Abstract
Purpose
Circadian rhythms are central to vision and retinal physiology. A circadian clock located within the retina controls various rhythmic processes including melatonin synthesis in photoreceptors. In the present study, we evaluated the rhythmic expression of clock genes and clock output genes in retinal explants maintained for several days in darkness.
Methods
Retinas were dissected from Wistar rats, either wild-type or from the Per1-luciferase transgenic line housed under a daily 12 h:12 h light-dark cycle (LD12/12), and put in culture at zeitgeber time (ZT) 12 on semipermeable membranes. Explants from wild-type rats were collected every 4 h over 3 days, and total RNA was extracted, quantified, and reverse transcribed. Gene expression was assessed with quantitative PCR, and the periodicity of the relative mRNA amounts was assessed with nonlinear least squares fitting to sine wave functions. Bioluminescence in explants from Per1-luciferase rats was monitored for several days under three different culture protocols.
Results
Rhythmic expression was found for all studied clock genes and for clock downstream targets such as c-fos and arylalkylamine N-acetyltransferase (Aanat) genes. Clock and output genes cycled with relatively similar periods and acrophases (peaks of expression during subjective night, except c-fos, which peaked around the end of the subjective day). Data for Per1 were confirmed with bioluminescence monitoring, which also permitted culture conditions to be optimized to study the retina clock.
Conclusions
Our work shows the free-running expression profile of multiple clock genes and potential clock targets in mammalian retinal explants. This research further strengthens the notion that the retina contains a self-sustained oscillator that can be functionally characterized in organotypic culture.
Introduction
Night/day transitions are major events to which living organisms have to adapt their physiology and behavior. Such daily changes rely on circadian rhythmicity in cellular and molecular events. These rhythms are generated by a hierarchical network of oscillators, comprising a central clock located in the suprachiasmatic nuclei (SCN) that is synchronized with daily environmental light cues via the retina, and a series of peripheral oscillators receptive to synchronizing information produced by the SCN [1]. At the molecular level, circadian oscillators involve the interconnection of transcriptional/translational feedback loops, involving clock genes such as Bmal1, Clock, Per1–2, Cry1–2, RevErbα, and Rorα or Rorβ, which have the property to entrain the expression of “clock-controlled genes” and thus to drive rhythmic gene expression programs [2]. Retinas possess endogenous timekeeping systems that ensure the adjustment of their function to daily changes in light intensity [3-5]. As a result, many cellular, biochemical, and physiologic processes in the retina, such as rod outer segment disc shedding and phagocytosis [6], synthesis and release of melatonin [7], and dopamine [8], are remarkably rhythmic [9,10]. Several clock-controlled genes have been identified in the retina, specifically in photoreceptors: Aanat, which encodes the rate-limiting enzyme in melatonin synthesis [11], c-fos [12], and visual pigment genes [13-15]. The retina was the first tissue outside the SCN to be shown to harbor a circadian clock, based on the ability of hamster retina cultures to display an autonomous and light-entrained rhythm of melatonin synthesis [7]. Accordingly, several clock genes analyzed at the level of the whole retina in vivo display circadian rhythmic patterns [16-21]. In addition, the Bmal1 gene was shown to be indispensable in the eye for optimal gene expression rhythms [14]. However, the mechanisms and localization of the retinal molecular clock(s) driving these rhythms have thus far remained elusive in mammals. Several studies analyzed the multilayered distribution of clock gene transcripts in the retina [19,20,22-25]. Localization of circadian oscillators was also addressed more recently by in vitro bioluminescence studies with transgenic animals carrying a luciferase reporter under the control of Period gene promoters, but these studies mainly concentrated on expression profiles of one clock gene [17,26].
Explanted tissues are well adapted to the investigation of mechanisms generating autonomous biological rhythms because they exclude other time-giving inputs, for instance, from the central clock. The retina is especially suited to such approaches because axonal lesions are limited to the optic nerve. In the present study, we asked whether whole retina explants from adult Wistar rats display rhythms in clock gene and clock output gene expression in constant conditions with qPCR analysis and bioluminescence recordings using Per1-luciferase rats. Our work describes the free-running expression profiles of the principal clock genes as well as selected outputs in rat retinal explants, and shows that the robustness of the retina clock in vitro strongly depends on culture conditions.
Methods
Animal care and handling
All animal procedures were performed at Chronobiotron UMS 3415 – CNRS, Strasbourg according to the rules of the French Department of Agriculture (license no. 67–67–298) and the European Committee Council Directive of 24 November 1986 (86⁄609⁄EEC) and in agreement with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Protocols were approved by the Animal Use and Care Committee from Strasbourg. Retinal explant cultures were prepared from male Wistar rats (5–6 weeks old), either wild-type or from the Per1-luciferase transgenic strain [27], housed under 12 h:12 h light (300 lux) -dark conditions (LD: lights off at zeitgeber time [ZT] 12; ZT0 defined as the moment lights were turned on), with water and food ad libitum. Animals were euthanized with CO2 (20% in an air-tight box) between ZT8 and ZT9, and eye globes immediately collected and processed under room light according to subsequent use.
