Abstract
Introduction
Frontal-subcortical cognitive and limbic feedback loops modulate higher cognitive functioning. The final step in these feedback loops is the thalamo-cortical projection through the anterior limb of the internal capsule (AL-IC). Using diffusion tensor imaging (DTI), we evaluated abnormalities in the AL-IC fiber tract in schizophrenia.
Methods
16 chronic schizophrenics and 19 male, normal controls group matched for handedness, age, and parental SES, underwent DTI on a 1.5 Tesla GE system. We measured the diffusion indices, fractional anisotropy (FA) mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD), and manually segmented, based on FA maps, AL-IC volume, normalized for intracranial contents (ICC).
Results
Results showed a significant reduction in the ICC corrected volume of the AL-IC, in schizophrenia, but did not show diffusion measure group differences in the AL-IC in FA, MD, RD or AD. In addition, results revealed in schizophrenics, AL-IC FA correlated positively with performance on measures of spatial and verbal declarative/episodic memory, and right AL-IC ICC corrected volume correlated positively with more perseverative responses on the Wisconsin Card Sort Test (WCST).
Discussion
We found a reduction in AL-IC ICC corrected volume in schizophrenia, without FA, MD, RD or AD group differences, implicating the presence of a structural abnormality in schizophrenia in this subcortical white matter region which contains important cognitive, and limbic feedback pathways which modulate prefrontal cortical function. Despite not demonstrating a group difference in FA, we found that AL-IC FA was a good predictor of spatial and verbal declarative/episodic memory performance in schizophrenia.
Keywords: Schizophrenia, Diffusion Tensor Imaging, Anterior Limb of the Internal Capsule, Thalamus, Prefrontal Cortex
1. Introduction
Frontal-subcortical cognitive and limbic feedback loops modulate higher cognitive functioning (Cummings 1993; Levitt et al 2002; Levy et al 1997; Manoach et al 2000). Of significance for the current study, the final step for all higher cognitive function prefrontal-subcortical feedback loops, both cognitive and limbic, is the white matter fiber tracts passing through the anterior limb of the internal capsule (ALIC) (Albin et al 1989; Alexander et al 1990; Alexander et al 1986; Cummings 1995; Middleton and Strick 2001). More specifically, discrete cognitive and limbic feedback circuits anatomically link the prefrontal cortex to the basal ganglia and thalamus forming fronto-striatal thalamic (FST) parallel, feedback loops (Albin et al 1989; Alexander et al 1990; Alexander et al 1986; Cummings 1995; Middleton and Strick 2001). Structural abnormalities in any of the gray and/or white matter core structures involved in this circuitry, including the ALIC, could functionally disconnect the cognitive and limbic feedback loops and consequently interfere with the output from this circuitry. In turn, this could produce cognitive and limbic symptoms similar to that produced by damaging the prefronatal cortex, itself, and could hence induce neuropsychiatric syndromes including schizophrenia. The mediodorsal nucleus which receives input, for example, from the dosolateral prefrontal cortex and the anterior nucleus which receives input from the hippocampus are both thalamic subnuclei which project out through the ALIC (Nolte 2002; Parent 1996). Hence, abnormalities in the ALIC could impair executive and memory functions subseved by such structures. Furthermore, two previous papers our ours (Nestor et al 2004; Nestor et al 2008) found that decreased FA in the cingulum and uncinate fasciculus, in chronic schizophrenic subjects, indeed, correlated respectively with worse performance on measures of executive function and verbal memory. The prefrontal targets of the cingulum and uncinate, the cingulate cortex and orbitofrontal cortex, both project to the striatum in FST subloops which, in turn, project back to the prefrontal cortex via thalamocortical projections through the ALIC. Hence, disturbances in the ALIC, via its disruption of prefrontal-subcortical loops, also could lead to impaired verbal memory and executive function. Using diffusion tensor imaging (DTI), we evaluated abnormalities in the AL-IC fiber tract in schizophrenia.
There are now a number of neuroimaging studies that suggest white matter abnormalities using DTI in schizophrenia (Buchsbaum et al 1998; Burns et al 2003; Kubicki et al 2002a; Kubicki et al 2003; Lim et al 1999; Wang et al 2004; Wolkin et al 2003). In particular, the diffusion measure of Fractional Anisotropy (FA) has been assessed. FA is an index of the degree of asphericity of water diffusion and is believed to be sensitive to the extent of anatomical connectivity between brain regions. More specifically, with regard to the AL-IC in schizophrenia, a number of studies, using a variety of MR imaging methods have reported FA abnormalities in schizophrenia. A number of approaches using MR-DTI have been employed. A reduction in FA in schizophrenia has been reported in the AL-IC based on the use of tract-based spatial statistics (Jeong et al 2009), tractography (Oh et al 2009), voxel-based morphometry (Sussmann et al 2009; Zhou et al 2003), and an automated “strereotactic ROI approach” (Mitelman et al 2007). FA asymmetry differences in schizophrenia including in the AL-IC has been reported using a voxel-based tensor analysis (Park et al 2004). However, a recent whole brain voxel-based morphometry (VBM) MR DTI study of paranoid schizophrenics with a history of auditory hallucinations did not find FA changes in the ALIC, although decreased FA was reported in a number of regions as well as increased FA in the arcuate fasiculus, only, in schizophrenic subjects (Rotarska-Jagiela et al 2009). In addition, MRI structural analysis of the AL-IC has also been performed in varying ways. Decreased volume of the AL-IC has been reported in schizophrenia based upon manual tracing methodology in first-episode and chronic schizophrenics (Lang et al 2006; Wobrock et al 2008; Zhou et al 2003) and based upon using VBM (Chua et al 2007; Zhou et al 2003). Additionally, using a combined manual and automatic methodology, decreased volume at “dorsal levels” of AL-IC in chronic schizophrenic patients with poor outcome has also been reported (Brickman et al 2006). Other morphometric measures of AL-IC have also been reported including decreased maximal cross-sectional area in the left AL-IC in first-episode schizophrenia (Wobrock et al 2009) and a shorter tract length connecting the AL-IC with the prefrontal cortex (Buchsbaum et al 2006).
Additional diffusion measures including RD, MD and AD have also been examined in schizophrenic subjects. Whitford et al (2010) from our group found a reduction in FA together with an increase in RD in frontal corpus callosum fibers, and the latter finding was interpreted as consistent with demyelination. Also, (Ashtari et al 2007) in adolescents with schizophrenia found decreased FA and increased RD and overall diffusion (Trace), after adjusting for premorbid intelligence, in the left inferior longitudinal fasciculus. Lastly, in a complex statistical analysis, Michael et al. (2008) examined the relationship of FA, MD, AD and RD in schizophrenic subjects with the PANNS, measures of symptom severity, and found that “…FA values of almost all regions” negatively correlated with PANNS scores indicating that more severe symptoms correlated with more disturbed white matter. They also found that “MD and AD, for most of the regions” positively correlated with PANNS scores, again, indicating that more disturbed white matter correlated with more severe symptoms, although RD “did not show strong correlations for any of the regions”.
