Abstract
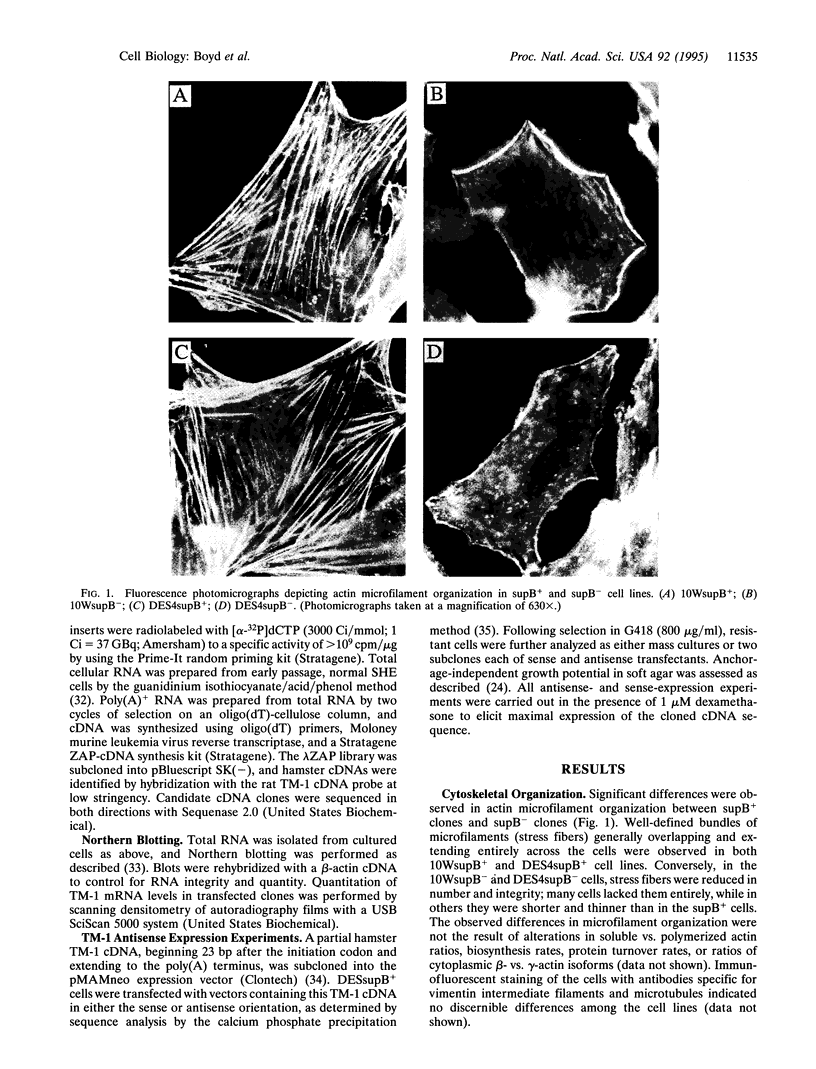
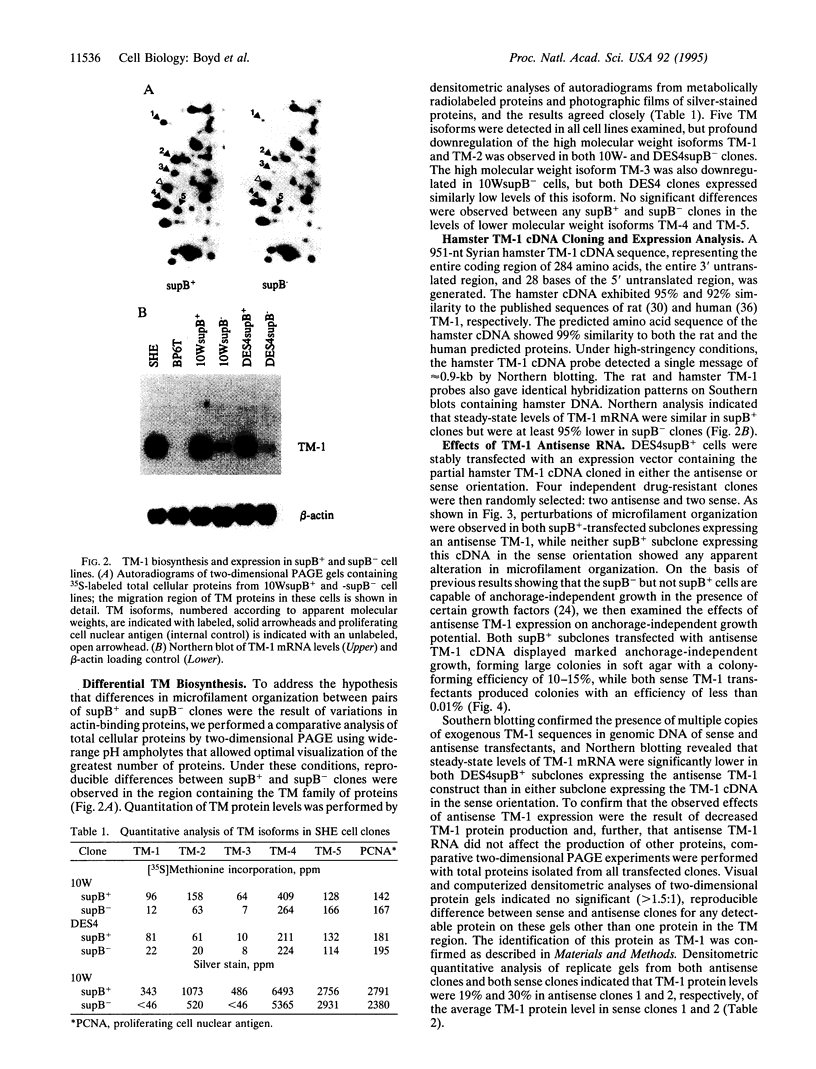
Variants of chemically immortalized Syrian hamster embryo cells that had either retained (supB+) or lost (supB-) the ability to suppress tumorigenicity when hybridized with a fibrosarcoma cell line were subcloned. Both supB cell types are nontumorigenic; however, the supB- but not supB+ cells exhibit conditional anchorage-independent growth. Alterations of actin microfilament organization were observed in supB- but not supB+ cells that corresponded to a significant reduction of the actin-binding protein tropomyosin 1 (TM-1) in subB- cells. To examine the possibility of a direct relationship between TM-1 expression and the subB- phenotype, subB+ cells were transfected with an expression vector containing the TM-1 cDNA in an antisense orientation. The antisense-induced reduction of TM-1 levels in supB+ clones caused a microfilament reorganization and conferred anchorage-independent growth potential that were indistinguishable from those characteristic of supB- cells. These data provide direct evidence that TM-1 regulates both microfilament organization and anchorage-independent growth and suggest that microfilament alterations are sufficient for anchorage-independent growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afshari C. A., Barrett J. C. Negative regulation of mitogen-stimulated, anchorage-independent cell growth by a tumor-suppressor gene function. Mol Carcinog. 1993;7(4):249–256. doi: 10.1002/mc.2940070407. [DOI] [PubMed] [Google Scholar]
- Antecol M. H., Darveau A., Sonenberg N., Mukherjee B. B. Altered biochemical properties of actin in normal skin fibroblasts from individuals predisposed to dominantly inherited cancers. Cancer Res. 1986 Apr;46(4 Pt 1):1867–1873. [PubMed] [Google Scholar]
- Ben-Ze'ev A. The cytoskeleton in cancer cells. Biochim Biophys Acta. 1985;780(3):197–212. doi: 10.1016/0304-419x(85)90003-4. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B., Prasad G. L., Valverius E. M., Salomon D. S., Cooper H. L. Tropomyosins of human mammary epithelial cells: consistent defects of expression in mammary carcinoma cell lines. Cancer Res. 1990 Apr 1;50(7):2105–2112. [PubMed] [Google Scholar]
- Bouck N., Stoler A., Polverini P. J. Coordinate control of anchorage independence, actin cytoskeleton, and angiogenesis by human chromosome 1 in hamster-human hybrids. Cancer Res. 1986 Oct;46(10):5101–5105. [PubMed] [Google Scholar]
