles. Jacobi et al. analyzed 1846 non-contrast chest CT studies without ECG gating. Fatty deposits were identified in 26 (1.4%) patients (14 were male, 12 were female, with a mean age of 69 years). Twelve patients were diagnosed with a 1-row CT scanner, 3 patients with a 4-row scanner, and 11 patients with a 16-row scanner. Fat was detected in the right ventricle in 27% of patients, in the left ventricle in 46% of patients, and biventricularly in 27% of patients. The patients with fatty metaplasia of the right ventricle were statistically older compared to the group of patients with fatty deposits in the left ventricle. Fifty percent of patients with fatty metaplasia of the left ventricle had myocardial infarction in the past. Only 1 patient in the analyzed group had histologically confirmed ARVD. In this case, fat was localized simultaneously in both ventricles. The authors concluded that fatty metaplasia of the right ventricle correlates with increased patient age, while fatty metaplasia of the left ventricle is associated with prior myocardial infarction. Raney et al. [27] analyzed the ECG-MSCT (64-slice scanner; Toshiba Aquilion, Tustin, CA) examinations of 100 healthy patients and 25 patients after myocardial infarction. They concluded that RV myocardial fat correlates with increased age in healthy populations, while LV myocardial fat most commonly was associated with myocardial infarction. They noticed that fatty foci in the left ventricle may also be present in healthy populations, but in these cases the optical density of fatty lesions is significantly higher compared to the infarcted group. It was also concluded that healthy men had a lower risk of fatty lesion within the left ventricle.
Our study determined that differences in distribution and morphology of fatty lesions were correlated with the patient’s clinical diagnosis. A significant association was found between the localization of the fatty lesions within the heart and a clinical diagnosis (p<0.001). In patients after myocardial infarction, fatty deposits were usually located within the left ventricle and in the interventricular septum. Among the patients with suspected coronary artery disease and diagnosed as “other”, fatty foci were mainly localized simultaneously in both ventricles and septum. A statistically significant relationship was also found between the localization of fatty foci within the heart wall and clinical diagnosis (p<0.001). In patients with advanced coronary artery disease, fatty lesions were located primarily subendocardially in the left ventricle. A similar dependence was described by Kimura et al. [12]. In patients with suspected coronary artery disease, fatty foci were observed mainly subpericardially in the right ventricular and papillary muscles. In patients with a diagnosis of “other”, fatty changes were most frequently localized subendocardially and subpericardially in the left ventricle and subendocardially in the right ventricle.
Of all locations of fatty deposits assessed within the heart, only fatty changes restricted to the interatrial septum can be directly applied to literature data. LHIS was found in 15 patients in our study group, representing 0.8% of the analyzed group. These data are most similar to the autopsy results presented by Gay et al. and Reyes et al., which confirmed the presence of LHIS in 1% of patients [8,17]. Heyer et al. detected LHIS in 28 patients (2.2%) in MSCT examination of the chest carried out during 2001–2002 in a group of 1292 patients.
Many authors have suggested a relationship between the occurrence of LHIS and high BMI index, as well as an age correlation [17,19]. Authors have shown that the disease is more common in females [8]. A similar relationship was found in our study, where 60% of patients with LHIS were women. Also, the average BMI in patients with LHIS was elevated (an average of 28.0 for women and 27.7 for men). The average age of patients with this disease was 57.3 years for women and 57.6 years for men. Beau et al. [29] found that in patients fed parenterally, there was an increase in the layer of fat within the interatrial septum. According Degott et al. [30], the probability that the interatrial septum will become a place for accumulation of adipose tissue increases with age, degree of obesity, and other factors affecting metabolism.
In cardiac imaging, LHIS has the characteristic shape of an hourglass. In all cases in our study, this morphology of the interatrial septum was noted. In evaluating MSCT studies, Heyer et al. indicated this morphology of the atrial septum in 92.6% of cases [28]. The average thickness of the septum in that study was 3.2cm (2.0–6.2 cm). In another study, Meaney et al. [31] reported an average thickness of 2.7cm (1.5–4.8).
In our study, the average thickness of the interatrial septum in patients with LHIS measured in the native phase was 23.8 mm (15.5–39 mm). In the diastolic phase, the average thickness of the interatrial septum was statistically lower and equaled 19.0 mm (15–34 mm). The optical density of the IAS was also determined before and after contrast administration. Average density was −73.3 HU in native scans. After administration of contrast medium, optical density was significantly higher, amounting to −25.0 HU.
