Abstract
Objective:
Peri-implantitis is one of the most common reasons for implant failure. Decontamination of infected implant surfaces can be achieved effectively by laser irradiation; although the associated thermal rise may cause irreversible bone damage and lead to implant loss. Temperature increments of over 10ºC during laser application may suffice for irreversible bone damage.
Purpose of Study:
The purpose of this study was to evaluate the temperature increment of implant surface during Er:YAG laser irradiation with different cooling systems.
Materials and Methods:
Three implants were placed in a resected block of sheep mandible and irradiated with Er:YAG laser with 3 different cooling systems namely water and air spray, air spray alone and no water or air spray. Temperature changes of the implant surface were monitored during laser irradiation with a K-type thermocouple at the apical area of the fixture.
Results:
In all 3 groups, the maximum temperature rise was lower than 10°C. Temperature changes were significantly different with different cooling systems used (P<0.001).
Conclusion:
Based on the results, no thermal damage was observed during implant surface decontamination by Er:YAG laser with and without refrigeration. Thus, Er:YAG laser irradiation can be a safe method for treatment of periimplantitis.
Keywords: Dental implants, Peri-implantitis, Laser irradiation, Temperature changes
INTRODUCTION
In the recent years, dental implants have become an important part of oral rehabilitation. Due to technological and technical advancements in the past decades, dental implant success rate has reached 90% and higher [1, 2]. However, successfully integrated implants are at risk of peri-implantitis. Peri-implantitis refers to the inflammatory disease of the peri-implant tissues leading to bone loss. If not treated or controlled, progressive bone loss may eventually lead to infected implant loss [3]. At the early stages of disease, the inflammatory response to colonization and formation of microbial plaque is limited to the soft tissues around the implant called peri-implant mucositis. Peri-implantitis is defined as an inflammatory process affecting the implant’s supporting bone [4, 5]. By the growing popularity of dental implants the prevalence of peri-implantitis has increased as well [6]. The basics of peri-implantitis treatment include elimination of inflammation by removing calculus and granulation tissue and decontamination of implant surface without modifying the surface structure [7]. Although treatment options such as mechanical debridement, use of local antiseptic agents, systemic and local antibiotics and surgical procedures for elimination of inflammation in the peri-implant tissues have been reported in the literature, there is no standard treatment for this condition [8]. Surgical treatment may be indicated in cases with severe bone loss and pocket depth greater than 5 mm [9]. Decontamination of implant surface without damaging it is a prerequisite for regeneration treatments [10]. Mechanical methods alone cannot eliminate all the pathogens on rough surfaces and adjunctive use of antimicrobial agents has been recommended to boost the decontamination efficacy of mechanical methods [11]. A new technique for implant surface decontamination is the use of laser energy with reportedly positive results [12, 13]. Fast healing, ease of use, bactericidal effect, effective ablation, hemostatic ability and adaptation with irregular implant surface are the main advantages of laser beam for treatment of peri-implantitis [14, 15, 16]. One major side effect of laser application on metal objects inserted in vital bone is the associated thermal increase. Eriksson et al. demonstrated that 10ºC temperature increase maintained for 60 seconds caused permanent damage to bone tissue [17]. In order to prevent thermal damage during laser irradiation on implant surfaces, suitable wavelength and parameters should be used.
One of the best laser systems for decontamination of implant surfaces is Er:YAG laser with 2.94 μm wavelength.
Due to its high absorbability in water and effective ablation of hard tissue, Er:YAG is among the most widely used laser systems in dentistry [18]. In comparison with Er;Cr:YSGG laser, Er:YAG has three times higher absorption in water. Studies have shown that Er:YAG irradiation at 100mJ/pulse and 10pps for 60 seconds was safe for use on implant surfaces and no microscopic changes occurred in surface structures. Using these parameters, bacterial load on HA implant surface decreased by up to 98% and adequate surface detoxification was achieved. Alternations in surface characteristics have been reported with energies exceeding 140–180 mJ/pulse [19].
Er:YAG laser can remove calculus and bacterial plaque on the implant surface with suitable power setting with no damage to the texture of titanium implant surfaces [20]. The advantages of Er:YAG laser such as its high bactericidal effect and excellent tissue ablation have been shown in previous studies [21, 22, 23]. With appropriate irradiation parameters, this laser may be used for therapeutic purposes. The purpose of the present study was to evaluate and compare the temperature increase during Er:YAG irradiation with air-water, air cooling system and without refrigeration.
