Abstract
Autonomic nervous system activation can induce significant and heterogeneous changes of atrial electrophysiology and induce atrial tachyarrhythmias, including atrial tachycardia (AT) and atrial fibrillation (AF). The importance of the autonomic nervous system in atrial arrhythmogenesis is also supported by circadian variation in the incidence of symptomatic AF in humans. Methods that reduce autonomic innervation or outflow have been shown to reduce the incidence of spontaneous or induced atrial arrhythmias, suggesting that neuromodulation may be helpful in controlling AF. In this review we focus on the relationship between the autonomic nervous system and the pathophysiology of AF, and the potential benefit and limitations of neuromodulation in the management of this arrhythmia. We conclude that autonomic nerve activity plays an important role in the initiation and maintenance of AF, and modulating autonomic nerve function may contribute to AF control. Potential therapeutic applications include ganglionated plexus ablation, renal sympathetic denervation, cervical vagal nerve stimulation, baroreflex stimulation, cutaneous stimulation, novel drug approaches and biological therapies. While the role of the autonomic nervous system has long been recognized, new science and new technologies promise exciting prospects for the future.
Keywords: Cardiac nerve sprouting, heart failure, myocardial infarction, neuromodulation
Autonomic nervous system activation can induce significant and heterogeneous changes of atrial electrophysiology and induce atrial tachyarrhythmias, including atrial tachycardia (AT) and atrial fibrillation (AF). The importance of the autonomic nervous system in atrial arrhythmogenesis is also supported by circadian variation in the incidence of symptomatic AF in humans.1 Methods that reduce autonomic innervation or outflow have been shown to reduce the incidence of spontaneous or induced atrial arrhythmias.2–5, 6 The latter studies suggest that neuromodulation may be helpful in controlling AF. In this review we focus on the relationship between the autonomic nervous system and the pathophysiology of atrial fibrillation (AF), and the potential benefit and limitations of neuromodulation in the management of this arrhythmia.
Cardiac autonomic innervation
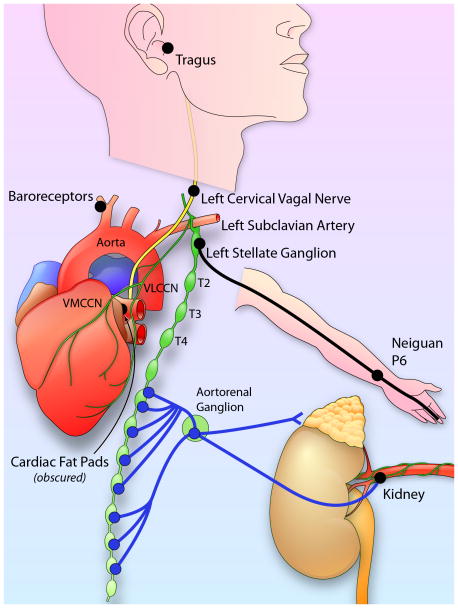
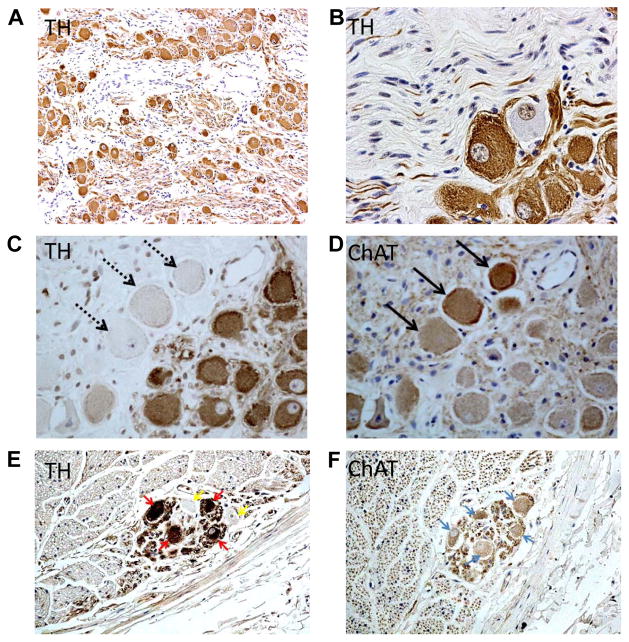
The heart is richly innervated by the autonomic nerves. The ganglion cells of the autonomic nerves are located either outside the heart (extrinsic) or inside the heart (intrinsic). Both extrinsic and intrinsic nervous systems are important for cardiac function and arrhythmogenesis.7–10 The vagal nerves include axons that come from various nuclei in the medulla. The extrinsic sympathetic nerves come from the paravertebral ganglia, including the superior cervical ganglion, middle cervical ganglion, the cervicothoracic (stellate) ganglion and the thoracic ganglia.11 The intrinsic cardiac nerves are found mostly in the atria, and are intimately involved in atrial arrhythmogenesis. Figure 1 is a highly simplified illustration of the cardiac autonomic innervation and sites reported to be relevant in neuromodulation to control atrial arrhythmia. Among them, the stellate ganglion is a major source of cardiac sympathetic innervation. The stellate ganglion connects with multiple intrathoracic nerves and structures as well as skin.12–15 Figure 2 shows immunohistochemical staining of the major autonomic structures that innervate the heart. The ganglion cells within the stellate ganglion mostly (> 90%) stain positive for tyrosine hydroxylase, the rate-limiting enzyme responsible for the synthesis of catecholamines (Figure 2A). However, there are also ganglion cells that are negative for that enzyme (Figure 2B). The negatively stained cells (Figure 2C) stain positively for choline acetyltransferase (Figure 2D),16 an enzyme responsible for the synthesis of the neurotransmitter acetylcholine. Tyrosine hydroxylase-positive ganglion cells are also found in the cervical vagal nerve of dogs (Figure 2E) and humans.17, 18 These findings suggest that the sympathetic components in the vagal nerve may serve as a source of sympathetic tone. Because cells that stain positive for tyrosine hydroxylase may also stain positive for choline acetyltransferase (Figure 2E), ganglion cells in the autonomic nerve structures are not only dedicated to produce catecholamines.
Figure 1.
Autonomic innervation and neuromodulation. VLCCN, ventral lateral cervical cardiac nerve; VMCCN, ventromedial cervical cardiac nerve. Neiguan P6 is an acupoint used in a clinical trial of AF.138 The black dots indicate sites used by various investigators for neuromodulation to control AF. See Neuromodulation section for details. Illustration Credit: Ben Smith.
Figure 2.
Presence of both adrenergic and cholinergic nerves structures in the extrinsic cardiac nervous system. Panel A is a low power view of the left stellate ganglion, showing numerous ganglion cells and nerve fibers stained positively for tyrosine hydroxylase. While most of the ganglion cells are tyrosine hydroxylase (TH) positive, some ganglion cells were negative (Panel B). Panels C shows tyrosine hydroxylase staining of a different stellate ganglion, showing tyrosine hydroxylase-negative cells (arrows). These same cells stained positive for cholineacetyltransferase (ChAT, arrows in Panel D). Some cells stain positive for both markers. These figures came from Shen et al,16 with permission. Panels E and F show tyrosine hydroxylase and cholineacetylesterase stains, respectively, of the canine left cervical vagal nerve. Arrows point to cells that stained positive for both markers. From Onkka et al,17 with permission.
Like the stellate ganglion, the vagal nerves also have a complex structure containing mixed nerve types. A large portion of the vagus trunk contains sensory and motor nerves.19 In addition to the parasympathetic structure that sends fibers to various parts of the body,20 a sympathetic component is known to be present in the vagal nerves based on physiological observations.21–23 These findings were subsequently confirmed with immunohistochemical staining that documented the presence of tyrosine hydroxylase-positive nerve fibers in human and canine vagal nerves.17, 18, 24–26 As shown in Figure 3, the tyrosine hydroxylase-positive nerves are distributed mostly in the periphery of the vagal nerve (Figures 3A–3E), but occasionally tyrosine hydroxylase-positive nerves can extend into the center of the vagal nerve (Figure 3F). Similar findings are found in the thoracic vagal nerves.25 Vagal nerve recordings in ambulatory dogs showed that in 3 dogs isolated vagal nerve activation induces tachycardia (Figure 3G), consistent with activation of the sympathetic component of the vagal nerves.
Figure 3.
Tyrosine hydroxylase (TH) and cholineacetyltransferase (ChAT) staining of the cervical vagal nerves. A: A low power view of the right cervical vagal nerve stained with tyrosine hydroxylase. There are 2 distinct nerve bundles in this nerve. B, D: The tyrosine hydroxylase stain of the smaller (B) and the larger (D) bundles in A. The brown color identifies the positively stained nerves. Note that tyrosine hydroxylase -positive nerves are located in the periphery of the nerve bundle. C, E: cholineacetyltransferase staining of the same structures as in panels B and D, respectively. Note that cholineacetyltransferase-positive components are widely distributed in the cervical vagal nerve. F: The tyrosine hydroxylase -positive nerve structure (red arrow) in the middle of the cervical vagal nerve. The objective lens used in panel A was 4X, with a calibration bar of 0.2 mm in length. The objective lens used in panels B–F was 20X, with a calibration bar of 0.2 mm in length. G shows the activation of vagal nerve alone is associated increased heart rate, a finding consistent with the activation of the sympathetic component of the vagal nerve. From Onkka et al,17 with permission.
In addition to these extrinsic cardiac nerves, the heart is also well innervated by the intrinsic cardiac nerves.9, 27 Histological study of human pulmonary vein (PV)-left atrium (LA) junction28 showed that numerous autonomic nerves are present. The nerve densities are the greatest in the left atrium within 5 mm of the PV-LA junction, and are higher in the epicardium than endocardium. Adrenergic and cholinergic nerves are strongly co-located at tissue and cellular levels. A significant proportion (30%) of ganglion cells expresses dual adrenocholinergic phenotypes (i.e., stain positive for both tyrosine hydroxylase and cholineacetyltransferase). Because these nerve structures are highly co-localized, it is difficult to perform radiofrequency catheter ablation that selectively eliminates purely sympathetic or parasympathetic arms of the autonomic nervous system.
Neuroplasticity
In addition to the complex anatomical and physiological interactions between various nerve structures, cardiac autonomic innervation is also constantly remodeling, especially during disease states. Pathological examinations of diseased hearts by Vracko et al showed findings consistent with cardiac neural remodeling.29, 30 Cao et al31 injected nerve growth factor (NGF) into the left stellate ganglion and induced robust cardiac nerve sprouting in normal canine hearts. The same effects are observed with low amplitude electrical stimulation of the left stellate ganglion.32 Zhou et al33 performed a study of the mechanisms of nerve sprouting using a canine model of myocardial infarction. The results show a persistent elevation of NGF levels in aorta and coronary sinus within 1 month after myocardial infarction. NGF and growth associated protein 43 are transported retrogradely to the left stellate ganglion through retrograde axonal transport. The increased NGF then triggers nerve sprouting at the non-infarcted ventricles and atria.34 Increased atrial sympathetic innervation is associated with increased incidence and duration of AF in those animals. These studies show that, while cardiac injury is limited to the ventricle, neural remodeling may occur throughout the heart. Cardiac diseases, such as myocardial infarction, can potentially increase nerve activities and promote the development of both atrial and ventricular arrhythmias.
