Abstract
Purpose
The aim of this study was to investigate radiation pneumonitis and its associated risk factors in patients with non-small-cell lung cancer treated with concurrent erlotinib and thoracic radiotherapy.
Materials and methods
We conducted an analysis of patients with nonoperable stage IIIA–IV non-small-cell lung cancer who were treated with concurrent thoracic radiotherapy and erlotinib (ClinicalTrials.gov identifier: NCT00973310). The Common Terminology Criteria for Adverse Events version 3.0 grading system was applied to evaluate the incidence of radiation pneumonitis. The lung dosimetric parameters were recorded in accordance with the treatment plan, and the study endpoint was radiation pneumonitis at grade 2 or more.
Results
Among the 24 selected clinical cases, nine were identified with radiation pneumonitis of grade 2 or above (37.5%). This included four cases with grade 2 (16.7%), two cases with grade 3 (8.3%), and three cases with grade 5 (12.5%). The results showed that the planning target volume was a significant factor affecting the incidence of radiation pneumonitis. All lung dosimetric parameters exhibited statistically significant differences between patients with pneumonitis and patients without pneumonitis. The receiver operating characteristic (ROC) curve analysis showed that all lung dosimetric parameters were useful in predicting the incidence of radiation pneumonitis. In addition, the threshold values of V5, V10, V15, V20, V30, and mean lung dose were >44%, >29%, >27%, >22%, >17% and >1,027 cGy, respectively.
Conclusion
Special attention should be paid to the adverse effects of radiation pneumonitis in concurrent thoracic radiotherapy and erlotinib treatment. Lung dosimetric parameters are important predictive factors in radiation pneumonitis.
Keywords: erlotinib, thoracic radiotherapy, radiation pneumonitis, dosimetric parameter, threshold value for lung radiation
Introduction
Erlotinib has achieved promising therapeutic efficacy in the treatment of non-small-cell lung cancer.1–3 Thoracic radiotherapy is also an important therapeutic approach in this setting.4 However, thoracic radiotherapy and erlotinib can induce interstitial lung damage.5,6 Limited studies detailing concurrent thoracic radiotherapy and erlotinib have been conducted. In addition, studies on concurrent erlotinib and thoracic radiotherapy as a cause of radiation pneumonitis and the threshold value for lung radiation are rarely reported.
In this study, we have summarized the clinical data from patients with stage III–IV non-small-cell lung cancer treated with concurrent thoracic radiotherapy and erlotinib. We analyzed the incidence of radiation pneumonitis, and we preliminarily investigated the predictive factors to provide values for concurrent thoracic radiotherapy and erlotinib in clinical practice.
Clinical data
This prospective study was conducted under the review, approval, and supervision of the Tianjin Medical University Cancer Institute and Hospital, People’s Republic of China. All enrolled patients met the ethical requirements. We collected the medical records of patients with stage III–IV non-small-cell lung cancer who received concurrent thoracic radiotherapy and erlotinib from September 2009 to June 2012 (ClinicalTrials.gov identifier: NCT00973310). The inclusion criteria were as follows: 1) patients with stage III–IV non-small-cell lung cancer; and 2) patients receiving concurrent thoracic radiotherapy and erlotinib. The exclusion criteria were as follows: 1) patients with non-small-cell lung cancer after receiving surgical intervention; 2) patients receiving re-irradiation therapy; and 3) patients with a history of interstitial lung disease. Thoracic radiotherapy was prescribed at a dose that depended on the treatment intention with 1.8–2.1 Gy/fraction for 5 days per week. Additionally, 150 mg of erlotinib was administered once daily from the first day of radiotherapy to the end of radiotherapy and then continued as maintenance therapy. If the patient presented with pneumonia (> grade 3), then the drug and radiotherapy were discontinued. The detailed clinical data for the patients are shown in Table 1.
Table 1.
