Abstract
Background
Adherence to treatment is an important issue in chronic disease management and an indicator of patients’ ability to self-manage their condition and treatment. Some drug-dispensing and drug-delivery devices have been designed to support patients’ medication-taking behavior by including dose-memory and combined dose-memory and dose-reminder functions, which electronically store, and visually display dose-history information, enabling the patient to review, monitor, and/or be actively reminded about their medication doses.
Purpose
This literature review explored the role and impact of these devices on patients’ treatment adherence, confidence with, and self-management of their condition and treatment.
Materials and methods
A search of MEDLINE, Embase, and PsycINFO was performed to identify articles published in English from 2003–2013 that studied the effect of devices with dose-memory and combined dose-memory and dose-reminder functions on treatment adherence and users’ (patients, health care professionals [HCPs], and caregivers) confidence, self-management behavior, and attitudes.
Results
The database searches yielded 940 abstracts from which 13 articles met the inclusion criteria and were retained. Devices with dose-memory and combined dose-memory and dose-reminder functions were found to improve self-reported and electronically monitored treatment adherence in chronic conditions such as asthma, diabetes, and HIV. The ability of the devices to provide dose-history information and active medication reminders was considered valuable in disease management by patients, caregivers, and HCPs. The devices were found to enhance patients’ confidence in, and motivation to manage their medication and condition, and help reduce forgotten or incorrect medication dosing.
Conclusion
The incorporation of dose-memory and combined dose-memory and dose-reminder functions in drug-delivery devices can improve patients’ adherence, confidence, and self-management behavior. They can target non-intentional barriers to adherence and can provide a means of improving disease control and clinical outcomes, thereby offering clinical and economic value. This review highlights the importance of conducting further qualitative and quantitative research to further understand the value and impact of these types of devices on patients’ long-term adherence to, and self-management of treatment.
Keywords: patient adherence, memory function, reminder function, self-management, drug-delivery devices
Introduction
Patient adherence (or lack thereof) to prescribed medication regimens is a complex and multidimensional behavior. Patient adherence is one of the most widely researched topics of recent times and attempts to understand, measure, predict, and enhance patients’ medication-taking behavior have been documented throughout the literature.1,2
Adherence can be defined as the degree to which a patient’s medication-taking behavior and/or executing of lifestyle changes (eg, following a diet) correspond with agreed recommendations from a health care professional (HCP) with respect to timing, dosage, and frequency.1,3 The term adherence is often used interchangeably with the term “compliance”;3 however, their connotations differ: adherence presumes that the patient is an active collaborator in the treatment process, whereas compliance suggests that the patient is not part of a therapeutic alliance and is passively following the orders of a HCP.4–6
Nonadherence to prescribed treatment regimens (eg, medications, screening, exercise, diet) is problematic, with estimates across empirical studies averaging 25%.7 Reviews have shown that nonadherence is a prevalent problem in patients with chronic conditions such as diabetes, asthma, hypertension, and HIV/AIDs. Adherence rates are typically lower within this population as compared to those with acute conditions, with patients with chronic conditions achieving an average of only 50% adherence to prescribed medication, regardless of prognosis or disease progression.8
Nonadherence to medication regimens, whether willful or inadvertent can include taking an incorrect dose, taking the medication at the wrong time, forgetting to take the medication, polypharmacy, and improper use of a medication administration device (eg, auto-injector or inhaler).5,9 Poor adherence compromises treatment efficacy and leads to suboptimal disease control and poor clinical outcomes such as preventable disease progression and complications, adverse events, reductions in health-related quality of life, disability, and even death.1,10 Poor adherence also results in poor economic outcomes, contributing to an increased use of health care services and expenditures.1,11,12 Within the US alone, nonadherence is estimated to account for 10% of hospitalizations and 23% of nursing home admissions,13 with resultant costs of approximately US$100 billion per year.14–16
The barriers to patient adherence are multidimensional and can include a complex interplay of patient-centered factors and external factors relating to the patients’ HCP, condition, and medication and/or society and economy.1,16,17 For example, the act of forgetting is one of the most frequently cited reasons for poor adherence,18 whether due to lifestyle factors such as having a busy routine or being tired, or clinical factors such as dementia. Fear of or experience of treatment side effects are also reported as major reasons for nonadherence.18 In addition, poor adherence is often observed among patients who have complex or variable treatment regimens, with adherence rates shown to decrease as the number of required daily medication doses increases.19 Other reasons for patient nonadherence include having poor communication and lack of a relationship with a HCP, having a lack of understanding and knowledge about their condition, strong cultural or lay beliefs, or a lack of self-confidence to manage their treatment regimen.5,20–23
Patients with chronic conditions play a large role in the management of their condition. Patients with diabetes, for example, provide close to 95% of their own care, which includes the integration of a series of complex daily actions such as measuring blood glucose levels, administering variable doses of insulin, and dietary control.24,25 Such self-management involves the medical, social, and emotional aspects of living with, adjusting to, and monitoring of a long-term chronic condition over a lifetime in a dynamic and continual state of self-regulation.26 Together, self-management and adherence encompass the activities that patients must carry out to regulate their illness and cope with the impact of their condition and treatment on themselves and others. Adherence to these complex regimens is a crucial factor in the success of a treatment, and therefore, the suboptimal adherence rates seen among patients with chronic conditions are seen as an indication of patients’ (in)ability to self-manage their condition and treatment.27