- Boyd J., Pienta K. J., Getzenberg R. H., Coffey D. S., Barrett J. C. Preneoplastic alterations in nuclear morphology that accompany loss of tumor suppressor phenotype. J Natl Cancer Inst. 1991 Jun 19;83(12):862–866. doi: 10.1093/jnci/83.12.862. [DOI] [PubMed] [Google Scholar]
- Boyd J., Risinger J. I. Analysis of oncogene alterations in human endometrial carcinoma: prevalence of ras mutations. Mol Carcinog. 1991;4(3):189–195. doi: 10.1002/mc.2940040305. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Thin filament regulatory proteins of smooth- and non-muscle cells. Nature. 1986 Jun 19;321(6072):726–727. doi: 10.1038/321726b0. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Ash J. F., Stanbridge E. J. Cytoskeletal and transmembrane interactions in the expression of tumorigenicity in human cell hybrids. J Cell Sci. 1981 Dec;52:151–166. doi: 10.1242/jcs.52.1.151. [DOI] [PubMed] [Google Scholar]
- Erba H. P., Gunning P., Kedes L. Nucleotide sequence of the human gamma cytoskeletal actin mRNA: anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986 Jul 11;14(13):5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattoum A., Hartwig J. H., Stossel T. P. Isolation and some structural and functional properties of macrophage tropomyosin. Biochemistry. 1983 Mar 1;22(5):1187–1193. doi: 10.1021/bi00274a031. [DOI] [PubMed] [Google Scholar]
- Folkman J., Greenspan H. P. Influence of geometry on control of cell growth. Biochim Biophys Acta. 1975 Dec 31;417(3-4):211–236. doi: 10.1016/0304-419x(75)90011-6. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. The QUEST system for quantitative analysis of two-dimensional gels. J Biol Chem. 1989 Mar 25;264(9):5269–5282. [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Multiple tropomyosin polypeptides in chicken embryo fibroblasts: differential repression of transcription by Rous sarcoma virus transformation. Mol Cell Biol. 1984 Sep;4(9):1823–1833. doi: 10.1128/mcb.4.9.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery W. R. Spatial distribution of messenger RNA in the cytoskeletal framework of ascidian eggs. Dev Biol. 1984 Jun;103(2):482–492. doi: 10.1016/0012-1606(84)90335-x. [DOI] [PubMed] [Google Scholar]