Despite the array of approaches to measuring the AL-IC in schizophrenia, we note that we did not find studies which measured both FA and total AL-IC volume, based on FA maps, which was the approach employed by us. We believe the simultaneous measurement of both volume and diffusion indices of the IC in a single study, helps to understand better the meaning of the reported diffusion measure findings including FA, mean diffusity, radial and axial diffusivity in schizophrenia. We used MR-DTI, instead of structural MRI, as DTI allows for the assessment of diffusion measures as well as volume (e.g., Kubicki et al 2002b), and may be more sensitive to the detection of the boundaries of fiber tracts, such as the AL-IC. We hypothesized both decreased FA and decreased volume in the AL-IC in schizophrenia. Given our FA hypothesis, we also measured mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD). MD can be thought of as an index of extracellular fluid (Hoptman et al 2002); it reflects overall diffusivity not dependent on direction, and specifically measures the mean of the tensor eigenvalues; AD can be thought of as indexing longitudinal axonal integrity; whereas, RD can be thought of as indexing myelin pathology and potentially inter-axonal extracellular fluid, or axonal packing (Song et al 2003; Song et al 2002). Finally, we predicted that FA and volume of the AL-IC white matter, in schizophrenic patients, would correlate with poorer performance on neuropsychological measures sensitive to prefrontal cortical functioning, as well as with greater severity on clinical measures of positive and negative symptoms of psychosis.
2. Methods
2.1. Subjects
Sixteen male patients with chronic schizophrenia were recruited from inpatient, day treatment, outpatient, and foster care programs at the VA Boston Healthcare System, Brockton, Mass. Assessments with the Structured Clinical Interview for DSM-IV—Patient Version (SCID) (First et al 1997) were used to make DSM-IV diagnoses, and the non-patient edition of the SCID (First et al 1995) was completed for 19 normal control subjects. The control subjects were recruited from the general community and were group-matched to the patients on age, sex, handedness (Oldfield 1971), and parental socioeconomic status (Hollingshead 1965). The inclusion criteria for all subjects were right-handedness (one subject was ambidextrous), age between 17 and 55 years, no history of electroconvulsive shock treatment, no history of neurological illness, no alcohol or drug abuse in the last 5 years, no medication with known effects on MR (such as steroids), a verbal IQ at least 70, and an ability and desire to cooperate with the procedures as evidenced by written informed consent. In addition, the control subjects were screened to exclude individuals who had a first-degree relative with an axis I disorder.
Mean age did not differ between patients and normal controls (39.4, SD=7.2, versus 42.4, SD 6.6). Also, there was no significant difference in parental socioeconomic status between groups (2.8, SD=1 versus 2.4, SD 1.2). There were, however, the expected differences in other indices including years of education (12.4, SD 2.0 versus 15.6, SD 2.4, t=4.1, p<0.001), IQ (86.2, SD 14.0 versus 109.0, SD 10.3, t=5.0, p<0.001) and personal socioeconomic status (4.3, SD 0.8 versus 2.2, SD 1.0, t=−6.5, p<0.001; See Table 1). Furthermore, the chronic schizophrenic subjects were quite ill as reflected in their scores on their clinical measures. As noted in Table 1, their mean scores on the PANNS total, Global SANS and Global SAPS, respectively, were 69.6 (±21.7), 10.5 (±5.7) and 9.6 (±3.3).
Table 1.
Demographic characteristics for Chronic Schizophrenic and Normal Control Subjects
| Variable | Schizophrenia Subjects (N=16) | Normal Controls (N=19) | t-test (two-tailed) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | |
| Age (years) | 39.4 | 7.2 | 42.4 | 6.7 | 1.3 |
| Education (years)a | 12.4 | 2 | 15.6 | 2.4 | 4.1 |
| WAIS-iii Verbal IQ b | 86.2 | 14 | 109 | 10.3 | 5 |
| SESc,d | 4.3 | 0.8 | 2.2 | 1 | −6.5 |
| Parental SESc,d | 2.8 | 1 | 2.4 | 1.2 | −0.95 |
| Handednesse | 0.842 | 0.14 | 0.725 | 0.25 | −1.65 |
| PANSS total sumf | 69.6 | 21.7 | NA | NA | |
| Global SANSf | 10.5 | 5.7 | NA | NA | |
| Global SAPSf | 9.6 | 3.3 | NA | NA | |
based on 16 Chronic Schizophrenic subjects and 17 Normal Controls
based on 14 Chronic Schizophrenic subjects and 15 Normal Controls
based on 16 Chronic Schizophrenic subjects and 18 Normal Controls
Socioeconomic Status (with lower numbers representing higher SES)
measured as the ratio of (Right − Left) to (Right + Left) on the Edinburgh Handedness Inventory
based on 13 Chronic Schizophrenic subjects
Written informed consent was obtained from all subjects after they received a complete description of the studies.
2.2. Neuropsychological and clinical measures
All subjects were evaluated using a neuropsychological test battery, where we selected tests that we believed were especially sensitive to frontal, particularly, prefrontal cortical functions (Lezak 1995; Nestor et al 1993; Nestor et al 1998). Hence, for neuropsychological measures, all subsets were evaluated on the Wisconsin Card Sorting Test (WCST), the FAS verbal fluency task, Trailmaking B, the WAIS-III scaled score on block design, digits forward and backward, digit span, spatial span, letter-number sequencing, the wais III working memory index and the wais III family pictures recall, and the Wechsler Memory Scale— 3rd ed. (WMS) LMI story recall. For clinical measures, all subjects were evaluated on The Positive and Negative syndrome scale (PANSS) (Kay et al 1987), the overall SANS (Andreasen 1981) and the overall SAPS (Andreasen 1984). We used the above tests to assess the psychopathological significance of the internal capsule in patients with schizophrenia.
2.3 MRI methods
2.3.1. Image acquisition and post-processing
All subjects were scanned using a line-scan diffusion tensor imaging (LSDI) acquisition, (Gudbjartsson et al 1996), which, unlike single-shot (Turner et al 1990) and navigated echo (Ordidge et al 1994) echo-planar imaging, the most commonly used MR diffusion imaging techniques, is largely insensitive to bulk motion and movement artifacts, producing relatively undistorted DWI images (Kubicki et al 2004). MR scans were performed with a quadrature head coil on a 1.5-T GE Echospeed system (General Electric Medical Systems, Milwaukee), which permits maximum gradient amplitudes of 40 mT/meter. The line-scan diffusion tensor imaging sequence was acquired in the coronal oblique orientation, parallel to the AC-PC line, and perpendicular to the interhemispheric fissure. For each line, six images with high diffusion weighting (1000 sec/mm2) along six directions were collected. For low diffusion weighting (5 sec/mm2) we collected only two images, since diffusion-related signal changes are minimal. The following scan characteristics were used: rectangular field of view, 220×165 mm; 128×128 scan matrix (256×256 image matrix); slice thickness, 4 mm; interslice distance, 1 mm; receiver bandwidth, ±4 kHz; TE (echo time), 64 msec; effective TR (repetition time), 2592 msec; scan time, 60 sec/slice section. We acquired a total of 31–35 coronal slices covering the entire brain, depending on brain size. The total scan time was 31–35 minutes. After reconstruction, the diffusion-weighted images were transferred to a workstation, where eigenvalue, eigenvector, and fractional anisotropy maps of the diffusion tensor were calculated and multiplied by 1000 for slicer display purposes.
