Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia in humans. The mechanisms that govern AF initiation and persistence are highly complex, of dynamic nature, and involve interactions across multiple temporal and spatial scales in the atria. This articles aims to review the mathematical modeling and computer simulation approaches to understanding AF mechanisms and aiding in its management. Various atrial modeling approaches are presented, with descriptions of the methodological basis and advancements in both lower-dimensional and realistic geometry models. A review of the most significant mechanistic insights made by atrial simulations is provided. The article showcases the contributions that atrial modeling and simulation have made not only to our understanding of the pathophysiology of atrial arrhythmias, but also to the development of AF management approaches. A summary of the future developments envisioned for the field of atrial simulation and modeling is also presented. The review contends that computational models of the atria assembled with data from clinical imaging modalities that incorporate electrophysiological and structural remodeling could become a first line of screening for new AF therapies and approaches, new diagnostic developments, and new methods for arrhythmia prevention.
Keywords: atrial fibrillation, arrhythmia, atrial models, simulation, atrial remodeling
1. Introduction
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia and an important contributor to population morbidity and mortality, affects over 2 million people in the United States alone; data suggests that its prevalence will continue to increase as the population ages.1,2 The mechanisms that govern AF initiation and persistence are highly complex, of dynamic nature, and involve interactions across multiple temporal and spatial scales in the atria, often leading to unpredictable outcomes and emergent phenomena at the organ level. Electrophysiological experimental investigations in cells, tissues and the whole animal, and the human patient3–8 have led to a rapid increase in the body of knowledge regarding the mechanisms underlying AF. In this quest, modeling and simulation of atrial electrophysiology and arrhythmias has played an important role, both in hypothesis-driven research at various levels of integration, but also in providing the framework for the unification of diverse experimental findings. With the increase in computer power over the last decades and the advancement in imaging technologies, multiscale, biophysically-detailed models of the atria have made the initial foray into clinical translation, as part of the emerging discipline of computational medicine,9 by evaluating therapeutic approaches and contributing the patient-specific optimization of cardiac care.
The present review article, part of a thematic series in Circulation Research on AF, provides a broad overview of the plethora of approaches in modeling atrial arrhythmias. The articles focuses on the important role mathematical approaches and computer simulations have played in our mechanistic understanding of AF, and discusses the emerging role of image-based simulation and modeling in assisting the clinical diagnosis and treatment of atrial arrhythmias.
2. Modeling Atrial Fibrillation: Overview of Approaches
Modeling AF, even in its most simple mathematical representation, involves propagation of an electrical impulse (atrial cell action potential, AP) in a network of cells. In their vast majority, models of AF involve biophysically-detailed atrial cell membrane kinetics, i.e. ionic currents, pumps and exchangers, the mathematical description of which is based on the formalism introduced by Hodgkin and Huxley.10 In the study of AF, cells either form a regular two- or three-dimensional (2D or 3D) network, or are arranged in a volumetric representation of atrial geometry and structure. Additionally, cellular automata models have been used in the study of atrial arrhythmias, most notably the first model of AF, by Moe et al11 in 1964, which suggested that AF is maintained my multiple meandering wavelets; this study has had a profound effect on AF research over many years, as well as on the concepts of arrhythmia and its therapy. This section reviews briefly the methodological basis and advancements in both cell automata and biophysically-based models of AF.
2.1 Cellular Automata Models
Cellular automata models involve regular grids of cells (typically 2D, and of square or hexagonal structure), where each cell is in one of a number of states. The behavior of a cell in the grid evolves at discrete time steps, following state update functions. Cell state updates are obtained by taking into account cells’ states in the local neighborhood only. In the classical paper by Moe et al,11 cells in the automaton existed in one of 5 states, an absolutely refractory state, three stages of partial refractoriness permitting firing after a delay of decreasing duration, and an excitable state. The duration of the absolute refractory period was varied, and was distributed randomly within the sheet. While simulation of AF using a cellular automaton is computationally inexpensive, its most significant drawbacks are lack of dynamic electrotonic interactions (since influences do not extend beyond a defined neighborhood) and inability to dynamically regulate ionic flows. Despite these significant limitations, cellular automata models have enjoyed resurgence in the last few years,12,13 driven by the need to employ rapidly-executable models in clinical applications and to provide a framework for quick interpretation of clinical observations.
2.2 Biophysically-detailed cell electrophysiology models
Biophysically-based cell models, typically following the Hodgkin-Huxley formulation, represent current flow through ion channels, pumps and exchangers as well as subcellular calcium (Ca) cycling, and are governed by a set of ordinary differential and algebraic equations; ionic models differ vastly in their level of complexity. For the atrial cell, a number of ionic models have been developed, as reviewed in recent papers.14–16 Here we briefly summarize these developments and highlight the newest advancements in representing atrial cell electrophysiology not covered by these reviews.
The earliest atrial cell models were based on measurements in frog17 and rabbit.18,19 Two human atrial cells models were subsequently developed, by Courtemanche et al20 and the Nygren et al21, and have enjoyed a wide use in AF multiscale simulations. While these models have been partially developed using the same human experimental measurements, the paucity of the latter had necessitated the use of additional experimental data obtained from mammalian hearts. Therefore, the two models differ by their AP shape and by their dynamics, and have different rate-dependent behavior and restitution, due to marked differences in Ca handling formulation (see reviews22,23). The Courtemanche model has been further extended to reproduce regional heterogeneities and electrophysiological remodeling in the human atria,24,25 account for acetylcholine-dependent influences,26 and simulate effects of drug delivery;24,27 a canine atrial cell model25 has also been developed based on it.
As new human data has recently become available, both models have been modified to improve their physiological accuracy. Maleckar et al28 re-implemented the Nygren model, with improved description of repolarization and rate dependence. Koivumaäki et al29 further extended the Nygren and Maleckar models by accounting for atria-specific characteristics of sarcoplasmic reticulum (SR) Ca uptake and release, and specifically, the delay between peripheral and central SR Ca release, characteristic of cells lacking t-tubules. Krummen et al30 modified the Courtemanche model to account for extracellular K accumulation during rapid pacing and to fit AP duration (APD) and its restitution to newly available clinical recordings.
A third lineage of human atrial cell models was commenced by Grandi et al.31 The model incorporated new Ca dynamics formulation based on data from atrial myocytes at physiological temperature obtained from patients, and also represented the contribution of beta-adrenergic and cholinergic stimulation. The model was recently modified32 to represent a mutation (E299V) in KCNJ2, the gene that encodes the inward rectifier K channel protein, and used to examine its effect on AP (it resulted in abbreviated APD).
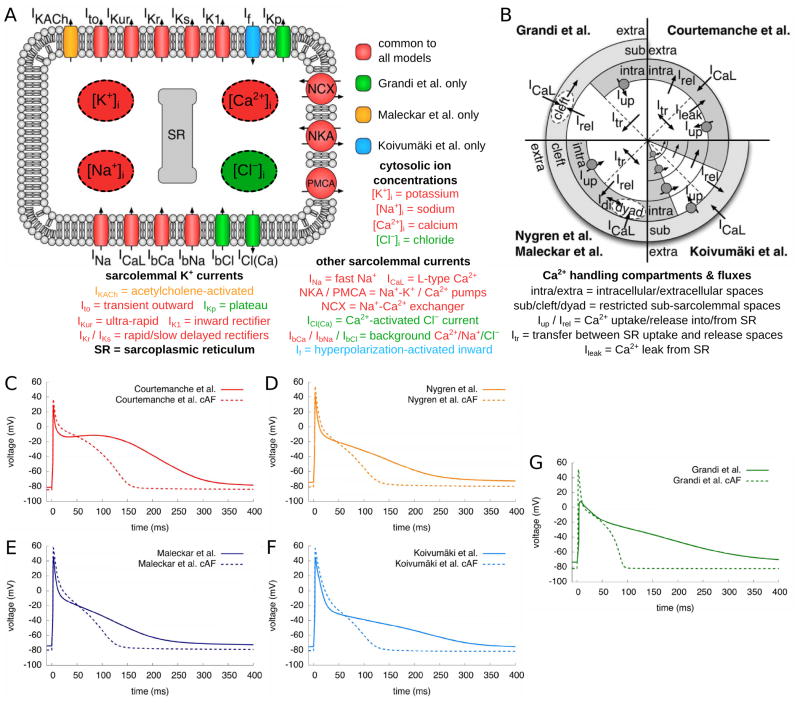
All three distinct human atrial cell models were further augmented to represent APs under chronic AF conditions. This effort has been based on seminal studies by the Allessie’s group,33 who proposed that “AF begets AF”, emphasizing that persistence of AF leads, in itself, to electrophysiological alteration in AP properties (predominantly via the altered expression of different ion channels34–36), which, in turn, increases the propensity to chronic AF (cAF). To represent remodeling under the conditions of cAF, human ionic channel measurements were used to construct the respective cAF versions of the three main human ionic models, as summarized in the review by Bers and Grandi16. The study by Wilhelms et al37 provided a systematic benchmarking of these three models, their corresponding augmented models, as described above, as well as their cAF versions. Fig. 1 illustrates the benchmarked AP models (paced at a frequency of 1Hz) as well as the differences between the ionic currents and Ca cycling in the models; the Krummen et al model was not included in this analysis since the benchmarking study preceded its publication. This benchmarking provided information that could be used to guide the selection of the cell model for a particular study. In terms of usefulness of the cell models in the study of AF, as tested by Wilhelms et al, reentrant arrhythmia was inducible with all cAF models, however the resulting spiral wave dynamics were highly divergent, underscoring the fact that the choice of cell model should be tailored to the application. For instance, the Courtemanche et al model was found unique in its ability to consistently produce stable beat-to-beat APD alternans for tissue-level simulations (and so is the Krummen et al model). The differences in ionic model properties may reflect inherent electrophysiological variations in human atrial myocytes behavior and/or regional electrophysiological differences in the human atria; dynamic parameter fitting and adjustments38 might offer a standardized approach in model development.
Figure 1.