Patient characteristics and treatment modalities
| Factors | Value |
|---|---|
| Sex, n (%) of cases | |
| Male | 9 (38%) |
| Female | 15 (62%) |
| Age (years) | |
| Median | 64 |
| Range | 33–85 |
| Location, n (%) | |
| Upper lobe | 14 (58%) |
| Middle and lower lobe | 10 (42%) |
| Clinical stage, n (%) | |
| IIIA | 11 (46%) |
| IIIB | 7 (29%) |
| IV | 6 (25%) |
| ECOG PS, n (%) | |
| 0 | 6 (25%) |
| 1 | 18 (75%) |
| Smoking history, n (%) | |
| Yes | 8 (33%) |
| No | 16 (67%) |
| FVCa (L) | |
| Median | 2.46 |
| Range | 1.4–4.2 |
| FVC1a (L) | |
| Median | 1.90 |
| Range | 0.9–3.2 |
| DLcoa (L) | |
| Median | 2.05 |
| Range | 1.2–4.7 |
| Pathology, n (%) | |
| Squamous | 5 (21%) |
| Adenocarcinoma | 17 (71%) |
| Adenosquamous carcinoma | 1 (4%) |
| Sarcomatoid carcinoma | 1 (4%) |
| Intention of the radiotherapy, n (%) | |
| Radical | 14 (58%) |
| Palliative | 10 (42%) |
| Radiotherapy dose (Gy) | |
| Median | 57 |
| Range | 46–66 |
| Dose per fraction (Gy) | |
| Median | 2 |
| Range | 1.8–2.1 |
| GTV size (mL) | |
| Median | 35.50 |
| Range | 8.6–454.4 |
| PTV size (mL) | |
| Median | 279.70 |
| Range | 32.9–794.1 |
| Neoadjuvant chemotherapy, n (%) | |
| Yesb | 13 (54%) |
| No | 11 (46%) |
| Adjuvant chemotherapy, n (%) | |
| Yesb | 12 (50%) |
| No | 12 (50%) |
| Taking time of erlotinib concurrent with RT (days) | |
| Median | 41.5 |
| Range | 30–45 |
| Total taking time of erlotinib (months) | |
| Median | 3.2 |
| Range | 1.2–27 |
| Time onset of radiation pneumonitis (weeks)c | |
| Median | 9.5 |
| Range | 4–15.5 |
Notes:
An AS-507 lung function tester (Autospiro-507; Minato Medical Science, Osaka, Japan) was used in the research
the most frequently used regimen for chemotherapy was cisplatin-based doublet
the time was from the beginning of radiotherapy to the onset of pneumonitis in the patients who had radiation pneumonitis.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FVC, forced vital capacity; FVC1, forced vital capacity in 1 second; DLco, diffusing capacity of the lung; GTV, gross tumor volume; PTV, planning target volume, RT, radiation therapy.
Ethics statement
The study was approved by the Tianjin Medical University Cancer Institute and Hospital ethics committee. All of the consents were given by the patients for their information to be stored in the hospital database and subsequently used for research. All of the participants provided their written consent to participate in this study. Further, at the time of patient follow-up, in order to document the process, the presence of a qualified physician, a staff member of the hospital ethics committee, and a staff member of the medical records department was required at the same time.
Therapeutic approach
All patients underwent a computed topography (CT) scan to determine the appropriate positioning, and a precise or pinnacle-planning system was applied to complete intensity-modulated radiotherapy. The gross tumor volume (GTV) is defined as the gross tumor volume. The clinical target volume is defined as a subclinical lesion. The internal target volume is defined as internal target volume production from four-dimensional (4D) CT images. The planning target volume (PTV) was expanded 0.5 cm from the internal target volume. The lung organ at risk is defined as the whole lung volume with the exception of the GTV. The fractionated dose was 1.8–2.1 Gy/fraction (median dose: 2 Gy). The dose for thoracic radiotherapy was 46–66 Gy, depending on the treatment regimen for each patient (eg, some patients presenting with stage IIIb–IV non-small-cell lung cancer received palliative therapy). All the intention and treatment strategies of the patients were determined before treatment.