Autonomic Remodeling and AF
There is an association between abnormal autonomic innervation and AF in both animal models and in humans. The abnormal autonomic innervation may be important in the mechanisms of AF.35–39 Jayachandran et al40 used [C-11] hydroxyephedrine to label sympathetic nerve terminals in dogs with pacing-induced AF and documented heterogeneously increased atrial sympathetic innervation. The increased sympathetic nerve densities were later confirmed by immunohistochemical staining using antibody against tyrosine hydroxylase in dogs with pacing-induced AF.41 Atrial nerve sprouting and sympathetic hyperinnervation also occur after ventricular myocardial infarction and are associated with increased incidence and duration of AF.34 Consistent with these results, atrial sympathetic nerve densities are also significantly increased in in patients with chronic AF.42 Multiple other studies have further documented the pathophysiological importance of autonomic remodeling in various animal models and in humans.43–46 In addition to atrial sympathetic hyperinnervation, diseases also cause remodeling of extracardiac nerve structures in both experimental animals and in humans.47–49
Cellular mechanisms of cardiac autonomic neurotransmission and signaling
Sympathetic neurotransmission results from the excitation of sympathetic nerve terminals via electrical impulses travelling down the efferent post-synaptic sympathetic nerves, which originate in sympathetic ganglia like the stellates. The production, release, reuptake and degradation of sympathetic neurotransmitters is an extremely complex and highly regulated process.50 This regulation is essential to ensure that the critically-important function of adrenergic control is well-tuned to physiological needs under a wide range of conditions. In brief, the principal neurotransmitter norepinephrine is synthesized in neural cell bodies, transported and concentrated in vesicles in nerve varicosities adjacent to adrenergic receptors, where it is released by nerve depolarization through a Ca2+-dependent process. In addition to norepinephrine, these vesicles contain smaller amounts of a variety of other biologically-active substances like opioids, chromogranin and other neuropeptides.50 Very rapid uptake mechanisms limit the amount of norepinephrine that can access adrenergic receptors, and norepinephrine is also rapidly degraded by a variety of enzymes like monoamine oxidase. In addition to reuptake and enzymatic degradation, norepinephrine action is controlled by negative feedback through presynaptic receptors, particularly alpha2-adrenergic, dopamine and muscarinic receptors.50 Systemically-circulating epinephrine released from the adrenal medulla also contributes to cardiac sympathetic activation, especially in conditions of generalized sympathetic activation.
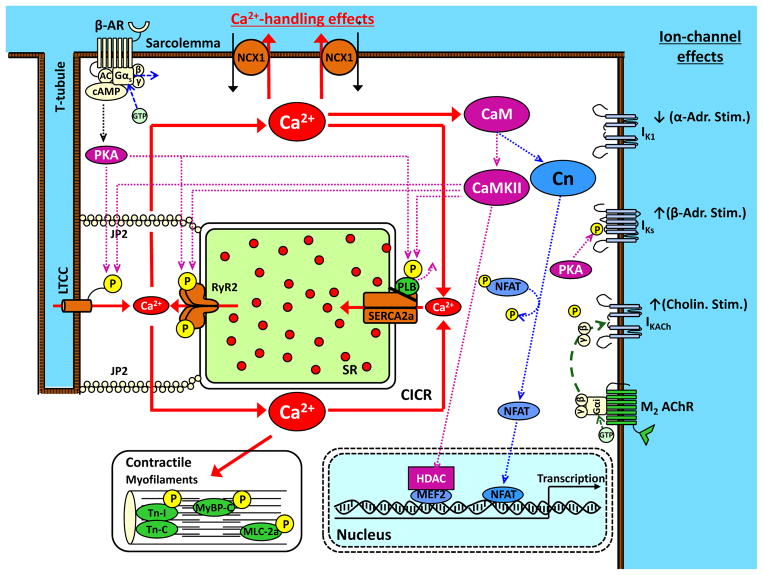
Norepinephrine interacts with a variety of adrenergic receptors on cardiomyocytes to execute adrenergic actions. The detailed biochemistry of adrenergic-receptor pharmacology is very complex and the interested reader is referred to an excellent recent review.51 Here, we will focus primarily on the beta-adrenergic receptor and its downstream signaling relevant to AF (Figure 4). The beta-adrenergic receptor is a member of the enormous family of 7-transmembrane domain G-protein coupled receptors (commonly abbreviated “GPCRs”), and includes 3 subtypes, beta1-3, of which beta1-receptors are most relevant to atrial arrhythmias. The G-protein system includes 3 subunits: α, β and γ. The Gβ and Gγ-subunits bind to each other and are often referred to together as the Gβγ-subunit. A variety of Gα-subunits exist, but the principal adrenergic Gα-subunit is the Gαs, or “stimulatory” subunit. When the beta-receptor is unoccupied, most Gαs is bound to Gβγ. Norepinephrine-binding to the beta-receptor leads to GTP-binding of the Gαs-subunit, lowering its affinity to Gβγ, which dissociates and allows the free Gαs subunit to activate adenylate cyclase, which converts ATP to cyclic-AMP (cAMP), the primary beta-adrenergic second messenger. cAMP activates protein-kinase A (PKA), which exerts a wide range of effects by phosphorylating membrane-proteins, including Ca2+-handling proteins and ion-channels.
Figure 4.
Molecular basis for autonomic contributions to AF substrate. Beta-adrenergic receptor (β-AR) activation causes GTP-binding to the Gαs-subunite, allowing it to dissociate from Gβ and γ subunits and activate adenylate cyclase (AC), which converts ATP to cyclic-AMP (cAMP). cAMP activates protein-kinase A (PKA), which phosphorylates a range of Ca2+-handling proteins including the L-type Ca2+-channel (LTCC), ryanodine-receptor (RyR2) and phospholamban (PLB). PLB-phosphorylation causes it to dissociate from the sarcoplasmic-reticulum (SR) Ca2+-ATPase, SERCA2a, removing SERCA2a from PLB-inhibition and activating SR Ca2+-uptake. RyR2-phosphorylation increases RyR2 open probability, enhancing the systolic Ca2+-transient but also enhancing diastolic Ca2+-leak. Adrenergic stimulation also increases Ca2+ binding to calmodulin (CaM), activating Ca2+/CaM-dependent kinase type-II (CaMKII), which phosphorylates many of the same proteins as PKA. Ca2+/CaM also activates calcineurin (Cn), which dephosphorylates nuclear factor of activated T-cells (NFAT), allowing it to translocate to the nucleus and activate hypertrophic and profibrotic gene-programs. LTCC-phosphorylation increases ICaL and shifts its voltage-dependence to cause larger window-currents. Adrenergic stimulation also inhibits inward-rectifier K+-current (IK1) and enhances slow delayed-rectifier K+-current (IKs). Cholinergic activation of muscarinic type-2 (M2) acetylcholine-receptors (AChRs) causing GTP-binding to Gαi, releasing Gβγ and allowing it to activate the acetylcholine-dependent K+-current (IKACh).
Acetylcholine (ACh) is synthesized from choline and acetylcoenzyme-A via choline acetyltransferase, primarily in cholinergic nerve terminals where it is concentrated in synaptic vesicles. Like sympathetic neurotransmitter production and release, ACh-biology is highly regulated and subject to feeback inhibition via presynaptic muscarinic receptors.52 Released ACh is rapidly broken down by acetylcholinesterase. Acetylcholinesterase is remarkably efficient at breaking down ACh, and greatly limits the spread of ACh from its release site. Consequently, the effects of ACh are very localized, allowing for spatial heterogeneity of ACh-effects under vagal activation, a property that is very important in AF.
The cardiac cholinergic receptor is an M2 type-2 muscarinic subtype. M2-ACh-receptors are also G-coupled, with the “inhibitory” G-protein Gαi being the principal subtype bound to Gβγ. When ACh interacts with the M2-receptor, Gαi-GTP interaction occurs, and as for adrenergic receptors this causes dissociation of Gβγ-subunits from Gαi (Figure 4). However, unlike adrenergic activation, which uses Gαs as the main signaling G-protein, cholinergic effects result predominantly from Gβγ activation of the ligand-gated K+-channel IKACh, composed of Kir3.1 and Kir3.4 subunits.53 IKACh-activation produces an outward K+-current that flows throughout the depolarized phases of the cardiac action-potential (AP), resulting in substantial reduction in AP-duration (APD).
