Abstract
Listeria monocytogenes is a ubiquitous food-borne pathogen found widely distributed in nature as well as an undesirable contaminant in a variety of fresh and processed foods. This ubiquity can be at least partly explained by the ability of the organism to grow at high osmolarity and reduced temperatures, a consequence of its ability to accumulate osmo- and cryoprotective compounds termed osmolytes. Single and multiple deletions of the known osmolyte transporters BetL, Gbu, and OpuC significantly reduce growth at low temperatures. During growth in brain heart infusion broth at 7°C, Gbu and OpuC had a more pronounced role in cryoprotection than did BetL. However, upon the addition of betaine to defined medium, the hierarchy of transporter importance shifted to Gbu > BetL > OpuC. Upon the addition of carnitine, only OpuC appeared to play a role in cryoprotection. Measurements of the accumulated osmolytes showed that betaine is preferred over carnitine, while in the absence of a functional Gbu, carnitine was accumulated to higher levels than betaine was at 7°C. Transcriptional analysis of the genes encoding BetL, Gbu, and OpuC revealed that each transporter is induced to different degrees upon cold shock of L. monocytogenes LO28. Additionally, despite being transcriptionally up-regulated upon cold shock, a putative fourth osmolyte transporter, OpuB (identified by bioinformatic analysis and encoded by lmo1421 and lmo1422), showed no significant contribution to listerial chill tolerance. Growth of the quadruple mutant LO28ΔBCGB (ΔbetL ΔopuC Δgbu ΔopuB) was comparable to the that of the triple mutant LO28ΔBCGsoe (ΔbetL ΔopuC Δgbu) at low temperatures. Here, we conclude that betaine and carnitine transport upon low-temperature exposure is mediated via three osmolyte transporters, BetL, Gbu, and OpuC.
The gram-positive food-borne pathogen Listeria monocytogenes accounts for almost 35% of all deaths in the United States due to known food-borne bacterial pathogens (23). A number of recent outbreaks have been associated with ready-to-eat foods that have been minimally processed (9). As the demand for fresh food products is increasing, cold storage of these products is becoming more widespread. This continuing trend toward minimal food processing and reliance on refrigeration as a preservation technique has in turn been accompanied by a steady increase in the incidence of food poisoning, particularly by psychrotrophic pathogens such as L. monocytogenes, which can grow at temperatures as low as −0.1°C (36).
Mechanisms that allow low-temperature growth of microorganisms involve the maintenance of the cellular membrane fluidity, structure stabilization of macromolecules such as ribosomes which are necessary for continued protein synthesis, and the uptake or synthesis of compatible solutes (17). For L. monocytogenes, the adaptation of membranes to low temperatures is accomplished by altering branching in the methyl end of the fatty acid from iso to anteiso and by shortening of the fatty acid chain length, resulting mainly in an increase of anteiso-C15:0 fatty acids (5). In L. monocytogenes, instability in the 70S ribosomal particle structure is found upon cold shock (6), which must be overcome to allow normal protein synthesis. In Escherichia coli, protein synthesis after cold shock is related to the synthesis of so-called cold shock proteins (CSPs) (26). In L. monocytogenes LO28, four cold shock proteins have been identified, two of which are produced in increased amounts following a cold shock from 30 to 10°C (37).
With the exception of proline (31), L. monocytogenes appears unable to synthesize osmolytes (22) either de novo or from precursor compounds (33). However, the transport of the principal osmolytes glycine betaine (N,N,N-trimethylglycine) and carnitine (β-hydroxy-γ-N-trimethyl aminobutyrate) was found to be increased 15- and 4.5-fold, respectively, upon low-temperature stress (3, 21).
To date, three osmolyte transporters, Gbu, OpuC, and BetL (3, 10, 14, 15, 20, 24, 30, 34; for a review, see reference 32), dedicated to betaine and carnitine uptake in L. monocytogenes, have been identified and characterized experimentally. However, recent in silico analysis of the L. monocytogenes EGD-e genome sequence (16) revealed a fourth putative osmolyte transporter with significant homology to the high-affinity choline uptake system OpuB of Bacillus subtilis. Consisting of two genes, lmo1421 and lmo1422, this operon is located approximately 2.4 kb downstream of opuC on the listerial chromosome (32). While a possible role for OpuB as a carnitine uptake system in Listeria has previously been suggested (11, 38), Angelidis and Smith (2) recently demonstrated that, at least in L. monocytogenes 10403S, carnitine uptake is mediated exclusively by OpuC and Gbu. However, the existence or potential impact of OpuB was not examined in this strain.