We measured mean FA and MD, RD and AD for region of interest (ROI) volumes. FA was calculated based upon the fraction of the magnitude of the tensor that can be ascribed to the anisotropic diffusion by manipulating tensor eigenvalues, as described by (Basser 1995). MD was calculated as representing the mean diffusivity of the 3 orthogonal tensor eigenvalues, λ1 and λ2 and λ3/3. Furthermore, AD was calculated as representing the value of the principal eigenvalue, Lambda, λ1, and RD represents the value of the mean of eigenvalues for Lambda, λ2 and λ3/2 (Song et al 2003; Song et al 2002). Volume was calculated by summing all voxels manually traced upon FA DTI maps, as described below, for left and right AL-IC. Volume measurements were normalized for head size by using the volume of Intracranial Contents (ICC) to calculate ROI linear regression residualized measures (See 2.4. Statistical analysis, below). An axial series of contiguous double-echo (proton density and T2-weighted) images (repetition time=3000 msec, echo time=30 and 80 msec, voxel dimensions=0.9375×0.9375×3.0mm) was employed to measure ICC volume, as previously described (Hirayasu et al 2000; Kasai et al 2003).
2.3.2. Manual segmentation: internal capsule anatomical landmarks
To quantify internal capsule FA, we used the 3D-Slicer (http://www.slicer.org) which provides tools for the manual tracing of Region of Interests (ROIs) including allowing the viewing of ROIs in all 3 planes simultaneously. ROIs were manually drawn using the following criteria.
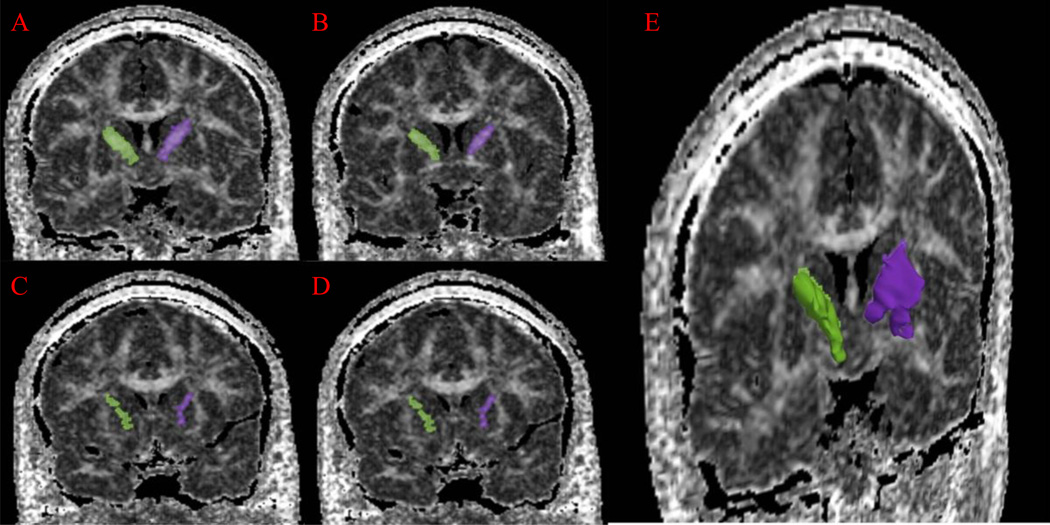
The AL-IC spanned approximately four coronal slices. The anterior boundary of the AL-IC was the coronal slice posterior to the emergence of the rostrum of the corpus callosum. The superior boundary of the AL-IC was formed by the line connecting the most medio-dorsal aspect of the putamen and the dorso-lateral aspect of the caudate nucleus where it is contiguous with the corpus callosum (easier to visualize using DTI FA Maps). The medial and lateral boundaries were formed by the lateral aspect of the caudate nucleus and the medial aspect of the lentiform nucleus, respectively. The inferior boundary was defined by a gray matter area that included the ventral striatum, basal forebrain nuclei and the globus pallidus. Lastly, the posterior boundary was formed by the most anterior slice where the column of the fornix was present (Figure 1).
Figure 1.
(A–D) Manual segmentations of left (shaded purple) and right (shaded green) AL-IC ROIs superimposed on MRI coronal slices in a subject. The slices are from posterior to anterior from A to D. (E) A Three-Dimensional rendering of left (purple) and right (green) AL-IC superimposed on an MRI coronal slice in a subject.
Interrater reliability for left and right AL-IC (rI =0.95; rI =0.93) were high. Inter-rater reliabilities, based on intraclass correlation coefficients, were computed by three raters on the brain scans of 5 schizophrenic and 5 normal control subjects, randomly selected from the total pool of subjects.
2.4. Statistical analysis
To correct for differences in brain size, a linear regression procedure was employed with absolute volume of each of the ROIs as the dependent variable and ICC as the independent variable, yielding residualized volumes which were then used in subsequent analyses. Repeated measures analysis of variance (ANOVA) with group (chronic schizophrenic versus normal controls) as a ‘between-subjects’ factor and side as a ‘within-subjects factor’, was used to test for group differences in AL-IC residualized volumes. Repeated measures analysis of variance (ANOVA), with group (chronic schizophrenic versus normal controls) as a ‘between-subjects’ factor and side as a ‘within-subjects factor’, was used to test for group differences in internal capsule diffusion FA. In the case of a significant main effect, planned, independent t-tests were used, with significance set at P < 0.05 (two-tailed) to compare group mean differences. Because of our relatively small sample size, and to avoid the undue influence of outliers, we employed nonparametric Spearman’s rho (r) tests, with 2-tailed p values, to test possible associations between internal capsule variables with cognitive and clinical measures of psychopathology.