Sample preparation and culture
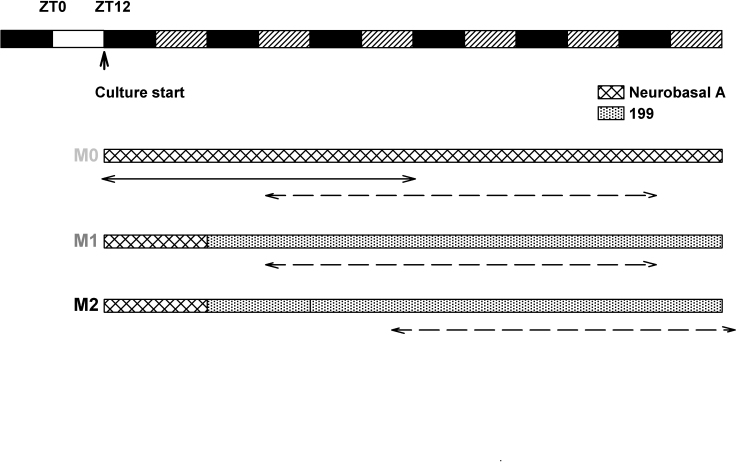
For qPCR analysis (wild-type Wistar rats), eye globes were placed in cold CO2-independent medium (Invitrogen, Carlsbad, CA). After the sclera, choroid, and retinal pigmented epithelial tissues were removed, the retina was teased away from the vitreous body, and four small radial cuts were made around the periphery of the retina to facilitate flattening. All successively dissected retinas were maintained flattened with the photoreceptor side down on a glass slide covered with cold CO2-independent medium in a humid chamber until ZT11. Between ZT11 and ZT12, each retina was transferred, the photoreceptor side down, onto a 35-mm culture dish that contained a Millicell organotypic insert (Millipore, Billerica, MA) and Neurobasal-A Medium (Invitrogen) supplemented with 2% B27 (Invitrogen), 2 mM L-glutamine, and antibiotics (10 U/ml penicillin and 10 µg/ml streptomycin). Retinal explants were incubated under constant darkness at 37 °C in a humidified 5% CO2 atmosphere starting at ZT12 (Figure 1, protocol M0) for up to 3 days. Explants (n = 3 per time point) were collected every 4 h under dim red light by using sterile forceps, snap frozen on dry ice, and stored at −80 °C until further processing. Four distinct experiments were performed: two in which sampling was done from 4 h to 48 h of culture and two in which sampling was done from 16 h to 72 h of culture.
Figure 1.
Schematic diagram showing the timing of retina explant cultures. Solid white and black boxes at the top left show the lighting schedule to which animals were exposed before sacrifice (white: 12 h light, black: 12 h dark). All cultures were started at zeitgeber time 12 (ZT12), corresponding to the beginning of the night period and were maintained in constant dark. Alternating black and hatched rectangles thereafter indicate the projections of the previous lighting schedule (black: dark, hatched; light). In the M0 protocol, retina explants were cultured on Neurobasal A medium (with luciferin when adequate) throughout the experiment, without any medium change. The time window corresponding to retina sampling for qPCR analysis is shown with a double arrow. In the M1 protocol, retinas were incubated on Neurobasal A for 24 h and then transferred to 199 medium supplemented with luciferin. In the M2 protocol, retinas were incubated on Neurobasal A during 24 h as in M1, transferred to 199 medium for 24 h, and the medium was again refreshed after 24 h and supplemented with luciferin. The time windows during which the bioluminescence data were analyzed are shown with the dashed double arrows.
For the bioluminescence studies (Per1-luciferase Wistar rats), eye globes were processed the same way as above but in cold Hank’s saline buffer (Sigma, St. Louis, MO), and the whole retina explants were either kept in the same conditions (Figure 1 protocol, M0: in this case the medium was supplemented with 100 µM luciferin (Promega, Fitchburg, WI) and saturated with 5% CO2 before transfer into Lumicycle) or transferred to 199 medium (Sigma) 24 h later, before bioluminescence recording started (Figure 1, protocol M1: 199 medium was supplemented with 20 mM glucose, 2% B27, 0.7 mM glutamine, 100 µM luciferin, 25 U/ml penicillin, and 25 µg/ml streptomycin), or transferred to 199 medium 24 h later. This medium was again renewed 24 h later before bioluminescence recording started (Figure 1, protocol M2).
Isolation of total RNA
Total RNA was extracted from cultured retinas using the Absolutely RNA Miniprep Kit (Stratagene, Santa Clara, CA) including RNase-Free DNase treatment to remove contaminating genomic DNA. Concentration and purity of isolated total RNA were measured with spectrophotometry (A260/A280 and A260/A230 values were between 1.8 and 2.0). Quantification was confirmed with agarose gel electrophoresis: RNA samples had sharp rRNA bands with no sign of degradation. No significant quantitative or qualitative differences between the different time point groups were visible.
Reverse transcription
Isolated RNA (0.5 μg) was transcribed into first-strand cDNA with 200 U of RevertAid H Minus M-MuLV Reverse Transcriptase (Fermentas, Burlington, Canada) and 0.2 µg of random hexamer primers in a total reaction volume of 20 μl. The resulting cDNA sample was stored at −80 °C.
Real-time quantitative PCR
Quantitative PCR was performed using the 7300 Real Time PCR System (Applied Biosystems, Foster City, CA) and hydrolyzed probe-based TaqMan chemistry. We used optimized TaqMan gene expression assays designed to specifically amplify mRNA (Applied Biosystems; Table 1). PCR reaction was performed in duplicates with 1 µl of cDNA at appropriate dilution supplemented with 10 µl of TaqMan Universal PCR Master Mix, No AmpErase UNG (2X; Applied Biosystems), 1 µl of 20X TaqMan Gene Expression Assay Mix, and 8 µl of RNase-free water. The thermal cycler conditions were as follows: 10 min at 95 °C, and then 40 cycles of 15 s at 95 °C and 1 min at 60 °C. No-template controls were included for each primer pair to check for any contaminant. For all gene expression assays, amplification efficiency was determined with a cDNA dilution curve corresponding to the ongoing experiment and had values between 1.8 and 2.0. Gene expression was measured as relative mRNA amounts by using the comparative Ct method and, as a calibrator, the 28 h time point (T28), a time point that was common to the four experiments. No single studied gene reached the criteria to be used as a reference gene, and thus, data are presented without reference to endogenous control genes [28].