Autonomic regulation of atrial cardiomyocyte electrophysiology
The principal molecular mechanisms by which autonomic influences affect AF-likelihood are illustrated in Figure 4. Please note that another paper in this compendium deals in detail with the cellular machinery underlying AF.54 In this article, we will limit ourselves to the specific mechanisms underlying autonomic AF-promotion. The principal arrhythmogenic targets of beta-adrenergic stimulation relate to cardiomyocyte Ca2+-handling. The main “business” of beta-adrenergic activation in the heart is to enhance cardiac output during “fight-or-flight” reactions. Accordingly, beta-adrenergic stimulation enhances virtually all process controlling Ca2+-entry, storage and release in the heart. These effects are initiated by PKA, and amplified by Ca2+-calmodulin dependent protein-kinase type-II, CaMKII. PKA and CaMKII phosphorylate many of the same proteins (albeit at different sites): the L-type Ca2+-channel (ICaL), the sarcoplasmic-reticulum (SR) Ca2+-release channel ryanodine-receptor (RyR2) and phospholamban.55 ICaL-phosphorylation increases voltage-dependent Ca2+-entry through the plasma-membrane. RyR2-phosphorylation amplifies Ca2+-dependent Ca2+-release from the SR. Together, these actions greatly augment the systolic Ca2+-transient and thereby contraction-strength. Phospholamban binds to and inhibits the SR Ca2+-transporter, SR Ca-ATP’ase (SERCA2a), the principal mechanism responsible for maintaining SR Ca2+-stores and restoring low diastolic Ca2+-levels following the systolic Ca2+-transient to allow diastolic relaxation/filling. Adrenergically-induced phospholamban-phosphorylation by PKA and CaMK2 dissociates phospholamban from SERCA2a, disinhibiting SERCA2a Ca2+-pumping into the SR. Under acute stress conditions, adrenergic activation provides an essential boost to Ca2+-dependent cardiac function. However, in under conditions predisposing to Ca2+-dependent triggered activity,56, 57 the enhanced Ca2+-loading/release conditions produced by adrenergic stimulation strongly promote arrhythmogenesis. In a canine model of chronic atrial ischemia, aberrant Ca2+-release responsible for ectopic activity requires adrenergic drive to manifest.58
Autonomic modulation has very significant effects on cardiac ion-channels. In addition to the ACh-induced activation of IKACh, a host of ion-channels are affected by adrenergic tone.59 The most important of these are ICaL, already discussed, the slow delayed-rectifier K+-current IKs, and the inward-rectifier IK1. IKs is strongly enhanced by adrenergically-induced PKA-phosphorylation,60 allowing it to offset the increased inward current resulting from adrenergic enhancement of ICaL and prevent EADs.61 IK1 is important in setting the resting potential, contributing to repolarization reserve62 and governing AF-dynamics.63 IK1 is typically inhibited via α-adrenergic receptor stimulation.64
Autonomic effects on mechanisms governing AF-occurrence
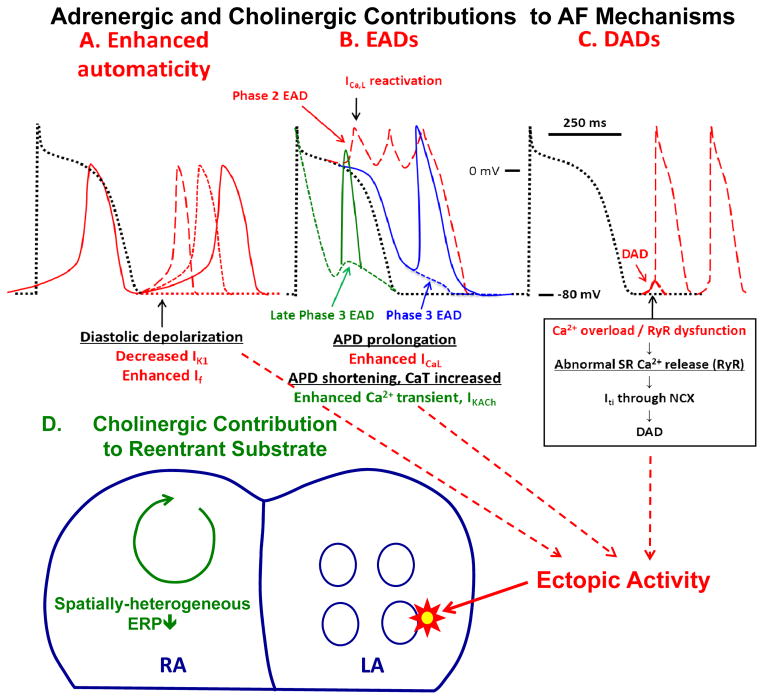
The potential basis for autonomic nervous system promotion of AF is summarized in Figure 5. AF can result from focal or reentrant mechanisms.65, 66 Focal mechanisms are important in 2 ways- they may act as a trigger on a susceptible substrate, or by firing rapidly provide an AF-maintaining driver. Adrenergic activation may promote focal activity via each of the principal cellular mechanisms: enhanced automaticity (Figure 5A), early afterdepolarizations (EADs, dashed tracings, Figure 5B) or delayed afterdepolarization-associated triggered activity (red dashed tracings, Figure 5C). IK1 provides a diastolic outward current that prevents spontaneous phase-4 depolarization to the threshold-potential by the pacemaker “funny” current that underlies spontaneous automaticity. Automaticity is enhanced by reduced IK1, which can result from α-adrenergic stimulation, or increased “funny” current, produced by β-adrenergic activation.67 Phase-2 EAD-induced ectopic activity (red dashed tracings, Figure 5B) likely underlies the increased risk of AF in patients with congenital long-QT syndrome.68 Beta-adrenergic activation enhances plateau ICaL (via PKA/CaMKII-phosphorylation), increasing EAD likelihood, particularly when adrenergic augmentation of IKs is deficient (e.g. in long-QT syndrome type 1). Phase-3 EADs can be associated with APD prolongation (blue tracing, Figure 5B). It may occur as the result of electrotonic current across steep repolarization gradients between phase-2 EAD and the adjacent repolarized tissues, or occur as the result of low IK1.69 In comparison, a late phase-3 EAD (green tracings, Figure 5B) is associated with shortened rather than prolonged APD.70 if there is simultaneous activation of the sympathetic nervous system that increases the intracellular Ca2+ transient and parasympathetic nervous system that activates IKAch, so that APD is shortened while the Ca2+ transient is large and long. A short APD and a large Ca2+ transient creates a condition for late phase-3 EADs, which can induce triggered activity and AF (solid blue tracing, Figure 5B).70, 71 Because pulmonary veins naturally have short APDs, they are particularly prone to develop these Ca2+ transient triggered arrhythmias.37, 39, 72 Delayed afterdepolarizations (DADs, Figure 5C) result from diastolic RyR2 Ca2+-leak, favored by β-adrenergic enhancement of cell Ca2+-loading and increased RyR2 open probability due to PKA/CaMKII-phosphorylation.
Figure 5.
Mechanisms by which autonomic tone can promote AF. Top: Action potential changes showing cellular mechanisms by which adrenergic activation can lead to focal ectopic firing. Black dotted tracings represent normal reference action potentials in each panel. A. Enhanced automaticity. B. Early afterdepolarizations (EADs). C. Delayed afterdepolarization (DADs). Contributions from adrenergic activation alone are shown by red tracings, while that from cholinergic activation (combined with adrenergic activation) by green tracings. Adrenergic stimulation in the setting of impaired repolarization reserve can cause phase-2 EADs (red dashed tracings in B). Most phase 3 EADs are also associated with prolonged APD (blue dashed tracings in B). Combined adrenergic/vagal discharge can produce late phase-3 EADs (green dashed tracings in B) due to a prolonged and enhanced Ca2+-transient that outlasts IKACh-induced accelerated repolarization. Bottom: Tissue-level arrhythmia mechanisms, with focal ectopic activity maintaining AF as a driver or acting on vulnerable reentrant substrates. Parasympathetic firing discharges acetylcholine, producing spatially-heterogeneous action-potential and refractory-period abbreviation that promotes the occurrence and maintenance of reentrant activity.
The precise details of mechanisms maintaining reentry (Figures 5D), such as the structure and number of circuits, role of rotors, etc. remain controversial.73 However, shortened refractoriness promotes functional reentry in all conceptual models. Vagal stimulation strongly abbreviates atrial refractoriness by augmenting IKACh. Furthermore, the refractoriness-abbreviating effects of vagal activation show strong regional variation, much more so than adrenergic effects; this regional variability underlies particularly-strong AF-promoting effects of vagal tone.74
Finally, structural remodeling is known to be an important contributor to AF-persistence.65 Increased Ca2+/calmodulin-binding caused by β-adrenergic stimulation activates the protein-phosphatase calcineurin (Figure 4). Calcineurin dephosphorylates the transcription-factor nuclear factor of activated T-cells, allowing it to translocate into the nucleus and alter gene-transcription, inducing hypertrophic and profibrotic gene-expression programs. Adrenergic stimulation also promotes structural remodeling via other actions, including actions mediated by CaMKII, oxidative stress and signaling via an alternate Gα-subunit, Gαq.51
Autonomic nerve activity and atrial arrhythmias
Direct recording of autonomic nerve activity can provide insight into its role in atrial arrhythmogenesis in animal models. Long term recording of nerve activity in ambulatory animals was first successfully performed by Barett et al.75 Stable cardiac nerve activity was then recorded in the heart, allowing for the relationships between neural activity and arrhythmogenesis.76
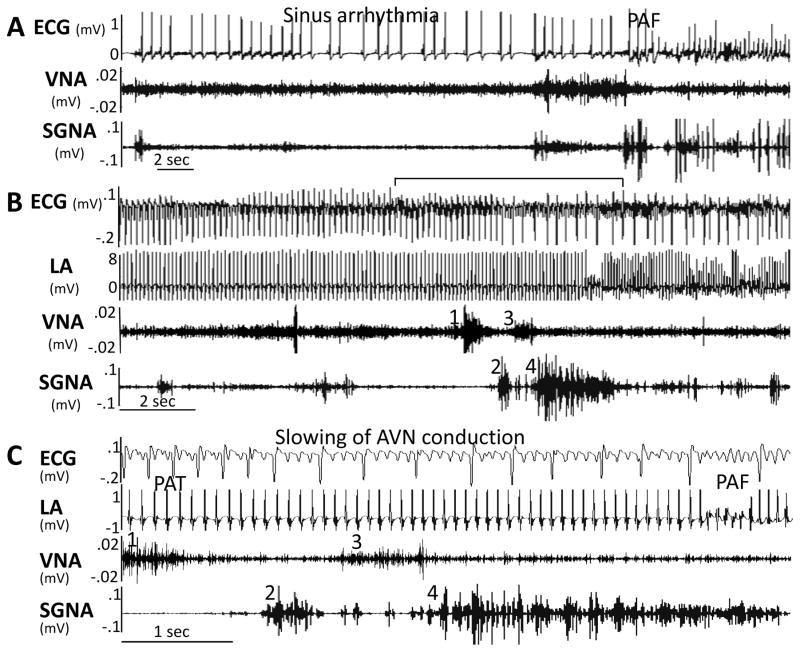
Stellate ganglion nerve activity (SGNA) and vagal nerve activity (VNA) increase after the induction of heart failure by ventricular tachypacing.25 Increased nerve activity was directly associated with paroxysmal atrial tachycardias (PAT) in these dogs. A canine model of intermittent atrial tachypacing was then developed, with rapid atrial pacing for 6–7 days, followed by 1 non-paced day to observe PAT and PAF without pacing artifacts. Intermittent left atrial (LA) tachypacing causes sympathetic hyperinnervation, PAF and PAT.4 Simultaneous sympathovagal discharges commonly precede arrhythmias, implicating them as triggers. Figure 6A shows a typical example of PAF, with sinus arrhythmia in the first 20-s, followed by an abrupt increase in SGNA and VNA and PAF. Figure 6B shows an example of PAT to PAF transition that occurs frequently both in this animal model, as in humans. Figure 6C is a 6-s close up of the same episode shown in Figure 6B, straddling the initiation of PAF. An initial increase in VNA (1) followed by increased SGNA (2) is followed by an acceleration of PAT from 521 bpm to 562 bpm. A second increase in VNA (3) followed closely by a massive burst of SGNA (4) precedes the onset of PAF by approximately 3-s. About 73% of PAT and PAF episodes were preceded by simultaneous sympathovagal discharges. Optical mapping data implicate Ca2+-initiated triggered activity in atrial arrhythmogenesis resulting from parasympathetic activation in transgenic mice that develop a fibrotic AF-substrate due to overexpression of constitutively-activated transforming growth-factor (TGF)-β1. These findings are consistent with a previous study77 that showed AF-induction by simultaneous acetylcholine and isoproterenol infusion into the sinus node artery of anesthetized dogs.
Figure 6.
Two examples of paroxysmal atrial fibrillation (PAF). (A) Sinus rhythm to AF conversion. (B) Atrial tachycardia to AF conversion. (C) Magnified from the center of Panel B (line segment above ECG), showing that the elevated vagal nerve activity (VNA) accelerated atrial rate, leading to paroxysmal reduction of ventricular rate (prolonged RR interval) before conversion from paroxysmal atrial tachycardia (PAT) to paroxysmal atrial fibrillation (PAF). From Tan et al,4 with permission.
Direct recordings from both the extrinsic nervous system (left stellate ganglion and left thoracic vagal nerve) and the intrinsic cardiac nervous system (including superior left ganglionated plexi and ligament of Marshall) were performed to distinguish their relative role in AF-development.74 After intermittent rapid atrial pacing, ambulatory dogs displayed spontaneous PATs before the development of persistent AF. Atrial tachyarrhythmias were invariably preceded by intrinsic cardiac nerve activity. These findings further support the importance of autonomic ganglia in the pathogenesis of AF associated with atrial-tachycardia remodeling.78 Because histological studies show extensive co-localization of adrenergic and cholinergic nerve structures in the intrinsic cardiac nerves,28 it is possible the simultaneous activation of these two arms of autonomic nervous system may be involved in arrhythmia initiation.