- Koi M., Afshari C. A., Annab L. A., Barrett J. C. Role of a tumor-suppressor gene in the negative control of anchorage-independent growth of Syrian hamster cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8773–8777. doi: 10.1073/pnas.86.22.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi M., Barrett J. C. Loss of tumor-suppressive function during chemically induced neoplastic progression of Syrian hamster embryo cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5992–5996. doi: 10.1073/pnas.83.16.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelovich L., Conlon S., Pollack R. Defective organization of actin in cultured skin fibroblasts from patients with inherited adenocarcinoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3019–3022. doi: 10.1073/pnas.74.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991 Sep;13(9):429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- Leonardi C. L., Warren R. H., Rubin R. W. Lack of tropomyosin correlates with the absence of stress fibers in transformed rat kidney cells. Biochim Biophys Acta. 1982 Apr 29;720(2):154–162. doi: 10.1016/0167-4889(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Liu H. P., Bretscher A. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 1989 Apr 21;57(2):233–242. doi: 10.1016/0092-8674(89)90961-6. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S., Lin J. J. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983 May 25;258(10):6636–6644. [PubMed] [Google Scholar]
- Merrick B. A., He C. Y., Craig W. A., Clark G. C., Corsini E., Rosenthal G. J., Mansfield B. K., Selkirk J. K. Two dimensional gel electrophoresis of cellular and secreted proteins from rat alveolar macrophages after lipopolysaccharide treatment. Appl Theor Electrophor. 1992;2(6):177–187. [PubMed] [Google Scholar]
- Nelson W. G., Pienta K. J., Barrack E. R., Coffey D. S. The role of the nuclear matrix in the organization and function of DNA. Annu Rev Biophys Biophys Chem. 1986;15:457–475. doi: 10.1146/annurev.bb.15.060186.002325. [DOI] [PubMed] [Google Scholar]
- Ng S. Y., Erba H., Latter G., Kedes L., Leavitt J. Modulation of microfilament protein composition by transfected cytoskeletal actin genes. Mol Cell Biol. 1988 Apr;8(4):1790–1794. doi: 10.1128/mcb.8.4.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimura M., Gilmer T. M., Barrett J. C. Nonrandom loss of chromosome 15 in Syrian hamster tumours induced by v-Ha-ras plus v-myc oncogenes. Nature. 1985 Aug 15;316(6029):636–639. doi: 10.1038/316636a0. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Moskowitz M. Arrest of 3T3 cells in G1 phase in suspension culture. J Cell Physiol. 1975 Dec;87(2):213–219. doi: 10.1002/jcp.1040870209. [DOI] [PubMed] [Google Scholar]