Across the literature, there is a consistent and unequivocal finding that adherence problems occur across all situations where the self-administration and self-management of treatment is required, regardless of disease type, disease severity, and access to health care resources.1 In chronic conditions such as type 1 diabetes for example, approximately one in three patients are nonadherent to insulin regimens, which can lead to poor glycemic control and an increase in hemoglobin A1c (HbA1c) above the target level of 6.5%.28,29 It has been demonstrated that forgetting or omitting 2.1 meal-related insulin injections per week can cause an increase in HbA1c of 0.3%–0.4% points, thus contributing to decreased glycemic control.29 To further illustrate the magnitude of poor glycemic control within this population, only 37% of US patients with diabetes are estimated to achieve the clinical outcome of less than 7% HbA1c.30 Similarly, across other chronic disease areas, nonadherence to hypertensive medication compromises the clinical goal of controlling high blood pressure to a level of <140/90 mmHg and doubles the risk of stroke, myocardial infarction, and cardiovascular mortality.1,31 Therefore, better understanding of nonadherence is important in order to accurately monitor, evaluate, and manage treatment outcomes and improve patient care.32 It has been suggested that “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.”8
A multitude of drug-dispensing, drug-delivery, and reminder devices have been developed over the years, aimed at monitoring and improving patients’ self-management and adherence behavior. At the forefront of adherence monitoring are medication event monitoring systems (MEMS), which are widely reported within the literature as the gold standard measure of patient adherence. MEMS typically compile the dose-history of patients prescribed oral medications via a microprocessor included in the medication bottle top/device, which provides time-stamped records of the numbers of opening or actuations. This information can then be transferred and analyzed via computer, enabling a HCP or researcher to track a patient’s adherence. MEMS do not, however, provide non-clinician–controlled “real time” dose-history information directly to the patient, enabling them to self-monitor their medication-taking behavior and facilitate adherence. Devices designed to directly improve adherence range from simple calendar pillboxes and blister packaging, aimed at assisting patients with medication scheduling, to electronic devices with inbuilt dose-count and dose-memory functions that provide the patient with predetermined audio and/or visual medication monitoring and dose-reminders, or information about the date, time, and volume of their last medication dose to facilitate successive dose-taking.2,8,33
A new wave of drug-delivery technology now exists, primarily in conditions requiring the use of auto-injectors such as diabetes (eg, NovoPen Echo®, Novo Nordisk A/S, Bagsværd, Denmark) and growth hormone deficiency (eg, Easypod®, Merck Serono, International SA, Geneva, Switzerland) which now feature inbuilt or aftermarket device dose-memory functions (eg, InsulCheck®, Innovation Zed Ltd., Dublin, Ireland) to facilitate treatment self-management and variable dose-monitoring. These devices enable the patient to directly record and monitor their own medication-taking behavior without involvement from a HCP. Use of electronic dose-memory and combined dose-memory and dose-reminder devices to facilitate patient self-management can also reduce the burden on caregivers and offer a solution for patients who are unintentionally nonadherent.32,33 In addition, such devices could potentially reduce the cognitive, emotional, and physical burdens associated with a condition that contribute to nonadherence, and promote increased confidence in patients by helping them deal with these barriers to adherence.32
A vast array of adherence literature exists relating to MEMS and medication reminder systems; however, despite new developments and the increasing recognition of the potential value devices with dose-memory functions may have, little has been done to consolidate evidence regarding their link with adherence as well as other benefits for patients and the wider health care system. Thus, a targeted literature review was conducted to explore the role and impact of medical devices with dose-memory and combined dose-memory and dose-reminder functions on patients’ treatment adherence, confidence with, and self-management of their condition and treatment.
Materials and methods
Published peer-reviewed articles were identified via searches performed in MEDLINE, Embase, and PsycINFO electronic bibliographic databases. Searches were performed across the three databases using device, memory, and patient-related terms combined using Boolean logic commands (Table 1). Searches were conducted on January 6, 2013, and limited to articles published between 2003 to 2013, published in the English language, and limited to humans.
Table 1.
Search terms for identification of peer-reviewed articles
| Search | Search terms | Command |
|---|---|---|
| 1 (device) | Device* OR medical device* OR equipment | AND |
| 2 (memory) | Memory OR monitor* OR memory function OR memory feature OR remind* OR alarm OR clock OR timer OR track* | AND |
| 3 (patient) | Compliance OR adherence OR satisfaction OR self medicat* OR self administr* OR self manag* |
Note:
Search command operator used to retrieve all possible suffix variations of the root search term (eg, monitor* retrieves monitor, monitors, monitoring).
All abstracts were reviewed by two independent researchers. For consideration for inclusion in the review, selected abstracts were required to make reference to the effects of patient-used medical devices with dose-memory or combined dose-memory and dose-reminder functions on treatment adherence within, but not limited to chronic conditions, device usability, and users’ (patients, HCPs, and caregivers) relationship and attitudes towards the devices. The reference lists of the selected articles were also reviewed to identify additional papers not retrieved from the database searches. The final list of abstract and articles selected for in-depth review was agreed following consensus between the authors. Key information on each selected article regarding the study design, study aims, sample characteristics, device type, methodology, and results were evaluated and summarized in data extraction tables.
Results
Searches in the electronic bibliographic databases returned a combined total of 940 abstracts; however, due to the niche area under investigation, 911 were excluded. Abstracts were excluded because they either failed to report on patient-used drug-dispensing or drug-delivery devices at all (eg, clinical guidelines, health service evaluation, and medication efficacy reviews) or reported on devices irrelevant to the current study (eg, MEMS, dose-reminder devices without dose-memory functions, clinician-used medical devices, and devices measuring physiological parameters [eg, high blood pressure or blood glucose monitors]). Abstracts were also excluded if they discussed general adherence and compliance monitoring, or non-medical content. A total of 29 articles were selected for full text review, and following review of their reference lists, a further eleven articles were selected for inclusion. Of the 40 articles identified for full text review, 27 were omitted as they did not contain relevant content to the concepts of interest as expected from their abstracts. A final total of 13 articles were selected for inclusion in this review and data extraction (Figure 1).34–46 Of the 13 included studies, eight (61.5%) utilized patient-used dose-memory function devices34,38–41,43,44,46 and five utilized combined dose-memory and dose-reminder functionalities (38.5%),35–37,42,45 which electronically store, and visually display dose-history information, enabling the patient to review, monitor, and/or be actively reminded about their medication doses.
Figure 1.
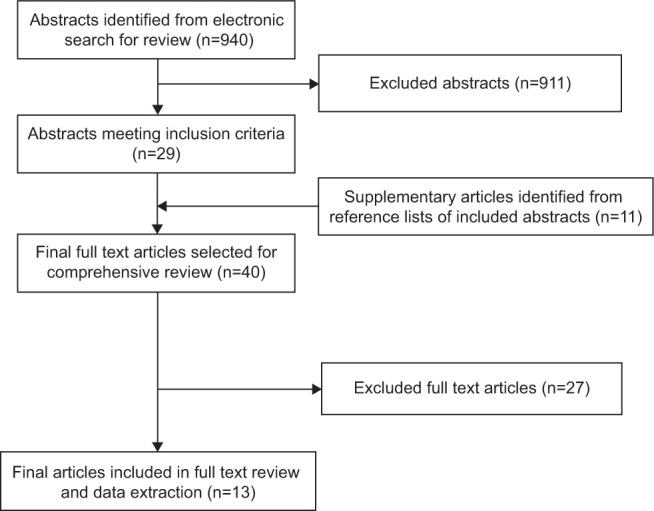
Article selection flow diagram.