The size and optical density of fatty changes in our study were analyzed before and after contrast administration. There are no reports in the literature on this issue to date. It was found that 66% of fatty foci were visible only in pre-contrast scanning. It was also noticed that the optical density of fatty foci increased after contrast administration; therefore, they became less visible in the post-contrast phase of ECG-MSCT examination. At most research centers, ECG-MSCT examinations of the coronary arteries are performed only after administration of contrast medium, without the review phase. This may be why fatty changes within the heart are so rarely reported by radiologists. As a result, many fatty foci remain unnoticed.
Conclusions
The incidental frequency of focal fatty replacement within the heart in patients diagnosed with the ECG-MSCT examinations is about 11%. Pre-contrast scanning is the most valuable for assessment of focal fatty replacement. Therefore, in studies focussing on diagnosis of fatty infiltration of the myocardium, as with suspected ARVD or LHIS, it is sufficient to perform these studies utilizing only the native scans. This prevents exposing patients to higher doses of ionizing radiation and reduces the risks associated with IV contrast administration.
Footnotes
Source of support: Departmental sources
References
- 1.Kiès P, Bootsma M, Bax J, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: screening, diagnosis, and treatment. Heart Rhythm. 2006;3:225–34. doi: 10.1016/j.hrthm.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 2.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic riht ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council an Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–18. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baroldi G, Silver MD, De Maria R, et al. Lipomatous metaplasia in left ventricular scar. Can J Cardiol. 1997;13:65–71. [PubMed] [Google Scholar]
- 4.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–57. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 5.Basso C, Barbazza R, Thiene G. Lipomatous hypertrophy of the atrial septum. Circulation. 1998;97:1423. doi: 10.1161/01.cir.97.14.1423. [DOI] [PubMed] [Google Scholar]
- 6.Burke AP, Litovsky S, Virmani R. Lipomatous hypertrophy of the atrial septum presenting as a right atrial mass. Am J Surg Pathol. 1996;20:678–85. doi: 10.1097/00000478-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham KS, Veinot JP, Feindel CM, Butany J. Fatty lesion of the atria and interatrial septum. Hum Pathol. 2006;37:1245–51. doi: 10.1016/j.humpath.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Gay JD, Guileyardo JM, Townsend-Parchman JK, Ross K. Clinical and morphologic features of lipomatous hypertrophy (“ massive fatty deposits”) of the interatrial septum. Am J Forensic Med Pathol. 1996;18:107–8. doi: 10.1097/00000433-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Adriaensen ME, Schaefer-Prokop CM, Duyndam DA, et al. Fatty foci in the myocardium in patients with tuberous sclerosis complex: common finding at CT. Radiology. 2009;253:359–63. doi: 10.1148/radiol.2533082118. [DOI] [PubMed] [Google Scholar]
- 10.Burke A, Virmani R. Tumors of the heart and great vessels. Washington (DC): Armed Forces Institute of Pathology; 1996. Benign tumors of fatty tissue. [Google Scholar]
- 11.Kim E, Choe YH, Han BK, et al. Right ventricular fat infiltration in asymptomatic subjects: observations from ECG-gated 16-slice multidetector CT. J Comput Assist Tomogr. 2007;31:22–28. doi: 10.1097/01.rct.0000236416.05267.6c. [DOI] [PubMed] [Google Scholar]
- 12.Kimura F, Matsuo Y, Nakajima T, et al. Myocardial fat at cardiac imaging: how can we differentiate pathologic from physiologic fatty infiltration? Radiographics. 2010;30:1587–602. doi: 10.1148/rg.306105519. [DOI] [PubMed] [Google Scholar]
- 13.Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 14.Kimura F, Sakai F, Sakomura Y, et al. Helical CT features of arrhythmogenic right ventricular cardiomyopathy. Radiographics. 2002;22:1111–24. doi: 10.1148/radiographics.22.5.g02se031111. [DOI] [PubMed] [Google Scholar]