Treatment strategies for stage IIIA–IIIB patients were done in accordance with the treatment plan evaluation. The treatments of stage IV patients were all palliative care. Only the enrolled patients of the trial were treated with erlotinib concurrently with radiotherapy, and it was not conducted as a routine treatment for other patients. The treatment plan was evaluated using dose-volume histograms. The dosimetric parameters, including V5, V10, V15, V20, V30, and mean lung dose (MLD) were recorded. Erlotinib was administered orally at 150 mg/day from the first day of treatment up to at least the end of the course of radiotherapy. Afterwards, the patients stopped or continued to take medications, dependent upon their condition. No adjuvant chemotherapy was given within a month for patients who stopped taking erlotinib after radiotherapy. The chemotherapy plan for each patient was developed according to a follow-up evaluation.
Evaluation of radiation pneumonitis
Once the symptoms of radiation pneumonitis were presented during radiotherapy, the patients received an immediate follow-up evaluation. The follow-up assessment of pulmonary function was conducted during the third week of the radiotherapy course and at 1 and 3 months after the completion of radiotherapy. The interval of the follow-up was determined by the presence of radiation pneumonitis and the subsequent disease condition. However, follow-up evaluations were performed every 3 months (or more often if recommended by the treating physician) for the first year after the radiotherapy course had concluded, and at the discretion of the treating physician thereafter. A chest X-ray or CT scan was performed to evaluate the patients’ pulmonary condition. Common Terminology Criteria for Adverse Events version 3.0 was used as a reference to evaluate radiation pneumonitis in the patients.7 Treatment for radiation pneumonitis was aimed at decreasing inflammation. Steroids, such as prednisone, were given until the inflammation regressed, and then slowly decreased over time.
Statistical analysis
Radiation pneumonitis of grade 2 or more was the primary endpoint in this study. The SPSS version 17.0 software program (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Logistic regression analysis was applied to investigate the relationship between clinical factors and radiation pneumonitis. The Student’s t-test was used to analyze the differences in lung dosimetric parameters between patients with radiation pneumonitis and patients without radiation pneumonitis. MedCalc software (MedCalc Software, Ostend, Belgium) was used to draw receiver operating characteristic (ROC) curves, and to analyze the predictive effect of lung dosimetric parameters. The threshold value for lung radiation in concurrent thoracic radiotherapy and erlotinib treatment was also calculated. An alpha value of P<0.05 was considered statistically significant.
Results
The incidence of radiation pneumonitis
Twenty-four patients were included in this study, with a median follow-up of 31.5 months (range, 7–48 months). Among the participants, there were nine patients (37.5%) with grade 2 or above radiation pneumonitis, including four cases (16.7%) with grade 2 radiation pneumonitis, two cases (8.3%) with grade 3 radiation pneumonitis, and three cases (12.5%) with grade 5 radiation pneumonitis.
Clinical factors
Single-factor logistic regression analysis was performed on the following factors: age, sex, pulmonary function, total lung capacity, GTV, PTV, radiation dose, duration of concurrent thoracic radiotherapy and erlotinib, total duration of erlotinib administration, chemotherapy before concurrent therapy, and chemotherapy after concurrent therapy. Multivariable analysis was not performed due to the sample size. The results showed that only PTV had a statistically significant effect on the incidence of radiation pneumonitis, while the remaining factors showed no significant effect (Table 2).
Table 2.