The principal betaine uptake system Gbu is a binding protein-dependent ATP-binding cassette (ABC) transporter homologous to OpuA in B. subtilis (20). The growth rate of L. monocytogenes 10403S in the absence of a functional Gbu transporter was significantly lower than that of the wild type at 7°C, with uptake rates for [14C]glycine betaine being reduced approximately eightfold in this mutant (20). Moreover, in vitro activation of the Gbu transport activity was demonstrated to occur in membrane vesicles at reduced temperatures (14). OpuC, the principal carnitine transporter, encoded by the opuCABCD operon (10, 34), is homologous to opuC and opuB in B. subtilis and is also an ABC transporter, coupling ATP hydrolysis to osmolyte transport across the membrane. An interesting feature of opuC is that it is preceded by a consensus σB-dependent promoter binding site (10, 12), which may also imply chill-stimulated osmolyte uptake since transcription of σB has itself been shown to be up-regulated in response to a temperature downshift (7). Indeed, a σB deletion mutant of L. monocytogenes 10403S exhibited reduced growth rates at 8°C in defined medium (DM) supplemented with either betaine or carnitine (0.011 and 0.010 h−1, respectively) compared with wild-type 10403S (0.018 and 0.017 h−1, respectively) (8). Finally, BetL, a secondary betaine uptake system that couples a Na+ motive force to solute transport across the membrane, is homologous to OpuD of B. subtilis and BetP of Corynebacterium glutamicum (30). The reduced growth rate for the 10403S σB deletion mutant at 8°C upon addition of betaine (8) might reflect the presence of a putative σB-dependent promoter binding site upstream of betL (33). However, the growth rate of a strain in which betL is functionally inactivated was not affected at 4°C (33); in addition, in vitro betaine transport via BetL in proteoliposomes does not appear to be activated by cold (15).
Previously, a set of mutants carrying deletions in the known osmolyte uptake systems Gbu, BetL, and OpuC was constructed and the role of these systems in listerial growth and survival at elevated osmolarity was determined (33, 34, 38). Here, we employ a similar strategy to analyze the role of osmolyte uptake in growth at refrigeration temperature (7°C). Recently, Angelidis et al. (4) characterized a Gbu mutant at low temperatures and predicted that additionally removing BetL may further impair growth, while deleting opuC from this background would possibly eliminate growth altogether under cold stress. Herein, we demonstrate that while the proposed triple mutant LO28ΔBCGsoe (ΔbetL ΔopuC Δgbu) is indeed severely affected at low temperatures (illustrating the importance of these systems to listerial chill tolerance), growth is not completely abolished, thus suggesting the existence of at least one additional cryoprotective system. However, removing OpuB, a putative fourth osmolyte uptake system, from the existing triple mutant background showed no further reduction in growth at low temperatures, thus ruling out a potential cryoprotective role for OpuB in Listeria.
MATERIALS AND METHODS
Strains, chemicals, and growth conditions.
Bacterial strains used in this study are listed in Table 1. Strains were grown either in brain heart infusion (BHI) broth (Oxoid, Basingstoke, United Kingdom) or in DM (27). Where indicated, carnitine and glycine betaine (Sigma Chemical Co., St. Louis, Mo.) were added to DM as filter-sterilized solutions to a final concentration of 1 mM. For growth of LO28ΔC (ΔopuC), LO28ΔCG (ΔopuC Δgbu), and LO28ΔBCG (ΔbetL ΔopuC Δgbu), erythromycin (10 μg/ml) was used as a selection marker. Cell growth was monitored spectrophotometrically by measuring the optical density at 620 nm (OD620), and growth rates were calculated by plotting the natural logarithm of the OD620 versus time (standard deviations varied within 10% of the values; experiments were performed in triplicate).
TABLE 1.
L. monocytogenes strains used in this study
| Strain | Relevant genotype or characteristic | Source or reference |
|---|---|---|
| LO28 (serotype 1/2c) | betL+gbu+opuC+opuB+ | P. Cossarta |
| LO28ΔB (formerly BSOE) | LO28 ΔbetL | 30 |
| LO28ΔC (formerly LO28C) | LO28 ΔopuC opuC::pCPL5 | 34 |
| LO28ΔG (formerly GSOE) | LO28 Δgbu | 38 |
| LO28ΔBG (formerly BGSOE) | LO28 ΔbetL Δgbu | 38 |
| LO28ΔCG | LO28 ΔopuC Δgbu | 38 |
| LO28ΔBCG | LO28 ΔbetL ΔopuC Δgbu opuC::pCPL5 | 38 |
| LO28ΔBCGsoe | LO28 ΔbetL ΔopuC Δgbu | This study |
| LO28ΔBCGB | LO28 ΔbetL ΔopuC Δgbu ΔopuB | Sleator et al.b |
Institute Pasteur, Paris, France.