The conditions in which these studies were implemented included diabetes (type 1 and/or 2) (n=7, 53.8%),34,39–41,43,44,46 hypertension (n=2, 15.4%),37,45 asthma (n=1, 7.7%),36 HIV/AIDS (n=1, 7.7%),35 growth hormone deficiency (n=1, 7.7%),38 and rhinoconjunctivitis (n=1, 7.7%).42
Different study designs were utilized across the 13 studies such as observational studies and randomized control trials. The studies included research conducted worldwide using ethnically and racially diverse samples and both adult (n=9, 69.2%)35,37–40,42,43,45,46 and adolescent (n=2, 15.4%),34,44 as well as combined age populations (n=2, 15.4%).36,41 The studies, the devices implemented, and their results are presented in Table 2.
Table 2.
Summary of included studies
| Author | Device type (M or C) | Description | Sample size | Study design | Results and conclusion |
|---|---|---|---|---|---|
| Adolfsson et al34 | NovoPen Echo® (Novo Nordisk A/S, Bagsvaerd, Denmark) (M) | Insulin pen designed for children. Records dose volume and hours since last injection. |
Diabetes type 1 Sample size: N=358 |
Observational, multicenter study |
Patient adherence: Fewer forgotten injections by patients/caregivers with NovoPen Echo® device (27% versus [vs] 51%, P<0.0001) compared to pre-study device. Proportion of children reporting self-injection using NovoPen Echo® was higher compared with pre-study device (71% vs 66%, P=0.006). Usability, functionality, and user/prescriber impact: Patients reported increased confidence and self-management using the NovoPen Echo® compared to the pre-study device. Conclusion: The dose-memory function of NovoPen Echo® may help reduce forgetfulness, a common form of nonadherence. Confidence and usability can be increased with this device, which could minimize double dosing and missed doses. |
| Andrade et al35 | DMAS (The John Hopkins University, Baltimore, ML, USA) (C) | Electronic device. Records dosing times and dates that prompt patients to take antiretroviral medication. | HIV Sample size: N=58 |
Randomized controlled trial |
Patient adherence: Patients with memory impairment who used the DMAS had significantly higher adherence rates (77%) compared to controls who did not (57%). Memory-intact patients who use the DMAS also demonstrated trends towards improved adherence (83%), compared to a control group; however, this finding was not significant. Conclusion: The DMAS significantly improved adherence for HIV patients with memory impairment but did not for memory-intact patients. |
| Charles et al36 | Smartlnhaler (Nexus6 Ltd, Auckland, New Zealand) (C) | Metered-dose inhaler with AVRF. Emits an audible reminder at preset designated times, as well as a visual cue that shows patients whether they have taken their inhaler during a designated period. | Asthma Sample size: N=110 |
Randomized open-label parallel group study |
Patient adherence: Median percentage of medication taken was higher in the Smartlnhaler with AVRF group compared to the control group (93% vs 74%). Absolute mean difference was 18% (P<0.001). The proportion of patients likely to take >50%, >80%, and >90% of their medication was higher in the AVRF group than the control group; 1.33 (95% CI: 1.10–1.61; P=0.003), 2.27 (95% CI: 1.56–3.3; P<0.0001), and 3.25 (95% CI: 1.74–6.1; P<0.000l), respectively. Conclusion: An AVRF can significantly improve adherence to therapy in adult asthma. |
| Christensen et al37 | Helping hand, (Bang and Olufsen Medicom, Struer, Denmark) (HH) (C) | Tablet blister card device. AVRF to indicate when it is time to take medication. Visual feedback signal indicating the regularity of intake during the past 7 days (green for good compliance and red for poor compliance). |
Hypertension Sample size: N= 1,194 |
Randomized controlled trial |
Usability, functionality, and user/prescriber impact: The traffic light feedback system received positive feedback from 75% of patients and 78% of HCPs. 65% of patients and 70.4% of HCPs felt the device influenced the regularity of their drug intake (P<0.00l). Conclusion: The device was well-accepted by the majority of patients and HCPs in the study. |
| Dahlgren et al38 | Easypod™ (Merck Serono International SA, Geneva, Switzerland) (M) | Auto-injector. The device digitally displays the dose pre-programmed by a physician, the dose administered, the amount remaining within the device within a dose log, and the date of the last injection and injection history in a calendar. | Patients using r-hGH. Sample size: HCPs: N=30 |
Open-label, uncontrolled study |
Usability, functionality, and user/prescriber impact: Display of last injection date was rated as “useful” or “very useful” by 96% of patients. 96% of patients rated the audible and visual signals as “useful” or “very useful”. 69% of patients rate the dose history as “useful” or “very useful”. Conclusion: The device may facilitate patients’ adherence to the regimen of daily injections. |
| Danne et al39 | HumaPen® Memoir™; (Eli Lilly and Company, Indianapolis, IN, USA) (M)HumaPen Luxura™ (Eli Lilly and Company) (M) |
Insulin pen. Pen features a dose-memory function that records the dose, date, and time of the past 16 injections. Insulin pen. Delivers single units from 1–60 units. |
Diabetes type 1 Sample size: N=257 |
Randomized, open-label study |
Physiological outcomes: There was no significant difference in least square mean changes in HbA1c up to week 24 between HumaPen® Memoir™ and HumaPen Luxura™ (0.43% vs 0.64%, P=0.669). There were no significant differences in hypoglycemic episodes between groups (P=0.982). Conclusion: Dose-memory function was associated with neither an additional improvement in glycemic control nor with a lower rate of hypoglycemia when compared with a conventional device pen, suggesting that adherence to injections was not improved in a relevant manner by this device. |
| Guo et al40 | NovoPen® 5 (Novo Nordisk A/S, Bagsvaerd, Denmark) | Insulin pen. Device includes a dose-memory function that records the dose and hours passed since the last injection. | Diabetes types I & II Sample size: Patients: N=278 HCPs: N= 102 |
Cross-over, multicenter usability study |
Usability, functionality, and use/prescriber impact: 65% of patients reported feeling more confident in managing their insulin injections using the NovoPen® 5 than their pre-study device because it allowed them to review the volume (42%) and time (39%) of their last dose. 75% of HCPs reported that the NovoPen® 5 was valuable for patients who forget their injections. Conclusion: NovoPen® 5 was preferred to other devices by most patients and HCPs. The simple dose-memory function and design functions may contribute to overall preference. |
| HumaPen® Memoir™ (Eli Lilly and Company) (M) |
Insulin pen. Pen features a dose-memory function that records the dose, date and time of the past 16 injections. | ||||
| HumaPen Luxura™ (Eli Lilly and Company) (M) ClikSTAR® (Sanofi-Aventis, Paris, France) (M) |
Insulin pen. Delivers single units from 1–60 units. Insulin pen. Delivers up to 80 units in 1 unit increments. |
||||
| Ignaut and Venekamp41 | HumaPen® Memoir™ (Eli Lilly and Company) (M) |
Insulin pen. Pen features a dose-memory function that records the dose, date, and time of the past 16 injections. | Diabetes types I & II Sample size: N=100 |
Observational study |