Autonomic Nerve Activity and Persistent AF
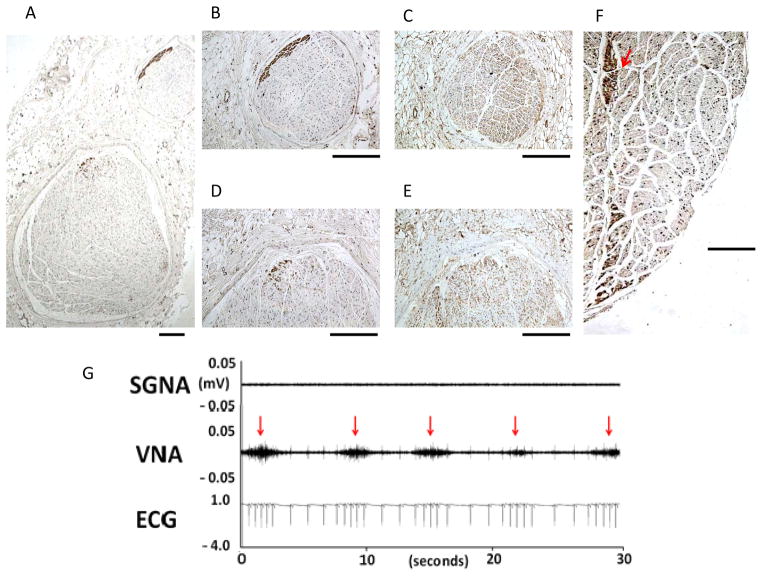
In most patients with AF, rate control is not inferior to rhythm control as a management strategy.79 It is known that the inferior vena cava-inferior atrial ganglionated plexus (also known as the inferior right or right inferior ganglionated plexi80) is important in modulating atrioventricular (AV) node conduction. Direct electrical stimulation of these ganglionated plexi may slow ventricular rate during AF in human patients.81 Ambulatory recordings of bilateral cervical VNA and inferior vena cava-inferior atrial ganglionated plexus nerve activity during persistent AF show that in most but not all dogs, the left vagal nerve controls the AV node while the right vagal nerve controls the sinus node.18 The only nerve structure that consistently controls AV-nodal conduction is the inferior vena cava-inferior atrial ganglionated plexus. Figure 7 shows an example in which inferior vena cava-inferior atrial ganglionated plexus nerve activity is associated with abrupt reduction of ventricular rate during persistent. Vagal nerve activity may sometimes be associated with acceleration of heart rate, probably due to activation of the sympathetic component within the vagal nerves.17, 26 Thus, the ventricular rate during sustained AF is controlled by collaboration among different nerve structures.
Figure 7.
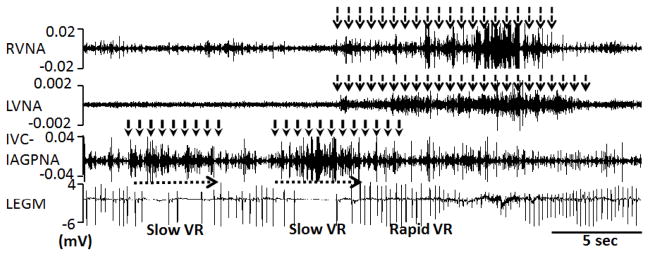
Local control of atrioventricular (AV) node conduction during persistent atrial fibrillation (AF). Slowing of ventricular rate (VR) was associated with inferior vena cava-inferior atrial ganglionated plexus nerve activity (IVC-IAGPNA) without either right vagal nerve activity (RVNA) or left vagal nerve activity (LVNA). Subsequent simultaneous activation of right vagal nerve activity and left vagal nerve activity resulted in a rapid ventricular rate. Because of the presence of abundant sympathetic nerves within the vagus,17 these observations suggest that sympathetic component within the vagal nerves have accelerated the ventricular rate. LEGM is the bipolar local electrogram showing ventricular activation. From Park et al,18 with permission.
Coordination Among Nerve Structures and the Development of AF
Detailed analysis and integration of nerve-activity over time has revealed a number of previously unappreciated patterns of nerve activation.18, 82–84 First, the correlation between SGNA and VNA was found to fall into two different basic patterns. In a minority of dogs, the two nerve structures would fire simultaneously (Group 1). In the remaining dogs, the SGNA and VNA fired separately, i.e., one would activate while the other was quiescent (Group 2). The Group 1 dogs, which tend to have simultaneous sympathovagal discharges, have more PAT episodes at baseline and faster induction of sustained AF by rapid pacing than the remaining (Group 2) dogs that had an L-shaped correlation indicating temporally-separate sympathetic and vagal activity. Perhaps because these dogs were followed for relatively short periods of time (weeks), each dog continued to show a consistent pattern of nerve-firing. However, in a subsequent study when one dog was followed for nearly 6 months, a switch from Group 1 to Group 2 was observed.84 If sympathovagal correlation is important in the development of atrial tachyarrhythmias and AF, the changing patterns of sympathovagal correlation suggest the possibility of dynamically varying arrhythmia susceptibility.
In addition to SGNA and VNA, both linear and L-shaped correlations have been observed between cervical VNA and the inferior vena cava-inferior atrial ganglionated plexus.18 In 5 of the 6 dogs studied, an ‘L’-shaped relationship was present between right VNA and left VNA during AF. In the remaining one dog, a linear correlation was noted between right and left VNA. These findings indicate that right and left cervical vagal nerves do not randomly activate relative to each other. Rather, most typically one would activate when the other is quiescent. In a small minority of dogs, they almost always activate together. Co-activation of these two nerves may be associated with rapid ventricular rate, suggesting that there might be co-activation of the sympathetic nervous system. Another important finding is that the intrinsic nerves (inferior vena cava-inferior atrial ganglionated plexus nerve activity) show a linear correlation with left VNA in a dog with L-shaped correlation between left and right VNA. This indicates that the left VNA almost always fire together with inferior vena cava-inferior atrial ganglionated plexus while the right VNA fires at a different time and does not control the inferior vena cava-inferior atrial ganglionated plexus. Observations such as these clearly indicate that extrinsic and intrinsic nervous systems do not activate randomly in ambulatory dogs. Rather, a high degree of coordination is present among these nerve structures.
Neuromodulation as a therapeutic approach
Because different autonomic nerve structures coordinate their activation with each other, interruption or modification of the activity in one structure may change the pattern of activation of another. These changes may convey therapeutic effects, including arrhythmia control. Some methods of neuromodulation are already in place in clinical use. Others are still being tested in the animal laboratory or clinical trials. Common sites for neuromodulation are labeled by black dots in Figure 1.
Sympathetic and vagal denervation
Because autonomic nerve activity can act as a direct trigger of PAF,4, 85 it is logical to test the hypothesis that stellate ganglion ablation can reduce the incidence of AF. Accordingly, cryoablation of the lower portion of both left and right stellate ganglia, sparing the upper portion of the stellate ganglia to prevent Horner’s syndrome,86 along with the T2–T4 thoracic sympathetic ganglia, was performed in dogs. The vagi were denervated by ablating the superior cardiac branch of the left thoracic vagal nerve. The locations of these structures are shown in Figure 1. One major consequence of cryoablation was a lack of heart rate response to SGNA and VNA. A second major consequence was a delay in the development of sustained AF in response to atrial tachypacing. Whereas control dogs developed sustained AF in 2–4 weeks, the group subjected to cryoablation required 3–12 weeks of atrial pacing to sustain AF.4 A third effect of cryoablation was a suppression of premature atrial contractions and elimination of episodes of PAT and PAF typically associated with intermittent rapid atrial pacing. These findings support the notion that simultaneous sympathovagal discharges contribute importantly to atrial arrhythmogenesis. Because cryoablation only delayed but did not prevent sustained AF, autonomic nerve activity is not the only factor determining AF maintenance. Dogs with pacing-induced heart failure develop both prolonged sinus pauses and PAT.25 Cryoablation of bilateral stellate and T2–T4 thoracic ganglia significantly reduces PAT and prolonged sinus pause episodes induced by sympathetic discharges in dogs with pacing-induced heart failure.87
The above studies suggest that cardiac sympathetic denervation might be useful in controlling PAT and PAF by reducing sympathetic outflow to the heart. However, these studies have multiple limitations. One limitation is that in the canine model PAT and PAF were induced by rapid pacing of either the atria or the ventricles. In contrast, the established risk factors for AF in humans include age, male gender, systolic and diastolic heart failure, valvular heart disease, myocardial infarction, hypertension, diabetes mellitus, obesity, and cigarette smoking.88 The canine model of PAT and PAF may not be applicable to humans. A second limitation is that the stellate ganglion and T2–T4 sympathetic ganglia are not easily accessible in humans. However, the invention of videoscopic left cardiac denervation89 may reduce the procedural complexity of this approach. A third limitation is that the nervous system is highly plastic. It is possible that reinnervation can occur after the denervation procedures and negate the effects of denervation. A fourth limitation is that surgical removal of the stellate ganglion causes irreversible changes of the sympathetic nervous system. The long-term effects of sympathetic denervation in AF patients are unknown.
Vagal Nerve Stimulation
Because of the above limitations, it is highly desirable to develop a neuromodulation method that can be easily terminated, without causing permanent damage to the autonomic structures. Transvenous parasympathetic nerve stimulation can be used as a method of ventricular rate control during atrial fibrillation.90 However, vagal nerve stimulation (VNS) can also be used in the animal laboratory as a method to induce or maintain sustained AF.91, 92 Many studies have documented the effects of neural stimulation or ablation in inducing or controlling cardiac arrhythmias.93–96 The effects of neural stimulation may not be limited to the area directly innervated by the modified nerve structures. For example, stimulating the afferent cervical vagal nerve in cats suppresses sympathetic discharges.97 Because cervical vagal nerves are accessible through surgical approaches, they are the prime target for neural modulation with the hope that their stimulation will achieve therapeutic effects distant beyond the nerves stimulated. A documented success is the use of left cervical VNS to suppress epilepsy in humans.98 Vanoli et al99 showed that chronic VNS can prevent ventricular fibrillation and sudden cardiac death in conscious dogs with a healed myocardial infarction. Others showed that VNS might be used to attenuate heart failure development in dogs,100 rats101 and humans.102–104 While most of these studies used stimulus strength sufficient to reduce heart rate, low-level VNS, defined by a stimulus strength 1 V below the threshold needed to reduce heart rate, is effective in suppressing AF induction in open-chest anesthetized dogs.105, 106 Because VNS opposes sympathetic actions at both pre and post-junctional levels,107, 108 VNS may achieve the therapeutic effects by suppressing sympathetic outflow to the heart. To test this hypothesis, Shen et al5 performed continuous low level VNS in a canine model of PAF while continuing to record SGNA and VNA. Consistent with the observations of Schwartz et al,97 VNS may immediately suppress SGNA when the stimulator is turned on. However, chronic VNS is associated with further reduction of SGNA. The effects of VNS are most apparent in the morning, when the SGNA is most active. The VNS reduced the number of sympathetic discharge episodes and shortened the average duration of discharges. Because of the reduced duration of sympathetic discharges, the SGNA caused less heart rate acceleration during VNS than at baseline. The effects of VNS are not permanent. Rather, SGNA normalizes at the cessation of low-level VNS. In addition to its effects on SGNA, low-level VNS also significantly reduces the number PAT episodes.