R. D. Sleator, University College Cork, Cork, Ireland, unpublished data.
Creation of the triple SOEing mutant LO28BCGsoe.
Creation of a SOEing (splicing by overlap extension) mutant was essentially performed as described by Horton et al. (18). For the creation of the triple SOEing mutant LO28BCGsoe, the SOE (oSOE-A, oSOE-B, oSOE-C, and oSOE-D) and forward and reverse (oSOE-F and oSOE-R) primers used were of the same sequence as those used by Angelidis and Smith (2). The hybrid 882-bp opuC construct (comprised of two regions in the 5′ and 3′ end of opuC) was digested with HindIII and BamHI, cloned into pKSV7, and transformed into E. coli DH5α to yield pCPL18. This construct was then electroporated into L. monocytogenes LO28ΔBG, and successful transformants were selected on BHI plates containing 10 μg of chloramphenicol/ml. Selection at 42°C of cells with chromosomal integration of pCPL18 followed by sequential passaging in BHI broth at 30°C in the absence of chloramphenicol facilitated the recovery of cells in which allelic exchange between the intact opuC operon and the 882-bp insert on pCPL18 had occurred, thus giving rise to LO28BCGsoe. Confirmation of the deletion event was obtained by PCR with the forward and reverse primers oSOE-F and oSOE-R.
Transcriptional analysis of the osmolyte transporters.
Reverse transcription (RT)-PCR experiments were performed essentially as described by Sleator et al. (33). L. monocytogenes cells were grown to mid-exponential phase at 37°C. Cells were then centrifuged and resuspended in 1 ml of BHI at 10 or 37°C (control temperature). After 15 and 30 min of incubation, cells were harvested and flash frozen at −80°C. RNA was extracted by using a hot acid phenol procedure as described by Ripio et al. (29), and cDNA was synthesized by adding 1 μl of total RNA to 4 μl of 5× RT buffer (Roche), 2 μl of 100 mM dithiothreitol, 0.5 μl of a deoxynucleoside triphosphate mix (10 mM [each] dATP, dCTP, dGTP, and dTTP), 0.25 μl of RNasin, 100 ng of the random primer p(dN)6, and 1 μl of Expand reverse transcriptase (Roche). The reaction mixture was incubated at 42°C for 9 h. PCR on cDNA was carried out by using the following primers: for gbu, gbu SOEA and gbu SOEB (38), targeting gbuB; for betL, betL SOEC and betL SOED (36); for opuC, opuC-F (5′-CACCAAAAGTAGCGAAC-3′) and opuC-R (5′-GAATTAAGTCTGGACGGTATAAG-3′), targeting opuCB; for opuB, opuB-F (5′-ATCTAGAAAATGACTTCGTTAAA-3′) and opuB-R (5′-ACAAGCTCACCTGAACTTTCC-3′), targeting lmo1421; and for 16S RNA, 16SRNA-E (5′-TTAGCTAGTTGGTAGGGT-3′) and 16SRNA-B (5′-AATCCGGACAACGCTTGC-3′). PCRs were carried out for 16, 22, and 30 cycles to allow optimal quantification of PCR products.
Uptake studies.
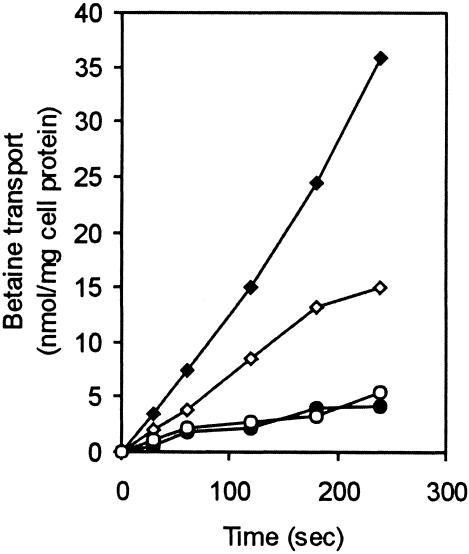
The L. monocytogenes LO28 wild type was grown in DM at 30°C until early exponential phase (OD620 of ∼0.25). Where indicated, a cold shock to 10°C was performed as previously described (37). Osmolyte uptake studies were carried out essentially as described by Verheul et al. (35). Cells were concentrated to an OD620 of 20 in 50 mM potassium phosphate buffer (pH 6.8) containing 5 mM MgSO4 and chloramphenicol (100 μg/ml) to inhibit protein synthesis. Cells (OD620 of 1) were preenergized at 37°C with 1% glucose for 5 min prior to the addition of radiolabeled osmolyte N,N,N-[1-14C]trimethylglycine (final concentration of 19 μM) purchased from Campo Scientific (Veenendaal, The Netherlands). Samples were withdrawn after 0.5, 1, 2, 3, and 4 min, and uptake was stopped by the addition of 2 ml of cold 50 mM potassium phosphate buffer (pH 6.8) containing 5 mM MgSO4. After the reaction was stopped, the cells were immediately collected on 0.2-μm-pore-size cellulose nitrate filters (Schleicher and Schuell GmbH, Dassell, Germany) under vacuum, the filters were washed with 2 ml of buffer, and the radioactivity trapped in the cells was measured with a scintillation counter (model 1600TR; Packard Instruments Co., Downers Grove, Ill.). Uptake of osmolytes by the cells was normalized to total cellular protein. The experiment was performed in duplicate, and the results of a typical experiment are displayed in Fig. 4.