Usability, functionality, and user/prescriber impact: 81.4% of patients preferred the HumaPen® Memoir™ to their pre-study device. Patients identified the HumaPen® Memoir’s™ dose-memory functions as important, including the ability to confirm that an injection was taken, as well as indicate the previous insulin dose, time of dosing, and previous 16 doses. HCPs also reported similar findings. 54% of patients and 75% of HCPs reported that they would recommend the device to other patients because of the dose-memory function. Conclusion: Most patients rated the dose-memory feature as being amongst the top reasons for recommending the device. The ability to confirm injections was considered the most important aspect of the dose-memory feature. |
| Jansen et al42 | Memozax® (ALK-Abelló A/S, Horsholm, Denmark) (C) | Tablet blister card device. Device stores and dispenses grass allergen tablets, reminds patients to take their medication, and keeps a record of compliance over the previous 7 days. | Rhinoconjunctivitis Sample size: N=7I |
Multicenter, randomized, open-label, controlled, parallel-group trial |
Usability, functionality, and user/prescriber impact: 79% of patients found the device easy to use. 46% of participants found that the compliance device made it easier or much easier to remember to take their medication. Only 32% of participants felt that the compliance feedback function made them feel motivated or highly motivated to take their tablets. Conclusion: Most patients reported that they used the device as a reminder to take their medication and found it easy to use. |
| Klausmann et al43 | NovoPen® 5 (Novo Nordisk A/S) (M) | Insulin pen. Device includes a memory function that records the dose and hours passed since the last injection. | Diabetes types I & II Sample size: Patients: N=300 HCPs:N=150 |
Interview study |
Usability, functionality, and user/prescriber impact: Significantly more patients rated NovoPen® 5 as their preferred pen (82%) compared with the HumaPen Luxura™ (17%) (P<0.001). The presence of a dose-memory function was cited by 56% of patients as their main reason for preferring the NovoPen® 5. |
| HumaPen Luxura™ (Eli Lilly and Company) (M) | Insulin pen. Delivers single units from 1–60 units. | 82% patients stated that using the NovoPen® 5 would give them more confidence in managing their insulin injections compared with the HumaPen Luxura™ (11%, P<0.001). The dose-memory function of the NovoPen® 5 was reported as the most helpful feature in this device. Patients also felt that a dose-memory function would improve the following: daily diabetes management, how often they would have to monitor their blood glucose, and keeping their blood sugar levels more stable. Conclusion: The results of this study indicate that the NovoPen® 5 insulin pen could be beneficial in everyday clinical practice. |
|||
| Olsen et al44 | NovoPen Echo® (Novo Nordisk A/S) (M) NovoPen® Junior (Novo Nordisk A/S) (M) HumaPen Luxura™ (Eli Lilly and Company) (M) |
Insulin pen designed for children. Records dose volume and hours since last injection. Insulin pen for children and adolescents. Delivers half unit dosing from 1–35 units. Insulin pen. Delivers single units from 1–60 units. |
Diabetes type 1 Sample size: Pediatric subjects: N=79 Parents: N=78 HCPs: N=48 |
Design: Observational study |
Usability, functionality and user/prescriber impact: 89% of pediatric subjects and 94% of parents rated the user-friendliness of the dose-memory function as “very easy/easy” to use. 87% of pediatric subjects and 81% of parents successfully activated the dose-memory function and read the amount and time of the last dose. Most pediatric subjects, parents, and HCPs considered the dose-memory function of the device as completely meeting their needs. Conclusion: The simple dose-memory function of the NovoPen Echo may contribute to the success of this pen in the pediatric setting. |
| Santschi et al45 | IDAS II (Bang and Olufsen Medicom) (C) | Tablet blister card device. Records the date and hour the drug was removed from the packet. | Hypertension Sample size: N=25 |
Randomized controlled trial, crossover study |
Patient adherence: Median adherence was 99.2% and did not differ between patients using the IDAS II and MEMS devices. Regularity of drug intake timing, however, was significantly higher with the IDAS II compared with the MEMS (P<0.00l). Usability, functionality, and user/prescriber impact: Most of the patients characterized the display information of the IDAS II (88%) and the MEMS devices (84%) as “easy to understand”. |
| The device has a visual reminder that indicates the time elapsed since the last dose and an audible sound that is triggered at a preset time for 1 minute or until the patient opens the device. | The MEMS LCD showed information indicating the number of doses taken and hours elapsed since the last dose, which was considered useful by 64% and 28% of patients, respectively. The IDAS digital display indicating time elapsed since last dose and visual and audio reminder were considered to be useful by 46%, 38%, and 42%, respectively. |
||||
| MEMS 6 SmartCap (Aardex Ltd., Zug, Switzerland) (C) | Device which records date and hour of each bottle opening. The cap also has a LCD display indicating the number of daily openings and the number of hours lapsed since the last opening. |
Drug intake: 56% of patients with the MEMS and 50% with the IDAS II reported that the device helped them maintain more regular drug dose intake. The majority of patients considered both devices to be a reliable reminder (84% MEMS; 75% IDAS II); however, more patients preferred the IDAS II as a supporting device over the MEMS. Nine patients did, however, suggest that neither device was helpful as they already had a strong daily routine for drug intake. Conclusion: IDAS II appears to be a well-accepted device. Use and acceptability of both devices were similar but IDAS II could be a useful tool for the management of long-term therapies. |
|||
| Venekamp et al46 | HumaPen® Memoir™ (Eli Lilly and Company) (M) |
Insulin pen. Pen features a dose-memory function that records the dose, date, and time of the past 16 injections. | Diabetes types I & II Sample size: N=300 |
Multinational, multicenter, open-label, single-arm study |
Usability, functionality, and user/prescriber impact: 81.4% of patients preferred the HumaPen® Memoir™ to their pre-study pen. The most important aspects of the dose-memory feature of the HumaPen® Memoir™ according to patients and HCPs were: the ability to view the number of previous insulin doses, ability to view the time of a previous dose, and confirm that an injection had been taken. 15% of patients, however, stated that the dose-memory feature was not important at all. 54% participants and 75% HCPs stated the dose-memory feature as a reason for recommending the device. Conclusion: The HumaPen® Memoir™ pen with dose-memory feature offers potential advantages over some currently available pens. |
Abbreviations: AVRF, audiovisual reminder function; C, combined dose-memory and dose-reminder function device; CI, confidence interval; DMAS, Disease Management Assistance System; HbA1c, glycated hemoglobin; HCP, health care professional; IDAS II, Intelligent Drug Administration System; LCD, liquid-crystal display; M, dose-memory function device; MEMS, medication event monitoring system; r-hGH, recombinant human growth hormone.