MATERIALS AND METHODS
Three super RBM surface implant (EZ Plus™ Internal fixture, MEGAGEN, Korea) were placed in a freshly resected block of sheep mandible using the standard surgical procedure. For simulating a typical lesion of periimplantitis and access to laser irradiation, a 2×6 mm vertical lesion was prepared at the buccal of each implant. In the apical third of each implant, a 1×1 mm hole was drilled to accommodate the thermocouple. A K-type digital thermocouple (NX4 K Type, HANYOUNG, Incheon, Korea) with 1mm diameter contact tip and error rate of 0.5% was used for recording temperature changes during laser irradiation. NX EV 1.1.0 (HANYOUNG, Incheon, Korea) software with the ability to record the temperature at one-second intervals was used to draw a graph simultaneously.
The specimens were placed in a water bath at 37°C.Er:YAG laser (2940D Plus DEKA™, Italy) with a wavelength of 2 94μm, an energy output of 100 mJ/pulse, repetition rates of 10pps and pulse duration of 230μs delivered with a non-contact handpiece (4mm above the surface) was used for 60 seconds.
The power setting and the irradiation process were similar to the typical decontamination procedure of periodontal pockets and periimplantitis lesions successfully used in clinical studies [24, 25]. The experiments were performed in three conditions: with air-water refrigeration system (group 1), with air-cooling (group 2) and without the cooling system (group 3). The temperature changes were recorded 10 times at each irradiation condition.
After each irradiation, the sample was allowed to cool-down to initial temperature (37°C). The data were analyzed by one-way ANOVA.
RESULTS
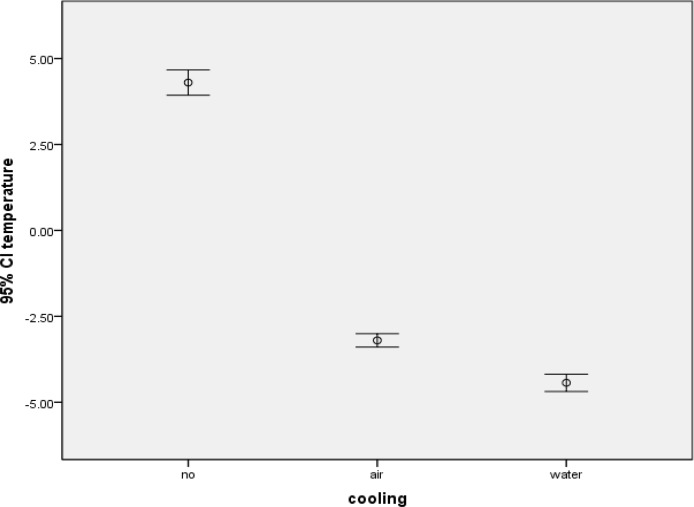
The mean± SD values of group 1, group 2 and group 3 were −4.43 ± 0.39, −3.20 ± 0.30 and 4.30 ± 0.57, respectively. Based on the results of this study, temperature changes between different Er:YAG laser cooling conditions were significant (P<0.001). The error bar and 95% confidence interval diagram of the results are shown in Figure
Fig 1.
The error bar and 95% confidence interval diagram of temperature changes
DISCUSSION
The main goal in treatment of peri-implantitis is the elimination of inflammation that leads to bone loss. To achieve this outcome, elimination of bacterial plaque and deposits and removal of inflammatory tissue at the site of peri-implantitis are necessary. Considering the inadequacy of conventional methods and shortcomings namely potential damage of implant surface and compromising the healing process at the bone-implant interface, use of laser as an alternative method for treatment of peri-implantitis has increased. Use of Er:YAG laser is recommended because of its decontamination and degranulation ability on infected implant surfaces. Clinical research supported the use of Er:YAG laser for treatment of peri-implantitis [26, 27]. However, for use in the clinical setting, the thermal effects of lasers must be considered. A bone temperature rise of over 47ºC for one minute can cause irreversible bone damage [28].