3. Results
3.1. AL-IC ROI volumes and diffusion measures
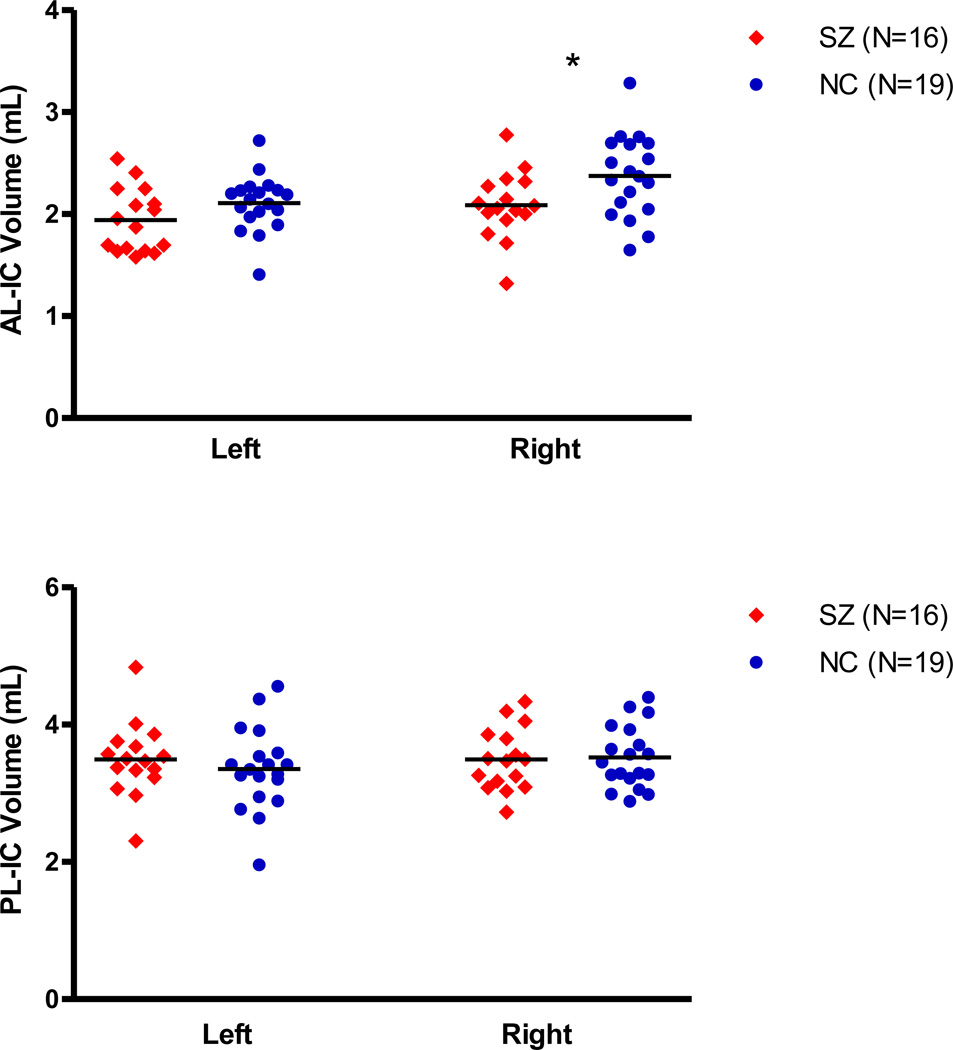
As shown in Figure 2 and Table 2, statistical tests of group difference were performed on both FA and on residualized volume measures of internal capsule ROIs as well as on total intracranial contents absolute volumes. There was no group difference in total intracranial volume (1488.02 ± 117.71 vs 1536.02 ± 121.69, t=1.18, df=33. p=0.25). We found that Repeated Measures ANOVA, using residualized measures of AL-IC volume, revealed a significant main effect for group (F=4.27, df=1,33, p=0.047) but not for side (F=0.010, df=1,33, p=0.92), with no group by side interaction (F=1.39, df=1,33, p=0.25). Follow-up t-tests revealed a significant decrease in residualized volume measures for the right (−0.15 ± 0.34 vs 0.12 ± 0.39 mls, t=2.13, df=33, p=0.04) with a trend decrease in volume for the left (−0.082 ± 0.31 vs 0.07 ± 0.27 mls, t=1.54, df=33, p=.13) AL-IC in schizophrenia. Of note, when we performed Repeated Measures ANOVA using absolute volumes of internal capsule ROIs and covaried for ICC, instead of using residualized volumes, we found similar results.
Figure 2.
Scatterplot of AL-IC (absolute) Volumes in Schizophrenia and Normal Controls. * P<0.05.
Table 2.
Values of Mean Absolute (mL) and Mean Residualized Volume (mL), Mean FA, and Mean MD of Internal Capsule Regions of Interest In Chronic Schizophrenic and Normal Comparison Subjects
| Variable | Schizophrenia Subjects (N=16) | Normal Controls (N=19) | t-test (two-tailed) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Total ICC (mL) | 1488.02 | 117.71 | 1536.02 | 121.69 | 1.18 | 33 | 0.25 |
| Right anterior IC | |||||||
| a absolute volume * | 2.09 | 0.33 | 2.37 | 0.40 | 2.26 | 33 | 0.03 |
| b Residualized volumes* | −0.15 | 0.34 | 0.12 | 0.39 | 2.13 | 33 | 0.04 |
| c FA | 0.55 | 0.03 | 0.56 | 0.02 | 1.76 | 33 | 0.09 |
| d MD | 0.710 | 0.02 | 0.701 | 0.02 | −1.30 | 33 | 0.20 |
| e RD | 0.484 | 0.05 | 0.468 | 0.05 | −0.90 | 33 | 0.37 |
| f AD | 1.185 | 0.05 | 1.154 | 0.10 | −1.09 | 33 | 0.28 |
| Left anterior IC | |||||||
| a absolute volume | 1.94 | 0.31 | 2.11 | 0.27 | 1.69 | 33 | 0.10 |
| b Resisualized volumes | −0.08 | 0.31 | 0.07 | 0.27 | 1.54 | 33 | 0.13 |
| c FA | 0.53 | 0.02 | 0.55 | 0.03 | 1.37 | 33 | 0.18 |
| d MD | 0.709 | 0.02 | 0.703 | 0.02 | −1.04 | 33 | 0.31 |
| e RD | 0.492 | 0.04 | 0.475 | 0.05 | −1.06 | 33 | 0.30 |
| f AD | 1.162 | 0.03 | 1.124 | 0.13 | −1.12 | 33 | −0.27 |
p<0.05
We found that Repeated Measures ANOVA for AL-IC diffusion FA revealed a trend main effect for group (F=2.18, df=1,33, p=0.084) a main effect for side (F=10.89, df=1,33, p=0.002) and no group by side interaction (F=0.06, df=1,33, p=0.81). Furthermore, repeated measures ANOVA for mean AL-IC MD revealed no main effect for group (F=1.44, df=1,33, p=0.239) or side (F=0.33, df=1,33, p=0.569), and no group by side interaction (F=1.11, df=1,33, p=0.299). Follow up t-tests showed no group differences between schizophrenics and normal controls in MD in the left (0.709±0.02 vs 0.703±0.02; t=−1.04, df=33, p=0.31) or right (0.710±.02 vs 0.701±0.02; t=−1.30, df=33, p=0.20) AL-IC. In addition, we found that Repeated Measures ANOVA for AL-IC radial diffusivity revealed no main effect for group (F=1.04, df=1,33, p=0.316) or for side (F=2.35 df=1,33, p=0.135), and no group by side interaction (F=0.015, df=1,33, p=0.903). Moreover, we found that Repeated Measures ANOVA for AL-IC axial diffusivity revealed no main effect for group (F=1.30, df=1,33, p=0.262), a main effect for side (F=10.16, df=1,33, p=0.003), and no group by side interaction (F=0.23, df=1,33, p=0.639).