The dose-memory function devices comprised three diabetes insulin pens (NovoPen Echo®, NovoPen® 5 [Novo Nordisk A/S], and HumaPen® Memoir™ [Eli Lilly and Company, Indianapolis, IN, USA]) and one recombinant human growth hormone (r-hGH) auto-injector (Easypod®, [Merck Serono]), utilized in seven34,39–41,43,44,46 and one study, respectively.38 These dose-memory function devices all included an electronic dose-history log that recorded the last dose volume and time since the last injection.
The five devices, which all incorporated a combined dose-memory and dose-reminder function, featured either an auditory reminder (n=4)35,36,42,45 or a combined auditory and visual reminder (n=1)37 that actively reminded the patient to take their medication. The dose-memory function capabilities of these devices included either a detailed dose-memory functionality (eg, electronic dose history log; such as the Disease Management Assistance System [DMAS; HIV/AIDS]35 and Intelligent Drug Administration System [IDAS II; Bang and Olufsen Medicom, Struer, Denmark; hypertension]),45 or provided general feedback about dosing adherence (eg, visual indicators of past adherence; such as the Smartinhaler [Nexus6 Ltd, Auckland, New Zealand; asthma],36 Helping Hand [hypertension; Bang and Olufsen Medicom],37 and Memozax®42 [ALK-Abelló A/S; Hørsholm, Denmark; rhinoconjunctivitis]).
Impact of dose-memory and dose-reminder devices on patient adherence
Five of the 13 (38.5%) studies explored the impact of dose-memory and combined dose-memory and dose-reminder functions on patient adherence: four based on objective measures of adherence35,36,39,45 using the HumaPen® Memoir™ in type 1 diabetes, or MEMS (eg, electronic-drug exposure caps; see Andrade et al and Santschi et al)35,45 in HIV, asthma, and hypertension, and one study based on subjective self-report questionnaires in type 1 diabetes.34
Objective assessments of adherence
Two of the four studies that used objective measures of adherence in HIV35 and asthma36 indicated that devices with combined dose-memory and dose-reminder functions improved patients’ adherence to medication when compared to a control group. For example, Charles et al36 assessed whether a metered dose inhaler (MDI) with an audiovisual reminder function (AVRF) improved adherence to inhaled corticosteroid use among asthma patients. A significant improvement in median adherence at 12 weeks (median difference: 18%, P<0.0001) was found among patients using the MDI with an AVRF (93%), compared to a control group using the MDI without an AVRF (74%). They also found that the proportion of patients taking >50%, >80%, and >90% of their medication was significantly higher in patients using the MDI with AVRF, with a ratio of proportions adherent of 1.33 (95% confidence interval [CI]: 1.10–1.61; P=0.003), 2.27 (95% CI: 1.56–3.3; P<0.0001), and 3.25 (95% CI: 1.74–6.1; P<0.0001), respectively. Furthermore, patients using the MDI with AVRF were significantly less likely to “dose dump” (ie, take multiple doses in a short time period) than the control group (0.25, 95% CI: 0.09–0.7; P=0.008).
The effects of combined dose-memory and dose-reminder devices on adherence to highly active antiretroviral therapy (HAART) were assessed in a study implementing the DMAS in HIV patients with clinically confirmed memory impairments.35 Andrade et al35 found significantly higher adherence rates among HIV patients with memory impairments who used the DMAS (77%), compared to a control group who did not (57%). Similarly, there was also a trend for improved adherence among memory-intact HIV patients who used the DMAS (83%), compared to those who did not (77%); however, this finding was not significant. These findings suggest that such devices can be beneficial for all types of patients who cite forgetting as a reason for nonadherence.
Santschi et al45 used an objective measure of adherence, whereby 24 patients with hypertension each used the IDAS II (with combined dose-memory and dose-reminder function) and a MEMS device, which recorded the number of medication bottle openings and time since last opening, for 2 months.45 Over the 4-month study period, adherence to antihypertensive medication was found to be excellent (99.2%), with comparable rates for both devices in terms of the percentage of doses taken, the percentage of days with correct dosing, and the percentage of correct intervals between doses. There was, however, significantly less variation in the regularity of drug intake timing when using the IDAS II (P<0.001) as demonstrated by a small timing distribution index of 0.60, compared to a distribution index of 1.03 when using the MEMS device. This finding indicates that patients using the IDAS II showed stricter adherence to taking their medication at the same time each day and stricter medication persistence, taking it for the duration of the study.
In the last study using objective measures of adherence, however, use of the HumaPen® Memoir™ with dose-memory function was not associated with improved adherence and superior glycemic control in patients with type 1 diabetes when compared to the HumaPen Luxura™ (Eli Lilly and Company) without dose-memory function.39 Although the two HumaPens had identical mechanical platforms and single dosing increments, this finding suggests that adherence to injection schedules was not improved by the additional dose-memory function of the HumaPen® Memoir™. Several limitations were present in this study, however, which affects the validity of the findings. Limitations include the fact that the number of missed injections and number of corrective actions taken based on the dose-memory function were not recorded; therefore, it is unclear how these were associated with increases and reductions in HbA1c, respectively. The HumaPen® Memoir™ was also only used for mealtime insulin injections, which in very poorly controlled diabetes patients, may be insufficient to achieve a relevant HbA1c reduction independently of fasting blood glucose control. Extremely noncompliant patients stand to benefit most from devices targeting improved adherence; however, this population was perhaps overrepresented in the study, as indicated by 40.1% of patients having very poor glycemic control (baseline HbA1c >9%).39 It is possible that this overrepresentation when combined with other methodological limitations of the study, contributed to the failure to discriminate between the assumed benefit of an injection device with dose-memory function, compared to one without.
Subjective assessments of adherence
Adolfsson et al34 explored the impact of the NovoPen Echo® (with a dose-memory function) on adherence to diabetes insulin injections in patients aged 2–18 years old. Forgotten injections administered by patients or their parents were reported for 27% of patients when using the NovoPen Echo®, as compared to 51% of patients using a pre-study insulin pen (unspecified by the authors), without a dose-memory function (P=0.0001). The authors also report that a higher proportion of children and adolescents self-injected rather than relying on parental administration when using the NovoPen Echo® (71%), as compared to their pre-study insulin pen (66%).