- Pienta K. J., Partin A. W., Coffey D. S. Cancer as a disease of DNA organization and dynamic cell structure. Cancer Res. 1989 May 15;49(10):2525–2532. [PubMed] [Google Scholar]
- Prasad G. L., Fuldner R. A., Cooper H. L. Expression of transduced tropomyosin 1 cDNA suppresses neoplastic growth of cells transformed by the ras oncogene. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7039–7043. doi: 10.1073/pnas.90.15.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad G. L., Meissner S., Sheer D. G., Cooper H. L. A cDNA encoding a muscle-type tropomyosin cloned from a human epithelial cell line: identity with human fibroblast tropomyosin TM1. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1068–1075. doi: 10.1016/0006-291x(91)90647-p. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Blau H. M. Genetic complementation reveals a novel regulatory role for 3' untranslated regions in growth and differentiation. Cell. 1993 Mar 26;72(6):903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Conboy M. J., Rando T. A., Blau H. M. Tumor suppression by RNA from the 3' untranslated region of alpha-tropomyosin. Cell. 1993 Dec 17;75(6):1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- Taneja K. L., Lifshitz L. M., Fay F. S., Singer R. H. Poly(A) RNA codistribution with microfilaments: evaluation by in situ hybridization and quantitative digital imaging microscopy. J Cell Biol. 1992 Dec;119(5):1245–1260. doi: 10.1083/jcb.119.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen D. G., Gilmer T. M., Annab L. A., Barrett J. C. Evidence for multiple steps in neoplastic transformation of normal and preneoplastic Syrian hamster embryo cells following transfection with Harvey murine sarcoma virus oncogene (v-Ha-ras). Cancer Res. 1985 Feb;45(2):726–732. [PubMed] [Google Scholar]
- Varma M., Leavitt J. Macromolecular changes accompanying immortalization and tumorigenic conversion in a human fibroblast model system. Mutat Res. 1988 Jun;199(2):437–447. doi: 10.1016/0027-5107(88)90220-5. [DOI] [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Helfman D. M. Isolation and characterization of cDNA clones encoding a low molecular weight nonmuscle tropomyosin isoform. J Biol Chem. 1987 Aug 5;262(22):10791–10800. [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Helfman D. M. Rat embryonic fibroblast tropomyosin 1. cDNA and complete primary amino acid sequence. J Biol Chem. 1985 Nov 25;260(27):14440–14445. [PubMed] [Google Scholar]