FIG. 4.
Glycine betaine transport in wild-type strain LO28 at 37°C for exponential-phase cells (⧫) and cells cold shocked for 4 h (◊) and betaine transport at 10°C for exponential-phase cells (•) and cells cold shocked for 4 h (○) are shown.
Accumulation studies.
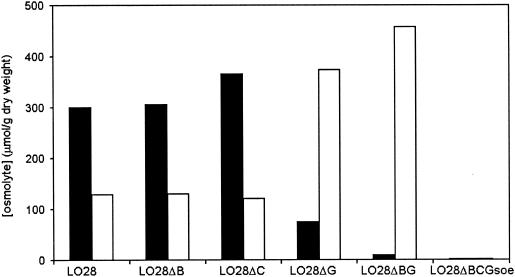
Cells were grown in BHI at 30°C or at 7°C until mid-exponential growth phase (OD620 of ∼0.4). One hundred milliliters of culture was pelleted and washed twice in potassium phosphate buffer (50 mM with 5 mM MgSO4, pH 6.8, 7°C) before being resuspended in 750 μl of water and freeze dried for determination of dry weight. The cells were extracted by the procedure described by Galinski and Herzog (13) using methanol and chloroform. Betaine and carnitine concentrations were determined as described by Verheul et al. (35). Betaine and carnitine were measured by refractive index after high-performance liquid chromatography by using a LiChrosphere 100-NH2 5-μm column (Merck, Darmstadt, Germany) at a flow rate of 1 ml/min at 45°C with a mobile phase of 80:20 (vol/vol) acetonitrile-20 mM potassium phosphate (pH 7.0). The concentrations of betaine and carnitine were calculated from the area under the respective peaks by using calibration curves. The experiment was performed in duplicate, and the results of a typical experiment are displayed in Fig. 5.
FIG. 5.
Internal concentration of betaine (black bars) and carnitine (white bars) in L. monocytogenes LO28 and mutants grown at 7°C in BHI.
RESULTS
Deletion of osmolyte transporters reduces growth at low temperatures.
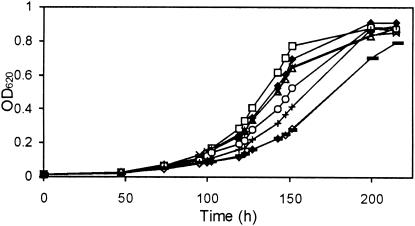
To identify the role of each osmolyte transporters in growth at low temperatures, we monitored the growth of various osmolyte uptake mutants in BHI at 7°C (Fig. 1). While growth of the wild-type and mutant strains in BHI at 30°C was similar (data not shown), the effect of deleting the various osmolyte transporters at 7°C was clearly observed. The growth rate of the wild type in BHI at 7°C was 0.028 h−1 (Table 2), and deleting gbu or opuC decreased growth rates to 0.026 h−1, whereas removing betL had no significant effect. Deleting all three transporters in combination had the most dramatic effect, resulting in a reduction in the growth rate of 0.028 to 0.018 h−1. Notably, growth of the quadruple mutant LO28ΔBCGB was similar to that of the triple mutant, suggesting that, at least under the conditions tested, OpuB is unlikely to contribute to listerial chill tolerance.
FIG. 1.
Growth of LO28 and osmolyte uptake mutants in BHI at 7°C. LO28 (♦), LO28ΔB (ΔbetL) (□), LO28ΔC (ΔopuC) (▵), LO28ΔG (Δgbu) (×), LO28ΔBG (ΔbetL Δgbu) (○), LO28ΔCG (ΔopuC Δgbu) (+), LO28ΔBCGsoe (ΔbetL ΔopuC Δgbu) (⋄), and LO28ΔBCGB (ΔbetL ΔopuC Δgbu ΔopuB) () are shown.