Table 1. TaqMan Gene Expression Assays used in the study.
| Target genes | TaqMan gene expression assay | RefSeq |
|---|---|---|
|
Clock |
Rn00573120_m1 |
NM_021856.1 |
|
Bmal1 |
Rn00577590_m1 |
NM_024362.2 |
|
Per1 |
Rn01496757_m1 |
NM_001034125.1 |
|
Per2 |
Rn01427704_m1 |
NM_031678.1 |
|
Cry1 |
Rn01649292_m1 |
NM_198750.2 |
|
Cry2 |
Rn00591457_m1 |
NM_133405.1 |
|
Rorβ |
Rn01451215_m1 |
NM_001270958 |
|
RevErbα |
Rn00595671_m1 |
NM_145775.1 |
|
Aanat |
Rn00563117_m1 |
NM_012818.1 |
| c-fos | Rn02396759_m1 | NM_022197.1 |
Reference (Applied Biosystems) and GenBank accession number (RefSeq) are given for each studied mRNA.
For a certain gene, the relative mRNA amount in sample x (RQx), expressed regarding the amount of mRNA at T28, was calculated as follows:
RQx = E-(Ctx - CtT28) where E = efficiency calculated with the group of samples regarding the TaqMan set, CtT28 = mean Ct value for the three samples at T28 (= calibrator), Ctx = the Ct value for sample x. This allowed the RQ values to be expressed for each individual sample regarding the 28 h time point, which mean RQ in the experiment was artificially set at 1.
RQ values were calculated that way in the four distinct experiments and were thus expressed relative to T28 that was common to all four experiments and artificially rescaled to 1. Since the general drift was similar in all experiments, this allowed us to include all RQ values in a unique analysis over 3 days in culture (see below), in which the mean RQ at T28 of the four experiments would still be 1.
Statistical analysis of qPCR results
The rhythmicity of gene expression was first evaluated on relative mRNA amounts (log) with the cosinor method (SigmaPlot V 12; Systat Software, San Jose, CA) after [29]. To better characterize rhythmic parameters, mathematical analysis of the time course of relative mRNA amounts (log) was performed with nonlinear least squares fitting to a sine wave equation added to a linear drift term, using SigmaPlot V 12 software (Systat Software):
y = y0 + b·t + c·cos [2π(t-φ)/τ], where τ is the endogenous period (h), φ is the phase (h), and c is the amplitude. Normality of distribution (Kolmogorov–Smirnov), lack of colinearity (VIF), and constancy of variance were checked with the tests provided in the software and were satisfactorily fulfilled.
Bioluminescence measurement and analysis
Explant cultures sealed with vacuum grease were placed in the Lumicycle (Actimetrics, Wilmette, IL) at ZT12, and Per1 activity was measured at 36.5 °C for up to 7 days. Bioluminescence was analyzed with the Lumicycle Analysis software (Actimetrics) to generate detrended curves (24 h moving average) and to calculate the rhythmic power [30] (recording interval from day 1.5 to 6.5) in the periodogram analysis [31]. To calculate the oscillation periods, baseline-subtracted bioluminescence curves covering four full cycles were fitted with cosine curves adapted to each protocol. In protocol M0, there was no obvious damping of the amplitude over the four studied cycles, and we used: y = y0 + c·cos[2π(t-φ)/τ] where τ is the endogenous period (days), φ is the phase (days), and c the amplitude (arbitrary units). The bioluminescence curves obtained under protocols M1 and M2 showed higher amplitude but damping oscillations and thus were fitted to cosine curves, including an exponential damping component: y = y0 + c·exp(-t/d)·cos[2π(t-φ)/τ] where d represents the time (days) needed for the amplitude to display a 63% decrease.
Statistical analyses of bioluminescence results
Differences in the period and rhythmic power of bioluminescence oscillations were analyzed by using one-way ANOVA (ANOVA) and post hoc comparisons with Fisher’s least significant difference (LSD) test (SigmaPlot V 12 software; Systat Software).
Histology
Retina explants were prepared according to protocol M2. Fixation (1 h at 4 °C) was performed either immediately after the explant was transferred onto the semipermeable membrane, or after 2, 4, or 9 days of culture, by replacing the medium with 4% paraformaldehyde in PBS (1X; 140 mM NaCl, 10 mM Phosphate, 3 mM KCl). Samples were embedded (Tissue-Tek; Sakura Finetek, Tokyo, Japan), and 10-µm sections were prepared and stored at −20 °C until ready for use. They were stained with Carazzi’s hematoxylin (6.67 mg/l solution for 5 min) and counterstained with eosin (0.25% in acetic acid 0.16%) for 30 s.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine-5'-triphosphate-fluorescein nick end labeling analysis
Apoptosis was assessed with a commercial kit (In situ Cell Death Detection Kit-Fluorescein; Roche Diagnostics, Basel, Switzerland) on sections prepared as for histology, pretreated 30 min at room temperature with Proteinase K (10 µg/ml in PBS, Roche), and permeabilized with Triton X-100 0.1%/sodium citrate 0.1%/PBS (8 min, room temperature). The solution mixture was placed on sections in a dark humid chamber at 37 °C for 1 h according to the manufacturer’s instructions, and positive and negative controls were treated likewise. Cell nuclei were stained with 4,6-diaminophenyl-indolamine (DAPI diluted at 4 µg/ml in PBS; Sigma), and sections were mounted with PBS/glycerol 1:1 and then observed under a fluorescent microscope (Nikon Optiphot 2). Images were obtained with charge-coupled device (CCD) camera video capture linked to a dedicated PC containing image analysis software (Nikon BIA).