3.2. Correlations between volume and diffusion of the AL-IC with measures of psychopathology, demographic variables and neuroleptic exposure in chronic schizophrenic subjects
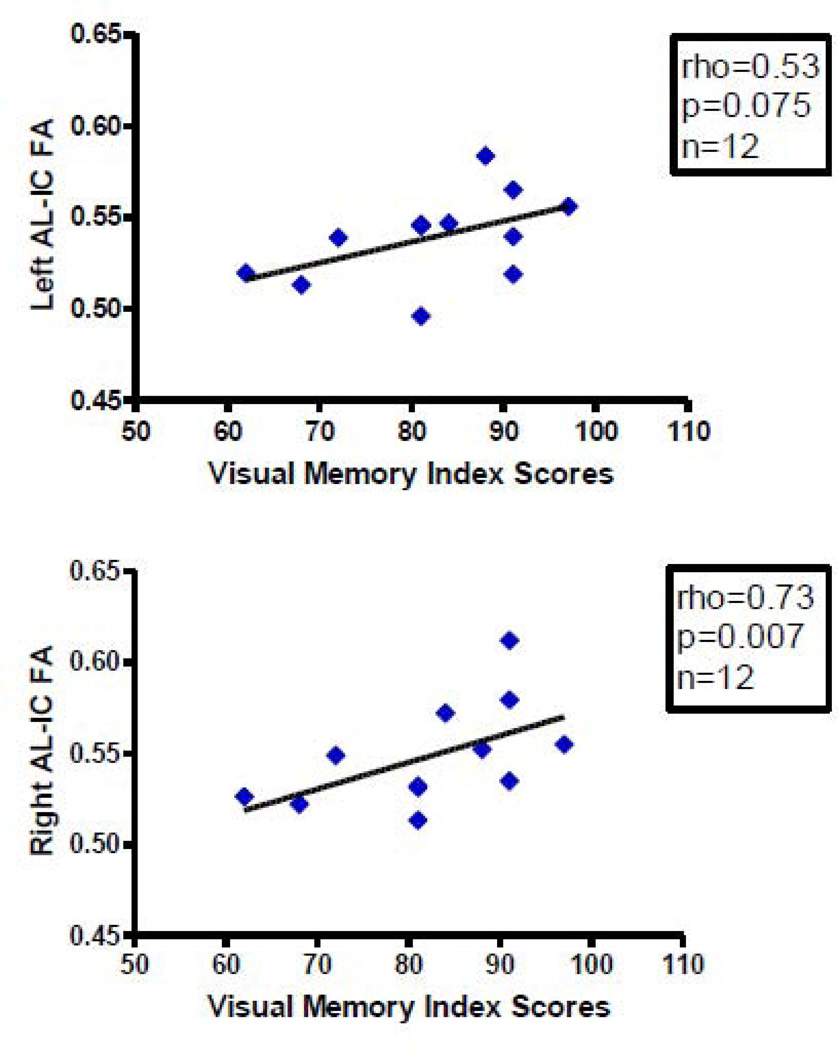
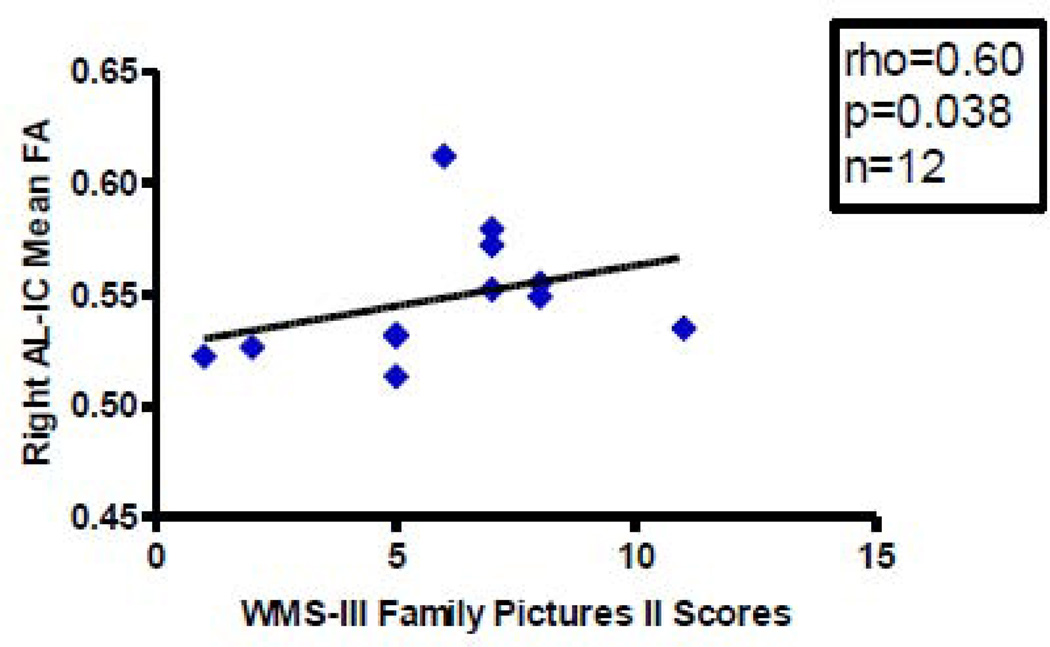
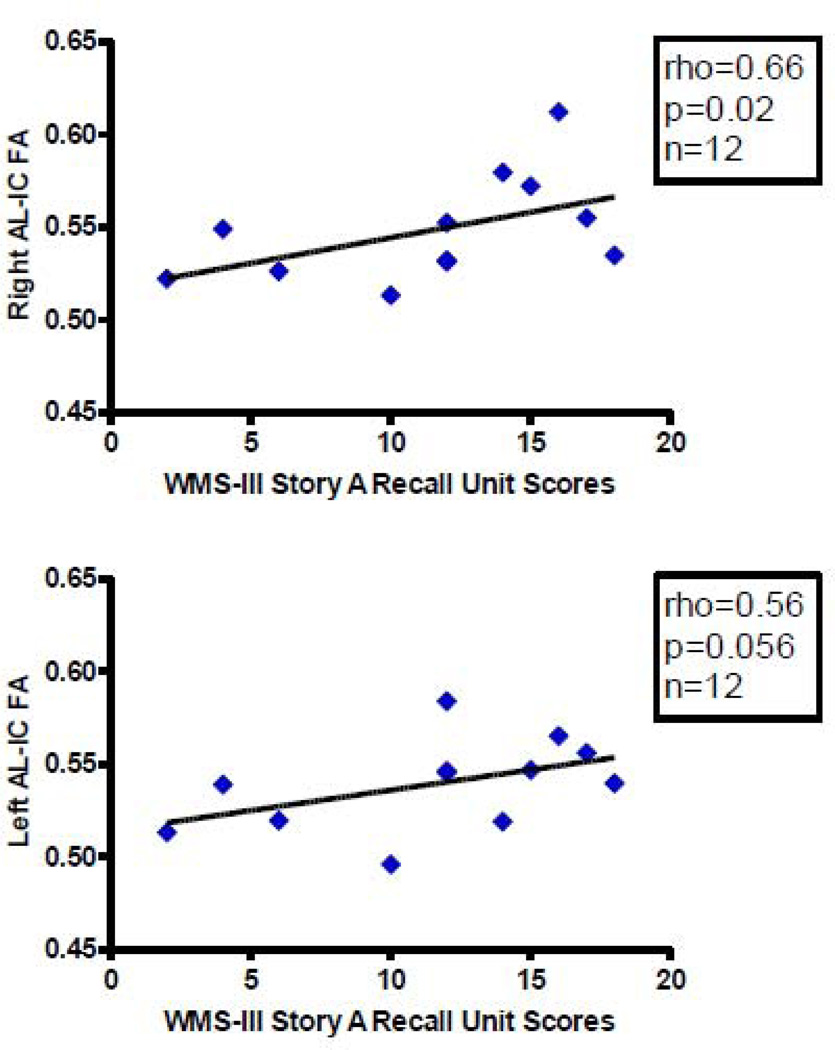
We found more significant correlations with psychopathology in patients diagnosed with schizophrenia with AL-IC FA measures than with volume measures. Overall, mean FA, primarily on the right, positively correlated with better performance on several spatial and verbal declarative/episodic memory tasks and positively correlated with one longitudinal clinical course measure but not with cross-sectional clinical measures. Specifically, we found, first, a positive correlation between right AL-IC mean FA (rho=0.73, N=12, p=0.007) and WAIS-III visual delayed index scores (Figure 3), a measure of spatial episodic memory. Second, we found a positive correlation between right AL-IC mean FA (rho=0.60, N=12, p=0.038) and WMS-III family pictures II scales scores (Figure 4), a second measure of visuospatial episodic memory. And third, we found a positive correlation between right AL-IC mean FA (rho=0.66, N=12, p=0.021) and a near positive correlation between left AL-IC mean FA (rho=0.56, N=12, p=0.056) and WMS-III story A recall unit scores (Figure 5), a measure of verbal episodic memory. With regard to clinical symptoms we found no correlations between left nor right AL-IC mean FA and cross-sectional clinical measures of sum of negative or positive PANNSS scores and SANS and SAPS scores (0.24<ps<0.78). Also, age of onset, age of subject at SCID date and CPZ Dosage equivalent did not correlate with our measures of left or right AL-IC FA (0.15<ps<0.70). There was, however, a positive correlation between right AL-IC mean FA (rho=0.51, N=16, p=0.045), but not left Al-IC mean FA (p=0.60), and the longitudinal clinical course measure of duration of illness from onset to date of SCID.