TABLE 2.
Growth rate of L. monocytogenes LO28 and osmolyte uptake mutants at 7°C in BHI
| Strain | Growth rate (h−1) |
|---|---|
| LO28 | 0.028 |
| LO28ΔB | 0.028 |
| LO28ΔC | 0.026 |
| LO28ΔG | 0.026 |
| LO28ΔBG | 0.024 |
| LO28ΔCG | 0.024 |
| LO28ΔBCG | 0.019 |
| LO28ΔBCGsoe | 0.018 |
| LO28ΔBCGB | 0.018 |
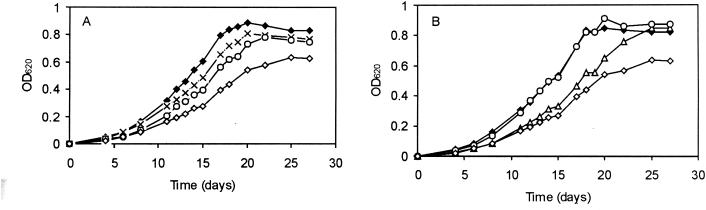
To study the individual contribution of betaine and carnitine to low-temperature adaptation, DM was used. Growth did not significantly differ at 7°C in DM. While the addition of 1 mM betaine resulted in a significant increase of growth of the wild type, the increase in growth of the mutants upon the addition of betaine was lower than that of the wild type (Fig. 2A). Given that growth was most affected following the deletion of gbu, it appears that the Gbu system is the most effective transporter during low-temperature growth. Interestingly, it has previously been demonstrated that for growth at elevated osmolarities (3% NaCl), Gbu also plays the dominant role (38). Upon the addition of carnitine to DM, the role of OpuC in the uptake of carnitine for cryoprotective purposes is apparent, as only those mutants deficient in their OpuC transporter show reduced growth compared to that of the wild type (Fig. 2B), indicating the importance of OpuC in carnitine uptake at low temperatures. Furthermore, the deletion of the two principal betaine uptake systems Gbu and BetL (strain LO28ΔBG) does not influence the growth characteristics at 7°C compared with those of the wild type upon the addition of carnitine to DM (Fig. 2B). In addition, growth of the triple mutant LO28ΔBCGsoe and the quadruple mutant LO28ΔBCGB was not induced upon the addition of betaine or carnitine to the medium (data not shown), supporting our proposal that OpuB is unlikely to play a role in betaine or carnitine uptake and subsequent cryoprotection.
FIG. 2.
Growth of LO28 and osmolyte uptake mutants. (A) Growth of LO28 (♦), LO28ΔG (Δgbu) (×), LO28ΔBG (ΔbetL Δgbu) (○), and LO28ΔBCGsoe (ΔbetL ΔopuC Δgbu) (⋄) in DM at 7°C with the addition of betaine. (B) Growth of LO28 (⋄), LO28ΔC (ΔopuC) (▵), LO28ΔBG (ΔbetL Δgbu) (○), and LO28ΔBCGsoe (ΔbetL ΔopuC Δgbu) (⋄) in DM at 7°C with the addition of carnitine.
Induced transcription of osmolyte transporter genes at low temperatures.
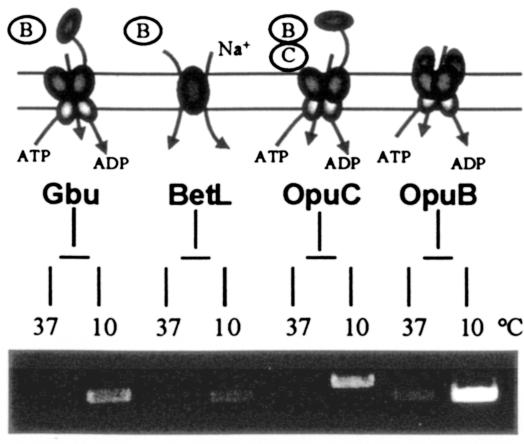
RT-PCR was performed to analyze the transcription of the different transporter genes following cold shock. Though expression levels of the genes at 37°C were at the detection limit, both gbu and betL, encoding the principal betaine uptake systems, were significantly up-regulated following exposure to 10°C for 30 min (Fig. 3). However, the level of gbu up-regulation appears greater than that of betL following cold shock. This result is consistent with the observation that Gbu provides significantly more cryoprotection than does BetL. The carnitine transporter-encoding genes were also clearly induced following cold shock. In addition, transcription of the operon encoding OpuB, a putative fourth osmolyte uptake system, was also significantly induced upon low-temperature exposure of L. monocytogenes LO28 cells.
FIG. 3.