Results
Expression patterns of clock genes and clock output genes in rat retina explants
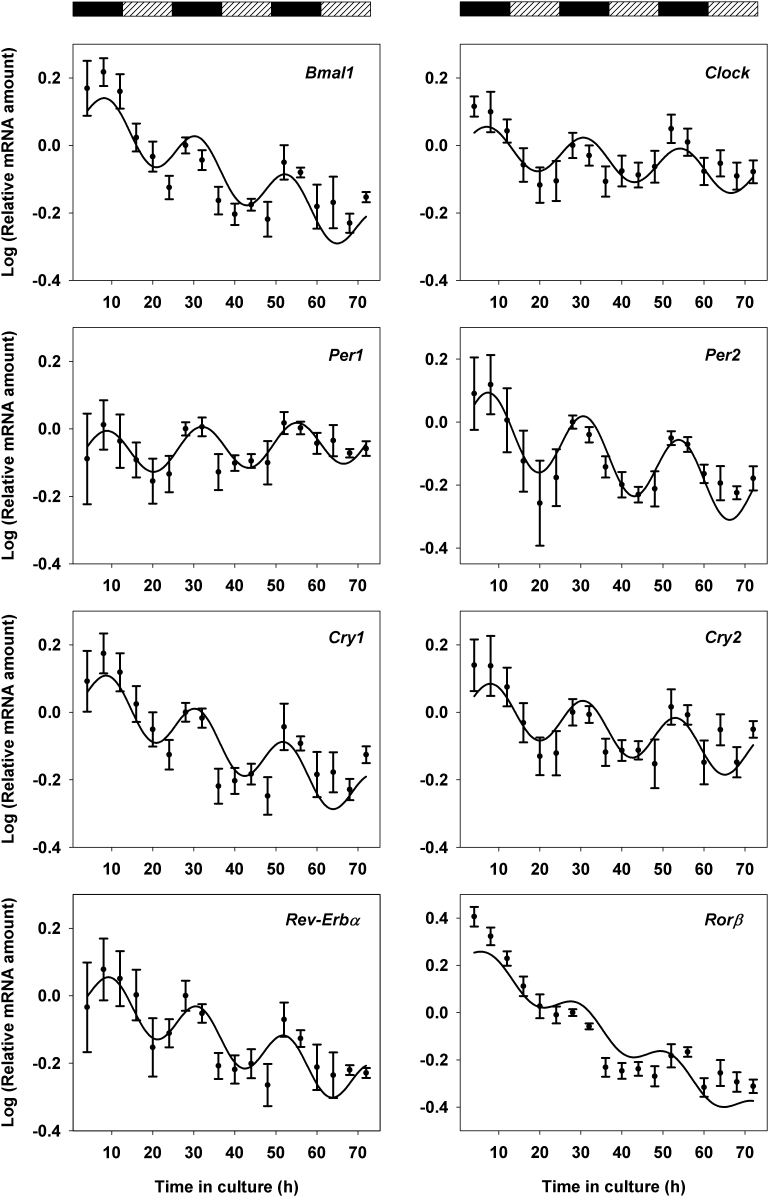
To investigate the capacity of the retina clock to work as an autonomous oscillator, we assessed the kinetics of clock gene expression in whole retina explants maintained in culture in the dark. Four independent experiments were performed, in which retina explants were sampled every 4 h (n = 3 for each time point), either between 4 and 48 h or between 16 and 72 h of culture, and relative mRNA amounts were measured with quantitative real-time PCR for eight clock genes: Bmal1, Clock, Per1, Per2, Cry1, Cry2, RevErbα, and Rorβ. A common calibration time point (28 h of culture) was used to quantify gene expression across the four sample series. Expression of all examined clock genes over 3 days of culture showed a cyclic profile (Figure 2) confirmed with cosinor analysis , which consists in fitting a 24 h-period sinusoid [29] (data not shown). Moreover, the data were fitted to an oscillating function coupled with a linear drift (Table 2) with calculated periods ranging from 21.29±1.08 h to 23.54±1.32 h. All calculated acrophases (Table 2) clustered between 7 and 10 h (time after culture start); indeed, all first peaks occurred during the second half of the subjective night (Figure 2). Taken together, these results show that the mRNA levels of all studied clock genes oscillate in a circadian manner in explanted retinas maintained in constant, dark conditions.
Figure 2.
Temporal profiles of clock gene transcripts in retina explants cultured for 72 h. Each graph shows data from four independent experiments (n = 3 per time point in each experiment). The mRNA amounts (log units) are expressed relative to the mean value found at a time point common to the four experiments: 28 h of culture. Mean values±standard error of the mean (SEM) are shown together with the best fitted sine wave curve. Alternating rectangles at the top indicate the projection of the lighting schedule (black: 12 h dark; hatched: 12 h light) to which the animals were exposed before they were euthanized.
Table 2. Mathematical analysis of clock gene and clock-controlled gene mRNA rhythmicity in retina explants cultured respectively for 72 and 48 h.
| Gene | c (amplitude) | φ (phase in h) | τ (period in h) | P value |
|---|---|---|---|---|
| Clock genes |
||||
|
Bmal1 |
0.07±0.02 |
9.02±1.25 |
22.02±1.54 |
<0.0001 |
|
Clock |
0.06±0.02 |
7.39±2.00 |
23.54±1.32 |
0.0009 |
|
Per1 |
0.06±0.02 |
8.72±1.53 |
23.17±1.04 |
0.0012 |
|
Per2 |
0.11±0.02 |
7.76±1.32 |
23.23±0.87 |
<0.0001 |
|
Cry1 |
0.07±0.02 |
9.34±1.34 |
21.55±0.91 |
<0.0001 |
|
Cry2 |
0.07±0.02 |
8.36±1.54 |
22.51±1.01 |
<0.0001 |
|
Reverbα |
0.07±0.02 |
9.86±1.61 |
21.29±1.08 |
<0.0001 |
|
Rorβ |
0.06±0.01 |
7.83±1.62 |
21.91±1.05 |
<0.0001 |
| Clock controlled genes |
||||
|
Aanat |
0.14±0.03 |
8.95±0.83 |
20.82±1.17 |
<0.0001 |
| c-fos | 0.12±0.03 | 24.27±2.22 | 25.23±2.04 | <0.0001 |
Data (relative mRNA amounts, in log units) were fitted to the equation y=y0 + b·t + c·cos [2π(t-φ)/τ] by non linear least squares regression.