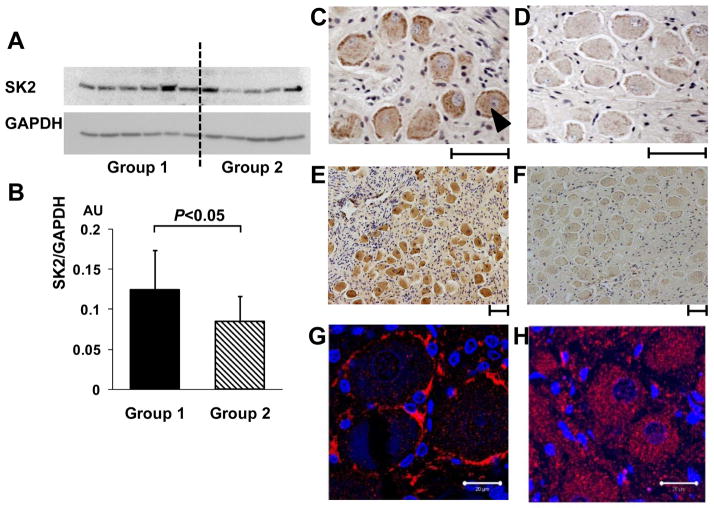
Because VNS has chronic effects on SGNA, VNS might have caused the remodeling of the stellate ganglion. Immunostaining of the left stellate ganglion in dogs with and without VNS showed that low-level VNS decreased the density of nerve structures (presumably sympathetic) staining positive for tyrosine hydroxylase. While a majority (>90%) of the ganglion cells normally stain positive for tyrosine hydroxylase, a small minority of cells show no tyrosine hydroxylase staining (Figure 2). There was a 3-fold increase in the prevalence of tyrosine hydroxylase negative cells in VNS group compared with controls. In a different group of dogs, small conductance calcium activated K channel subtype 2 (SK2) protein expression in the VNS group was found to be nearly 50% higher than in the control group.16 Immunostaining also showed that the density of nerve structures stained with SK2 antibody was higher in VNS group than in the control group. There was significantly increased SK2 protein staining in the periphery of ganglion cells compared with the cell center. This was not observed in normal control dogs. In addition, there were significantly more ganglion cells without immunoreactivity to tyrosine hydroxylase in dogs with VNS (average 11.4%) than in control (4.9%), again showing a roughly 2–3 fold increase of the tyrosine hydroxylase negative cells in the VNS group. Furthermore, a high percentage of tyrosine hydroxylase-negative cells stained positive for choline acetyltransferase. The increased percentage of these cells suggests that VNS might cause phenotypic switching between adrenergic and cholinergic nerves. Figure 8 shows a summary of the stellate ganglion remodeling induced by VNS. The chronic effects of VNS can be partially explained by stellate ganglion remodeling, including increased SK2 proteins and the reduction of tyrosine hydroxylase positive ganglion cells.
Figure 8.
Changes of type 2 small conductance calcium activated K (SK2) protein in the left stellate ganglion (LSG) with low level vagal nerve stimulation (VNS). A, Representative western blots show that the signal ratio of SK2 protein to GAPDH of vagal nerve stimulation dogs (Group 1) was significantly higher than that of control (Group 2). B, There is an upregulation of SK2 protein level in the LSG in Group 1 dogs after being normalized to GAPDH. C–D, Representative immunostaining of SK2 protein in the left stellate ganglion. The density of SK2-positive nerve structures (as pointed by a black arrowhead) is significantly higher in Group 1 dogs (Panel C), compared to Group 2 dogs (Panel D). E–F, Representative low-power view of immunostaining of SK2 protein in the LSG, that clearly demonstrates higher SK2 density in Group 1 dogs (Panel E), compared to Group 2 dogs (Panel F). G and H show immunofluorescence confocal microscope images of the LSG from Group 1 and Group 2 dogs, respectively. Blue colored dots show the nuclei stained with 4′,6-diamidino-2-phenylindole. Red color marks the SK2 protein. Note a significantly increased SK2 staining in the periphery of ganglion cells but decrease in the cytosol of Group 1 (G). In contrast, in Group 2 LSG, the SK2 staining was homogeneous (H). SK2, Small conductance calcium-activated potassium channels subtype 2; GAPDH, glyceraldehydes-3-phosphate-dehydrogenase; AU, arbitrary units. Calibration bar = 50 μm for C–F and 20 μM for G and H.
Baroreflex stimulation and Exercise
Exercise-training results in functional modulation of autonomic balance. Exercise may activate parasympathetic nervous system through changes of plasma volume (baroreflex stimulation),109 or via augmented baroreflex responsiveness and increased cardiomyocyte sensitivity to cholinergic stimulation.110 In the case of exercise training, enhanced sensitivity to ACh appears to be due to reduced expression of a family of proteins called Regulators of G-protein Signaling,109 which have GTP’ase activity and terminate ACh-induced IKACh-activation by breaking down Gαs-associated GTP Endurance exercise-training increases AF-susceptibility in rats via increased parasympathetic tone accompanied by atrial dilation and mild fibrosis.110 These observations parallel clinical observations of an importantly increased prevalence of AF in endurance-athletes.111 However, chronic exercise training may be beneficial for the management of AF by improving rate control.112 It is possible to use implantable devices to directly stimulate the carotid sinus and activate the baroreflex.113, 114 Similar to VNS, baroreflex stimulators can sharply decrease sympathetic nerve activity and lower blood pressure among responders.115 The reduced sympathetic nerve activity may be in part responsible for the improved rate control during AF. While strong baroreflex stimulation may reduce atrial effective refractory period and promote AF, low-level baroreflex stimulation only causes moderate shortening of atrial effective refractory period.116, 117 Further studies are needed to determine whether low level baroreflex stimulation can be used to control cardiac arrhythmias by reducing sympathetic tone without massively shortening the atrial effective refractory period.
Ganglionated plexus ablation
Intrinsic cardiac nerve activity invariably precedes the onset of AF in ambulatory dogs.85 If these findings are applicable to humans, then ablation of the ganglionated plexi of the intrinsic cardiac nervous system with surgical or catheter ablation techniques may be effective in controlling AF. Earlier non-randomized observational studies showed that pulmonary vein denervation may enhance the long term outcome of circumferential ablation of PAF.118 These findings enhanced the theory that hyperactivity of local cardiac ganglionated plexi plays a role in the generation and maintenance of AF.38 One approach to ganglionated plexus ablation is to use high-frequency stimulation to identify ganglionated plexi before ablation.119 Others used an anatomically based approach without high-frequency stimulation.120, 121 Because ganglionated plexus ablation is a new procedure, it is possible that there is a bias in favor of reporting positive results. Katritsis et al122 performed a prospective randomized clinical trial, exposing 242 patients with PAF to pulmonary vein isolation alone, ganglionated plexus ablation alone (anatomical approach) and pulmonary vein isolation plus ganglionated plexus ablation. After 2 years of follow-up, freedom from AF or AT was achieved in 56%, 48%, and 74% of patients in the pulmonary vein isolation, ganglionated plexus ablation, and pulmonary vein isolation+ganglionated plexus ablation groups, respectively (p=0.0036). The authors concluded that the addition of ganglionated plexus ablation to pulmonary vein isolation confers a significantly higher success rate compared with either pulmonary vein isolation or ganglionated plexus ablation alone in patients with PAF. In addition to catheter ablation, minimally invasive surgical procedures have been used for pulmonary vein isolation and ganglionated plexus ablation, with significant improvement in the outcome.123 The clinical evidence so far seems to support the use of ganglionated plexus ablation as an adjunctive procedure in AF ablation.
Renal Sympathetic denervation
Preliminary clinical trials conducted by various investigators suggest that renal sympathetic denervation through an endovascular approach is effective in controlling drug resistant hypertension.124, 125 Other work showed that renal sympathetic denervation can reduce sympathetic nerve activity.126 Because sympathetic nerve activity is important in blood pressure control,75, 84 reduction of sympathetic outflow may in part explain the reduction of blood pressure in some patients. The same effects may also be useful in controlling AF. There are ongoing clinical studies testing the hypothesis that concomitant renal denervation may improve the outcomes from catheter ablation of AF.127, 128 Renal sympathetic denervation has also been used for ventricular rate control in AF and for reduction of AF episodes in patients with sleep apnea.127–129 Preclinical studies suggest that long-term renal denervation may be beneficial in treating rats with heart failure induced by myocardial infarction.130 It is possible that renal sympathetic denervation may benefit cardiac arrhythmic control by improving myocardial function in heart failure. The latter hypothesis is being tested by a number of studies listed in clinicaltrials.org. The results of those studies should advance the field by defining the benefits and risks of renal sympathetic denervation. It remains to be seen if successful treatment of heart failure can also result in reduced incidence of AF in those trials. Recently, the first large scale randomized clinical trial incorporating a sham procedure control group (SYMPLICITY HTN-3)131 failed to document the efficacy of renal denervation in patients with resistant hypertension.132 The implications of this outcome for the concept and application of renal sympathetic denervation are certainly major, and will undoubtedly motivate careful reflection and additional investigation.133
Somatic sensory stimulation for neuromodulation
Various forms of somatic sensory stimulation can produce autonomic reflex responses, depending on the visceral organs and somatic afferents that are stimulated.134 Yu et al135 developed a noninvasive transcutaneous approach to deliver low-level VNS to the tragus of the ear to treat cardiac arrhythmias such as AF. The authors found that low-level tragus stimulation can reverse pacing induced atrial remodeling and suppress AF inducibility, suggesting possible value in treatment of AF. An alternative approach to neuromodulation is acupuncture, which is widely practiced for pain control, although the clinical efficacy remains unproven.136, 137 Lomuscio et al138 showed that acupuncture using Neiguan, Shenmen, and Xinshu spots might prevent arrhythmia recurrences in patients with persistent AF after electrical cardioversion. These two studies applying cutaneous stimulation raise the possibility of using somatic sensory stimulation to achieve neuromodulation. A possible mechanistic rationale is that the somata of the skin sympathetic nerves originate from the middle cervical and stellate ganglion, the same ganglia that innervate the heart.13 However, the limitations of these studies are considerable, and extensive further investigations and clinical trials will be needed to optimize and test the efficacy of cutaneous neuromodulation in the management of AF.
Effects of neuromodulation on the structure and function of the heart
In addition to changes in the structure and function of the nervous systems, neuromodulation may also exert direct effects on the structure and function of the heart. Chronic norepinephrine infusion in dogs can reduce cardiac sympathetic nerve density, decrease myocardial norepinephrine uptake activity and downregulates cardiac beta adrenoceptors, reproducing that which occurs in heart failure.139, 140 Successful treatment of heart failure may result in the improvement of cardiac norepinephrine uptake and attenuate sympathetic nerve terminal abnormalities.141, 142 Because neuromodulation methods may reduce sympathetic outflow, it may help normalize the cardiac sympathetic innervation and improve receptor function in diseased hearts. In addition to suppressing sympathetic outflow, vagal nerve and epicardial ganglionated plexi stimulations may be anti-inflammatory100, 143, 144 and may improve LA function and suppress the development of LA fibrosis.145 Renal sympathetic denervation may control AF through modification of the atrial substrates.6 These findings suggest that neuromodulation may achieve its therapeutic effects in part by causing beneficial structural and functional remodeling in the heart.