The purpose of this study was to evaluate and compare the temperature increment of implant surface during Er:YAG laser irradiation with different cooling systems. In this study, we simulated the clinical decontamination process used for treatment of peri-implantitis. The protocol applied in this study was similar to the clinical setting. In this study, the mandible of sheep was used as the model with density, bone marrow and heat conduction properties similar to those of human mandible. In all groups, the temperature rise was not high enough to damage the adjacent bone. Although the use of Er;YAG was safe without refrigeration, a 4.30°C increase in temperature was observed; while the use of air and air-water refrigeration eliminated the risk of possible thermal damage. Kreisler et al. reported that temperature elevation did not exceed 47°C after 120s of Er:YAG laser irradiation with a pulse energy between 60 and 120 mJ and frequency of 10 Hz [29]. In agreement with our results, Gómez-Santos et al. evaluated temperature changes during irradiation with Er;Cr:YSGG laser system with and without refrigeration. The mean temperature rise in the group without cooling system was 5.02°C; however, when laser irradiation was accompanied by water spray a decrease in temperature was reported [30]. In contrast, Geminiani et al. showed that the application of CO2 and Er:YAG lasers in continuous mode for 10 seconds generated high temperature above the critical threshold [31]. Also, Leja et al, assessing temperature rise after using diode, CO2 and Er:YAG lasers on dental implants concluded that irradiation for 18 seconds increased the temperature by up to 10ºC [32]. More studies are needed to evaluate thermal changes on different implant surfaces after laser irradiation to find the appropriate protocol for safe use in the clinical setting.
CONCLUSION
The findings of the present in-vitro study suggested that the heat generated by the application of Er:YAG laser with and without refrigeration is not high enough to compromise the integrity of the peri-implant bone. Further studies are required to assess the bactericidal effects of laser and possible associated implant surface structural damage.
REFERENCES
- 1.Albrektsson TA. Multicenter report on osseointegrated oral implants. J Prosthet Dent. 1988;60:75–84. doi: 10.1016/0022-3913(88)90355-1. [DOI] [PubMed] [Google Scholar]
- 2.Buser D, Mericske-Stern R, Dula K, Lang NP. Clinical experience with one-stage, non-submerged dental implants. Adv Dent Res. 1999;13:153–161. doi: 10.1177/08959374990130010501. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Isidor F. Consensus report of session IV. In: Lang NP, Karring T, editors. Proceedings of the 1st European Workshop on Periodontology. London: Quintessence Publishing Co Ltd; 1994. pp. 365–369. [Google Scholar]
- 4.Mombelli A, van Oosten MA, Schurch E, Lang NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987 Dec;2(4):145–51. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 5.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998 Jun;17:63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 6.Kotsovilis S, Karoussis IK, Trianti M, Fourmousis I. Therapy of peri-implantitis: a systematic review. J Clin Periodontol. 2008 Jul;35(7):621–9. doi: 10.1111/j.1600-051X.2008.01240.x. [DOI] [PubMed] [Google Scholar]
- 7.Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002;28:177–89. doi: 10.1034/j.1600-0757.2002.280107.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilson V. An insight into periimplantitis: a systematic literature review. Prim Dent J. 2013 Apr;2(2):69–73. doi: 10.1308/205016813806144209. [DOI] [PubMed] [Google Scholar]
- 9.Schou S, Berglundh T, Lang NP. Surgical treatment of peri-implantitis. Int J Oral Maxillofac Implants. 2004;19(Suppl):140–9. [PubMed] [Google Scholar]
- 10.Schwartz Z, Kieswetter K, Dean DD, Boyan BD. Underlying mechanisms at the bone-surface interface during regeneration. J Periodontal Res. 1997 Jan;32(1 Pt 2):166–71. doi: 10.1111/j.1600-0765.1997.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 11.Mombelli A, Lang NP. Antimicrobial treatment of peri-implant infections. Clin Oral Implants Res. 1992 Dec;3(4):162–8. doi: 10.1034/j.1600-0501.1992.030402.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz F1, Jepsen S, Herten M, Sager M, Rothamel D, Becker J. Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. J Clin Periodontol. 2006 Aug;33(8):584–95. doi: 10.1111/j.1600-051X.2006.00956.x. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz F, Bieling K, Nuesry E, Sculean A, Becker J. Clinical and histological healing pattern of peri-implantitis lesions following non-surgical treatment with an Er:YAG laser. Lasers Surg Med. 2006 Aug;38(7):663–71. doi: 10.1002/lsm.20347. [DOI] [PubMed] [Google Scholar]