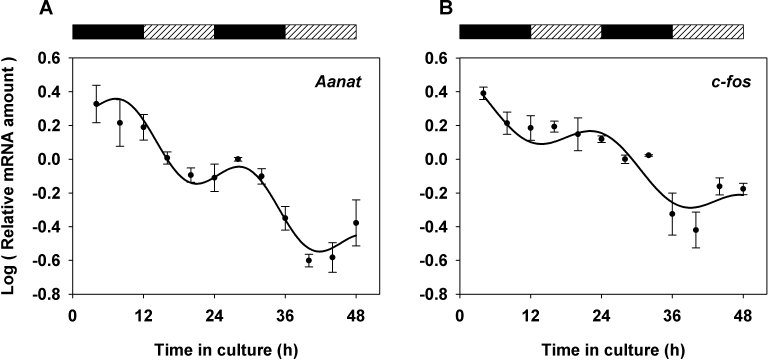
Previous studies have revealed a subset of genes potentially regulated by the retinal clock, including Aanat, which encodes the rate-limiting enzyme of melatonin synthesis, and c-fos, both of which display strong amplitude oscillations [11,12,19]. We studied the expression of these clock output genes in a 48-h culture with qPCR analysis. By using the same mathematical analysis as above, we found that expression of c-fos and Aanat was rhythmic (Figure 3) with periods that were also in the circadian range, 25.23±2.04 h and 20.82±1.17 h, respectively (Table 2), and with the Aanat expression peaks delayed compared to those for c-fos (Table 2 and Figure 3).
Figure 3.
Temporal profiles of clock-controlled gene transcripts in retina explants cultured for 48 h. Aanat (A) and c-fos (B) mRNA amounts (log units) are expressed relative to the 28 h time point (one experiment; n = 3 per time point). Mean values±standard error of the mean (SEM) are shown together with the best fitted sine wave curve. Alternating rectangles at the top indicate the projection of the lighting schedule (black: 12 h dark; hatched: 12 h light) to which animals were exposed before they were euthanized.
Rhythmic expression of Per1 is confirmed with bioluminescence analysis in retina explants
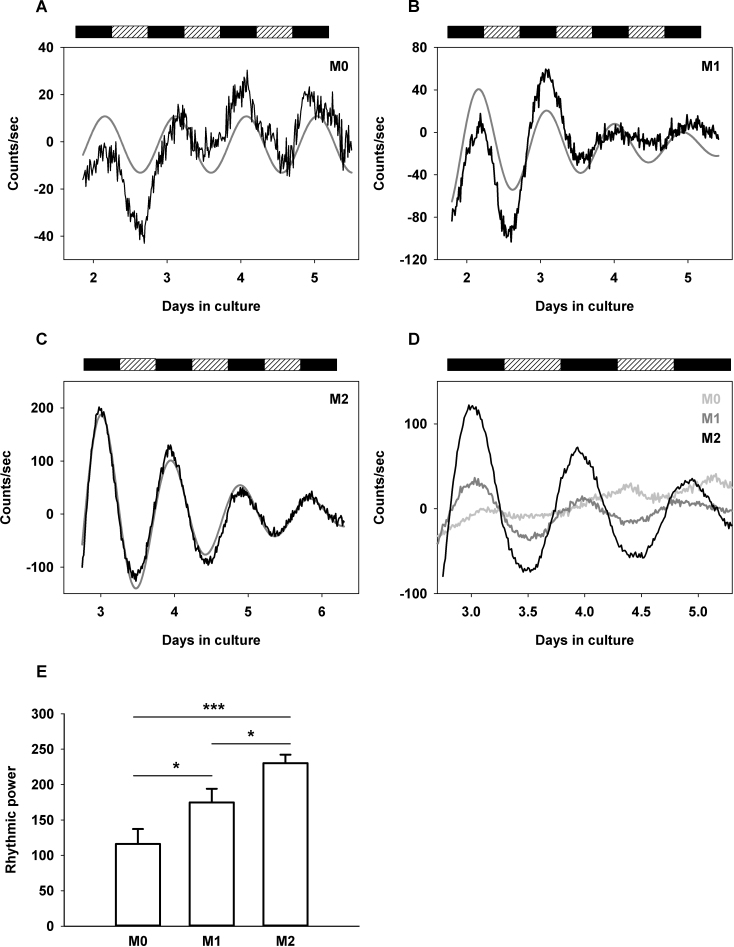
We used the Per1-luciferase transgenic strain of Wistar rats to further investigate the capacity of the retina circadian clock to oscillate in vitro. Whole retinas (n = 4) from Per1-luciferase rats were explanted and cultured similar to above, with the exception that 0.1 mM beetle luciferin was added and dishes were placed into the Lumicycle (Actimetrics). Detrended bioluminescence recordings displayed low amplitude, noisy oscillations that could nevertheless be fitted with cosine curves, and a mean period of 22.36±1.27 h (n = 4; Figure 4A). Moreover, the peaks of the detrended curves occurred during the subjective nights, as previously observed for Per1 with qPCR analysis. Thus, bioluminescence analysis of Per1 gene expression confirmed that a circadian clockwork is active in whole retina explants, albeit with low amplitude.