A. Schematic of the atrial cell, including representations of ionic channels and intracellular ion concentrations used in the various human atrial cell models. B. Schematic of Ca handling with different compartments and currents in the various atrial cell models. Each of the models’ Ca handling is represented in one quarter of the schematic. White spaces in the central region denote the SR, with straight broken lines representing the division between SR uptake and release regions. Gray spaces represent the other compartments within the cell and outside of SR, with the “cleft” and “dyad” sub-spaces shown in white. Arrows indicate directions of Ca flow between the different compartments. The SR Ca pump is indicated by circle and arrow. The Koivumaki et al model29 also incorporates additional compartments representing peripheral and central SR regions and the current flow between these regions and the intracellular space. Additional detail regarding Ca handling in the models can be found in the original publications.20,21,28,29,31 D–G. Control and cAF APs of five atrial cell models paced at a frequency of 1Hz. Modified with permission from.37
Two very recent developments in single atrial cell models left the realm of the Hodgkin-Huxley formalism. The goal of the modeling effort by Voigt et al39 was to help ascertain the mechanisms of SR Ca-release events in human paroxysmal AF (rather than cAF); experimental data from right atrium (RA) appendages (RAAs) from sinus rhythm patients and patients with paroxysmal AF were used for model development. The model was based on that by Grandi et al,31 but included a spatial representation of Ca handling and stochastic gating of ryanodine receptors (RyRs). Model results demonstrated that both RyR dysregulation and enhanced SERCA2a activity promote increased SR Ca leak and SR Ca release events, causing delayed after-depolarizations (DADs) in paroxysmal AF atrial cells.
The second model development40 entailed a different mathematical modeling approach that allowed characterization of Ca movement within the (idealized) 3D volume of an atrial myocyte. Novel model aspects included the geometrically realistic representation of Ca release sites within the cell, allowing for exploration of their interaction, as well as Ca wave initiation and propagation. The study explored the generation of centripetal Ca waves during excitation–contraction coupling, and the effect of positive inotropic stimulation on the spatial profile of Ca signals. It remains unknown, however, whether such modeling approaches could be incorporated in tissue-level atrial models and used in the study of AF.
2.3. Multiscale Modeling of Propagation
In tissue, atrial myocytes are electrically connected via low-resistance gap junctions. Ionic current can flow from cell to cell via this pathway, in addition to the current exchange between intracellular and extracellular spaces through cell membrane proteins, as described above. Propagation in excitable media, such as atrial tissue, is typically modeled using spatially continuous models that are viewed as resulting from a local spatial homogenization of behavior in tissue compartments (membrane, intra- and extracellular spaces). Current flow in the tissue structure is typically governed by the monodomain reaction-diffusion partial differential equation, PDE, over the tissue or organ volume, with the use of conductivity tensor fields. Simultaneous solution of the PDE(s) with the set of ionic model equations represents simulation of electrical wave propagation in the heart. The conductivity tensor fields used in these continuous models integrate all the information about the distribution of gap junctions over the cell membranes as well as the fiber, sheet and other microstructure organization in the atria.
The methodology for modeling atrial propagation is the same as that for modeling wave propagation in the ventricles, thus for detailed information on the various approaches we direct the reader to comprehensive reviews on the subject14,15,41,42.
2.4. Geometric and Image-based Atrial Modeling
Cellular automata and biophysically-detailed models have both been used with simple 2D geometries (sheets of atrial tissue).11,12,26,43–47 Because of the smaller atrial wall thickness as compared to the ventricles, 2D geometries rather than 3D slabs of tissue have been typically employed in simplified AF simulations.
In contrast, geometrical atrial models include those of high structural detail of an atrium part, such as RAA, pulmonary veins (PVs), crista terminalis, or pectinate muscles (PM), of different animal species48,49, or geometrical models of at least one atrial chamber. Development of high resolution geometrical models of isolated atrial structures has been motivated by the notion that specific atrial structural substrates are more likely to be involved in sustaining AF.5
3D models of at least one of the atria have predominantly aimed to represent organ anatomy in the study of human AF. The exception is the high-resolution structural model of the sheep atria acquired by serial surface imaging50,51; the complex structure of this model is shown in Fig. 2A. The group of models based on geometric representations of at least one of the human atrial chambers can further be sub-classified into surface and volumetric models. Surface models represent atrial geometry in 3D but neglect wall thickness;52–55 the latter is not true for volumetric models.56–62 Furthermore, human atrial models incorporate either idealized atrial shapes representing closed-surface organ properties52,55,63 (spheroidal shape of the atrium), including the topology of the insertions of veins and valves, or atrial geometries based on image acquisition53,54,61,62 employing a variety of imaging modalities.
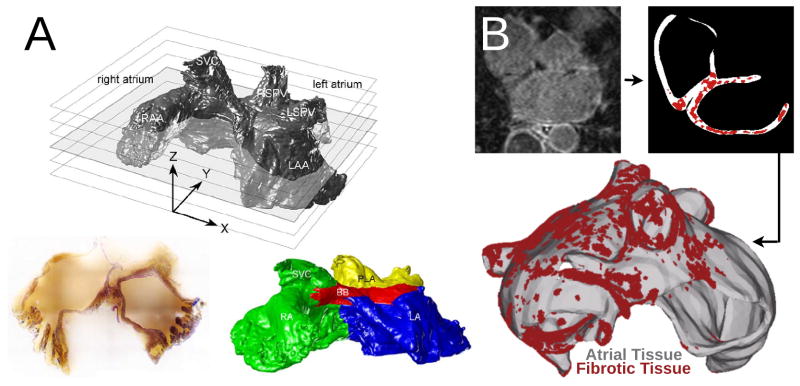
Figure 2.
Geometrical models of the atria. A: Volume image of the sheep atria acquired by serial surface imaging (resolution 50 microns), with a representative slice. Subdivision of atria into different regions as represented by the different colors: RA—green, LA—blue, Bachman’s bundle (BB)—red, posterior left atrium (PLA)—yellow. Images reproduced with permission from50,51. B: A model of the fibrotic human atria generated from a patient LGE-MRI scan (top left) following segmentation (top right) into normal and fibrotic tissue (fibrotic lesions in red). With permission from62.
Human image-based atrial model development commenced with the use of the atrial geometry dataset56–58,64–67 (see also table of models in14) resulting from the Visible Human project.68 Subsequently, atrial geometries used in electrophysiological simulations were acquired using magnetic resonance imaging (MRI)53,54,61,62,69 (Fig. 2B) as well as computed tomography (CT)70; refer to71 for a review of image acquisition. An informative comparison between some of the different geometries used in human atrial modeling can be found in Fig. 5 of the review by Jacquemet et al.15 Additionally, a comprehensive listing of human atrial models is provided in Table 1 of Doessl et al; models not represented in that table include those recently published by McDowell et al61,62, and Tobon et al.67 Fiber structure has also been represented in these models either manually or using a semi-automatic rule-based approach14 (Fig. 2C) since diffusion tensor imaging of the thin atrial walls does not provide reliable information about atrial fiber architecture. 3D atrial models have often incorporated add-on representations of atrial structures such as the Bachman’s bundle, crista terminalis, PMs, and the coronary sinus sheath.
Recently, organ-level atrial models have begun to represent fibrotic structural remodeling associated with persistent AF. Atrial fibrosis is imaged using late gadolinium enhancement (LGE) MRI.72 McDowell et al61,62 created the first model of patient atria with fibrotic remodeling (Fig. 2B) by segmenting out the enhanced regions in the LGE MRI scans; similar approaches followed.73 McDowell et al.61,62 used a sophisticated model of fibrotic remodeling in the LGE enhanced regions of the patient atria, accounting for (i) connexin downregulation/hypophosphorylation and lateralization, (ii) collagen deposition, and (iii) myofibroblast infiltration. Myofibroblasts in the fibrotic regions, represented by the ionic model of MacCannell et al74 were coupled to myocytes, as described in Maleckar et al.75 The electrophysiological representation of fibrotic remodeling in the human atrial models remains, however, controversial because of the lack of experimental data. Similarly, the segmentation of the LGE MRI fibrotic regions and even segmentation of the geometry of the thin atria from clinical MRI is fraught with uncertainty and is an area of intense image-processing research.
3. Exploring Atrial Fibrillation Mechanisms: Insights from Modeling
3.1 Mechanisms Landscape
Despite a significant body of basic and clinical research, the fundamental mechanisms governing AF initiation and maintenance are incompletely understood.7 As a result, treatment of AF remains ineffective, presenting a significant potential for improvement. Excellent recent reviews offer a detailed overview of the long history of mechanistic exploration of AF mechanisms.3,6 It is now accepted conceptually that the clinical progression of AF involves evolution from paroxysmal to persistent and permanent forms of the arrhythmia, and that it reflects progressive electrophysiological and structural remodeling35,76,77 caused by the downward spiraling impact of the arrhythmia itself and the progression of the underlying heart disease.4,78 Note that the described progression is seen in only part of the patient population, with the issue of AF evolution remaining the subject of intense research.
Our understanding today is that paroxysmal AF is typically driven by rapid focal activity (either early or delayed afterdepolarizations) or local reentry in the cardiac muscle sleeves around the PVs.79,80 Accordingly, PV isolation via RF ablation eliminates paroxysmal AF in 70%-80% of the treated patient population.81 Persistent AF is thought to arise from electrophysiological remodeling of the atria resulting from altered protein expression and/or function of cardiac ion channels, often caused by AF itself.5 Its hallmark is the decrease in APD, often accompanied by increased DAD risk due to Ca overload.5,82 The overarching persistent AF mechanism is currently thought to be functional reentry: one or more rapidly rotating spirals, the emitted waves of which interact with anatomic and functional obstacles, leading to wavefront fragmentation and fibrillatory conduction.83 Furthermore, autonomic neural remodeling contributes to AF recurrence and maintenance in both paroxysmal and persistent AF forms.84,85
Electrophysiological remodeling itself accelerates the progression from paroxysmal to permanent AF;4,78 the latter is also associated with irreversible structural changes, particularly fibrosis,86,87 rendering the remodeled atria as a substrate for both functional and anatomical reentry. While the multiple wavelet hypothesis,11 according to which AF is the result of randomly propagating multiple electrical wavelets changing in number and direction, has been a dominant mechanistic model of permanent AF (and even persistent AF), recent clinical evidence88 (albeit limited) has demonstrated that in humans permanent AF may also be the result of a small number of persistent rotors with fibrillatory conduction to the surrounding atrium.
While not all atrial modeling efforts to uncover AF mechanisms are classifiable along the lines of AF progression as described above, they have nonetheless addressed mechanisms that could be pertinent to any form of AF. A review of the most significant mechanistic contributions made by atrial simulations is provided below.