(Top) Schematic of the transcription of the four osmolyte transporters in L. monocytogenes after cold shock. B, betaine; C, carnitine. (Bottom) RNA was isolated from mid-exponential-phase cells prior to cold shock (lanes labeled 37) and from cells exposed to 10°C for 30 min (lanes labeled 10). The results after 30 cycles are shown for gbu (Gbu), betL (BetL), opuC (OpuC), and opuB (OpuB).
Betaine and carnitine accumulation at low temperatures.
Increased transcription of the transporters upon cold shock (Fig. 3) suggests an increased uptake of osmolytes at low temperatures. However, when L. monocytogenes LO28 wild-type cells, cold shocked to 10°C, were tested for betaine uptake after 4 h at 10°C, no increase in betaine uptake at low temperatures was observed within the time frame measured (Fig. 4). Furthermore, the uptake rate at 37°C for cold-shocked cells was lower than that for exponential-phase cells grown at 37°C. Notably, the intracellular concentrations of betaine and carnitine during growth of wild-type LO28 in BHI at 7°C were 300 and 120 μmol/g (dry weight) of cells, respectively (Fig. 5), whereas during growth at 30°C, the betaine concentration was 70 μmol/g (dry weight) of cells, while carnitine was not accumulated to detectable levels (38). This indicates that L. monocytogenes LO28 cells do accumulate betaine and carnitine at low temperatures. For the single mutants LO28ΔB and LO28ΔC, accumulation levels similar to that for the wild type were found (Fig. 5). However, in LO28ΔG, the betaine concentration is much lower (∼70 μmol/g [dry weight] of cells), which is apparently compensated for by the accumulation of carnitine. In strain LO28ΔBG (ΔbetL Δgbu), OpuC is the remaining transporter responsible for the accumulated carnitine (about 450 μmol/g [dry weight] of cells). Analysis of the triple mutant LO28ΔBCGsoe following growth at 7°C in BHI revealed no accumulated betaine or carnitine (with a detection limit of about 20 μmol/g [dry weight] of cells). Thus, at least under the conditions tested, BetL, Gbu, and OpuC appear to be the only betaine and carnitine uptake systems effective during low-temperature growth in L. monocytogenes.
DISCUSSION
L. monocytogenes is a food-borne pathogen capable of growth at low temperatures. Given the increasing reliance on refrigeration as a method of food preservation (1), the psychrotropic nature of this pathogen is an especially important consideration from a food safety perspective. A major factor contributing to low-temperature growth of L. monocytogenes is the ability of the organism to accumulate compatible solutes such as betaine and carnitine. Using a set of mutants with deletions in the known osmolyte transporters BetL, Gbu, and OpuC, we were able to determine the individual contribution of betaine and carnitine and their transporters to the low-temperature growth of L. monocytogenes.
In wild-type LO28, growth at low temperatures is clearly stimulated by the addition of betaine and/or carnitine to DM, in which betaine is more successful than carnitine. This result suggests that betaine functions as a more effective cryoprotectant than carnitine, an observation which also appears to extend to their respective osmoprotective effects (38), a phenomenon which Peddie et al. (25) attributed to differences in hydrocarbon chain length. Upon the addition of betaine to DM at 7°C, the hierarchy of transporter importance is Gbu > BetL > OpuC. However, when betaine is replaced by carnitine, the role of OpuC at low temperatures becomes apparent in that only those mutants deficient in their OpuC transporter are reduced in their growth compared to that of the wild type. This is consistent with the findings of Becker et al. (8), who showed that the growth of L. monocytogenes after a cold shock from 37 to 8°C is stimulated upon addition of carnitine to DM, thus proving a role for carnitine in low-temperature growth. Moreover, it was found that the uptake of carnitine via OpuC seems to be regulated by the alternative sigma factor σB, a stress-related sigma factor, which is itself induced at low temperatures (7, 12).
The increased transcription of the transporters upon cold shock (Fig. 3) suggests an increased uptake of osmolytes at low temperatures. However, L. monocytogenes LO28 wild-type cells, cold shocked to 10°C for 4 h, did not show an increase in betaine uptake at low temperatures within the time frame measured (Fig. 4). Furthermore, the uptake rate at 37°C for cold-shocked cells was lower than that for exponential-phase cells grown at 37°C. In contrast, Ko et al. (21) and Mendum and Smith (24) showed that L. monocytogenes cells (Scott A and 10403S, respectively) grown at low temperatures have an increased betaine uptake at this temperature compared to cells grown and tested at 30°C. In addition, cold-shocked 10403S cells tested by Becker et al. (8) were found to have comparable betaine uptake levels at 8 and 25°C. These apparent differences might be explained by different experimental setups and strain differences.