Satisfaction with the usability, functionality, and user/prescriber impact of dose-memory and dose-reminder devices
A total of nine studies reported patients’ and clinicians’ attitudes towards the usability, functionality, and impact of devices with dose-memory and combined dose-memory and dose-reminder device functions.37,38,40–46
Attitudes to dose-memory devices
Favorable responses were reported for devices with a dose-memory function among patients with diabetes and those requiring r-hGH. Guo et al40 found that patients reported feeling considerably more confident managing their daily insulin injections using the NovoPen® 5 (with dose-memory function) than the HumaPen Luxura™ and ClikSTAR® (Sanofi-Aventis, Paris, France) (both without dose-memory function) because the NovoPen® 5 allowed them to review the volume (42%) and time (39%) of their last dose. This was supported by HCPs, with 75% agreeing that the dose-memory device function was particularly valuable for patients who tended to forget to perform injections.
The NovoPen® 5 (with dose-memory function) was also rated by patients and HCPs as their preferred insulin pen (82% and 79%, P<0.0001, respectively) versus the HumaPen Luxura®, which does not contain a dose-memory function (17% and 19%, P<0.0001, respectively).43 The dose-memory function was cited by 56% of patients as their primary reason for preferring the NovoPen® 5. Patients reported feeling that the NovoPen® 5 would improve their daily diabetes management and enable them to feel more confident about the time and volume of their last dose. Patients also felt that the device would help promote successful control of their blood glucose levels.
Olsen et al44 also concluded that the dose-memory function of the NovoPen® 5 “completely met” the needs of patients with diabetes (including children and adolescents) as well as the needs of their parents and HCPs. In addition, 89% of all participants in the study preferred the NovoPen® 5 compared with the NovoPen® Junior (Novo Nordisk A/S) and HumaPen® Luxura™ HD (Eli Lilly and Company), which did not have a dose-memory function. Participants reported that they found the NovoPen® 5 easier to use, making them feel more certain that they had administered a full dose of their injection.
Preference for an insulin pen that included a dose-memory function (HumaPen Memoir®) was also found in a study reported by Venekamp et al46 and Ignaut and Venekamp.41 In this study, 54% of patients and 75% of HCPs reported that they would recommend this device to other patients because of the dose-memory function.46 The dose-memory function was considered beneficial by HCPs because patients could confirm that an injection had been taken, view the units of the previous dose, and view the time of the previous dose. Only 15% of patients in this study felt that the dose-memory function was not an important feature at all.
Similarly, in a study that explored the acceptability of the Easypod® auto-injector with dose-memory function for r-hGH, 96% of patients reported having a display of their last injection date “useful” or “very useful” and 69% reported having access to their dose history “useful” or “very useful”.38
Attitudes to combined dose-memory and dose-reminder devices
Similar findings were found in studies looking at devices with combined dose-memory and dose-reminder functions. For example, hypertensive patients rated the dose-memory function of the IDAS II as a contributing factor for their medication adherence. Specifically, 64% of patients commented that it was useful to know the number of doses they had previously taken and 46% reported that knowing how much time had elapsed since their last dose was valuable.45
Combined devices that include visual feedback about the regularity of medication dosing have also been studied and have received mixed results in terms of their acceptability. In one study, the majority of patients with hypertension (75%) felt that a traffic light visual feedback system, indicated by a colored light, was helpful.37 Furthermore, Jansen et al42 found that just under one-third (32%) of patients in their study with rhinoconjunctivitis reported that the Memozax® with traffic light function motivated them to keep taking their medication. The majority of patients (79%) within this study also found the device easy to use and 46% of patients felt that it made remembering to take their medication “easier” or “much easier”.
In contrast, in a study by Santschi et al45 who trialed the effect of both the IDAS II (with combined dose-memory and dose-reminder function) and a MEMS device on patient adherence to hypertension medication, half of their patients reported that a combined dose-memory and dose-reminder device helped them maintain a more regular dose intake. Similar findings were reported in a study by Christensen et al37 in which 65% of patients with hypertension commented that the combined dose-memory and dose-reminder device positively influenced the regularity of their drug intake. Christensen et al37 also found that clinicians were generally positive about the functions of a combined dose-memory and dose-reminder function, and overall, were more positive than patients were. For example, a significantly greater proportion of clinicians positively rated the dose-reminder function of the device (83%, P<0.001) and its feedback functions (78%, P<0.001), than patients (78% and 75%, respectively). In addition, a significantly greater proportion of clinicians felt that the device would influence the regularity of patients’ drug intake versus the patients themselves (64.9% of patients versus 70.4% of clinicians, P<0.001).
Impact on patients’ confidence with and self-management of their treatment and condition
As well as exploring the impact of these devices on treatment adherence and attitudes, some studies have also examined the potential benefits on other areas of health and well-being, particularly in terms of self-confidence. In one study, patients with diabetes reported feeling more confident that they would not miss their injections and would better manage their daily medication when using the NovoPen Echo® versus their previous device (unspecified by the authors), which did not have a dose-memory function.34 In a different study where very similar findings were revealed, Klausmann et al43 report that patients attribute this increased confidence to the NovoPen Echo’s® ability to provide dose-history information.
Finally, Guo et al40 found that more patients with diabetes felt “very confident” managing their daily insulin injections using the NovoPen® 5 compared with their previous device because the visual confirmation of the dose and the audible end-of-dose sound provided reassurance that they had injected the full dose.
Discussion
The purpose of this review was to explore the impact of drug-delivery devices with dose-memory or combined dose-memory and dose-reminder functions on patients’ treatment adherence, confidence with, and self-management of their condition and treatment. Drug-delivery devices with dose-memory or combined dose-memory and dose-reminder functions, capable of recording and displaying dose-history information, and actively reminding patients to take their medication (eg, inhalers and auto-injectors) are available for patients being treated for a range of chronic conditions such as asthma, HIV, and diabetes.
This review provides evidence for the effectiveness and benefits of these device functions in improving patients’ medication adherence, their attitude towards the device, confidence in managing their condition, and ultimately, the value these products can have to patients, clinicians, and the wider health care system.