Figure 3.
Scatterplots between Visual Memory Index Scores and Left and Right AL-IC FA in Schizophrenia. Although we used Spearman’s rho for testing statistical significance because of our small N, we have plotted a least squares line for the convenience of the reader. Although we used Spearman ρ for testing statistical significance because of our small sample size, we have plotted a least squares line for the convenience of the reader.
Figure 4.
Scatterplot between WMS-III Family Pictures II Scale Scores and Right AL-IC FA in Schizophrenia. Although we used Spearman’s rho for testing statistical significance because of our small N, we have plotted a least squares line for the convenience of the reader. Although we used Spearman ρ for testing statistical significance because of our small sample size, we have plotted a least squares line for the convenience of the reader.
Figure 5.
Scatterplots between WMS-III Story A Recall Unit Scores and Right and Left AL-IC FA. Although we used Spearman’s rho for testing statistical significance because of our small N, we have plotted a least squares line for the convenience of the reader.
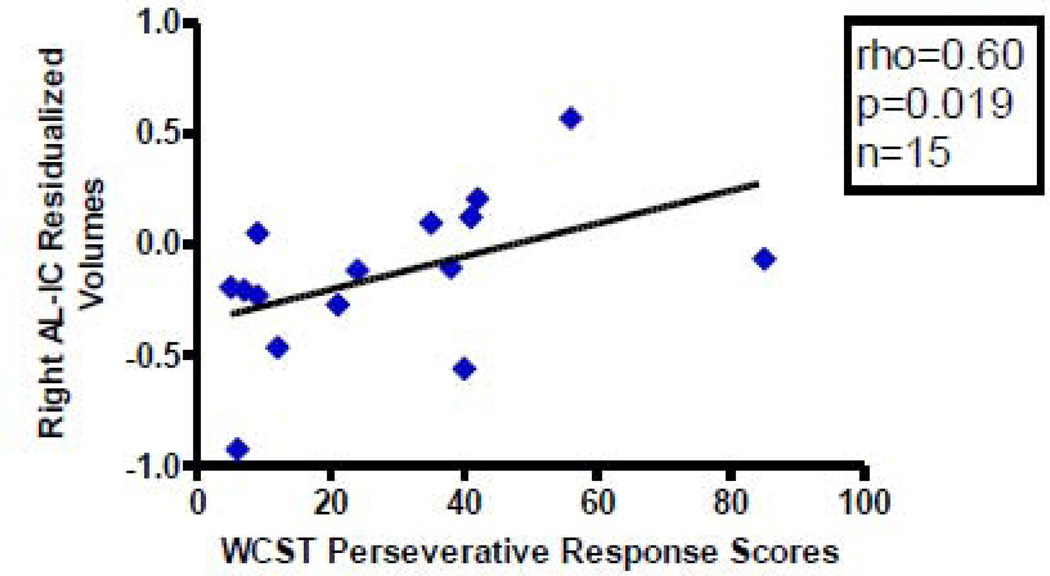
With regard to residualized AL-IC ROI volumes, we found a significant positive correlation between right AL-IC residualized volume measures (rho=0.60, N=15, p=0.019) and WCST perseverative response scores (Figure 6). Conversely, the sum of the negative or positive PANNSS scores and the SANS and SAPS did not significantly correlate with our measures of residualized left and right AL-IC Volumes (0.20<ps<0.92). Furthermore, clinical measures including age of onset, age of subject at SCID date, duration of illness and CPZ dosage equivalent also did not correlate with our measures of residualized left and right AL-IC volumes (0.50<ps>0.99).