Univariate analysis of factors related to radiation pneumonitis (≥ grade 2)
| Factors | RR value | 95% CI | P-value |
|---|---|---|---|
| Female versus male | 0.327 | 0.050–2.120 | 0.241 |
| Age | 0.438 | 0.079–2.437 | 0.345 |
| Smoking history | 2.200 | 0.385–12.573 | 0.375 |
| Location | 1.200 | 0.225–6.388 | 0.831 |
| Pre-RT FVC | 1.429 | 0.271–7.518 | 0.674 |
| Pre-RT FEV1 | 0.914 | 0.174–4.811 | 0.916 |
| Pre-RT DLco | 0.750 | 0.133–4.224 | 0.744 |
| Whole lung volume | 2.500 | 0.458–13.629 | 0.290 |
| RT dose | 0.429 | 0.271–7.518 | 0.377 |
| GTV size | 5.000 | 0.806–31.002 | 0.084 |
| PTV size | 1.007 | 1.001–1.013 | 0.025 |
| Taking time of erlotinib concurrent with radiotherapy | 0.533 | 0.100–2.839 | 0.461 |
| Total taking time of erlotinib | 0.333 | 0.059–1.877 | 0.213 |
| With versus without neoadjuvant chemotherapy | 1.904 | 0.208–5.756 | 0.916 |
| With versus without adjuvant chemotherapy | 2.909 | 0.271–31.904 | 0.378 |
Abbreviations: RR, relative risk; CI, confidence interval; RT, radiation therapy; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLco, diffusing capacity of the lung; GTV, gross tumor volume; PTV, planning target volume.
Lung dosimetric parameters
The lung dosimetric factors V5, V10, V15, V20, V30, and MLD were calculated in patients with or without radiation pneumonitis at grade 2 or above. The results are presented as mean ± standard deviation. In addition, the Student’s t-test was performed on the parameters of both groups. The results showed statistically significant differences in all of the dosimetric parameters between the two groups, indicating that the lung dosimetric parameters were important predictive factors for the incidence of radiation pneumonitis in patients receiving concurrent thoracic radiotherapy and erlotinib (Table 3).
Table 3.
Lung dosimetric parameters
| Variable | Radiation pneumonitis | Non-radiation pneumonitis | P-value |
|---|---|---|---|
| MLD (cGy) | 1,334.6±236.3 | 919.6±221.9 | 0.001 |
| V5 (%) | 45.4±9.6 | 34.5±8.2 | 0.012 |
| V10 (%) | 35.9±7.4 | 27.3±6.8 | 0.012 |
| V15 (%) | 28.1±5.8 | 21.2±5.2 | 0.010 |
| V20 (%) | 24.7±4.4 | 16.9±4.1 | 0.001 |
| V30 (%) | 19.0±4.0 | 13.0±3.1 | 0.002 |
Notes: Parameters in patients with radiation pneumonitis and in patients without radiation pneumonitis (mean ± standard deviation).
Abbreviation: MLD, mean lung dose.
ROC curve analysis
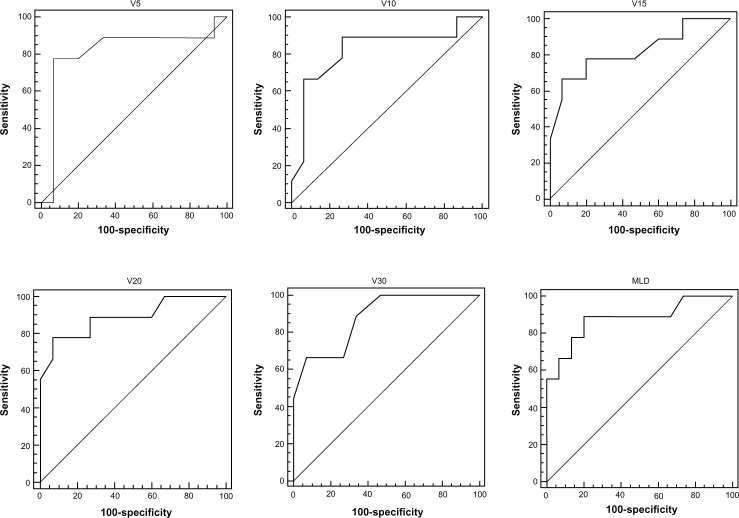
The ROC curve was generated according to the effects of the lung dosimetric parameters V5, V10, V15, V20, V30, and MLD on radiation pneumonitis. The area under the curve (AUC) was also calculated. The results showed that all dosimetric parameters were statistically significant, and there was no significant difference between the parameters. In addition, the threshold value and each corresponding dosimetric parameter were analyzed to determine the predictive effects of each parameter on radiation pneumonitis. The results showed that the threshold values of V5, V10, V15, V20, V30, and MLD were >44%, >29%, >27%, >22%, >17%, and 1,027 cGy, respectively (Table 4, and the ROC curves of each parameter, Figure 1).