The number of published studies reporting adherence data was quite limited; however, devices with dose-memory and combined dose-memory and dose-reminder functions were found to improve objective and subjective adherence to daily medication when compared to either a control group or pre-study drug-delivery device without a dose-memory or dose-reminder function.34–36 From a methodological standpoint, these studies are reflective of adherence data collected over a 6-month period or less. However, patients who are initially adherent can become nonadherent over time and adherence rates are subject to dramatic decline and/or variability within the first 6 months of treatment.3,8,47 Therefore, in order to assess the true long-term impact and value of these device functions, they need to be tested longitudinally.
In addition, a limitation of the reviewed studies is that they failed to consider how other features of the study devices may also impact adherence, beyond obtaining usability or preference data. For example, many of the reviewed devices, most notably insulin pens, had identical or comparable features such as mechanical platforms; however, some insulin pens offered slightly different functionalities such as the number of dosing increments that patients could select. Insulin pens such as the HumaPen® Memoir™ and HumaPen Luxura® offered single-unit dose increments, whereas the NovoPen Echo® offered half-unit dose increments. It is unclear from the reviewed literature what impact such additional and differentiating devices features have on adherence and this is something that should be explored further in future studies in this area.
This literature review has revealed that these devices have widespread value among those involved in the administration of medications in the management of chronic conditions. They have been shown to be beneficial for patients who self-administer their medication and parents/caregivers who administer medication for a patient. These devices have also been shown to be beneficial to patients susceptible to unintentional nonadherence (eg, forgetting), whether they are memory-intact patients who experience forgetfulness due to lifestyle factors or patients with clinical memory impairments. For example, Andrade et al35 found that HIV patients who had memory impairments were significantly more adherent when using the DMAS device with combined functions than memory-intact patients.35 These devices may also be of value to younger patients transitioning from assisted care to self-management, who may need additional reassurance and positive reinforcement about the time and volume of their last medication dose,34 as well as the elderly and those with multiple chronic conditions requiring polypharmacy. This review suggests that devices with dose-memory and combined dose-memory and dose-reminder functions may be most useful in modifying the behavior of patients who are unintentionally nonadherent, and therefore, further targeted research within this population may be of value.
As well as improving adherence to treatment, devices with combined functions have been shown to significantly reduce the potentially dangerous practice of dose dumping, thus demonstrating the capability of such devices to enhance patients’ safety in the self-management of their treatment and reduce patients’ susceptibility to adverse events.1,36
Taken together, these devices may represent additional value for parents and caregivers by reducing the burden and expectation on these individuals to care for the patient. For example, following use of a device with a combined dose-memory and dose-reminder function, caregivers in one study reported reduced burden as the device promoted better self-management by the patient themselves.35 This highlights a need for clinicians to consider the impact on caregivers when making decisions about the patients’ treatment options, especially caregivers at risk of experiencing high levels of burden.
Psychological benefits were also salient in the literature review, with dose-memory and combined dose-memory and dose-reminder functions seen as important and useful attributes of the product for both patients and clinicians. For example, patients felt that being able to review the time and volume of their last dose was valuable and useful37,38,45 in that it positively influenced the regularity of their medication intake.37,45 The devices were found to make patients feel more confident in managing their treatment and condition by assuring them that a dose had been taken correctly,34,40,43,44 thus providing peace of mind and security. The devices were also found to motivate patients to keep taking their medication.42
Similarly, HCPs considered dose-memory function devices to be beneficial to their patients and of particular value to those patients who forget to take their medication.37,41,46 Indeed, HCPs in some cases put greater emphasis on the importance of the dose-memory and dose-reminder functions on patient self-management than the patients themselves.37 This highlights a need for increased and improved concomitant patient education in improving adherence and promoting the value and benefit of such devices on patients’ self-management behavior and well-being.
The literature review also revealed a potential for discordance between patients’ perceptions of their adherence/compliance and the clinicians’ understanding of adherence/compliance. The lack of robust and systematic adherence data reflects both measurement limitations (ie, how best to measure adherence, objectively and subjectively) and a lack of real world and longitudinal research in this area. Ultimately, understanding, monitoring, and evaluating patients’ adherent/nonadherent behavior in an ecological or epidemiological study is the best approach to determine the true impact of a device on patients’, caregivers’, and clinicians’ disease management.
Conclusion
This literature review has provided supportive evidence that dose-memory and combined dose-memory and dose-reminder function devices that enable the patient to record, monitor, and/or be actively reminded about their dose-history, can improve patients’ adherence to treatment and self-management of their condition. The evidence suggests that the incorporation of such functions into drug-delivery devices can work to target some non-intentional barriers to adherence such as forgetting, whether caused by lifestyle factors, such as having a busy routine, or clinical factors, such as dementia.
These devices therefore offer clinical and economic value by helping to improve disease control (eg, lowering high blood pressure), clinical outcomes (eg, reducing risk factors associated with a condition such as stroke in the case of hypertension), as well as patients’ health-related quality of life, and self-management skills. These devices also have the potential to reduce patients’ exposure to adverse events and reduce the number of avoidable clinician visits and hospital admissions caused by nonadherence.
It is apparent that whilst the incorporation of dose-memory functions in drug-delivery devices is presently limited, they may provide a valuable addition for patients who require long-term treatment regimens and who self-manage their condition. There does, however, remain considerable scope for further targeted quantitative and qualitative research in this area, particularly in terms of assessing the effect these devices can have on adherence from real world device use outside of the study environment, their effect on long-term adherence, and their impact on patients’ confidence with and self-management of their treatment and condition.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization (WHO) Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Accessed April 23, 2014]. Available from: http://www.who.int/chp/knowledge/publications/adherence_report/en/ [Google Scholar]
- 2.Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence. 2012;6:127–135. doi: 10.2147/PPA.S26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 4.Gould E, Mitty E. Medication adherence is a partnership, medication compliance is not. Geriatri Nurs. 2010;31(4):290–298. doi: 10.1016/j.gerinurse.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 5.American Society on Aging and American Society of Consultant Pharmacist Foundation Adult Meducation: Improving Medication Adherenced in Older Adults: Medication Adherence – Where We Are Today? 2006. [Accessed January 7, 2013]. Available from: http://www.adultmeducation.com/downloads/Adult_Meducation.pdf.