3.2 The Normal Atria: Intrinsic Atrial Structural and Electrophysiological Heterogeneities Predispose to Atrial Arrhythmias
Even in the structurally and electrophysiologically normal atria, the complex closed-surface geometry of the chambers,89 with a set of distinct structural features such as orifices and discrete bundles, presents a substrate that predisposes to arrhythmia initiation under conditions of source-sink mismatch, and also often determines the specific (anatomic) reentrant pathways of the ensuing arrhythmia, as found by modeling studies.48,49,52,60,63,90–93 Following the seminal experimental-simulation work by Spach and co-workers,94 atrial modeling results43,48,49,58,60,70 have similarly demonstrated that intrinsic differences in APD in the various atrial structures additionally predispose the atria to rhythm disorders. Local variation of wall thickness resulting from the presence of PMs has been shown90,93,95 to increase the downstream load on a propagating wavefront and result in wave break-up. Furthermore, PMs have been found to play a role in the conversion between AF and flutter by anchoring spiral waves.90,92 Modeling studies43,52, 91,93 have implicated the highly anisotropic conduction and longer APD in the crista terminalis in setting up the conditions for reentry generation. Using anatomical models of the rabbit RA and of the pig RA appendage, respectively, both based on histological reconstructions, studies by Aslanidi et al49 and Zhao et al48 demonstrated that because electrotonic coupling transverse to fibers in the crista terminalis is weak, high-frequency pacing at the border between the crista and PMs results in a reduced safety factor, leading to unidirectional block and subsequent generation of reentry. Based on results from a model of the human atria,60 Fig. 3 shows the excitation wave on the epicardial surface of the atria during normal sinus rhythm as well as activation patterns in AF, the result of rapid pacing near the crista.
Figure 3.
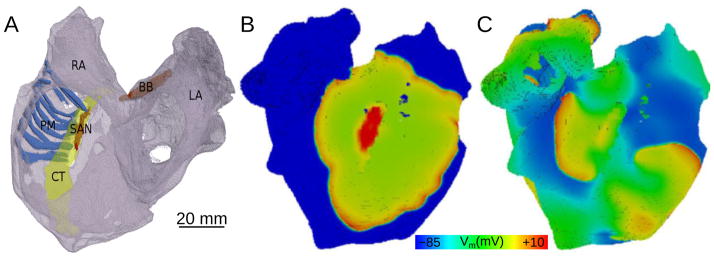
Membrane potential distribution on the epicardial surface of the human atria. A: Spontaneous normal rhythm. B: Pacing-induced AF resulting from different electrophysiological properties of the crista terminalis. With permission from60.
Other aspects of normal structure and electrophysiology have also been implicated, in simulation studies, in predisposing the atria to arrhythmias. Vigmond et al52 showed that the muscular sheath of the coronary sinus could act as a pathway for reentry and to also stabilize reentrant circuits utilizing the isthmus near the inferior vena cava. Using a model of sheep atrial geometry and myofiber orientations also reconstructed from serial section images, Zhao et al51 demonstrated that the complex myocyte arrangement in the posterior LA contributes to dispersion in activation times in the region adjacent to the PVs, and to increased vulnerability to arrhythmia following ectopic beats originating in PV sleeves; the arrhythmia vulnerability was exacerbated by spatial variation in APD across this region.50 Another study70 examined the effect of APD differences in the canine atria (LA vs. RA as well as increased APD shortening with increased distance from the sino-atrial node), and found that the APD gradients increase the propensity of wave break, spiral wave core meander, and quasi-stable reentry. Finally, the openings of the inferior and superior venae cavae52 and that of the tricuspid valve63 were shown to serve as anchors of reentry (atrial flutter).
The parasympathetic nervous system also plays a role in creating a substrate for AF: through the release of acetylcholine (ACh), vagal stimulation causes a significant reduction in effective refractory period and rate adaptation loss.96,97 This effect creates APD non-uniformity over the atria due to the sparsely distributed vagal nerve endings (ACh release sites). Using a new formulation of ACh-dependent K current, Kneller et al26 demonstrated, in a 2D atrial model with periodic variations in ACh concentrations, that sufficiently large vagally-induced APD gradients may be established, causing a reentrant wave to break up and the activity to transition into a cholinergic form of AF; the specific arrhythmia morphology was found to depend on substrate size.98 The arrhythmogenic conditions associated with non-uniform vagal stimulation were further explored,55 providing a detailed analysis of vulnerability windows for ectopic beats under different levels and patterns of vagal activity. Finally, Atienza et al99 simulated left–right differences in ACh-dependent K current, finding that this difference resulted in faster rates of the LA rotor, driving RA activity.
3.3 Paroxysmal AF Initiation
The demonstration by Haissaguerre et al79 of the importance of PV foci in initiating arrhythmia constitutes an important advancement in our understanding of AF etiology. Different cellular mechanisms have been proposed100 for the generation of spontaneous activity in the cardiomyocyte sleeves of the PV, including automaticity and afterdepolarizations. In early simulations,101 PV automaticity has been represented by the addition of the hyperpolarization-activated inward current If to the human atrial AP; a PV cardiomyocyte-specific cell model was subsequently developed.102 The possibility of microreentry within the PV sleeves of varying diameter and length was explored by the modeling study of Cherry et al103; it demonstrated that the electrical and microstructural characteristics of the PVs, distinct from those of the LA, result in heterogeneous and anisotropic conduction and PV reentry. As presented in Fig. 4A, a single premature LV activation invading the PV establishes PV reentry, which in turn continuously re-excites LA, presenting a “focal source” of LA activation.
Figure 4.
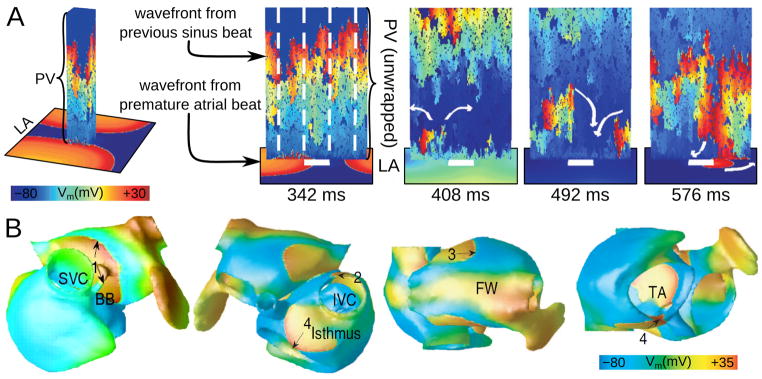
A: Continuous PV re-activation of the LA due to heterogeneous venous conduction and reentry following a single ectopic beat in the LA (with permission from103). The sinus beat propagates heterogeneously along the vein (wrapped and unwrapped views, 342ms). Vein length is 1 cm, and circumference is 2cm, with 30% longitudinal and 65% transverse cellular disconnections. A single premature activation originating somewhere in LA invades PV following the sinus beat (342ms and 408ms), encountering block (408ms) and establishing PV reentry (492ms). This reentry continuously re-excites the LA, serving as “focal source” for LA activations (576ms; propagation entering LA at bottom of image). B: A snapshot of membrane voltage in the human atria at a single time point during AF (different views of the atria are shown), in which numbered arrows (1–4) indicate multiple reentrant wavelets. AF was induced by PV ectopic beats (with permission from64). SVC and IVC–superior and inferior vena cava; BB--Bachman’s bundle; FW–free wall; TA—tricuspid annulus.
While simulation studies have not been conducted exploring how a biophysically and structurally detailed model of ectopy in the PV sleeves would drive the atria into paroxysmal AF, the onset of paroxysmal AF has been explored by modeling the delivery of trains of pacing stimuli in the PV region. Gong et al64 demonstrated that spontaneous firings of ectopic foci, coupled with sinus activity, produced dynamic spatial dispersions of repolarization, including discordant alternans, which caused conduction block and led to atrial flutter and AF (see Fig. 4B as an example of the distribution of atrial transmembrane potential, including several distinct wavefronts). While the likelihood of reentry induction varied depending on ectopic foci locations and timing, ectopy from the PV region resulted in the largest vulnerable window.
A combined clinical-simulation study by Krummen et al30 examined the mechanisms behind the clinical interventions that induce AF, namely the administration of isoproterenol or adenosine, as well as rapid pacing. Isoproterenol and rapid pacing both steepened maximum APD restitution slope promoting AF initiation, although via distinct mechanisms, as demonstrated by the simulations. APD restitution steepening in the former intervention arose from the alteration of Ca dynamics, while restitution steepening in the latter stemmed from K accumulation. Adenosine did not steepen APD restitution, and AF propensity remained unchanged.
Finally, lone forms of paroxysmal AF have been found to be the result of inherited ion channel dysfunction. A missense gain-in-function KCNQ1 S140G mutation has been implicated in a familial form of AF, with simulation research,104 using a model of the human atrium, establishing the causal link between mutation and genesis of AF: increased IKs current arising from the mutation abbreviated APD, facilitated the conduction of high rate atrial excitation waves, and stabilized reentry. Similarly, numerical experiments32 have elucidated how a mutation (E299V) in KCNJ2, the gene that encodes the strong inward rectifier K channel protein (Kir2.1), results in AF.
3.4 “AF begets AF”33: Electrophysiological Remodeling in Persistent AF
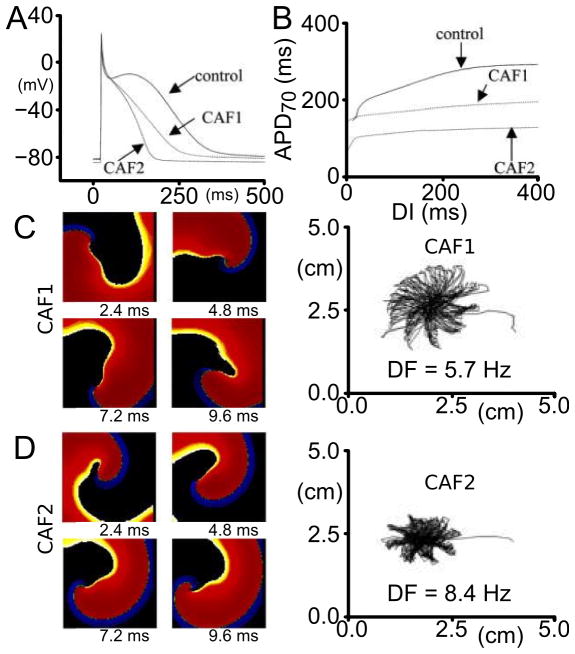
AF and very rapid tachyarrhythmias cause, over time, electrical remodeling of the atria; the latter can also be induced by other conditions, most commonly congestive heart failure (HF).105 Electrical remodeling is manifested as altered ion channel expression and/or function in a way that further promotes AF.4,106 The triggering mechanisms, chiefly among which is increased Ca load, lead to the onset of a chain of protective mechanisms resulting in electrophysiological remodeling of the tissue. Models of the atrial cell under the conditions of persistent AF have been developed, as described in section 2.2 (termed cAF models, since they were used to represent electrical remodeling in both persistent and permanent forms of AF; see next section for latter), and have made contributions to understanding the dynamic interplay between the various remodeled ionic channels/currents24,31 that give rise to diminished Ca transients and shorter APDs in persistent AF. A resulting characteristic of global atrial activity in persistent AF is rotor stabilization, which increases AF vulnerability and sustainability. This phenomenon has been investigated by modeling studies at the tissue level. Pandit et al45 examined the behavior of a stable but meandering rotor, and demonstrated that increasing the magnitude of the inward rectifier current K current (IK1) resulted in reduced meandering of the rotor (Fig. 5). A recent modeling paper44 analyzed systematically the relative importance of ionic currents and transporters in modulating excitability, refractoriness, and rotor dynamics in human atrial tissue. Results underscored the important role of the Na/K pump in modulating APD, restitution, and dominant frequency (DF) of the reentrant activity, providing comparisons between behavior in sinus rhythm and persistent AF; IK1 and INa were both found to strongly affect rotor stability.