In the wild-type strain, it is mainly betaine that is accumulated during growth at low temperatures. However, in strain LO28ΔG, more carnitine than betaine is accumulated. This increase in internal carnitine concentration upon the deletion of Gbu was also observed for L. monocytogenes 10403S at low temperatures (4) and for LO28 grown at high osmolarity (38). Thus, carnitine was accumulated to high levels only in those strains with impaired ability to transport betaine via Gbu. The ability of betaine to suppress the accumulation of other osmolytes is typical of bacterial species that can accumulate betaine together with other osmolytes (19, 28). Angelidis and Smith (2) have recently proposed that betaine and carnitine uptake in Listeria is mediated exclusively by BetL, Gbu, and OpuC. An earlier triple mutant created in our laboratory, LO28ΔBCG (38), displayed low levels of carnitine and betaine uptake which we attributed to the existence of a possible fourth transporter, which we and others (11) suggested to be OpuB, a potential osmolyte uptake system identified by in silico analysis of the completed genome sequence (32). Angelidis and Smith (2) suggested that the residual uptake observed may also be explained by the nature of the OpuC mutation, which was created by plasmid integration rather than SOEing mutagenesis. To resolve this issue, in this study we recreated the triple mutant by using SOEing to eliminate the OpuC gene. The resulting mutant, designated LO28ΔBCGsoe, was found to exhibit no detectable betaine or carnitine accumulation either at reduced temperatures or at elevated osmolarities. Thus, it appears that betaine and carnitine uptake in L. monocytogenes is indeed mediated by Gbu, BetL, and OpuC alone, both at elevated osmolarities as shown by Angelidis and Smith (2) and at refrigeration temperatures.
RT-PCR analysis revealed that the genes encoding all three transporters, Gbu, BetL, and OpuC, are significantly up-regulated following a rapid decrease in temperature from 37 to 10°C. Interestingly, the induced transcription of opuB after cold shock also suggests a role for this transporter at low temperatures. However, no difference in betaine or carnitine accumulation was observed between the triple and quadruple mutants LO28ΔBCGsoe and LO28ΔBCGB, confirming that OpuB is not involved in betaine or carnitine uptake. Furthermore, given that the growth characteristics of both mutants at low temperatures were similar, OpuB is unlikely to play a significant role in listerial cryotolerance.
In high-salt environments, compatible solutes serve to balance the osmotic disturbance. Although their functions at low temperatures are largely unknown, some functions of compatible solutes at low temperatures have been proposed: they can serve as stabilizers of enzyme function and as such may function in the stabilization of membrane bilayers (22). What is interesting in regard to this perspective is the possible overlapping function with CSPs. These proteins have been suggested to play a role in the stabilization of macromolecules at low temperatures (and during other stresses). Induction of two of the four CSPs in LO28 after cold shock was described previously (37). We have also analyzed CSP production 20 h after cold shock to 10°C of wild-type LO28 as well as the triple mutant LO28ΔBCG. However, no changes in CSP production between the two strains could be detected (results not shown), indicating that increasing the CSP levels does not compensate for osmolyte depletion.
Acknowledgments
We acknowledge the financial assistance of the Irish Government under National Development Plan 2000-2006. R.D.S. is funded by an IRCSET postdoctoral fellowship.
REFERENCES
- 1.Abee, T., and J. A. Wouters. 1999. Microbial stress response in minimal processing. Int. J. Food Microbiol. 50:65-91. [DOI] [PubMed] [Google Scholar]
- 2.Angelidis, A. S., and G. M. Smith. 2003. Three transporters mediate uptake of glycine betaine and carnitine by Listeria monocytogenes in response to hyperosmotic stress. Appl. Environ. Microbiol. 69:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelidis, A. S., L. T. Smith, L. M. Hoffman, and G. M. Smith. 2002. Identification of OpuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2644-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelidis, A. S., L. T. Smith, and G. M. Smith. 2002. Elevated carnitine accumulation by Listeria monocytogenes impaired in glycine betaine transport is insufficient to restore wild-type cryotolerance in milk whey. Int. J. Food Microbiol. 75:1-9. [DOI] [PubMed] [Google Scholar]
- 5.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayles, D. O., M. H. Tunick, T. A. Foglia, and A. J. Miller. 2000. Cold shock and its effect on ribosomes and thermal tolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4351-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker, L. A., M. S. Çetin, R. W. Hutkins, and A. K. Benson. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker, L. A., S. N. Evans, R. W. Hutkins, and A. K. Benson. 2000. Role of σB in adaptation of Listeria monocytogenes to growth at low temperature. J. Bacteriol. 182:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly, C. W. 2001. Listeria monocytogenes: a continuing challenge. Nutr. Rev. 59:183-194. [DOI] [PubMed] [Google Scholar]