- 6.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 8.Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002;(2):CD000011. doi: 10.1002/14651858.CD000011. [DOI] [PubMed] [Google Scholar]
- 9.Wroe AL. Intentional and unintentional nonadherence: a study of decision making. J Behav Med. 2002;25(4):355–372. doi: 10.1023/a:1015866415552. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54( Suppl 1):S57–S60. doi: 10.1016/s0895-4356(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 11.Elliott RA, Shinogle JA, Peele P, Bhosle M, Hughes DA. Understanding medication compliance and persistence from an economics perspective. Value Health. 2008;11(4):600–610. doi: 10.1111/j.1524-4733.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 12.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 13.National Pharmaceutical Council Noncompliance with medication regimens . An economic tragedy Emerging issues in pharmaceutical cost containing. Washington, DC: National Pharmaceutical Council; 1992. pp. 1–16. [Google Scholar]
- 14.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–665. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 15.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26(5):331–342. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 17.Krueger KP, Berger BA, Felkery B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22(4):313–356. doi: 10.1007/BF02850081. [DOI] [PubMed] [Google Scholar]
- 18.The Boston Consulting Group . The Hidden Epidemic: Finding a Cure for Unfilled Prescriptions and Missed Doses. Boston, MA: The Boston Consulting Group; 2003. [Accessed April 23, 2014]. pp. 1–8. Available at: http://www.bcg.com/documents/file14265.pdf. [Google Scholar]
- 19.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 20.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22(10):1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 21.Zgibor JC, Simmons D. Barriers to blood glucose monitoring in a multiethnic community. Diabetes Care. 2002;25(10):1772–1777. doi: 10.2337/diacare.25.10.1772. [DOI] [PubMed] [Google Scholar]
- 22.Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Family Med. 2003;1(1):15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onwudiwe NC, Mullins CD, Winston RA, et al. Barriers to self-management of diabetes: a qualitative study among low-income minority diabetics. Ethn Dis. 2011;21(1):27–32. [PubMed] [Google Scholar]
- 24.Funnell MM, Anderson RM. MSJAMA: the problem with compliance in diabetes. JAMA. 2000;284(13):1709. [PubMed] [Google Scholar]
- 25.McNabb WL. Adherence in diabetes: can we define it and can we measure it? Diabetes Care. 1997;20(2):215–218. doi: 10.2337/diacare.20.2.215. [DOI] [PubMed] [Google Scholar]
- 26.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 27.Walker EA, Usher JA. Understanding and enhancing adherence in adults with diabetes. Curr Diab Rep. 2003;3(2):141–148. doi: 10.1007/s11892-003-0038-5. [DOI] [PubMed] [Google Scholar]
- 28.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 29.Randløv J, Poulsen JU. How much do forgotten insulin injections matter to hemoglobin A1c in people with diabetes? A simulation study. J Diabetes Sci Technol. 2008;2(2):229–235. doi: 10.1177/193229680800200209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 31.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Fry A. Electronically enabled drug-delivery devices: are they part of the future? Ther Deliv. 2012;3(7):805–807. doi: 10.4155/tde.12.57. [DOI] [PubMed] [Google Scholar]
- 33.Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704. doi: 10.1136/amiajnl-2011-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adolfsson P, Veijola R, Huot C, Hansen HD, Lademann JB, Phillip M. Safety and patient perception of an insulin pen with simple memory function for children and adolescents with type 1 diabetes – the REMIND study. Curr Med Res Opin. 2012;28(9):1455–1463. doi: 10.1185/03007995.2012.698258. [DOI] [PubMed] [Google Scholar]
- 35.Andrade AS, McGruder HF, Wu AW, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis. 2005;41(6):875–882. doi: 10.1086/432877. [DOI] [PubMed] [Google Scholar]
- 36.Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. J Allergy Clin Immunol. 2007;119(4):811–816. doi: 10.1016/j.jaci.2006.11.700. [DOI] [PubMed] [Google Scholar]
- 37.Christensen A, Christrup LL, Fabricius PE, et al. Survey of patient and physician assessment of a compliance reminder device in the treatment of hypertension. Blood Press. 2009;18(5):280–285. doi: 10.3109/08037050903289598. [DOI] [PubMed] [Google Scholar]
- 38.Dahlgren J, Veimo D, Johansson L, Bech I. Patient acceptance of a novel electronic auto-injector device to administer recombinant human growth hormone: results from an open-label, user survey of everyday use. Curr Med Res Opin. 2007;23(7):1649–1655. doi: 10.1185/030079907x210589. [DOI] [PubMed] [Google Scholar]
- 39.Danne T, Forst T, Deinhard J, Rose L, Moennig E, Haupt A. No effect of insulin pen with memory function on glycemic control in a patient cohort with poorly controlled type 1 diabetes: a randomized open-label study. J Diabetes Sci Technol. 2012;6(6):1392–1397. doi: 10.1177/193229681200600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X, Sommavilla B, Vanterpool G, Qvist M, Bethien M, Lilleøre SK. Evaluation of a new durable insulin pen with memory function among people with diabetes and healthcare professionals. Expert Opin Drug Deliv. 2012;9(4):355–356. doi: 10.1517/17425247.2012.671808. [DOI] [PubMed] [Google Scholar]
- 41.Ignaut DA, Venekamp WJ. HumaPen Memoir: a novel insulin-injecting pen with a dose-memory feature. Expert Rev Med Devices. 2007;4(6):793–802. doi: 10.1586/17434440.4.6.793. [DOI] [PubMed] [Google Scholar]
- 42.Jansen A, Andersen KF, Brüning H. Evaluation of a compliance device in a subgroup of adult patients receiving specific immunotherapy with grass allergen tablets (GRAZAX) in a randomized, open-label, controlled study: an a priori subgroup analysis. Clin Ther. 2009;31(2):321–327. doi: 10.1016/j.clinthera.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Klausmann G, Hramiak I, Qvist M, Mikkelsen KH, Guo X. Evaluation of preference for a novel durable insulin pen with memory function among patients with diabetes and health care professionals. Patient Prefer Adherence. 2013;7:285–292. doi: 10.2147/PPA.S41929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen BS, Lilleøre SK, Korsholm CN, Kracht T. Novopen Echo® for the delivery of insulin: a comparison of usability, functionality and preference among pediatric subjects, their parents, and health care professionals. J Diabetes Sci Technol. 2010;4(6):1468–1475. doi: 10.1177/193229681000400622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santschi V, Wuerzner G, Schneider MP, Bugnon O, Burnier M. Clinical evaluation of IDAS II, a new electronic device enabling drug adherence monitoring. Eur J Clin Pharmacol. 2007;63(12):1179–1184. doi: 10.1007/s00228-007-0364-7. [DOI] [PubMed] [Google Scholar]
- 46.Venekamp WJ, Kerr L, Dowsett SA, et al. Functionality and acceptability of a new electronic insulin injection pen with a memory feature. Curr Med Res Opin. 2006;22(2):315–325. doi: 10.1185/030079906X80477. [DOI] [PubMed] [Google Scholar]
- 47.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]