Figure 5.
A: Simulated APs in control and chronic AF conditions (CAF1, CAF2). CAF1 is an AF cell model with Ito and ICaL reduced, without IK1 upregulation; CAF2 is the same model with IK1 increased. B: Electrical restitution plotted as APD–70 versus the diastolic interval (DI) in control and chronic AF cases. C and D: Spiral waves (phase movie snapshots) and tip meander in chronic AF conditions CAF1 and CAF2. Phase movies are shown at four distinct times. The figure demonstrates that IK1 stabilizes and accelerates reentry, as manifested by the reduced tip meander. Modified with permission from45.
Simulations of electrophysiological remodeling were also extended to 3D modeling of the human atria.107 Colman et al107, incorporating heterogeneity in electrical remodeling across the structures of the human atria, found that remodeling abbreviated atrial APD non-uniformly in the various atrial structures, resulting in relatively short APDs coexisting with marked regional differences in the APD at junctions of the crista terminalis/PMs and PVs/LA. The increased electrophysiological heterogeneity stabilized and accelerated reentrant excitation waves, leading to rapid and sustained AF.
A different remodeling effect of AF has been hypothesized to stem from the fact that under AF conditions the atria become dilated. Atrial dilation exerts a mechano-electric influence through stretch-activated ion channels; the effect is diastolic depolarization, abbreviated refractory periods, triggered activations, and increased dispersion in electrophysiological properties.108,109 Simulation studies in the human atria110 demonstrated that under dilation, focal sources near the PV initiated AF, resulting from electrophysiological alterations due to the heterogeneous stretch throughout the atria. Similarly, the experimental-modeling study by Yamazaki et al111 highlighted the stabilization of meandering spiral wave filaments at locations with large gradients in myocardial thickness, and thus stretch, leading to heterogeneous distribution of stretch-activated channels activation and their influence through mechano-electric feedback.
Finally, a model of heterogeneous electrical remodeling in the human atria was used66 to assess the spatiotemporal organization of the activity in persistent AF using different signal analysis techniques, such as DF112 and the organization index (OI, ratio of signal spectral power to total power of the spectrum)113. The ability of DF and OI maps to localize AF sources of high frequency was compared, with the results suggesting that a better localization might be obtained using OI maps.
3.5 Fibrotic Remodeling and Permanent AF
Structural remodeling, and specifically fibrosis, is a hallmark of permanent AF.86,87 Fibrotic remodeling of atrial tissue involves processes that occur in parallel across multiple scales: at the membrane level, gap junction remodeling due to connexin 43/40 (Cx43/40) protein downregulation/hypophosphorylation and lateralization114,115, at the cellular level, fibroblast proliferation and phenotype switching,86,116 and at the tissue level, the deposition of excess collagen87,117, both from reactive interstitial fibrosis separating muscle bundles, and from reparative fibrosis replacing dead cardiomyocytes, both interfering with electric continuity and slowing conduction.3,117 Thus, structural remodeling, combined with remodeling at the ion channel level as described above, gives rise to complex interactions at the organ level, setting the stage for AF initiation and maintenance in the fibrotic atria.
Models of the fibrotic atria have accounted for different aspects of fibrotic remodeling, in an attempt to elucidate the mechanisms leading to altered conduction and those responsible for the drivers and organization of permanent AF. The simplest model representation of atrial structural remodeling was based on the assumption that a component of structural remodeling, gap junction remodeling (Cx43/40 downregulation/hypophosphorylation and lateralization), occurs throughout the atria in a uniform fashion. Two such studies have been conducted thus far: one65 assumed that the coupling strength between computational cells was decreased (Cx43/40 downregulation/hypophosphorylation only), while the other118 modeled increased anisotropy throughout the LA (representing both aspects of Cx43/40 remodeling). The simulations showed65 that decreasing the coupling between cells slowed conduction and decreased the wavelength, further perpetuating AF. Plank et al118 demonstrated that increased anisotropy throughout the fibrotic human LV was an additional mechanism for the breakup of PV ectopic waves into multiple reentrant circuits; higher anisotropy ratios resulted in sustained reentrant activity even though the ectopic focus was no longer present. Similar conclusions were obtained from a human atrial model73 where the locations of the fibrotic (i.e. high-anisotropy-ratio) regions were implemented from patient MRI-LGE scans.
The next component of fibrosis, collagen deposition, has been represented in models as insulating barriers, and in several ways: (i) by removing randomly the electrical connections between two 2D layers of atrial tissue, the endocardial and the epicardial, in order to model an increased level of dissociation between these two layers (a form of reactive interstitial fibrosis), mimicking experimental observations in goats;119 (ii) by introducing a set of random collagenous septa disconnecting cardiac fibers in the transverse direction120 (reactive interstitial fibrosis again); and (iii) by incorporating non-conductive or non-excitable regions of various sizes throughout the tissue115,121,122 (reparative fibrosis), either randomly throughout the atria, or based on imaging data. Endo-epicardial dissociation resulted119 in a number of AF reentrant waves that was significantly higher than that in the case without dissociation, exacerbating AF complexity. The increase in collagen content in the interstitial spaces between fibers was not found to affect longitudinal conduction,61,62,115 but caused slowed propagation in transverse direction, with the degree of slowing dependent on the length of the collagenous septa.120
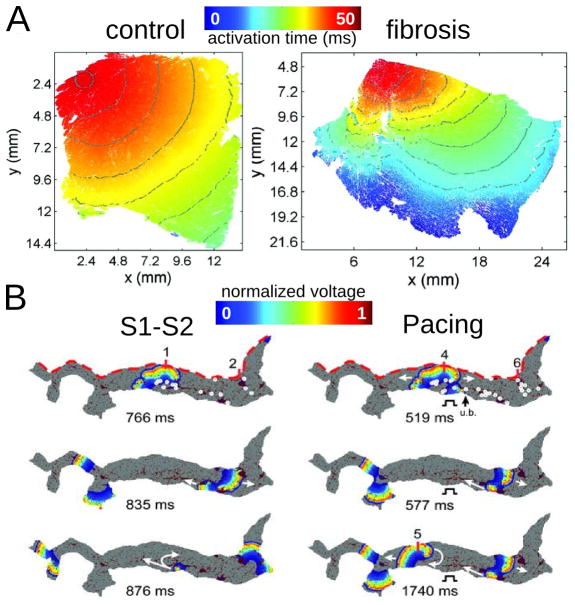
Atrial models incorporating transverse collagen deposition115,121,122 (as in reparative fibrosis) have highlighted the significant interruption and disarray in atrial conduction patterns caused by it. Importantly, collagen deposition rather than Cx43 remodeling was found to be the major factor in atrial conduction disturbances under HF conditions115 (Fig. 6A). Furthermore, it was established that not only the total amount, but also the specific distribution of collagen deposition (as generated by a stochastic algorithm) governed the occurrences of conduction block.121 To evaluate the consequences of HF remodeling (ionic and structural) on AF dynamics, Tanaka et al122 used 2D models of transmural posterior LA sections generated from histological data; patchy distributions of collagen were also reconstructed from that data (Fig. 6B, top image). Simulations demonstrated that whether the mechanism sustaining AF was reentrant or focal (generated by an S1–S2 protocol or pacing, Fig. 6B), fibrous patches of large size were the major factor responsible for the different dynamics of AF waves in failing versus control hearts. The patches anchored reentrant circuits (see white circles representing wavebreak, the locations of which, when associated with large collagen patches, remained the same regardless of AF induction protocol) and impaired wave propagation to generate delays and signal fractionation.
Figure 6.
Modeling fibrosis as regions of collagen presence. Collagen is represented as an insulator. A: Simulations of propagation in 2D tissue sections (control, left, and fibrosis, right). With permission from115. B: Simulations in LA transmural slices for HF conditions. Snapshots at several timeframes for cross-field stimulation (left) and pacing at a frequency of 6Hz (right). Colors indicate transmembrane voltage from low (blue) to high (red). The site of unidirectional block(ub.) is indicated by a black arrow. White circles on the upper voltage maps indicate sites of wavebreak. With permission from122.
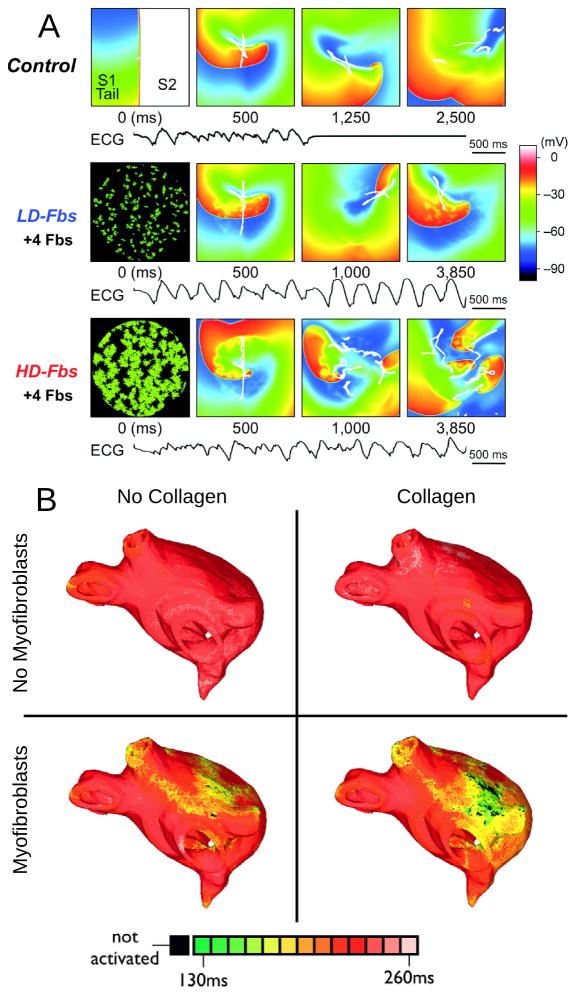
The third major component of fibrotic remodeling, fibroblast proliferation and phenotype switching, has also been represented in computational models of the atria, particularly in view of the fact that fibroblasts, in addition to being part of the structural remodeling of the atria, can also exert electrophysiological influences on neighboring myocytes, possibly either through electrical coupling,123 or via paracrine effects.124 The first study to explicitly incorporate fibroblast presence as a representation of fibrotic remodeling was the 2D atrial model by Asihara et al.46 Within the fibrotic region, coupling of fibroblasts (kinetics governed by a fibroblast ionic model) to atrial myocytes caused shorter APD, slower conduction, and lower excitability as well as spiral wave breakups, similar to experimental results125 in neonatal rat monolayers. This effect was exacerbated when fibroblast density increased (Fig. 7A). Interestingly, when fibroblasts were substituted by collagen in the model, wave breakups were not observed. While this study presented intriguing mechanistic insight, it is important to acknowledge that myofibroblast-myocyte coupling needs additional evidence of its existence in the intact heart.