Autonomic nervous system targets for antiarrhythmic drug therapy
Given the apparent importance of the autonomic nervous system in AF, it should be possible to identify autonomic targets for drug therapy. Beta-blockade has moderate but statistically-significant effects to prevent AF-recurrence after electrical cardioversion.146 With further research, it may be possible to identify patients to target based on particularly-important autonomic contributions to their AF. One such group is patients undergoing cardiac surgery, for which there is evidence of an important role of Ca2+-homeostasis abnormalities in post-operative AF.147 Prophylactic beta-blockers are particularly effective in preventing post-operative AF,148 illustrating the applicability of the concept. Based on the importance of IKACh in AF, selective blockers are being developed, with some success in preclinical studies.149 Biological therapies targeting G-proteins have been applied to modulate AV-nodal function and control the ventricular response in AF,150 as well as to prevent AF-induction in a vagal model.151 These studies offer a proof of principle for biological therapies targeting specific components of G-protein autonomic effectors, with possible greater specificity and efficacy in the future.
Conclusions
Autonomic nerve activity plays an important role in the initiation and maintenance of AF, and modulating autonomic nerve function may contribute to AF control. Potential therapeutic applications include ganglionated plexus ablation, renal sympathetic denervation, cervical VNS, baroreflex stimulation, cutaneous stimulation, novel drug approaches and biological therapies. While the role of the autonomic nervous system has long been recognized, new science and new technologies promise exciting prospects for the future.
Supplementary Material
Acknowledgments
Financial Support: Research reported in this manuscript was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award number P01HL78931, R01HL71140, R21HL106554, a Piansky Endowment (M.C.F.), a Medtronic-Zipes Endowment (P.-S.C.), the Canadian Institutes of Health Research (6957, 43565, S.N.) and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
We thank Medtronic, St Jude, Boston Scientific and Cyberonics Inc. for donating equipment to our research laboratories.
A list of nonstandard abbreviations
- ACh
Acetylcholine
- AF
atrial fibrillation
- AP
action potential
- APD
action potential duration
- AT
atrial tachycardia
- cAMP
cyclic adenosine monophosphate
- GPCRs
G-protein coupled receptors
- LA
left atrium
- NGF
nerve growth factor
- PAT
paroxysmal atrial tachycardia
- PKA
protein-kinase A
- PV
pulmonary vein
- SERCA2a
sarcoplasmic reticulum Ca-ATP’ase
- SGNA
stellate ganglion nerve activity
- SK2
small conductance calcium activated K channel subtype 2
- SR
sarcoplasmic-reticulum
- TGF
transforming growth-factor
- VNA
vagal nerve activity
- VNS
vagal nerve stimulation
Footnotes
Disclosures: Dr Chen has the following patents relevant to the materials described in this review: U.S. Patents 6,351,668; 6,353,757; 6,398,800; 6,487,450; 6,824,538; 7,266,410
References
- 1.Viskin S, Golovner M, Malov N, Fish R, Alroy I, Vila Y, Laniado S, Kaplinsky E, Roth A. Circadian variation of symptomatic paroxysmal atrial fibrillation. Data from almost 10 000 episodes. European Heart Journal. 1999;20:1429–1434. doi: 10.1053/euhj.1999.1632. [DOI] [PubMed] [Google Scholar]
- 2.Leiria TL, Glavinovic T, Armour JA, Cardinal R, de Lima GG, Kus T. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Autonomic neuroscience: basic & clinical. 2011;161:68–74. doi: 10.1016/j.autneu.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Richer LP, Vinet A, Kus T, Cardinal R, Ardell JL, Armour JA. Alpha-adrenoceptor blockade modifies neurally induced atrial arrhythmias. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;295:R1175–1180. doi: 10.1152/ajpregu.00840.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF, Chen PS. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Zhao Q, Huang H, Tang Y, Xiao J, Dai Z, Yu S, Huang C. Effect of renal sympathetic denervation on atrial substrate remodeling in ambulatory canines with prolonged atrial pacing. PloS one. 2013;8:e64611. doi: 10.1371/journal.pone.0064611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janes RD, Johnstone DE, Brandys JC, Armour JA. Functional and anatomical variability of canine cardiac sympathetic efferent pathways: Implications for regional denervation of the left ventricle. Canadian journal of physiology and pharmacology. 1986;64:958–969. doi: 10.1139/y86-165. [DOI] [PubMed] [Google Scholar]
- 8.Janes RD, Brandys JC, Hopkins DA, Johnstone DE, Murphy DA, Armour JA. Anatomy of human extrinsic cardiac nerves and ganglia. American Journal of Cardiology. 1986;57:299–309. doi: 10.1016/0002-9149(86)90908-2. [DOI] [PubMed] [Google Scholar]
- 9.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Ardell JL. The cardiac neuronal hierarchy and susceptibility to arrhythmias. Heart Rhythm. 2011;8:590–591. doi: 10.1016/j.hrthm.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol (Berl) 2005;209:425–438. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- 12.Ellison JP, Williams TH. Sympathetic nerve pathways to the human heart, and their variations. The American journal of anatomy. 1969;124:149–162. doi: 10.1002/aja.1001240203. [DOI] [PubMed] [Google Scholar]
- 13.Baron R, Janig W, With H. Sympathetic and afferent neurones projecting into forelimb and trunk nerves and the anatomical organization of the thoracic sympathetic outflow of the rat. Journal of the autonomic nervous system. 1995;53:205–214. doi: 10.1016/0165-1838(94)00171-f. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi T, Morimoto M, Taniguchi Y, Takasaki M, Totoki T. Cutaneous distribution of sympathetic postganglionic fibers from stellate ganglion: A retrograde axonal tracing study using wheat germ agglutinin conjugated with horseradish peroxidase. J Anesth. 1994;8:441–449. doi: 10.1007/BF02514624. [DOI] [PubMed] [Google Scholar]
- 15.Page PL, Dandan N, Savard P, Nadeau R, Armour JA, Cardinal R. Regional distribution of atrial electrical changes induced by stimulation of extracardiac and intracardiac neural elements. The Journal of thoracic and cardiovascular surgery. 1995;109:377–388. doi: 10.1016/S0022-5223(95)70400-0. [DOI] [PubMed] [Google Scholar]
- 16.Shen MJ, Hao-Che Chang X, Park HW, George Akingba A, Chang PC, Zheng Zhang X, Lin SF, Shen C, Chen LS, Chen Z, Fishbein MC, Chiamvimonvat N, Chen PS. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10:910–915. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onkka P, Maskoun W, Rhee KS, Hellyer J, Patel J, Tan J, Chen LS, Vinters HV, Fishbein MC, Chen PS. Sympathetic nerve fibers and ganglia in canine cervical vagus nerves: Localization and quantitation. Heart Rhythm. 2013;10:585–591. doi: 10.1016/j.hrthm.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park HW, Shen MJ, Han S, Shinohara T, Maruyama M, Lee YS, Shen C, Hwang C, Chen LS, Fishbein MC, Lin SF, Chen PS. Neural control of ventricular rate in ambulatory dogs with pacing-induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:571–580. doi: 10.1161/CIRCEP.111.967737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foly JO, DuBois FS. Quantitative studies of the vagus nerve in the cat. I. The ratio of sensory and motor fibers. Journal of Comparative Neurology. 1937;67:49–67. [Google Scholar]
- 20.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59:S3–S14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 21.Randall WC, Priola DV, Pace JB. Responses of individucal cardiac chambers to stimulation of the cervical vagosympathetic trunk in atropinized dogs. Circ Res. 1967;20:534–544. doi: 10.1161/01.res.20.5.534. [DOI] [PubMed] [Google Scholar]
- 22.Armour JA, Randall WC. Functional anatomy of canine cardiac nerves. Acta Anat (Basel) 1975;91:510–528. doi: 10.1159/000144411. [DOI] [PubMed] [Google Scholar]
- 23.Armour JA, Hopkins DA. Anatomy of the extrinsic efferent autonomic nerves and ganglia innervating the mammalian heart. In: Randall WC, editor. Nervous control of cardiovascular function. New York: Oxford University Press; 1984. pp. 21–45. [Google Scholar]
- 24.Kawagishi K, Fukushima N, Yokouchi K, Sumitomo N, Kakegawa A, Moriizumi T. Tyrosine hydroxylase-immunoreactive fibers in the human vagus nerve. J Clin Neurosci. 2008;15:1023–1026. doi: 10.1016/j.jocn.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MCLH, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. Journal of the American College of Cardiology. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 26.seki A, Tan J, Chen PS, Fishbein MC. Are there sympathetic nerve fibers in human cervical and thoracic vagus nerves? Circulation. 2013;128:A12365. doi: 10.1016/j.hrthm.2014.04.032. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 28.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: Implications for catheter ablation of atrial-pulmonary vein junction. Journal of the American College of Cardiology. 2006;48:132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Vracko R, Thorning D, Frederickson RG. Nerve fibers in human myocardial scars. Hum Pathol. 1991;22:138–146. doi: 10.1016/0046-8177(91)90035-n. [DOI] [PubMed] [Google Scholar]
- 30.Vracko R, Thorning D, Frederickson RG. Fate of nerve fibers in necrotic, healing, and healed rat myocardium. Lab Invest. 1990;63:490–501. [PubMed] [Google Scholar]
- 31.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circulation Research. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 32.Swissa M, Zhou S, Gonzalez-Gomez I, Chang CM, Lai AC, Cates A, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS. Long-term subthreshold electrical stimulation of the left stellate ganglion and a canine model of sudden cardiac death. Journal of the American College of Cardiology. 2004;43:858–864. doi: 10.1016/j.jacc.2003.07.053. [DOI] [PubMed] [Google Scholar]
- 33.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 34.Miyauchi Y, Zhou S, Miyauchi M, Omichi C, Okuyama Y, Hamabe A, Hayashi H, Mandel WJ, Fishbein MC, Chen LS, Chen PS, Karagueuzian HS. Induction of atrial sympathetic nerve sprouting and increased vulnerability to atrial fibrillation by chronic left ventricular myocardial infarction. Circulation. 2001;104:II-77. [Google Scholar]
- 35.Arora R. Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: Implications for therapies targeting the atrial autonomic nervous system. Circ Arrhythm Electrophysiol. 2012;5:850–859. doi: 10.1161/CIRCEP.112.972273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volders PG. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm. 2010;7:1900–1906. doi: 10.1016/j.hrthm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Patterson E, Jackman WM, Beckman KJ, Lazzara R, Lockwood D, Scherlag BJ, Wu R, Po S. Spontaneous pulmonary vein firing in man: Relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J Cardiovasc Electrophysiol. 2007;18:1067–1075. doi: 10.1111/j.1540-8167.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 38.Scherlag BJ, Patterson E, Po SS. The neural basis of atrial fibrillation. J Electrocardiol. 2006;39:S180–S183. doi: 10.1016/j.jelectrocard.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–1191. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 41.Chang CM, Wu TJ, Zhou SM, Doshi RN, Lee MH, Ohara T, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–25. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen BL, Fishbein MC, Chen LS, Chen PS, Masroor S. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm. 2009;6:454–460. doi: 10.1016/j.hrthm.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng J, Villuendas R, Cokic I, Schliamser JE, Gordon D, Koduri H, Benefield B, Simon J, Murthy SN, Lomasney JW, Wasserstrom JA, Goldberger JJ, Aistrup GL, Arora R. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: Creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–396. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arora R, Ulphani JS, Villuendas R, Ng J, Harvey L, Thordson S, Inderyas F, Lu Y, Gordon D, Denes P, Greene R, Crawford S, Decker R, Morris A, Goldberger J, Kadish AH. Neural substrate for atrial fibrillation: Implications for targeted parasympathetic blockade in the posterior left atrium. Am J Physiol Heart Circ Physiol. 2008;294:H134–H144. doi: 10.1152/ajpheart.00732.2007. [DOI] [PubMed] [Google Scholar]