- 10.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser, K. R., and C. P. O'Byrne. 2002. Osmoprotection by carnitine in a Listeria monocytogenes mutant lacking the OpuC transporter: evidence for a low affinity carnitine uptake system. FEMS Microbiol. Lett. 211:189-194. [DOI] [PubMed] [Google Scholar]
- 12.Fraser, K. R., D. Sue, M. Wiedmann, K. Boor, and C. P. O'Byrne. 2003. Role of σB in regulating the compatible solute uptake systems of Listeria monocytogenes: osmotic induction of opuC is σB dependent. Appl. Environ. Microbiol. 69:2015-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galinski, E. A., and R. M. Herzog. 1990. The role of trehalose as a substitute for nitrogen-containing compatible solutes. Arch. Microbiol. 153:607-613. [Google Scholar]
- 14.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 2000. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J. Bacteriol. 182:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 1996. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J. Bacteriol. 178:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Domínguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueño, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J.-C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 17.Hill, C., P. D. Cotter, R. D. Sleator, and C. G. M. Gahan. 2002. Bacterial stress response in Listeria monocytogenes: jumping the hurdles imposed by minimal processing. Int. Dairy J. 12:273-283. [Google Scholar]
- 18.Horton, R. M., Z. Cai, S. N. Ho, L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528-534. [PubMed] [Google Scholar]
- 19.Kempf, B., and E. Bremer. 1998. Stress responses of Bacillus subtilis to high osmolarity environments: uptake and synthesis of osmoprotectants. J. Biosci. 23:447-455. [Google Scholar]
- 20.Ko, R., and L. T. Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippert, K., and E. A. Galinski. 1992. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37:61-65. [Google Scholar]
- 23.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendum, M. L., and L. T. Smith. 2002. Characterization of glycine betaine porter I from Listeria monocytogenes and its roles in salt and chill tolerance. Appl. Environ. Microbiol. 68:813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peddie, B. A., M. Lever, C. M. Hayman, K. Randall, and S. T. Chambers. 1994. Relationship between osmoprotection and the structure and intracellular accumulation of betaines by Escherichia coli. FEMS Microbiol. Lett. 120:125-132. [DOI] [PubMed] [Google Scholar]
- 26.Phadtare, S., J. Alsina, and M. Inouye. 1999. Cold-shock response and cold-shock proteins. Curr. Opin. Microbiol. 2:175-180. [DOI] [PubMed] [Google Scholar]
- 27.Premaratne, R. J., W. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randall, K., M. Lever, B. A. Peddie, and S. T. Chambers. 1995. Competitive accumulation of betaines by Escherichia coli K-12 and derivative strains lacking betaine porters. Biochim. Biophys. Acta 1245:116-120. [DOI] [PubMed] [Google Scholar]
- 29.Ripio, M.-T., J.-A. Vázquez-Boland, Y. Vega, S. Nair, and P. Berche. 1998. Evidence for expressional crosstalk between the central virulence regulator PrfA and the stress response mediator ClpC in Listeria monocytogenes. FEMS Microbiol. Lett. 158:45-50. [DOI] [PubMed] [Google Scholar]
- 30.Sleator, R. D., C. G. M. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2001. Identification and disruption of the proBA locus in Listeria monocytogenes: role of proline biosynthesis in salt tolerance and murine infection. Appl. Environ. Microbiol. 67:2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2003. A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 69:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sleator, R. D., C. G. M. Gahan, B. O'Driscoll, and C. Hill. 2000. Analysis of the role of betL in contributing to the growth and survival of Listeria monocytogenes LO28. Int. J. Food Microbiol. 60:261-268. [DOI] [PubMed] [Google Scholar]
- 34.Sleator, R. D., J. Wouters, C. G. M. Gahan, T. Abee, and C. Hill. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verheul, A., E. Glaasker, B. Poolman, and T. Abee. 1997. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J. Bacteriol. 179:6979-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker, S. J., P. Archer, and J. G. Banks. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157-162. [DOI] [PubMed] [Google Scholar]
- 37.Wemekamp-Kamphuis, H. H., A. K. Karatzas, J. A. Wouters, and T. Abee. 2002. Enhanced levels of cold shock proteins in Listeria monocytogenes LO28 upon exposure to low temperature and high hydrostatic pressure. Appl. Environ. Microbiol. 68:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wemekamp-Kamphuis, H. H., J. A. Wouters, R. D. Sleator, C. G. M. Gahan, C. Hill, and T. Abee. 2002. Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl. Environ. Microbiol. 68:4710-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]