Figure 7.
Modeling fibroblast proliferation in the regions of fibrosis. A: Effect of myocyte- fibroblast coupling on spiral wave behavior in a myocardial sheet of size 4.5×4.5 cm. Top, control case without fibroblasts. Middle and bottom, models of low-density and high-density fibroblast proliferation (LD-Fbs and HD-Fbs) in a central circular region of the sheet. In the LD-Fbs and HD-Fbs models, atrial myocytes (100pF), each connecting to 4 fibroblasts (6.3pF) within the Fb-Area, account for 12.5% and 50.0% of that area, respectively. The simulated ECG in each case is shown at the bottom. With permission from46. B: Maps of APD in four human atrial models (same atrial geometry). Fibrotic lesions are modeled with (bottom row) and without (top row) myofibroblast infiltration (and coupling to myocytes), as well as with (right column) and without (left column) diffuse collagen deposition for both sets of maps. All models include gap-junction remodeling in the fibrotic lesions. With permission from61.
All three elements of fibrotic remodeling (gap-junction remodeling, collagen deposition, and myofibroblast proliferation), in addition to cAF ionic remodeling, were combined together in the LA model generated from MRI-LGE data of a patient with permanent AF,61,62 capturing accurately both the atrial geometry and the distribution of fibrotic lesions. Here, fibroblast proliferation was represented in two ways: via fibroblast coupling to myocites or via the paracrine effects on ionic channels, acknowledging the paucity of evidence regarding myocyte-fibroblast coupling in fibrotic regions. The model was used to examine the mechanisms for AF initiation by PV ectopic stimulation. The study found that for fibrotic lesions typical of human remodeled atria under the conditions of persistent AF, gap junction remodeling in the fibrotic lesions was a necessary but not sufficient condition for the development of AF following a PV ectopic beat. The sufficient condition was myofibroblast proliferation in these lesions, where myofibroblasts exerted either electrotonic or paracrine influences on myocytes within the lesions. Deposition of collagen in the lesions assisted the myofibroblasts’ paracrine or electrotonic effects by additionally shortening APD there (Fig. 7B).
4. Atrial Fibrillation Management: Can We Learn from Atrial Models?
The ability to construct multiscale models of the electrical functioning of the atria, representing integrative behavior from the molecule to the entire organ, has paved the way for the use of these models in AF management. Specifically, modeling work has been conducted to determine molecular targets for pharmacological rate control, and optimize antitachycardia pacing and AF ablation, as reviewed below.
4.1 Pharmacological Control of Atrial Rate
Anti-arrhythmic drugs constitute the main treatment option for AF. However, atrial rhythm control pharmacotherapy has been limited due to its inadequate effectiveness and adverse side effects.3 Additionally, such drugs are associated with risk of life-threatening ventricular proarrhythmia.126 Accordingly, research on pharmacological control of atrial rate has been directed towards finding drug targets that are atria-specific.127 The role of atrial modeling in this field of research has been to uncover the mechanisms for drug action, or the lack thereof. An experimental/simulation investigation of this type is represented by the study of Pandit et al.47 The objective was to probe the effectiveness of block of the atria-specific current IKur and to exploit the inherent differences between atrial and ventricular Na channel steady-state inactivation properties (by manipulating extracellular K concentration) in terminating cholinergic AF in pigs. Experimental results indicated that IKur was not a viable anti-arrhythmic target, and simulations shed light on the mechanisms, showing that in cholinergic AF the contribution of IKur was dwarfed by the large magnitude of IKACh. Furthermore, simulations determined that the lower availability of the atrial Na current at depolarized potentials could partly explain the earlier termination of AF compared with ventricular fibrillation during hyperkalemia. A recent attempt128 to further explore the therapeutic strategy of blocking IKur demonstrated that the antiarrhythmic effects of IKur inhibitors are dependent on kinetic properties of the blockade.
Block of Na channel conductance by ranolazine displays marked atrial selectivity; Nesterenko et al129 developed a Markovian model of Na channel gating that elucidated the mechanisms underlying ranolazine’s potent atrial selectivity. The possibility to develop Na-channel blockers with maximal actions on fibrillating atrial tissue and minimal actions on ventricular tissue at resting heart rates was also probed.130 A model of state-dependent Na-channel blocking (class I antiarrhythmic drug) action was used in simulations of AF and ventricular proarrhythmia. The study found that drugs that target inactivated channels are AF-selective, whereas drugs that target activated channels are not. Such simulation methodology has a strong potential to contribute to rational approaches to defining optimal Na-channel blocker properties.
Finally, simulation research44 has provided mechanistic explanations regarding the lower efficacy of pharmacological treatment in patients with long- term versus short-term AF, and of the antiarrhythmic properties of amiodarone and digitalis for AF treatment.
4.2 Anti-arrhythmia Pacing for AF Termination
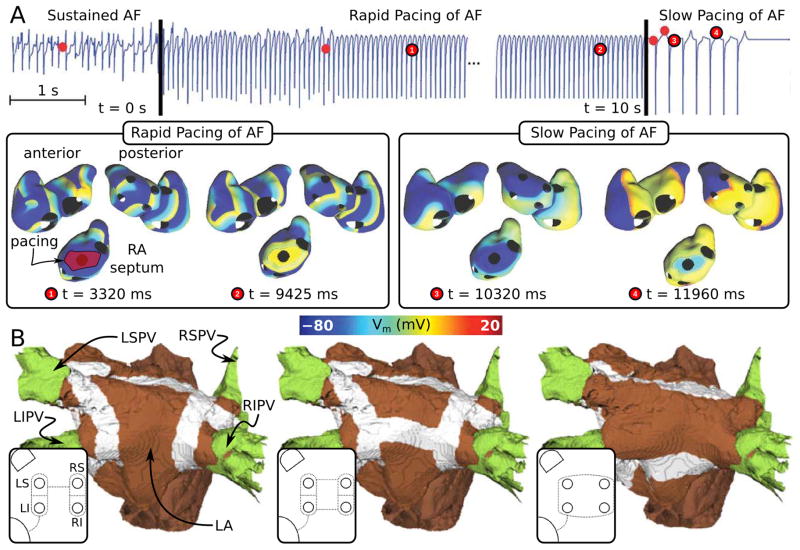
Pacemaker-based therapy for AF has been recognized as a possible alternative to drug therapy; today many pacemakers and implantable defibrillators include pacing algorithms for AF prevention and termination.131 Most existing pacing algorithms deliver preventive therapies aimed to suppress AF triggers and reduce dispersion in atrial refractoriness.132 Uldry et al133 recognized that with the use of an atrial model, a better understanding of the degree of local capture by pacing can be achieved, which might have important implications for the development of pacing algorithms for AF termination. The authors used a 3D surface model of the human atria and rapidly paced it at a cycle length shorter than that of the detected arrhythmia, from a single site, in an attempt to terminate AF. Results demonstrated that the septum was the only pacing site that yielded AF capture in both atria. However, capture was sporadic, and overall, did not result in AF termination or permanent changes in AF pattern. A new pacing scheme, shown in Fig. 8A, was subsequently devised,134 where the initial rapid septal pacing phase, this time from a large septal area (shown in red in the septal area, Fig. 8A left), was followed by a slow septal pacing phase from the same location (at a cycle length longer than that of the detected arrhythmia) aimed at lengthening the APD and thus eliminating any residual fibrillating wavelets that might have survived in areas distant from the septum during the rapid pacing phase. The new algorithm could suppress AF reentries in a more robust way than single site rapid pacing, with AF termination rate increasing from 10.2 to 20.2%. This simulation research provided a classical example of how realistic models of the atria can be used to generate new ideas and approaches to AF management optimization.
Figure 8.
Simulations of AF management. A: Dual stage septal pacing algorithm with successful atrial fibrillation termination in a 3D surface model of the human atria (with permission from134). B: Modeling lines of ablation in the atria. Tissue targeted by ablation is shown in white. Modified with permission from59.
4.3 Optimizing Atrial Ablation
Catheter-based ablation, the delivery of heat to destroy the ability of cardiac tissue to generate and conduct electrical signals locally, has emerged as a promising AF treatment option. The objective of AF ablation is to either abolish foci generating ectopic beats, or to create zones of conduction block, eliminating reentry. The procedure has successfully targeted AF triggers via PV electrical isolation.135 Aggressive ablation strategies, such as Maze III procedure,136 were found to be the most effective when the underlying AF involves turbulent activity with multiple wavelets (presumably permanent AF). Human atrial models have been used to optimize AF ablation, suggesting strategies to minimize the size of ablation lesions, and to study the effect of gaps in ablation lines. An example59 of simulated ablation lesions in a human atrial model are shown in Fig. 8B. A set of studies54,137 explored the effectiveness of ablation line patterns that are less invasive than Maze III procedure, and demonstrated that any such pattern needs to include ablation lines in both RA and LA so that a multiple-wavelet AF can be successfully terminated. Specifically, Maze III could be simplified while achieving the same success by diminishing RA ablation severity to a single line joining both vena cavae. Additionally, simulations54 showed that imperfect ablation lines in the Maze III procedure decreased success rate by up to 28%, with the rate depending on the location of lesion imperfection.
Recent ablation strategies have begun to target the LA wall in an attempt to alter the arrhythmogenic substrate.88 Both 2D cellular automata models13 as well as biophysically-detailed patent-specific models of fibrosis in the human atria138 have shown preliminary success in providing guidance in AF substrate ablation. There is a high expectation that atrial models can be employed to predict the optimal AF ablation strategies in a patient-specific manner.