- 45.Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: Neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol. 2013;305:H1031–1040. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H1838–1846. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajijola OA, Wisco JJ, Lambert HW, Mahajan A, Stark E, Fishbein MC, Shivkumar K. Extracardiac neural remodeling in humans with cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:1010–1116. doi: 10.1161/CIRCEP.112.972836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen BL, Li H, Fishbein MC, Lin SF, Gaudio C, Chen PS, Chen LS. Acute myocardial infarction induces bilateral stellate ganglia neural remodeling in rabbits. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2011 doi: 10.1016/j.carpath.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han S, Kobayashi K, Joung B, Piccirillo G, Maruyama M, Vinters HV, March K, Lin SF, Shen C, Fishbein MC, Chen PS, Chen LS. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol. 2012;59:954–961. doi: 10.1016/j.jacc.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francis GS. Modulation of peripheral sympathetic nerve transmission. J Am Coll Cardiol. 1988;12:250–254. doi: 10.1016/0735-1097(88)90382-8. [DOI] [PubMed] [Google Scholar]
- 51.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oberhauser V, Schwertfeger E, Rutz T, Beyersdorf F, Rump LC. Acetylcholine release in human heart atrium: Influence of muscarinic autoreceptors, diabetes, and age. Circulation. 2001;103:1638–1643. doi: 10.1161/01.cir.103.12.1638. [DOI] [PubMed] [Google Scholar]
- 53.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 54.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance and progression. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 55.Nattel S, Dobrev D. The multidimensional role of calcium in atrial fibrillation pathophysiology: Mechanistic insights and therapeutic opportunities. Eur Heart J. 2012;33:1870–1877. doi: 10.1093/eurheartj/ehs079. [DOI] [PubMed] [Google Scholar]
- 56.Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008;1:93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 57.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishida K, Qi XY, Wakili R, Comtois P, Chartier D, Harada M, Iwasaki YK, Romeo P, Maguy A, Dobrev D, Michael G, Talajic M, Nattel S. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011;123:137–146. doi: 10.1161/CIRCULATIONAHA.110.972778. [DOI] [PubMed] [Google Scholar]
- 59.Zicha S, Tsuji Y, Shiroshita-Takeshita A, Nattel S. Beta-blockers as anti-arrhythmic agents. In: Kass RS, Clancy CC, editors. Handbook of experimental pharmacology – basis and treatment of cardiac arrhythmias. Springer Publishing; Heidelberg, Germany: 2005. pp. 235–266. [Google Scholar]
- 60.Bartos DC, Giudicessi JR, Tester DJ, Ackerman MJ, Ohno S, Horie M, Gollob MH, Burgess DE, Delisle BP. A kcnq1 mutation contributes to the concealed type 1 long qt phenotype by limiting the kv7.1 channel conformational changes associated with protein kinase a phosphorylation. Heart Rhythm. 2014;11:459–468. doi: 10.1016/j.hrthm.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han W, Wang Z, Nattel S. Slow delayed rectifier current and repolarization in canine cardiac purkinje cells. Am J Physiol Heart Circ Physiol. 2001;280:H1075–1080. doi: 10.1152/ajpheart.2001.280.3.H1075. [DOI] [PubMed] [Google Scholar]
- 62.Fink M, Giles WR, Noble D. Contributions of inwardly rectifying k+ currents to repolarization assessed using mathematical models of human ventricular myocytes. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2006;364:1207–1222. doi: 10.1098/rsta.2006.1765. [DOI] [PubMed] [Google Scholar]
- 63.Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-d model of human atrial cells during simulated chronic atrial fibrillation. Biophysical journal. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato R, Koumi S. Modulation of the inwardly rectifying k+ channel in isolated human atrial myocytes by alpha 1-adrenergic stimulation. The Journal of membrane biology. 1995;148:185–191. doi: 10.1007/BF00207274. [DOI] [PubMed] [Google Scholar]
- 65.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.303211. in press. [DOI] [PubMed] [Google Scholar]
- 66.Nattel S. Paroxysmal atrial fibrillation and pulmonary veins: Relationships between clinical forms and automatic versus re-entrant mechanisms. The Canadian journal of cardiology. 2013;29:1147–1149. doi: 10.1016/j.cjca.2013.07.797. [DOI] [PubMed] [Google Scholar]
- 67.Difrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–446. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 68.Lemoine MD, Duverger JE, Naud P, Chartier D, Qi XY, Comtois P, Fabritz L, Kirchhof P, Nattel S. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long qt syndrome model. Cardiovasc Res. 2011;92:67–74. doi: 10.1093/cvr/cvr166. [DOI] [PubMed] [Google Scholar]
- 69.Maruyama M, Lin SF, Xie Y, Chua SK, Joung B, Han S, Shinohara T, Shen MJ, Qu Z, Weiss JN, Chen PS. Genesis of phase 3 early afterdepolarizations and triggered activity in acquired long-qt syndrome. Circ Arrhythm Electrophysiol. 2011;4:103–111. doi: 10.1161/CIRCEP.110.959064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 71.Choi EK, Chang PC, Lee YS, Lin SF, Zhu W, Maruyama M, Fishbein MC, Chen Z, Rubart-von der Lohe M, Field LJ, Chen PS. Triggered firing and atrial fibrillation in transgenic mice with selective atrial fibrosis induced by overexpression of tgf-beta1. Circulation journal: official journal of the Japanese Circulation Society. 2012;76:1354–1362. doi: 10.1253/circj.cj-11-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the ca2+ transient: An arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 73.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–862. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: Role of refractoriness heterogeneity. The American journal of physiology. 1997;273:H805–816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 75.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: A role for angiotensin ii and baroreflexes? Circ Res. 2003;92:1330–1336. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- 76.Jung BC, Dave AS, Tan AY, Gholmieh G, Zhou S, wang DC, Akingba G, Fishbein GA, Montemagno C, Lin SF, Chen LS, Chen PS. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm. 2006;3:78–85. doi: 10.1016/j.hrthm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 77.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 78.Nishida K, Maguy A, Sakabe M, Comtois P, Inoue H, Nattel S. The role of pulmonary veins vs. Autonomic ganglia in different experimental substrates of canine atrial fibrillation. Cardiovasc Res. 2011;89:825–833. doi: 10.1093/cvr/cvq332. [DOI] [PubMed] [Google Scholar]
- 79.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 80.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: Effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 81.Rossi P, Bianchi S, Barretta A, Della Scala A, Kornet L, De Paulis R, Bellisario A, D’Addio V, Pavaci H, Miraldi F. Post-operative atrial fibrillation management by selective epicardial vagal fat pad stimulation. Journal of interventional cardiac electrophysiology: an international journal of arrhythmias and pacing. 2009;24:37–45. doi: 10.1007/s10840-008-9286-2. [DOI] [PubMed] [Google Scholar]
- 82.Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin SF, Chen PS. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–589. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinohara T, Shen MJ, Han S, Maruyama M, Park HW, Fishbein MC, Shen C, Chen PS, Lin SF. Heart failure decreases nerve activity in the right atrial ganglionated plexus. J Cardiovasc Electrophysiol. 2011;23:404–412. doi: 10.1111/j.1540-8167.2011.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hellyer J, Akingba A, Rhee K-S, Tan AY, Lane KA, Shen C, Patel J, Fishbein M, Chen P-S. Autonomic nerve activity and blood pressure in ambulatory dogs. Heart Rhythm. 2013;11:307–313. doi: 10.1016/j.hrthm.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi E-K, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin S-F, Chen P-S. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long qt syndrome. A worldwide report. Circulation. 1991;84:503–511. doi: 10.1161/01.cir.84.2.503. [DOI] [PubMed] [Google Scholar]
- 87.Ogawa M, Tan AY, Song J, Kobayashi K, Fishbein MC, Lin SF, Chen LS, Chen Cryoablation of extrinsic cardiac sympathetic nerves markedly reduces atrial arrhythmias in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2008;5:S54. doi: 10.1016/j.hrthm.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: Report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coleman MA, Bos JM, Johnson JN, Owen HJ, Deschamps C, Moir C, Ackerman MJ. Videoscopic left cardiac sympathetic denervation for patients with recurrent ventricular fibrillation/malignant ventricular arrhythmia syndromes besides congenital long-qt syndrome. Circ Arrhythm Electrophysiol. 2012;5:782–788. doi: 10.1161/CIRCEP.112.971754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schauerte P, Scherlag BJ, Scherlag MA, Goli S, Jackman WM, Lazzara R. Ventricular rate control during atrial fibrillation by cardiac parasympathetic nerve stimulation: A transvenous approach. J Am Coll Cardiol. 1999;34:2043–2050. doi: 10.1016/s0735-1097(99)00471-4. [DOI] [PubMed] [Google Scholar]
- 91.Goldberger AL, Pavelec RS. Vagally-mediated atrial fibrillation in dogs: Conversion with bretylium tosylate. International journal of cardiology. 1986;13:47–55. doi: 10.1016/0167-5273(86)90078-1. [DOI] [PubMed] [Google Scholar]
- 92.Wang Z, Page P, Nattel S. Mechanism of flecainide’s antiarrhythmic action in experimental atrial fibrillation. Circ Res. 1992;71:271–287. doi: 10.1161/01.res.71.2.271. [DOI] [PubMed] [Google Scholar]
- 93.Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, Jackman WM. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000;102:2774–2780. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- 94.Scherlag BJ, Yamanashi WS, Schauerte P, Scherlag M, Sun YX, Hou Y, Jackman WM, Lazzara R. Endovascular stimulation within the left pulmonary artery to induce slowing of heart rate and paroxysmal atrial fibrillation. Cardiovasc Res. 2002;54:470–475. doi: 10.1016/s0008-6363(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 95.Elvan A, Huang XD, Pressler ML, Zipes DP. Radiofrequency catheter ablation of the atria eliminates pacing-induced sustained atrial fibrillation and reduces connexin 43 in dogs. Circulation. 1997;96:1675–1685. doi: 10.1161/01.cir.96.5.1675. [DOI] [PubMed] [Google Scholar]
- 96.Elvan A, Pride HP, Eble JN, Zipes DP. Radiofrequency catheter ablation of the atria reduces inducibility and duration of atrial fibrillation in dogs. Circulation. 1995;91:2235–2244. doi: 10.1161/01.cir.91.8.2235. [DOI] [PubMed] [Google Scholar]
- 97.Schwartz PJ, Pagani M, Lombardi F, Malliani A, Brown AM. A cardiocardiac sympathovagal reflex in the cat. Circ Res. 1973;32:215–220. doi: 10.1161/01.res.32.2.215. [DOI] [PubMed] [Google Scholar]
- 98.Terry R. Vagus nerve stimulation: A proven therapy for treatment of epilepsy strives to improve efficacy and expand applications. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2009;2009:4631–4634. doi: 10.1109/IEMBS.2009.5332676. [DOI] [PubMed] [Google Scholar]