- 14.Ando Y, Aoki A, Watanabe H, Ishikawa I. Bactericidal effect of erbium YAG laser on periodontopathic bacteria. Lasers Surg Med. 1996;19(2):190–200. doi: 10.1002/(SICI)1096-9101(1996)19:2<190::AID-LSM11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Romanos G, Nentwig GH. Diode laser (980 nm) in oral and maxillofacial surgical procedures: Clinical observations based on clinical applications. J Clin Laser Med Surg. 1999;17(5):193–197. doi: 10.1089/clm.1999.17.193. [DOI] [PubMed] [Google Scholar]
- 16.Eberhard J, Ehlers H, Falk W, Acil Y, Albers HK, Jepsen S. Efficacy of subgingival calculus removal with Er:YAG laser compared to mechanical debridement: An in situ study. J Clin Periodontol. 2003;30(6):511–518. doi: 10.1034/j.1600-051x.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101–7. doi: 10.1016/0022-3913(83)90174-9. [DOI] [PubMed] [Google Scholar]
- 18.Takasaki AA1, Aoki A, Mizutani K, Kikuchi S, Oda S, Ishikawa I. Er:YAG laser therapy for peri-implant infection:a histological study. Lasers Med Sci. 2007 Sep;22(3):143–57. doi: 10.1007/s10103-006-0430-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Herr Y, Chung JH, Shin SI, Kwon YH. The effect of erbium-doped: yttrium, aluminium and garnet laser irradiation on the surface microstructure and roughness of double acid-etched implants. J Periodontal Implant Sci. 2011 Oct;41(5):234–41. doi: 10.5051/jpis.2011.41.5.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuyama T1, Aoki A, Oda S, Yoneyama T, Ishikawa I. Effects of the Er:YAG laser irradiation on titanium implant materials and contaminated implant abutment surfaces. J Clin Laser Med Surg. 2003;21:7–17. doi: 10.1089/10445470360516680. [DOI] [PubMed] [Google Scholar]
- 21.Folwaczny M, Mehl A, Aggstaller H, Hickel R. Antimicrobial effects of 2.94 micron Er:YAG laser radiation on root surfaces: an in vitro study. J Clin Periodontol. 2002;29:73–78. doi: 10.1034/j.1600-051x.2002.290111.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz F, Aoki A, Sculean A, Georg T, Scherbaum W, Becker J. In vivo effects of an Er:YAG laser, an ultrasonic system and scaling and root planing on the biocompatibility of periodontally diseased root surfaces in cultures of human PDL fibroblasts. Lasers Surg Med. 2003;33:140–147. doi: 10.1002/lsm.10201. [DOI] [PubMed] [Google Scholar]
- 23.Aoki A, Miura M, Akiyama F, Nakagawa N, Tanaka J, Oda S, Watanabe H, Ishikawa I. In vitro evaluation of Er:YAG laser scaling of subgingival calculus in comparison with ultrasonic scaling. J Periodontal Res. 2000;35:266–277. doi: 10.1034/j.1600-0765.2000.035005266.x. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of periimplantitis: A randomized controlled clinical study. J Clin Periodontol. 2011 Mar;38(3):276–84. doi: 10.1111/j.1600-051X.2010.01690.x. [DOI] [PubMed] [Google Scholar]
- 25.Renvert S, Lindahl C, Roos Jansåker AM, Persson GR. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol. 2011 Jan;38(1):65–73. doi: 10.1111/j.1600-051X.2010.01646.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J. Clinical evaluation of an Er:YAG laser fornonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Implants Res. 2005;16:44–52. doi: 10.1111/j.1600-0501.2004.01051.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz F, Bieling K, Nuesry E, Sculean A, Becker J. Clinical and histological healing pattern of peri-implantitis lesions following non-surgical treatment with an Er:YAG laser. Lasers Surg Med. 2006;38:663–671. doi: 10.1002/lsm.20347. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson AR, Albrektsson T. Temperature threshold levels forheat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101–7. doi: 10.1016/0022-3913(83)90174-9. [DOI] [PubMed] [Google Scholar]
- 29.Kreisler M, Al Haj H, d’Hoedt B. Temperature changes at the implant-bone interface during simulated surface decontamination with an Er:YAG laser. Int J Prosthodont. 2002 Nov-Dec;15(6):582–7. [PubMed] [Google Scholar]
- 30.Gómez-Santos L, Arnabat-Domínguez J, Sierra-Rebolledo A, Gay-Escoda C. Thermal increment due to ErCr: YSGG and CO2 laser irradiation of different implant surfaces. A pilot study. Med Oral Patol Oral Cir Bucal. 2010;15(5):782–787. doi: 10.4317/medoral.15.e782. [DOI] [PubMed] [Google Scholar]
- 31.Geminiani A, Caton JG, Romanos GE. Temperature increase during CO(2) and Er:YAG irradiation on implant surfaces. Implant Dent. 2011 Oct;20(5):379–82. doi: 10.1097/ID.0b013e3182310d57. [DOI] [PubMed] [Google Scholar]
- 32.Leja C, Geminiani A, Caton J, Romanos GE. Thermodynamic effects of laser irradiation of implants placed in bone: an in vitro study. Lasers Med Sci. 2013 Nov;28(6):1435–40. doi: 10.1007/s10103-012-1215-z. [DOI] [PubMed] [Google Scholar]