5. The Future of Simulation Research on AF Mechanisms and Atrial Arrhythmia Management
As this review demonstrates, mathematical modeling and computer simulations of atrial electrophysiology have made major contributions to the interpretation of an array of experimental data and to the dissection of the fundamental mechanisms and relationships underlying AF initiation and persistence. As this trend will continue in the future, atrial modeling as a tool will necessitate continuous adaptation and integration of new elements, including model re-design and evaluation, improvements in the execution time of biophysically detailed atrial models, implementation of consistent strategies for comparison with experimental measurements, and investing in efforts to ensure repeatability and consistency of modeling results. The advancement of atrial modeling will continue to be strongly dependent on developments in experimental methodologies, which provide data to constrain, enrich, and validate the models. Of particular importance will be the capability to better resolve the pathophysiological structure of the atria and to fully characterize the complex electrophysiological and fibrotic remodeling in disease. Major challenges that lie ahead for computer models of AF include, among others, elucidating the dynamics human AF and detecting rotor locations, and well as understanding the multitude of factors that drive progression of AF in some, but not all, patients.
The use of atrial models in personalized diagnosis, treatment planning, and prevention of AF will also slowly become a reality; initial efforts in this direction are reviewed here. The feasibility of subject-specific AF modeling has been demonstrated through the use of atrial models reconstructed from clinical MRI scans. Biophysically-detailed models of the atria assembled with data from clinical imaging modalities that incorporate electrophysiological and structural remodeling in cardiac disease are poised to become a first line of screening for new AF therapies and approaches, new diagnostic developments, and new methods for arrhythmia prevention. There are number of important challenges that lie ahead: development and clinical translation of methodologies for personalized AF ablation planning; development of new and effective approaches for anti-arrhythmia pacing; and devising improved methodologies for AF rate control. Finally, implementing patient-specific cardiac simulations at the patient bedside for AF therapy and management could become a thrilling example of computational approaches in translational medicine.
Supplementary Material
Acknowledgments
Funding Sources
The author acknowledges support by NIH Director’s Pioneer Award.
Non-standard Abbreviations and Acronyms
- AF
Atrial Fibrillation
- 2D
Two-dimensional
- 3D
Three-dimensional
- AP
Action Potential
- PDE
Partial Differential Equation
- MRI
Magnetic Resonance Imaging
- CT
Computed Tomography
- APD
Action Potential Duration
- Ca
Calcium
- cAF
Chronic Atrial Fibrillation
- RA
Right Atrium
- LA
Left Atrium
- RyR
Ryanodine Receptor
- SR
Sarcoplasmic Reticulum
- DAD
Delayed Afterdepolarization
- RAA
Right Atrial Appendage
- PV
Pulmonary Vein
- PM
Papillary Muscle
- LGE
Late Gadolinium Enhancement
- ACh
Acetylcholine
- DF
Dominant Frequency
- OI
Organization Index
- HF
Heart Failure
Footnotes
Disclosures: None
References
- 1.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Archives of internal medicine. 1995;155:469–473. [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 4.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circulation. Arrhythmia and electrophysiology. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 5.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiological reviews. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 6.Atienza F, Martins RP, Jalife J. Translational research in atrial fibrillation: A quest for mechanistically based diagnosis and therapy. Circulation. Arrhythmia and electrophysiology. 2012;5:1207–1215. doi: 10.1161/CIRCEP.111.970335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovascular research. 2011;89:766–775. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packer DL. Evolution of mapping and anatomic imaging of cardiac arrhythmias. Journal of cardiovascular electrophysiology. 2004;15:839–854. doi: 10.1046/j.1540-8167.2004.04275.x. [DOI] [PubMed] [Google Scholar]
- 9.Winslow RL, Trayanova N, Geman D, Miller MI. Computational medicine: Translating models to clinical care. Science translational medicine. 2012;4:158rv111. doi: 10.1126/scitranslmed.3003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of physiology. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. American heart journal. 1964;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 12.Correa de Sa DD, Thompson N, Stinnett-Donnelly J, Znojkiewicz P, Habel N, Muller JG, Bates JH, Buzas JS, Spector PS. Electrogram fractionation: The relationship between spatiotemporal variation of tissue excitation and electrode spatial resolution. Circulation. Arrhythmia and electrophysiology. 2011;4:909–916. doi: 10.1161/CIRCEP.111.965145. [DOI] [PubMed] [Google Scholar]
- 13.Carrick RT, Benson B, Habel N, Bates OR, Bates JH, Spector PS. Ablation of multiwavelet re-entry guided by circuit-density and distribution: Maximizing the probability of circuit annihilation. Circulation. Arrhythmia and electrophysiology. 2013;6:1229–1235. doi: 10.1161/CIRCEP.113.000759. [DOI] [PubMed] [Google Scholar]
- 14.Dossel O, Krueger MW, Weber FM, Wilhelms M, Seemann G. Computational modeling of the human atrial anatomy and electrophysiology. Medical & biological engineering & computing. 2012;50:773–799. doi: 10.1007/s11517-012-0924-6. [DOI] [PubMed] [Google Scholar]
- 15.Jacquemet V, Kappenberger L, Henriquez CS. Modeling atrial arrhythmias: Impact on clinical diagnosis and therapies. IEEE reviews in biomedical engineering. 2008;1:94–114. doi: 10.1109/RBME.2008.2008242. [DOI] [PubMed] [Google Scholar]
- 16.Bers DM, Grandi E. Human atrial fibrillation: Insights from computational electrophysiological models. Trends in cardiovascular medicine. 2011;21:145–150. doi: 10.1016/j.tcm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmusson RL, Clark JW, Giles WR, Robinson K, Clark RB, Shibata EF, Campbell DL. A mathematical model of electrophysiological activity in a bullfrog atrial cell. The American journal of physiology. 1990;259:H370–389. doi: 10.1152/ajpheart.1990.259.2.H370. [DOI] [PubMed] [Google Scholar]
- 18.Hilgemann DW, Noble D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: Reconstruction of basic cellular mechanisms. Proc R Soc B. 1987;230:163–205. doi: 10.1098/rspb.1987.0015. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad DS, Murphey CR, Clark JW, Giles WR. A model of the action potential and underlying membrane currents in a rabbit atrial cell. The American journal of physiology. 1996;271:1666–1696. doi: 10.1152/ajpheart.1996.271.4.H1666. [DOI] [PubMed] [Google Scholar]
- 20.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. The American journal of physiology. 1998;275:301–321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 21.Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: The role of k+ currents in repolarization. Circulation research. 1998;82:63–81. doi: 10.1161/01.res.82.1.63. [DOI] [PubMed] [Google Scholar]
- 22.Nygren A, Leon LJ, Giles WR. Simulations of the human atrial action potential. Phil Trans R Soc A. 2001;359:1111–1125. [Google Scholar]
- 23.Cherry EM, Evans SJ. Properties of two human atrial cell models in tissue: Restitution, memory, propagation, and reentry. Journal of theoretical biology. 2008;254:674–690. doi: 10.1016/j.jtbi.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: Insights from a mathematical model. Cardiovascular research. 1999;42:477–489. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez RJ, Nattel S, Courtemanche M. Mathematical analysis of canine atrial action potentials: Rate, regional factors, and electrical remodeling. American journal of physiology. Heart and circulatory physiology. 2000;279:1767–1785. doi: 10.1152/ajpheart.2000.279.4.H1767. [DOI] [PubMed] [Google Scholar]
- 26.Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circulation research. 2002;90:73–87. doi: 10.1161/01.res.0000019783.88094.ba. [DOI] [PubMed] [Google Scholar]
- 27.Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, Vigmond EJ, Leon LJ, Nattel S, Jalife J. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circulation research. 2005;96:35–47. doi: 10.1161/01.RES.0000160709.49633.2b. [DOI] [PubMed] [Google Scholar]
- 28.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. American journal of physiology. Heart and circulatory physiology. 2009;297:1398–1410. doi: 10.1152/ajpheart.00411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koivumaki JT, Korhonen T, Tavi P. Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: A computational study. PLoS computational biology. 2011;7:1001067. doi: 10.1371/journal.pcbi.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, Trayanova NA, Narayan SM. Mechanisms of human atrial fibrillation initiation: Clinical and computational studies of repolarization restitution and activation latency. Circulation. Arrhythmia and electrophysiology. 2012;5:1149–1159. doi: 10.1161/CIRCEP.111.969022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and ca2+ model: Sinus rhythm and chronic atrial fibrillation. Circulation research. 2011;109:1055–1066. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deo M, Ruan Y, Pandit SV, Shah K, Berenfeld O, Blaufox A, Cerrone M, Noujaim SF, Denegri M, Jalife J, Priori SG. Kcnj2 mutation in short qt syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4291–4296. doi: 10.1073/pnas.1218154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 34.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward k+ current densities and kv1.5 expression are reduced in chronic human atrial fibrillation. Circulation research. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 35.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovascular research. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 36.Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, Christ T, Schuler S, Ravens U. Molecular basis of downregulation of g-protein-coupled inward rectifying k+ current (ik,ach) in chronic human atrial fibrillation: Decrease in girk4 mrna correlates with reduced ik,ach and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelms M, Hettmann H, Maleckar MM, Koivumaki JT, Dossel O, Seemann G. Benchmarking electrophysiological models of human atrial myocytes. Frontiers in physiology. 2012;3:487. doi: 10.3389/fphys.2012.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar AX, Christini DJ, Sobie EA. Exploiting mathematical models to illuminate electrophysiological variability between individuals. The Journal of physiology. 2012;590:2555–2567. doi: 10.1113/jphysiol.2011.223313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.006641. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thul R, Coombes S, Roderick HL, Bootman MD. Subcellular calcium dynamics in a whole-cell model of an atrial myocyte. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2150–2155. doi: 10.1073/pnas.1115855109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trayanova NA. Whole-heart modeling: Applications to cardiac electrophysiology and electromechanics. Circulation research. 2011;108:113–128. doi: 10.1161/CIRCRESAHA.110.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plank G, Zhou L, Greenstein JL, Cortassa S, Winslow RL, O’Rourke B, Trayanova NA. From mitochondrial ion channels to arrhythmias in the heart: Computational techniques to bridge the spatio-temporal scales. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2008;366:3381–3409. doi: 10.1098/rsta.2008.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo SR, Trayanova NA. Action potential morphology heterogeneity in the atrium and its effect on atrial reentry: A two-dimensional and quasi-three-dimensional study. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2006;364:1349–1366. doi: 10.1098/rsta.2006.1776. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez C, Corrias A, Bueno-Orovio A, Davies M, Swinton J, Jacobson I, Laguna P, Pueyo E, Rodriguez B. The na+/k+ pump is an important modulator of refractoriness and rotor dynamics in human atrial tissue. American journal of physiology. Heart and circulatory physiology. 2012;302:1146–1159. doi: 10.1152/ajpheart.00668.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-d model of human atrial cells during simulated chronic atrial fibrillation. Biophysical journal. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashihara T, Haraguchi R, Nakazawa K, Namba T, Ikeda T, Nakazawa Y, Ozawa T, Ito M, Horie M, Trayanova NA. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: Implications for electrogram-based catheter ablation. Circulation research. 2012;110:275–284. doi: 10.1161/CIRCRESAHA.111.255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandit SV, Zlochiver S, Filgueiras-Rama D, Mironov S, Yamazaki M, Ennis SR, Noujaim SF, Workman AJ, Berenfeld O, Kalifa J, Jalife J. Targeting atrioventricular differences in ion channel properties for terminating acute atrial fibrillation in pigs. Cardiovascular research. 2011;89:843–851. doi: 10.1093/cvr/cvq359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao J, Trew ML, Legrice IJ, Smaill BH, Pullan AJ. A tissue-specific model of reentry in the right atrial appendage. Journal of cardiovascular electrophysiology. 2009;20:675–684. doi: 10.1111/j.1540-8167.2008.01420.x. [DOI] [PubMed] [Google Scholar]
- 49.Aslanidi OV, Boyett MR, Dobrzynski H, Li J, Zhang H. Mechanisms of transition from normal to reentrant electrical activity in a model of rabbit atrial tissue: Interaction of tissue heterogeneity and anisotropy. Biophysical journal. 2009;96:798–817. doi: 10.1016/j.bpj.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao J, Butters TD, Zhang H, LeGrice IJ, Sands GB, Smaill BH. Image-based model of atrial anatomy and electrical activation: A computational platform for investigating atrial arrhythmia. IEEE transactions on medical imaging. 2013;32:18–27. doi: 10.1109/TMI.2012.2227776. [DOI] [PubMed] [Google Scholar]
- 51.Zhao J, Butters TD, Zhang H, Pullan AJ, LeGrice IJ, Sands GB, Smaill BH. An image-based model of atrial muscular architecture: Effects of structural anisotropy on electrical activation. Circulation. Arrhythmia and electrophysiology. 2012;5:361–370. doi: 10.1161/CIRCEP.111.967950. [DOI] [PubMed] [Google Scholar]
- 52.Vigmond EJ, Ruckdeschel R, Trayanova N. Reentry in a morphologically realistic atrial model. Journal of cardiovascular electrophysiology. 2001;12:1046–1054. doi: 10.1046/j.1540-8167.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- 53.Virag N, Jacquemet V, Henriquez CS, Zozor S, Blanc O, Vesin JM, Pruvot E, Kappenberger L. Study of atrial arrhythmias in a computer model based on magnetic resonance images of human atria. Chaos. 2002;12:754–763. doi: 10.1063/1.1483935. [DOI] [PubMed] [Google Scholar]
- 54.Dang L, Virag N, Ihara Z, Jacquemet V, Vesin JM, Schlaepfer J, Ruchat P, Kappenberger L. Evaluation of ablation patterns using a biophysical model of atrial fibrillation. Annals of biomedical engineering. 2005;33:465–474. doi: 10.1007/s10439-005-2502-7. [DOI] [PubMed] [Google Scholar]
- 55.Vigmond EJ, Tsoi V, Kuo S, Arevalo H, Kneller J, Nattel S, Trayanova N. The effect of vagally induced dispersion of action potential duration on atrial arrhythmogenesis. Heart rhythm: the official journal of the Heart Rhythm Society. 2004;1:334–344. doi: 10.1016/j.hrthm.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 56.Freudenberg J, Schiemann T, Tiede U, Hohne KH. Simulation of cardiac excitation patterns in a three-dimensional anatomical heart atlas. Computers in biology and medicine. 2000;30:191–205. doi: 10.1016/s0010-4825(00)00005-6. [DOI] [PubMed] [Google Scholar]
- 57.Harrild D, Henriquez C. A computer model of normal conduction in the human atria. Circulation research. 2000;87:25–36. doi: 10.1161/01.res.87.7.e25. [DOI] [PubMed] [Google Scholar]
- 58.Seemann G, Hoper C, Sachse FB, Dossel O, Holden AV, Zhang H. Heterogeneous three-dimensional anatomical and electrophysiological model of human atria. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2006;364:1465–1481. doi: 10.1098/rsta.2006.1781. [DOI] [PubMed] [Google Scholar]
- 59.Reumann M, Bohnert J, Seemann G, Osswald B, Dossel O. Preventive ablation strategies in a biophysical model of atrial fibrillation based on realistic anatomical data. IEEE transactions on bio-medical engineering. 2008;55:399–406. doi: 10.1109/TBME.2007.912672. [DOI] [PubMed] [Google Scholar]
- 60.Aslanidi OV, Colman MA, Stott J, Dobrzynski H, Boyett MR, Holden AV, Zhang H. 3d virtual human atria: A computational platform for studying clinical atrial fibrillation. Progress in biophysics and molecular biology. 2011;107:156–168. doi: 10.1016/j.pbiomolbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, Macleod RS, Trayanova NA. Mechanistic inquiry into the role of tissue remodeling in fibrotic lesions in human atrial fibrillation. Biophysical journal. 2013;104:2764–2773. doi: 10.1016/j.bpj.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, MacLeod RS, Trayanova NA. Methodology for patient-specific modeling of atrial fibrosis as a substrate for atrial fibrillation. Journal of electrocardiology. 2012;45:640–645. doi: 10.1016/j.jelectrocard.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray RA, Jalife J. Ventricular fibrillation and atrial fibrillation are two different beasts. Chaos. 1998;8:65–78. doi: 10.1063/1.166288. [DOI] [PubMed] [Google Scholar]
- 64.Gong Y, Xie F, Stein KM, Garfinkel A, Culianu CA, Lerman BB, Christini DJ. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: A simulation study. Circulation. 2007;115:2094–2102. doi: 10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- 65.Krogh-Madsen T, Abbott GW, Christini DJ. Effects of electrical and structural remodeling on atrial fibrillation maintenance: A simulation study. PLoS computational biology. 2012;8:1002390. doi: 10.1371/journal.pcbi.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tobon C, Rodriguez JF, Ferrero JM, Jr, Hornero F, Saiz J. Dominant frequency and organization index maps in a realistic three-dimensional computational model of atrial fibrillation. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012;14:25–32. doi: 10.1093/europace/eus268. [DOI] [PubMed] [Google Scholar]
- 67.Tobon C, Ruiz-Villa CA, Heidenreich E, Romero L, Hornero F, Saiz J. A three-dimensional human atrial model with fiber orientation. Electrograms and arrhythmic activation patterns relationship. PloS one. 2013;8:50883. doi: 10.1371/journal.pone.0050883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spitzer VM, Whitlock DG. The visible human dataset: The anatomical platform for human simulation. The Anatomical record. 1998;253:49–57. doi: 10.1002/(SICI)1097-0185(199804)253:2<49::AID-AR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 69.Jacquemet V, Virag N, Kappenberger L. Wavelength and vulnerability to atrial fibrillation: Insights from a computer model of human atria. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2005;7 (Suppl 2):83–92. doi: 10.1016/j.eupc.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Ridler ME, Lee M, McQueen D, Peskin C, Vigmond E. Arrhythmogenic consequences of action potential duration gradients in the atria. The Canadian journal of cardiology. 2011;27:112–119. doi: 10.1016/j.cjca.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Pennell DJ. Cardiovascular magnetic resonance. Circulation. 2010;121:692–705. doi: 10.1161/CIRCULATIONAHA.108.811547. [DOI] [PubMed] [Google Scholar]
- 72.Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, Kuppahally S, Badger T, Burgon N, Haslam T, Kholmovski E, Macleod R, Marrouche N. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: A de-mri guided approach. Journal of cardiovascular electrophysiology. 2011;22:16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krueger MW, Rhode KS, O’Neill MD, Rinaldi CA, Gill J, Razavi R, Seemann G, Doessel O. Patient-specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation. Journal of electrocardiology. doi: 10.1016/j.jelectrocard.2013.11.003. (in press) [DOI] [PubMed] [Google Scholar]
- 74.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophysical journal. 2007;92:4121–4132. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. Electrotonic coupling between human atrial myocytes and fibroblasts alters myocyte excitability and repolarization. Biophysical journal. 2009;97:2179–2190. doi: 10.1016/j.bpj.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voigt N, Maguy A, Yeh YH, Qi X, Ravens U, Dobrev D, Nattel S. Changes in ik, ach single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovascular research. 2008;77:35–43. doi: 10.1093/cvr/cvm051. [DOI] [PubMed] [Google Scholar]
- 77.Caballero R, de la Fuente MG, Gomez R, Barana A, Amoros I, Dolz-Gaiton P, Osuna L, Almendral J, Atienza F, Fernandez-Aviles F, Pita A, Rodriguez-Roda J, Pinto A, Tamargo J, Delpon E. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. Journal of the American College of Cardiology. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 78.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. Journal of the American College of Cardiology. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 79.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England journal of medicine. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 80.Nattel S. Paroxysmal atrial fibrillation and pulmonary veins: Relationships between clinical forms and automatic versus re-entrant mechanisms. The Canadian journal of cardiology. 2013;29:1147–1149. doi: 10.1016/j.cjca.2013.07.797. [DOI] [PubMed] [Google Scholar]
- 81.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. Arrhythmia and electrophysiology. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 82.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 83.Berenfeld O, Mandapati R, Dixit S, Skanes AC, Chen J, Mansour M, Jalife J. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the langendorff-perfused sheep heart. Journal of cardiovascular electrophysiology. 2000;11:869–879. doi: 10.1111/j.1540-8167.2000.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 84.Gould PA, Yii M, McLean C, Finch S, Marshall T, Lambert GW, Kaye DM. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing and clinical electrophysiology: PACE. 2006;29:821–829. doi: 10.1111/j.1540-8159.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 85.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovascular research. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. Journal of the American College of Cardiology. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 88.Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. Journal of cardiovascular electrophysiology. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho SY, Anderson RH, Sanchez-Quintana D. Atrial structure and fibres: Morphologic bases of atrial conduction. Cardiovascular research. 2002;54:325–336. doi: 10.1016/s0008-6363(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 90.Wu TJ, Yashima M, Xie F, Athill CA, Kim YH, Fishbein MC, Qu Z, Garfinkel A, Weiss JN, Karagueuzian HS, Chen PS. Role of pectinate muscle bundles in the generation and maintenance of intra-atrial reentry: Potential implications for the mechanism of conversion between atrial fibrillation and atrial flutter. Circulation research. 1998;83:448–462. doi: 10.1161/01.res.83.4.448. [DOI] [PubMed] [Google Scholar]