Figure 4.
Bioluminescence analysis of Per1-luciferase rat retina explants. A: Detrended bioluminescence recording (black line) from a representative explant cultured under protocol M0. Data were fitted with a cosine curve (y = y0 + c·cos[2π(t-φ)/τ]) shown in gray, to calculate corresponding periods. B: Detrended bioluminescence recording (black line) from a representative explant cultured under protocol M1. Data were fitted with a damped cosine curve (in gray; y = y0 + c·exp(-t/d)·cos[2π(t-φ)/τ]) to calculate the oscillation periods. C: Detrended bioluminescence recording (black line) from a representative explant cultured under protocol M2. Data were fitted with a damped cosine curve (in gray) as in B. D: Composite graph showing averaged traces on the same scale and during three cycles, from samples within each treatment group (n = 4 for M0, in light gray; n = 4 for M1, in gray; n = 7 for M2, in black). In panels A–D, the alternating rectangles at the top indicate the projection of the lighting schedule (black: 12 h dark; hatched: 12 h light) to which animals were exposed before they were euthanized. E: Rhythmic power in cultures under protocols M0, M1, and M2. Mean values±standard error of the mean (SEM) are shown. * p<0.05; *** p<0.001.
Robustness of the clock in retina explants is enhanced upon medium change
Oscillations of the circadian clock present in the retina of the PER2::LUC mice can be successfully analyzed in vitro, provided the explants are submitted to a specific protocol comprising 24 h of culture in Neurobasal-A-medium, followed by transfer to 199 medium before the bioluminescence is recorded [17]. We applied a similar protocol (called M1 in Figure 1) to the Per1-luciferase whole retina explants and observed that more sustained circadian oscillations in bioluminescence were detected (Figure 4B). The oscillation periods obtained by fitting the data with a damped cosine curve over four cycles lasted 22.44±0.61 h (n = 4). We repeated the experiment but made another round of 199 medium change 24 h later, before starting the bioluminescence recording (protocol M2 in Figure 1). This led to robust oscillations with reduced noise (Figures 4B-D) and mean periods of 22.84±0.20 h (n = 7). The increase in robustness conferred by one or two changes in the medium (protocols M1 and M2, respectively) was further supported by the analysis of rhythmic power (Figure 4E, effect of medium change: p = 0.001), which proved significantly increased upon each supplementary medium renewal. In addition, peaks of Per1 expression in protocols M1 and M2, as assessed with bioluminescence, again occurred during subjective nights, as was observed under the M0 protocol (Figure 4C,D). There was no significant difference between the periods calculated in the three culture conditions.
Optimal conditions for culturing whole retina explants
We examined the histological structure of whole retina explants maintained in culture according to protocol M2 over 2, 4, and 9 days. The retina structure was grossly conserved, with an obvious increase in fragility over time and an overall decrease in thickness, notably in plexiform layers (Figure 5A–D). Terminal deoxynucleotidyl transferase-mediated deoxyuridine-5'-triphosphate-fluorescein nick end labeling (TUNEL) analysis at similar time points showed absence of apoptosis until 4 days of culture, but some patches of apoptotic cells were detected after 9 days of culture, most notably in the photoreceptor layer (Figure 5E–H), based on the comparison to DAPI staining (Figure 5I–L). Thus, the culture conditions delineated as protocol M2 are appropriate for identifying and analyzing the properties of the rat retina clock.
Figure 5.
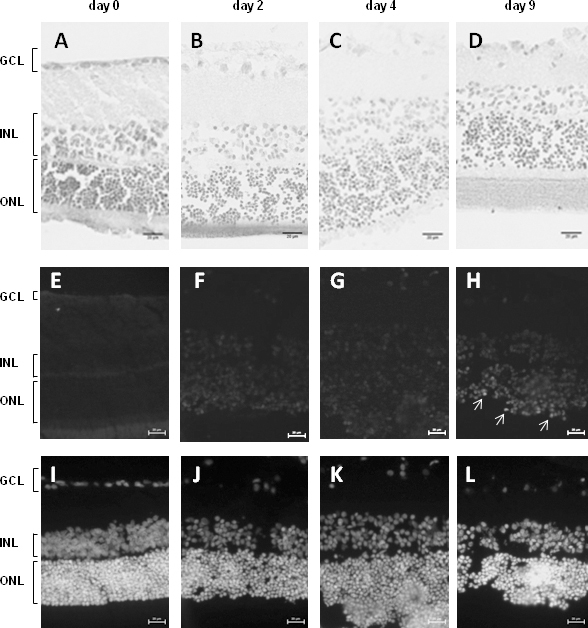
Retina explants cultured up to 9 days do not undergo massive cell death. A–D: Histological sections of explanted retinas cultured according to the M2 protocol and sampled at day 0 (A), 2 (B), 4 (C), or 9 (D) show that the tissues did not undergo massive changes. E–H: Representative sections showing the results of terminal deoxynucleotidyl transferase-mediated deoxyuridine-5'-triphosphate-fluorescein nick end labeling (TUNEL) staining in retina explants prepared as in A–D and kept in culture for 0 (E), 2 (F), 4 (G), or 9 (H) days. TUNEL staining does not reveal any apoptosis until at least day 4 and becomes visible in day 9 samples, mainly in the ONL (white arrows). I–L: 4,6-diaminophenyl-indolamine (DAPI) staining of the sections analyzed with TUNEL assay in retinas sampled at day 0 (I), 2 (J), 4 (K), and 9 (L). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars = 20 µm.