Figure 6.
Scatterplot between WCST Perseverative Response Scores and Right AL-IC Residualized Volumes in Schizophrenia. Although we used Spearman ρ for testing statistical significance because of our small sample size, we have plotted a least squares line for the convenience of the reader.
4. Discussion
There are several important findings in our study. First, using manual tracing of the entire white matter AL-IC, we found a significant group difference, more pronounced on the right, in AL-IC volume between patients diagnosed with chronic schizophrenia and normal controls. Second, and not expected, we did not detect a group difference in mean FA for AL-IC; nor, did we find group differences in MD, AD or RD. Third, despite the absence of a group difference in FA, we found in schizophrenics, important right-sided positive correlations between mean FA in the AL-IC and neuropsychological performance on visuospatial memory and a more bilateral positive correlation between mean FA and verbal declarative/episodic memory. With regard to our volumetric measures of the AL-IC, we reported a single positive, right-sided, correlation between volume and WCST perseverative responses.
Our finding of reduced AL-IC volume supports a structural abnormality in subcortical WM in schizophrenia in this important region. The absence of a group difference in MD and, also in particular, RD, supports the absence of an increase in extracellular fluid between axons in schizophrenic subjects. Though the microstructual implication of diffusion indices have yet to be fully elucidated, animal studies by Song and colleagues have shown that RD and AD can be used to discriminate between myelin and axonal damage. Song et al. (2002) studied “congenitally dysmylelinated shiverer mutant mice” and found using DTI that in these mice that RD (water diffusivity perpendicular to axonal fiber tracts) was significantly increased in a number of central nervous system white matter tracts, whereas AD (water diffusivity parallel to axonal fiber tracts) was not compared with wild type control mice. Furthermore Song et al. (2003) showed using DTI that in a model of retinal ischemia in mice” where axonal degeneration precedes myelin degeneration, the time course of change in AD and RD were consistent with AD changes reflecting axonal damage and RD changes reflecting myelin damage. Lastly, Song et al. (2005) used DTI to assess RD and AD in the corpus callosum using the cuprizone mouse model of demyelination and remylelination found that the time course of increase and subsequent re-normalization of RD, but not AD, was consistent with demyelination and remyelination in mice fed fist with cupriozone causing myelin degeneration and then fed normal chow allowing for myelin regeneration. This again supports that RD and AD can be used to discriminate between myelin degeneration and axonal damage. Hence, no group change in RD suggests similar water diffusivity perpendicular to axons, or between axons, in both groups and is consistent with an absence of an increase in extracellular fluid between axons in schizophrenics patients compared with controls. It should also be noted, that in contrast to RD and AD, FA does not discriminate between myelin and axonal damage.
Two possible interpretations of our findings are as follows. The volume reduction we found in the AL-IC in schizophrenia could represent fewer fibers with normal packing density; or, normal fiber numbers which have drawn closer together and, hence, have increased packing density. A reduction in oligodendroglia cells in schizophrenia has been reported by Hof et al. (2002) and a potential disturbance in the Myelin related gene, neuregulin-1, also has been noted in schizophrenia (Corfas et al 2004), both of which could, in turn, lead to reduced myelin sheath thickness without necessarily reducing fiber number. This would support the second interpretation for our findings; that is, if AL-IC fibers have thinner myelin in schizophrenia, and draw closer together leading to increased packing density, this may maintain a relatively normal amount of extracellular fluid yielding decreased volume, with greater preservation of FA, which is what we found. The latter idea is analogous to the proposed explanation for volume reduction in gray matter in schizophrenia of neuropil reduction with increased neuronal density of Selemon and Goldman-Rackic (1999). To our knowledge, the first interpretation of fewer fibers in the AL-IC in schizophrenia has not been demonstrated. A more definitive answer to the above the issue of packing density will require future careful EM studies assessing this issue in the AL-IC in schizophrenia. An additional possible explanation for our not finding a group difference in FA might be a potential normalizing effect of medication in the schizophrenic patients. We note, however, that we did not find that CPZ dosage equivalence correlated with either our measures of AL-IC FA or with AL-IC volume.
Our findings further support the importance of simultaneously measuring FA and volume. FA can be explained by a number of different processes and can reflect a combination of multiple potential factors including fiber size, density, or myelination, and tract coherence versus disorganization (McIntosh et al 2008; Park et al 2004). Thus an FA group difference is subject to a number of interpretations. Knowing volume in addition to FA for a given structure should help in clarifying which of multiple factors contributes. For example, decreased FA without volume change may point more to problems of tract coherence; whereas, in the presence of volume reduction this may point more to issues regarding fiber size, density and myelination. As our failure to find decreased FA may represent a type II error, due to a relatively small sample size, our study does not exclude the possibility of a reduction in both volume and FA in the AL-IC in schizophrenia. As described in our introduction, decreased AL-IC FA, utilizing different techniques, has been reported in a number of studies in schizophrenia. Given the above described notion that AL-IC fibers may draw closer together in schizophrenia, this may explain why in our study manually traced volume may have been a more sensitive measure to detect a group difference than FA.
Of further importance, despite not demonstrating a group difference in FA, our FA-cognitive correlations in schizophrenia suggest the value of AL-IC FA as a predictor of cognitive function in schizophrenia and the need for future studies using DTI in schizophrenia. More specifically, our correlative findings support the interpretation that AL-IC FA is an important measure in schizophrenics in predicting diminished declarative/episodic memory. Furthermore, chlorpromazine mg-equivalent dosages, in our study, did not correlate either with mean FA or with the volume of left or right AL-IC (0.33<Ps<0.78) suggesting our findings were not due to the effect of medication. Our finding of a positive correlation between right AL-IC volume and WCST perseverative responses at first is surprising as decreased FA and/or decreased volume might be thought to be associated with worse neuropsychological performance. Nonetheless, in a condition such as obsessive compulsive disorder, for which abnormal striatal structure and function have been described (Graybiel and Rauch 2000), it has been hypothesized that an overactive prefrontal-subcortical circuit may lead to repetitive behaviors (Saxena et al 1998), which is consistent with our finding of increased AL-IC volume leading to more perseverative, i.e., repetitive, behaviors. We do acknowledge, however, as this is one of few studies to assess AL-IC in relation to neuropsychological disturbance in schizophrenia, we ran a number of exploratory correlations to see how predictive volume and FA might be. While the number of computed correlations increased the risk of Type I error, the potential benefit lies in exploring possible helpful clinical correlations that subsequent studies can either confirm or disconfirm. Therefore, the observed neuropsychological-anatomical correlations should be considered as preliminary findings that will need to be replicated in independent studies.