- 15.Bomma C, Dalal D, Tandri H, et al. Evolving role of multidetector computed tomography in evaluation of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2007;100:99–105. doi: 10.1016/j.amjcard.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 16.Villa A, Di Guglielmo L, Salerno J, et al. Arrhythmogenic dysplasia of the right ventricle. Evaluation of 7 cases using computerized tomography. Radiol Med. 1988;75:28–35. [PubMed] [Google Scholar]
- 17.Reyes CV, Jablokow VR. Lipomatous hypertrophy of the cardiac interatrial septum: a report of 38 cases and review of the literature. Am J Clin Pathol. 1979;72:785–88. doi: 10.1093/ajcp/72.5.785. [DOI] [PubMed] [Google Scholar]
- 18.Agbamu DA, McMahon RF. Lipomatous hamartoma of the interatrial sepum. Am J Cardiovasc Pathol. 1993;4:371–73. [PubMed] [Google Scholar]
- 19.Shirani J, Roberts WC. Clinical, electrographic and morphologic features of massive fatty deposits (“lipomatous hypertrophy”) in the atrial septum. Am Coll Cardiol. 1993;22:226–38. doi: 10.1016/0735-1097(93)90839-s. [DOI] [PubMed] [Google Scholar]
- 20.Chhabra A, Aronow WS, Ahn C, et al. Incidence of new cardiovascular events in patients with and without peripheral arterial disease seen in a vascular surgery clinic. Med Sci Monit. 2012;18(3):CR131–34. doi: 10.12659/MSM.882517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winer-Muram HT, Tann M, Aisen AM, et al. Computed tomography demonstration of lipomatous metaplasia of left ventricle following myocardial infarction. J Computed Assist Tomogr. 2004;28:455–58. doi: 10.1097/00004728-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Su L, Siegel JE, Fishbein MC. Adipose tissue in myocardial infarction. Cardiovasc Pathol. 2004;13:98–102. doi: 10.1016/S1054-8807(03)00134-0. [DOI] [PubMed] [Google Scholar]
- 23.Araoz PA, Mulvagh SL, Tazelaar HD, et al. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20:1303–19. doi: 10.1148/radiographics.20.5.g00se121303. [DOI] [PubMed] [Google Scholar]
- 24.Hori Y, Funabashi N, Uehara M, et al. Positive influence of aging on the occurrence of fat replacement in the right ventricular myocardium determined by multislice-CT in subjects with atherosclerosis. Int J Cardiol. 2010;142:152–58. doi: 10.1016/j.ijcard.2008.12.187. [DOI] [PubMed] [Google Scholar]
- 25.Jacobi AH, Gohari A, Zalta B, et al. Ventricular myocardial fat: CT findings and clinical correlates. J Thorac Imaging. 2007;22:130–35. doi: 10.1097/01.rti.0000213576.39774.68. [DOI] [PubMed] [Google Scholar]
- 26.Imada M, Funabashi N, Asano M, et al. Epidemiology of fat replecement of the right ventricular myocardium determined by multislice computed tomography using a logistic regression model. Int J Cardiol. 2007;119:410–13. doi: 10.1016/j.ijcard.2006.07.174. [DOI] [PubMed] [Google Scholar]
- 27.Raney AR, Saremi F, Kenchaiah S, et al. Multidetector computed tomography shows intramyocardial fatdeposition. J Cardiovasc Comput Tomogr. 2008;2:152–63. doi: 10.1016/j.jcct.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Heyer CM, Kagel T, Lemburg SP, et al. Lipomatous hypertrophy of the interatrial septum: a prospective study of incidence, imaging findings, and clinical symptoms. Chest. 2003;124:2068–73. doi: 10.1378/chest.124.6.2068. [DOI] [PubMed] [Google Scholar]
- 29.Beau P, Michel P, Coisne D, Morichau-Beauchant M. Lipomatous hypertrophy of the cardiac interatrial septum: an unusual complication in long-term home parentereral nutrition in adult patients. J Parenter Enteral Nutr. 1991;15:659–62. doi: 10.1177/0148607191015006659. [DOI] [PubMed] [Google Scholar]
- 30.Degott C, Messing B, Moreau D, et al. Liver phospholipidosis induced by parenteral nutrition: histological, histochemical, and ultrastructural investigations. Gastroenterology. 1988;95:183–91. doi: 10.1016/0016-5085(88)90309-5. [DOI] [PubMed] [Google Scholar]
- 31.Meaney JF, Kazerooni EA, Jamadar DA, Korobkin M. CT appearance of lipomatous hypertrophy of the interatrial septum. Am J Roentgenol. 1997;168:1081–84. doi: 10.2214/ajr.168.4.9124119. [DOI] [PubMed] [Google Scholar]