Discussion
The mammalian retina was demonstrated about 15 years ago to harbor a circadian clock [7], but the way in which it works is still a matter of debate. In search of an in vitro model for analyzing retinal circadian properties in mammals, we describe free-running expression profiles of clock genes and clock targets in the isolated rat retina. Using various investigated genes and the complementarity of bioluminescence analysis, this study demonstrates that the retina contains a self-sustaining oscillator that is sensitive to culture conditions.
The occurrence of a circadian clock and of clock gene expression has been investigated in the retina of various mammalian, avian, and amphibian species [10]. Previous studies led to the conclusion that most clock genes are expressed in this tissue in mammals, without reaching any consensus concerning cellular localization and description of the retinal clockwork pathways [14,18-20,26,32,33]. In the present analysis, retinas taken from rats euthanized at the end of the light period were put in culture into constant darkness without any other stimulation, to follow the kinetics of clock gene expression with qPCR. Occurrence of rhythmic expression patterns was shown for all clock genes that displayed periods around 21–24 h, as repeatedly observed in in vitro studies [17,27,30]. These results demonstrate the capacity of the isolated rat retina to generate circadian rhythms, in agreement with previous data that showed the capacity of explanted rat retinas to synthesize and release melatonin in a cyclic manner [34]. Interestingly, the phase of Per1 expression assessed with qPCR coincided roughly with the peaks of bioluminescence driven by the Per1-luciferase reporter; both occurred during subjective nights, which validates the qPCR data. Curiously, the phases were fairly similar for all examined clock genes. The first peaks in vitro clustered during the second half of the subjective night (maximal phase difference of about 2 h when rescaled to a common period). This does not conform to predictions made from the working model for circadian oscillators, in which rhythms of Per genes should be in antiphase with that of Bmal1 [2,35]. The retina is a heterogeneous tissue in which individual cell types are likely to harbor distinct oscillators that show specific phases [10]. In this context, clock gene oscillations measured at the level of the whole tissue probably reflect phases of the expression rhythms with the largest amplitudes, even if they occur in distinct oscillators. In support of that hypothesis, Schneider et al. [20] recently found Clock, Bmal1, Per2, and Per3 oscillating in phase in the retina of rats kept in constant darkness. Similar coincidental phase patterns of clock gene expression were reported in the retina of a diurnal mammal [13], in mouse retinal cones [33] as well as in more peripheral oscillators such as human blood mononuclear cells [36] and mouse bone marrow [37]. Conversely, we cannot exclude that some of the observed oscillations might be relevant to a transitory stage preceding the final oscillatory mechanism of the clock in explants, which might be set later than the third day. Such transitory kinetics have been described for fibroblast cultures that are submitted to synchronizing temperature cycles and whose clock and clock controlled gene expression profiles shift to their final, stable phases within 3–4 days [38]. In this context, transient cycles of Bmal1 and Cry1 expression might be specifically and transiently induced by the process of preparing explants, even if these clock genes do not show cyclic activity in the retina in vivo, in constant conditions [19]. Understanding which one of these hypotheses is correct will require further analysis by using more gene-specific bioluminescence models and/or bioluminescence imaging to visualize clock kinetics at least at the level of retina layers.
Robust rhythmic functions have been described in photoreceptors, including melatonin release and c-fos transcription [10,15,39]. Analysis of mRNA profiles over 48 h in whole retina explants (Figure 3) showed rhythmic expression for c-fos and Aanat, in agreement with the robust rhythms previously described for these genes in vivo [11,12,19], suggesting that in the explants, photoreceptors are able to maintain, at least to a certain extent, rhythmicity in physiologic functions. The peak phases for the expression of the two genes did not occur at the same time, ruling out the possibility that phase clustering of clock genes could be due to a sampling artifact.
Initial bioluminescence investigation in the Per1-luciferase rats did not lead to conclusive results about the capacity of retina explants to display circadian oscillations [26], whereas similar analysis in the PER2::LUC mouse revealed robust cycles in bioluminescence, in specific culture conditions [17]. Our results strongly agree with both studies because the rhythms in clock gene expression assessed with qPCR displayed low amplitude, as could also be concluded from the bioluminescence recordings in the M0 protocol conditions. However, when the culture medium was replaced by 199 medium after 24 h (protocol M1, as in [17]) circadian oscillations in Per1 expression could be seen quite clearly, and this was even more evident when the medium was replaced twice, at 24 and 48 h. Calculation of rhythmic power as a hallmark of clock robustness confirmed that each medium change conferred increased coherence to the retina clock oscillations, as also suggested by the decreasing dispersion of the calculated periods. We do not know how the initial 24 h incubation in Neurobasal A (saturated with 5% CO2) contributes to the overall rhythmicity. Based on the study by Ruan et al. [17], incubation of retina explants directly in 199 medium yields only small amplitude oscillations, which we also observed with the rat explants. However, maintaining retinas on Neurobasal A throughout the recordings (protocol M0) does not promote rhythmicity, based on the present study. Thus, we propose that the medium change, likely in addition to the initial incubation in Neurobasal A, is critical regarding the amplitude of the oscillations. Changing the culture medium has been reported to resynchronize oscillators in vitro and increase the amplitude of bioluminescence oscillations [30,40]. Successive medium changes might also contribute to the health and survival of the explants in culture. Our histological and TUNEL analyses indicate that retina explants are healthy in culture across the bioluminescence recording window. Similarly, explants maintained for several days on Neurobasal-A-medium did not show any apoptosis (data not shown). We speculate that the successive medium changes contribute to increased rhythmic power by facilitating synchronization of individual circadian oscillators located within the retina. This stands in striking contrast to other tissues in which bioluminescent oscillations can be easily monitored directly after dissection, such as the SCN or lung [27]. Although it has been reported that rhythms of clock gene expression in the retina in vivo display low amplitudes regarding other peripheral organs such as the liver [41], we do not know whether it is directly linked to the low amplitudes that we observed in vitro.