Possible limitations of our study include first, that our sample size may be too small for detecting a group difference in our measure of FA. For example, our calculated Cohen d effect size for our right and left AL-IC FA were 0.59 and 0.47 SDs (Cohen 1977) with schizophrenics showing non-significantly reduced FA for both right and left AL-IC (See Table 2). This raises the possibility that our negative results regarding AL-IC FA may have been because of insufficient power. In fact, our repeated measures analysis comparing groups for FA resulted in a near significant p value for FA of 0.08. Second, although our FA maps are very useful in detecting the edge of white matter fiber bundles, and, hence, we believe were effective for tracing the AL-IC, the resolution in our DTI study was 0.8594 by 0.8594 by 4 mm with 1 mm gaps between slices. Nonetheless, we believe that our use of LSDI, due to better protection from movement artifact, greatly improved the accuracy of the data. We previously demonstrated that even though acquired at relatively low resolution, LSDI is characterized by smaller error, and smaller intersubject and intersession variability, thus, providing more accurate data than comparable EPI sequence (Kubicki et al 2004). Lastly, newer methods to assess white matter tracts employing various approaches to tractography are clearly of great importance but are not without potential methodologic issues. Thus, manual tracing, though labor intensive, we believe remains an important complementary approach to assessing white matter tracts, particularly anatomically well defined, discrete tracts such as the AL-IC, whose anatomic boundaries are especially well defined within the confines of the basal ganglia and the thalamus.
In summary, we found that the AL-IC, a key subcortical white matter component of prefrontal corticalsubcortical circuits relevant for modulating cognitive and emotional functions, is abnormally reduced in volume in chronic schizophrenia, but we did not detect group differences in the diffusion measures of FA, MD, RD and AD. These findings are consistent with a reduction in the number of fibers, or a reduction in the thickness of the myelin sheath of the fibers together with some degree of increased axonal fiber packing density, in the AL-IC in schizophrenia. Lastly, despite not detecting a group difference in FA, we found several important correlations with decreased AL-IC FA predicting poorer performance in spatial and verbal declarative/episodic memory in schizophrenia.
Acknowledgements
This study was supported, in part, by a Milton Award (JJL), by the Department of Veterans Affairs Merit Awards (JJL, MES, RWM), by a VA Schizophrenia Center grant (RWM, MES), by a Middleton Award (RWM) from the Department of Veterans Affairs, by grants from the National Institute of Health (K05 MH 700047 and R01 MH 50747 to MES, R01 MH 40799 to RWM, RO3 MH068464-01 to MK). The authors gratefully acknowledge the administrative support of Marie Fairbanks and the research assistant support of Doug Markant, B.A., and Lillian Hsu, B.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor,"prefrontal" and "limbic" functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1981. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64:1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusionweighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, et al. Internal capsule size in good-outcome and poor-outcome schizophrenia. The Journal of neuropsychiatry and clinical neurosciences. 2006;18:364–376. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, Chu KW, Mitelman S, et al. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Annals of general psychiatry. 2006;5:19. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89:12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis of the Behavioral Sciences, Revised Edition. Orlando, Florida: Academic Press, Inc.; 1977. [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - (SCID-I) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV - Non-Patient Edition (SCID-NP, version 1.0) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson H, Maier SE, Mulkern RV, Morocz IA, Patz S, Jolesz FA. Line scan diffusion imaging. Magn Reson Med. 1996;36:509–519. doi: 10.1002/mrm.1910360403. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochemical research. 2002;27:1193–1200. doi: 10.1023/a:1020981510759. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two Factor Index of Social Position. New Haven: Yale Station; 1965. [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Jeong B, Wible CG, Hashimoto R, Kubicki M. Functional and anatomical connectivity abnormalities in left inferior frontal gyrus in schizophrenia. Human brain mapping. 2009;30:4138–4151. doi: 10.1002/hbm.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Maier SE, Westin CF, Mamata H, Ersner-Hershfield H, Estepar R, et al. Comparison of single-shot echo-planar and line scan protocols for diffusion tensor imaging. Academic radiology. 2004;11:224–232. doi: 10.1016/s1076-6332(03)00563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002a;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, et al. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatry. 2002b;10:324–336. doi: 10.1080/10673220216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, et al. Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr Res. 2006;87:89–99. doi: 10.1016/j.schres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, et al. MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry. 2002;159:1190–1197. doi: 10.1176/appi.ajp.159.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Molecular psychiatry. 2008;13:1054–1059. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- Michael AM, Calhoun VD, Pearlson GD, Baum SA, Caprihan A. Correlations of diffusion tensor imaging values and symptom scores in patients with schizophrenia. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5494–5497. doi: 10.1109/IEMBS.2008.4650458. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. A Revised Neuroanatomy of Frontal-Subcortical Circuits. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York London: The Guilford Press; 2001. pp. 44–58. [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, et al. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O'Donnell B, et al. Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia [see comments] Am J Psychiatry. 1993;150:1849–1855. doi: 10.1176/ajp.150.12.1849. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, Wible C, Hokama H, O'Donnell BF, Law S, et al. A neuropsychological analysis of schizophrenic thought disorder. Schizophr Res. 1998;29:217–225. doi: 10.1016/s0920-9964(97)00101-1. [DOI] [PubMed] [Google Scholar]
- Nolte J. The Human Brain: An Introduction to Its Functional Anatomy. 5th Edition ed. St. Louis: Mosby, Inc.; 2002. [Google Scholar]
- Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, et al. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Human brain mapping. 2009;30:3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ordidge RJ, Helpern JA, Qing ZX, Knight RA, Nagesh V. Correction of motional artifacts in diffusion-weighted MR images using navigator echoes. Magn Reson Imaging. 1994;12:455–460. doi: 10.1016/0730-725x(94)92539-9. [DOI] [PubMed] [Google Scholar]
- Parent A. Carpenter's Human Neuroanatomy. 9th Edition ed. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, et al. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. NeuroImage. 2004;23:213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A, et al. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2009;174:9–16. doi: 10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998:26–37. [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Maniega SM, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar disorders. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990;177:407–414. doi: 10.1148/radiology.177.2.2217777. [DOI] [PubMed] [Google Scholar]
- Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161:573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68:70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Gruber O, Schneider-Axmann T, Wolwer W, Gaebel W, Riesbeck M, et al. Internal capsule size associated with outcome in first-episode schizophrenia. European archives of psychiatry and clinical neuroscience. 2009;259:278–283. doi: 10.1007/s00406-008-0867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Kamer T, Roy A, Vogeley K, Schneider-Axmann T, Wagner M, et al. Reduction of the internal capsule in families affected with schizophrenia. Biol Psychiatry. 2008;63:65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160:572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, et al. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol Psychiatry. 2003;54:427–436. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]