- 91.Ellis WS, SippensGroenewegen A, Auslander DM, Lesh MD. The role of the crista terminalis in atrial flutter and fibrillation: A computer modeling study. Annals of biomedical engineering. 2000;28:742–754. doi: 10.1114/1.1289456. [DOI] [PubMed] [Google Scholar]
- 92.Wieser L, Fischer G, Nowak CN, Tilg B. Fibrillatory conduction in branching atrial tissue--insight from volumetric and monolayer computer models. Computer methods and programs in biomedicine. 2007;86:103–111. doi: 10.1016/j.cmpb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 93.Spach MS, Miller WT, 3rd, Dolber PC, Kootsey JM, Sommer JR, Mosher CE., Jr The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circulation research. 1982;50:175–191. doi: 10.1161/01.res.50.2.175. [DOI] [PubMed] [Google Scholar]
- 94.Spach MS, Dolber PC, Heidlage JF. Interaction of inhomogeneities of repolarization with anisotropic propagation in dog atria. A mechanism for both preventing and initiating reentry. Circulation research. 1989;65:1612–1631. doi: 10.1161/01.res.65.6.1612. [DOI] [PubMed] [Google Scholar]
- 95.Spach MS, Heidlage JF, Dolber PC, Barr RC. Mechanism of origin of conduction disturbances in aging human atrial bundles: Experimental and model study. Heart rhythm: the official journal of the Heart Rhythm Society. 2007;4:175–185. doi: 10.1016/j.hrthm.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schuessler RB, Grayson TM, Bromberg BI, Cox JL, Boineau JP. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circulation research. 1992;71:1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 97.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: Role of refractoriness heterogeneity. The American journal of physiology. 1997;273:805–816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 98.Zou R, Kneller J, Leon LJ, Nattel S. Substrate size as a determinant of fibrillatory activity maintenance in a mathematical model of canine atrium. American journal of physiology. Heart and circulatory physiology. 2005;289:1002–1012. doi: 10.1152/ajpheart.00252.2005. [DOI] [PubMed] [Google Scholar]
- 99.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 100.Nattel S. Basic electrophysiology of the pulmonary veins and their role in atrial fibrillation: Precipitators, perpetuators, and perplexers. Journal of cardiovascular electrophysiology. 2003;14:1372–1375. doi: 10.1046/j.1540-8167.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- 101.Kuijpers NH, Keldermann RH, ten Eikelder HM, Arts T, Hilbers PA. The role of the hyperpolarization-activated inward current if in arrhythmogenesis: A computer model study. IEEE transactions on bio-medical engineering. 2006;53:1499–1511. doi: 10.1109/TBME.2006.877801. [DOI] [PubMed] [Google Scholar]
- 102.Seol CA, Kim J, Kim WT, Ha JM, Choe H, Jang YJ, Shim EB, Youm JB, Earm YE, Leem CH. Simulation of spontaneous action potentials of cardiomyocytes in pulmonary veins of rabbits. Progress in biophysics and molecular biology. 2008;96:132–151. doi: 10.1016/j.pbiomolbio.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Cherry EM, Ehrlich JR, Nattel S, Fenton FH. Pulmonary vein reentry--properties and size matter: Insights from a computational analysis. Heart rhythm: the official journal of the Heart Rhythm Society. 2007;4:1553–1562. doi: 10.1016/j.hrthm.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Kharche S, Adeniran I, Stott J, Law P, Boyett MR, Hancox JC, Zhang H. Pro-arrhythmogenic effects of the s140g kcnq1 mutation in human atrial fibrillation - insights from modelling. The Journal of physiology. 2012;590:4501–4514. doi: 10.1113/jphysiol.2012.229146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 106.Shiroshita-Takeshita A, Mitamura H, Ogawa S, Nattel S. Rate-dependence of atrial tachycardia effects on atrial refractoriness and atrial fibrillation maintenance. Cardiovascular research. 2009;81:90–97. doi: 10.1093/cvr/cvn249. [DOI] [PubMed] [Google Scholar]
- 107.Colman MA, Aslanidi OV, Kharche S, Boyett MR, Garratt CJ, Hancox JC, Zhang H. Pro-arrhythmogenic effects of atrial fibrillation induced electrical remodelling- insights from 3d virtual human atria. The Journal of physiology. 2013 doi: 10.1113/jphysiol.2013.254987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trayanova NA, Rice JJ. Cardiac electromechanical models: From cell to organ. Frontiers in physiology. 2011;2:43. doi: 10.3389/fphys.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trayanova NA, Constantino J, Gurev V. Electromechanical models of the ventricles. American journal of physiology. Heart and circulatory physiology. 2011;301:H279–286. doi: 10.1152/ajpheart.00324.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuijpers NH, Potse M, van Dam PM, ten Eikelder HM, Verheule S, Prinzen FW, Schotten U. Mechanoelectrical coupling enhances initiation and affects perpetuation of atrial fibrillation during acute atrial dilation. Heart rhythm: the official journal of the Heart Rhythm Society. 2011;8:429–436. doi: 10.1016/j.hrthm.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 111.Yamazaki M, Mironov S, Taravant C, Brec J, Vaquero LM, Bandaru K, Avula UM, Honjo H, Kodama I, Berenfeld O, Kalifa J. Heterogeneous atrial wall thickness and stretch promote scroll waves anchoring during atrial fibrillation. Cardiovascular research. 2012;94:48–57. doi: 10.1093/cvr/cvr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 113.Everett THt, Kok LC, Vaughn RH, Moorman JR, Haines DE. Frequency domain algorithm for quantifying atrial fibrillation organization to increase defibrillation efficacy. IEEE transactions on bio-medical engineering. 2001;48:969–978. doi: 10.1109/10.942586. [DOI] [PubMed] [Google Scholar]
- 114.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovascular research. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 115.Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, Nattel S. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circulation research. 2009;105:1213–1222. doi: 10.1161/CIRCRESAHA.108.183400. [DOI] [PubMed] [Google Scholar]
- 116.Rohr S. Myofibroblasts in diseased hearts: New players in cardiac arrhythmias? Heart rhythm: the official journal of the Heart Rhythm Society. 2009;6:848–856. doi: 10.1016/j.hrthm.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 117.Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, Odim J, Laks H, Sen L. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–368. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 118.Plank G, Prassl AJ, Wang JI, Seeman G, Scherr D, Sanchez-Quintana D, Calkins H, Trayanova N. Atrial fibrosis promotes the transition of pulmonary vein ectopy into reentrant arrhythmias. Heart Rhythm. 2008;5:S78. [Google Scholar]
- 119.Eckstein J, Maesen B, Linz D, Zeemering S, van Hunnik A, Verheule S, Allessie M, Schotten U. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovascular research. 2011;89:816–824. doi: 10.1093/cvr/cvq336. [DOI] [PubMed] [Google Scholar]
- 120.Jacquemet V, Henriquez CS. Genesis of complex fractionated atrial electrograms in zones of slow conduction: A computer model of microfibrosis. Heart rhythm: the official journal of the Heart Rhythm Society. 2009;6:803–810. doi: 10.1016/j.hrthm.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Comtois P, Nattel S. Interactions between cardiac fibrosis spatial pattern and ionic remodeling on electrical wave propagation. Conf Proc IEEE Eng Med Biol Soc. 2011:4669–4672. doi: 10.1109/IEMBS.2011.6091156. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS, Landas S, Guiraudon G, Jalife J, Berenfeld O, Kalifa J. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circulation research. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 123.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: Structural and functional identification of homogeneous and heterogeneous cell coupling. Circulation research. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 124.Pedrotty DM, Klinger RY, Kirkton RD, Bursac N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovascular research. 2009;83:688–697. doi: 10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophysical journal. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Page RL, Roden DM. Drug therapy for atrial fibrillation: Where do we go from here? Nature reviews. Drug discovery. 2005;4:899–910. doi: 10.1038/nrd1876. [DOI] [PubMed] [Google Scholar]
- 127.Ehrlich JR, Nattel S. Atrial-selective pharmacological therapy for atrial fibrillation: Hype or hope? Current opinion in cardiology. 2009;24:50–55. doi: 10.1097/HCO.0b013e32831bc336. [DOI] [PubMed] [Google Scholar]
- 128.Scholz EP, Carrillo-Bustamante P, Fischer F, Wilhelms M, Zitron E, Dossel O, Katus HA, Seemann G. Rotor termination is critically dependent on kinetic properties of ikur inhibitors in an in silico model of chronic atrial fibrillation. PloS one. 2013;8:83179. doi: 10.1371/journal.pone.0083179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nesterenko VV, Zygmunt AC, Rajamani S, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of na(+) channels by ranolazine: Ii. Insights from a mathematical model. American journal of physiology. Heart and circulatory physiology. 2011;301:H1615–1624. doi: 10.1152/ajpheart.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aguilar-Shardonofsky M, Vigmond EJ, Nattel S, Comtois P. In silico optimization of atrial fibrillation-selective sodium channel blocker pharmacodynamics. Biophysical journal. 2012;102:951–960. doi: 10.1016/j.bpj.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Redfearn DP, Yee R. Pacing delivered rate and rhythm control for atrial fibrillation. Current opinion in cardiology. 2006;21:83–87. doi: 10.1097/01.hco.0000210302.56736.60. [DOI] [PubMed] [Google Scholar]
- 132.Ellenbogen KA. Pacing therapy for prevention of atrial fibrillation. Heart rhythm: the official journal of the Heart Rhythm Society. 2007;4:S84–87. doi: 10.1016/j.hrthm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 133.Uldry L, Virag N, Jacquemet V, Vesin JM, Kappenberger L. Optimizing local capture of atrial fibrillation by rapid pacing: Study of the influence of tissue dynamics. Annals of biomedical engineering. 2010;38:3664–3673. doi: 10.1007/s10439-010-0122-3. [DOI] [PubMed] [Google Scholar]
- 134.Uldry L, Virag N, Lindemans F, Vesin JM, Kappenberger L. Atrial septal pacing for the termination of atrial fibrillation: Study in a biophysical model of human atria. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012;14:112–120. doi: 10.1093/europace/eus279. [DOI] [PubMed] [Google Scholar]
- 135.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 136.Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, Smith PK, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. Ii. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. The Journal of thoracic and cardiovascular surgery. 1991;101:406–426. [PubMed] [Google Scholar]
- 137.Ruchat P, Dang L, Virag N, Schlaepfer J, von Segesser LK, Kappenberger L. A biophysical model of atrial fibrillation to define the appropriate ablation pattern in modified maze. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2007;31:65–69. doi: 10.1016/j.ejcts.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 138.McDowell KS, Vadakkumpadan F, Blauer J, MacLeod R, Trayanova N. Virtual electrophysiology study to predict and explain how fibrotic remodeling mediates atrial fibrillation, and to determine optimal ablation targets. Heart Rhythm. 2014 (in revision) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.