- 99.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circulation Heart failure. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 101.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 102.De Ferrari GM, Sanzo A, Schwartz PJ. Chronic vagal stimulation in patients with congestive heart failure. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2009;2009:2037–2039. doi: 10.1109/IEMBS.2009.5334414. [DOI] [PubMed] [Google Scholar]
- 103.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 104.De Ferrari GM, Schwartz PJ. Vagus nerve stimulation: From pre-clinical to clinical application: Challenges and future directions. Heart Fail Rev. 2011;16:195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 105.Li S, Scherlag BJ, Yu L, Sheng X, Zhang Y, Ali R, Dong Y, Ghias M, Po SS. Low-level vagosympathetic stimulation: A paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:645–651. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 106.Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, Zhang Y, Jackman WM, Lazzara R, Jiang H, Po SS. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: Direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2010;22:455–463. doi: 10.1111/j.1540-8167.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- 107.Vanhoutte PM, Levy MN. Prejunctional cholinergic modulation of adrenergic neurotransmission in the cardiovascular system. The American journal of physiology. 1980;238:H275–281. doi: 10.1152/ajpheart.1980.238.3.H275. [DOI] [PubMed] [Google Scholar]
- 108.Takahashi N, Zipes DP. Vagal modulation of adrenergic effects on canine sinus and atrioventricular nodes. Am J Physiol. 1983;244:H775–H781. doi: 10.1152/ajpheart.1983.244.6.H775. [DOI] [PubMed] [Google Scholar]
- 109.Stanley J, Peake JM, Buchheit M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sports medicine. 2013;43:1259–1277. doi: 10.1007/s40279-013-0083-4. [DOI] [PubMed] [Google Scholar]
- 110.Guasch E, Benito B, Qi X, Cifelli C, Naud P, Shi Y, Mighiu A, Tardif JC, Tadevosyan A, Chen Y, Gillis MA, Iwasaki YK, Dobrev D, Mont L, Heximer S, Nattel S. Atrial fibrillation promotion by endurance exercise: Demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 111.Sorokin AV, Araujo CG, Zweibel S, Thompson PD. Atrial fibrillation in endurance-trained athletes. British journal of sports medicine. 2011;45:185–188. doi: 10.1136/bjsm.2009.057885. [DOI] [PubMed] [Google Scholar]
- 112.Reed JL, Mark AE, Reid RD, Pipe AL. The effects of chronic exercise training in individuals with permanent atrial fibrillation: A systematic review. The Canadian journal of cardiology. 2013;29:1721–1728. doi: 10.1016/j.cjca.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 113.Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T, de Leeuw PW. Novel baroreflex activation therapy in resistant hypertension: Results of a european multi-center feasibility study. J Am Coll Cardiol. 2010;56:1254–1258. doi: 10.1016/j.jacc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 114.Tordoir JH, Scheffers I, Schmidli J, Savolainen H, Liebeskind U, Hansky B, Herold U, Irwin E, Kroon AA, de Leeuw P, Peters TK, Kieval R, Cody R. An implantable carotid sinus baroreflex activating system: Surgical technique and short-term outcome from a multi-center feasibility trial for the treatment of resistant hypertension. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2007;33:414–421. doi: 10.1016/j.ejvs.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 115.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier AM, Luft FC, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 116.Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, Bohm M. Effects of electrical stimulation of carotid baroreflex and renal denervation on atrial electrophysiology. J Cardiovasc Electrophysiol. 2013;24:1028–1033. doi: 10.1111/jce.12171. [DOI] [PubMed] [Google Scholar]
- 117.Linz D, Ukena C, Mahfoud F, Neuberger HR, Bohm M. Atrial autonomic innervation: A target for interventional antiarrhythmic therapy? J Am Coll Cardiol. 2014;63:215–224. doi: 10.1016/j.jacc.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 118.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 119.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–1189. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 120.He B, Scherlag BJ, Nakagawa H, Lazzara R, Po SS. The intrinsic autonomic nervous system in atrial fibrillation: A review. ISRN cardiology. 2012;2012:490674. doi: 10.5402/2012/490674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y, Wang Z, Zhang Y, Wang W, Wang J, Gao M, Hou Y. Efficacy of cardiac autonomic denervation for atrial fibrillation: A meta-analysis. J Cardiovasc Electrophysiol. 2012;23:592–600. doi: 10.1111/j.1540-8167.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- 122.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: A randomized clinical trial. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 123.Zheng S, Li Y, Han J, Zhang H, Zeng W, Xu C, Jia Y, Wang J, Guo K, Jiao Y, Meng X. Long-term results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. PloS one. 2013;8:e79755. doi: 10.1371/journal.pone.0079755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA for the Symplicity HTNI. Renal sympathetic denervation for treatment of drug-resistant hypertension: One-year results from the symplicity htn-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 125.Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 126.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 127.Ahmed H, Miller MA, Dukkipati SR, Cammack S, Koruth JS, Gangireddy S, Ellsworth BA, D’Avila A, Domanski M, Gelijns AC, Moskowitz A, Reddy VY. Adjunctive renal sympathetic denervation to modify hypertension as upstream therapy in the treatment of atrial fibrillation (h-fib) study: Clinical background and study design. J Cardiovasc Electrophysiol. 2013;24:503–509. doi: 10.1111/jce.12095. [DOI] [PubMed] [Google Scholar]
- 128.Hogarth AJ, Dobson LE, Tayebjee MH. During ablation for atrial fibrillation, is simultaneous renal artery ablation appropriate? Journal of human hypertension. 2013 doi: 10.1038/jhh.2013.75. [DOI] [PubMed] [Google Scholar]
- 129.Linz D, Hohl M, Nickel A, Mahfoud F, Wagner M, Ewen S, Schotten U, Maack C, Wirth K, Bohm M. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767–774. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 130.Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, Inoue H. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart and vessels. 2002;16:51–56. doi: 10.1007/s380-002-8317-8. [DOI] [PubMed] [Google Scholar]
- 131.Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: Rationale and design of the symplicity htn-3 trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Medtronic I. Medtronic announces uS Renal denervation pivotal trial fails to meet primary efficacy endpoint while meeting primary safety endpoint. 2014;2014 [Google Scholar]
- 133.Floras JS. Renal denervation for drug resistant hypertension: Suffering its original sin, seeking redemption. Canadian Journal of Cardiology. 2014 doi: 10.1016/j.cjca.2014.02.008. in press. [DOI] [PubMed] [Google Scholar]
- 134.Kimura A, Sato A. Somatic regulation of autonomic functions in anesthetized animals--neural mechanisms of physical therapy including acupuncture. The Japanese journal of veterinary research. 1997;45:137–145. [PubMed] [Google Scholar]
- 135.Yu L, Scherlag BJ, Li S, Fan Y, Dyer J, Male S, Varma V, Sha Y, Stavrakis S, Po SS. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: A noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10:428–435. doi: 10.1016/j.hrthm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 136.White AR, Ernst E. A systematic review of randomized controlled trials of acupuncture for neck pain. Rheumatology. 1999;38:143–147. doi: 10.1093/rheumatology/38.2.143. [DOI] [PubMed] [Google Scholar]
- 137.Ezzo Mendelson G, et al. Is acupuncture effective for the treatment of chronic pain? A systematic review. pain. 2000;86:217–225. doi: 10.1016/S0304-3959(99)00304-8. [DOI] [PubMed] [Google Scholar]; Pain. 2001;93:198–200. [Google Scholar]
- 138.Lomuscio A, Belletti S, Battezzati PM, Lombardi F. Efficacy of acupuncture in preventing atrial fibrillation recurrences after electrical cardioversion. J Cardiovasc Electrophysiol. 2011;22:241–247. doi: 10.1111/j.1540-8167.2010.01878.x. [DOI] [PubMed] [Google Scholar]
- 139.Himura Y, Felten SY, Kashiki M, Lewandowski TJ, Delehanty JM, Liang CS. Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation. 1993;88:1299–1309. doi: 10.1161/01.cir.88.3.1299. [DOI] [PubMed] [Google Scholar]
- 140.Dong E, Yatani A, Mohan A, Liang CS. Myocardial beta-adrenoceptor down-regulation by norepinephrine is linked to reduced norepinephrine uptake activity. European journal of pharmacology. 1999;384:17–24. doi: 10.1016/s0014-2999(99)00652-4. [DOI] [PubMed] [Google Scholar]
- 141.Kawai H, Fan TH, Dong E, Siddiqui RA, Yatani A, Stevens SY, Liang CS. Ace inhibition improves cardiac ne uptake and attenuates sympathetic nerve terminal abnormalities in heart failure. Am J Physiol. 1999;277:H1609–H1617. doi: 10.1152/ajpheart.1999.277.4.H1609. [DOI] [PubMed] [Google Scholar]
- 142.Cha YM, Redfield MM, Shah S, Shen WK, Fishbein MC, Chen PS. Effects of omapatrilat on cardiac nerve sprouting and structural remodeling in experimental congestive heart failure. Heart Rhythm. 2005;2:984–990. doi: 10.1016/j.hrthm.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 143.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: A critical review. Autonomic neuroscience: basic & clinical. 2013 doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 144.Rossi P, Ricci A, De Paulis R, Papi E, Pavaci H, Porcelli D, Monari G, Maselli D, Bellisario A, Turani F, Nardella S, Azzolini P, Piccirillo G, Quaglione R, Valsecchi S, Bianchi S. Epicardial ganglionated plexus stimulation decreases postoperative inflammatory response in humans. Heart Rhythm. 2012;9:943–950. doi: 10.1016/j.hrthm.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 145.Kusunose K, Zhang Y, Mazgalev TN, Van Wagoner DR, Thomas JD, Popovic ZB. Impact of vagal nerve stimulation on left atrial structure and function in a canine high-rate pacing model. Circulation. Heart failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.113.000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nergardh AK, Rosenqvist M, Nordlander R, Frick M. Maintenance of sinus rhythm with metoprolol cr initiated before cardioversion and repeated cardioversion of atrial fibrillation: A randomized double-blind placebo-controlled study. Eur Heart J. 2007;28:1351–1357. doi: 10.1093/eurheartj/ehl544. [DOI] [PubMed] [Google Scholar]
- 147.Nattel S. Ionic determinants of atrial fibrillation and ca2+ channel abnormalities: Cause, consequence, or innocent bystander? Circ Res. 1999;85:473–476. doi: 10.1161/01.res.85.5.473. [DOI] [PubMed] [Google Scholar]
- 148.Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, Whitlock RP. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. The Cochrane database of systematic reviews. 2013;1:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Machida T, Hashimoto N, Kuwahara I, Ogino Y, Matsuura J, Yamamoto W, Itano Y, Zamma A, Matsumoto R, Kamon J, Kobayashi T, Ishiwata N, Yamashita T, Ogura T, Nakaya H. Effects of a highly selective acetylcholine-activated k+ channel blocker on experimental atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:94–102. doi: 10.1161/CIRCEP.110.951608. [DOI] [PubMed] [Google Scholar]
- 150.Bauer A, McDonald AD, Nasir K, Peller L, Rade JJ, Miller JM, Heldman AW, Donahue JK. Inhibitory g protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation. 2004;110:3115–3120. doi: 10.1161/01.CIR.0000147185.31974.BE. [DOI] [PubMed] [Google Scholar]
- 151.Aistrup GL, Cokic I, Ng J, Gordon D, Koduri H, Browne S, Arapi D, Segon Y, Goldstein J, Angulo A, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted nonviral gene-based inhibition of galpha(i/o)-mediated vagal signaling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8:1722–1729. doi: 10.1016/j.hrthm.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.