In conclusion, our study complements previous reports demonstrating that the rat retina contains a circadian clock(s) able to operate in vitro and to drive cyclic functions relevant to photoreceptor function. Thus, organotypic cultures constitute an appropriate model for investigating the control of rhythmic processes in the retina, and we provide suitable protocols for this purpose.
Acknowledgments
The authors thank Drs. Sophie Reibel and Dominique Sage for animal care, Jean-Georges Lorentz for technical assistance and Adrien Bonneau for assistance in statistical analysis. They also thank Dr. Michael Menaker for providing the Per1-luciferase rats. This work was supported by grants from Retina France and fellowship n°210011/2006–8 from National Council for Scientific and Technological Development (CNPq/Brazil) to DCB.
References
- 1.Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21:494–506. doi: 10.1177/0748730406293889. [DOI] [PubMed] [Google Scholar]
- 2.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 3.Bassi CJ, Powers MK. Daily fluctuations in the detectability of dim lights by humans. Physiol Behav. 1986;38:871–7. doi: 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- 4.Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1998;18:4775–84. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–4. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 7.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–21. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 8.Pozdeyev NV, Lavrikova EV. Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. J Mol Neurosci. 2000;15:1–9. doi: 10.1385/JMN:15:1:1. [DOI] [PubMed] [Google Scholar]
- 9.Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- 10.Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–56. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto K, Ishida N. Circadian expression of serotonin N-acetyltransferase mRNA in the rat retina. Neurosci Lett. 1998;245:113–6. doi: 10.1016/s0304-3940(98)00189-x. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 1993;10:1049–54. doi: 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]
- 13.Bobu C, Sandu C, Laurent V, Felder-Schmittbuhl MP, Hicks D. Prolonged light exposure induces widespread phase shifting in the circadian clock and visual pigment gene expression of the Arvicanthis ansorgei retina. Mol Vis. 2013;19:1060–73. [PMC free article] [PubMed] [Google Scholar]
- 14.Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–41. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Schantz M, Lucas RJ, Foster RG. Circadian oscillation of photopigment transcript levels in the mouse retina. Brain Res Mol Brain Res. 1999;72:108–14. doi: 10.1016/s0169-328x(99)00209-0. [DOI] [PubMed] [Google Scholar]
- 16.Kamphuis W, Cailotto C, Dijk F, Bergen A, Buijs RM. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem Biophys Res Commun. 2005;330:18–26. doi: 10.1016/j.bbrc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- 17.Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci USA. 2006;103:9703–8. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandu C, Hicks D, Felder-Schmittbuhl MP. Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci. 2011;34:507–16. doi: 10.1111/j.1460-9568.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneider K, Tippmann S, Spiwoks-Becker I, Holthues H, Wolloscheck T, Spatkowski G, Engel L, Frederiksen U, Spessert R. Unique clockwork in photoreceptor of rat. J Neurochem. 2010;115:585–94. doi: 10.1111/j.1471-4159.2010.06953.x. [DOI] [PubMed] [Google Scholar]
- 21.Tosini G, Kasamatsu M, Sakamoto K. Clock gene expression in the rat retina: effects of lighting conditions and photoreceptor degeneration. Brain Res. 2007;1159:134–40. doi: 10.1016/j.brainres.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, Rey C, Bennis M, Cooper HM. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013;70:3435–47. doi: 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci USA. 1998;95:6097–102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon D, Silver R. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci. 2003;23:7670–6. doi: 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–71. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–5. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 28.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 29.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–23. [PubMed] [Google Scholar]
- 30.Ruan GX, Gamble KL, Risner ML, Young LA, McMahon DG. Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators. PLoS ONE. 2012;7:e38985. doi: 10.1371/journal.pone.0038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–60. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 32.Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24:573–80. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS ONE. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto K, Liu C, Tosini G. Circadian rhythms in the retina of rats with photoreceptor degeneration. J Neurochem. 2004;90:1019–24. doi: 10.1111/j.1471-4159.2004.02571.x. [DOI] [PubMed] [Google Scholar]
- 35.Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- 36.Teboul M, Barrat-Petit MA, Li XM, Claustrat B, Formento JL, Delaunay F, Levi F, Milano G. Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med. 2005;83:693–9. doi: 10.1007/s00109-005-0697-6. [DOI] [PubMed] [Google Scholar]
- 37.Granda TG, Liu XH, Smaaland R, Cermakian N, Filipski E, Sassone-Corsi P, Levi F. Circadian regulation of cell cycle and apoptosis proteins in mouse bone marrow and tumor. FASEB J. 2005;19:304–6. doi: 10.1096/fj.04-2665fje. [DOI] [PubMed] [Google Scholar]
- 38.Saini C, Morf J, Stratmann M, Gos P, Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012;26:567–80. doi: 10.1101/gad.183251.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto K, Liu C, Kasamatsu M, Iuvone PM, Tosini G. Intraocular injection of kainic acid does not abolish the circadian rhythm of arylalkylamine N-acetyltransferase mRNA in rat photoreceptors. Mol Vis. 2006;12:117–24. [PubMed] [Google Scholar]
- 40.Nishide SY, Honma S, Nakajima Y, Ikeda M, Baba K, Ohmiya Y, Honma K. New reporter system for Per1 and Bmal1 expressions revealed self-sustained circadian rhythms in peripheral tissues. Genes Cells. 2006;11:1173–82. doi: 10.1111/j.1365-2443.2006.01015.x. [DOI] [PubMed] [Google Scholar]
- 41.Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, Foster RG. Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem Biophys Res Commun. 2006;351:800–7. doi: 10.1016/j.bbrc.2006.10.118. [DOI] [PubMed] [Google Scholar]