Table 4.
Lung dosimetric parameters to predict radiation peneumonitis with an ROC curve
| AUC | 95% CI | Z-statistic | P-value (area =0.5) | Youden index J | Associated criterion | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| MLD (cGy) | 0.878±0.0829 | 0.680–0.975 | 4.559 | <0.0001 | 0.6889 | >1,027 | 88.9 | 80.0 |
| V5 (%) | 0.815±0.113 | 0.605–0.942 | 2.782 | 0.0054 | 0.7111 | >44 | 77.8 | 93.3 |
| V10 (%) | 0.819±0.104 | 0.609–0.944 | 3.055 | 0.0022 | 0.6222 | >29 | 88.9 | 73.3 |
| V15 (%) | 0.822±0.0985 | 0.613–0.947 | 3.270 | 0.0011 | 0.6000 | >27 | 66.7 | 93.3 |
| V20 (%) | 0.889±0.0771 | 0.694–0.979 | 5.044 | <0.0001 | 0.7111 | >22 | 77.8 | 93.3 |
| V30 (%) | 0.881±0.0692 | 0.684–0.976 | 5.514 | <0.0001 | 0.6000 | >17 | 66.7 | 93.3 |
Abbreviations: ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval; MLD, mean lung dose.
Figure 1.
The receiver operating characteristic (ROC) curves of lung dosimetric parameters.
Abbreviation: MLD, mean lung dose.
Discussion
The preliminary results of this study indicated that special attention should be paid to the potential development of radiation pneumonitis during concurrent treatment with thoracic radiotherapy and erlotinib. In addition, our results showed that lung dosimetric parameters were important predictive factors for radiation pneumonitis.
The combined effects of radiation and erlotinib on the pulmonary interstitium might contribute markedly to radiation pneumonitis that was induced by concurrent thoracic radiotherapy and erlotinib. Radiation pneumonitis was caused by radiation damage to type II pneumocytes and the alveolar septum. The radiation also caused swelling of the capillary endothelium, interstitial congestion, and alveolar edema, thereby resulting in the infiltration of inflammatory cells, desquamation of the alveolar epithelial lining, and protein substance exudation due to interstitial inflammation of the lung.8–10 Moreover, many studies have reported that erlotinib has adverse effects on the pulmonary interstitium.11–13 Although the mechanisms have not yet been fully identified, and are awaiting further studies, both erlotinib and radiation can damage the pulmonary interstitium. Concurrent therapy can lead to a synergistic reaction between radiation and erlotinib, which further increases the risk of radiation pneumonitis.
A high level of radiation exposure during radiotherapy is the primary cause of radiation pneumonitis. Therefore, the most important preventive approach is to reduce the radiation dose to the lung tissues. During concurrent chemoradiotherapy, radiation pneumonitis is effectively prevented by strengthening the limits of the dosimetric parameters for pulmonary radiotherapy.14–16 Similarly, by alleviating pulmonary damage due to thoracic radiotherapy, and thereby reducing the synergistic reaction between thoracic radiotherapy and erlotinib on lung tissue during concurrent therapy, it is theoretically possible to reduce the risk of radiation pneumonitis. Therefore, it is important to investigate the differences in the dosimetric parameters of the lung in concurrent erlotinib and thoracic radiotherapy between patients who present with radiation pneumonitis and patients who present without radiation pneumonitis. It is also necessary to study the predictive effects of dosimetric parameters for radiation pneumonitis, and the threshold values of lung dosimetric parameters required for radiotherapy.
The results of the study confirmed the above mechanism, and provided preliminary values for lung dosimetric parameters of concurrent erlotinib and thoracic radiotherapy in clinical practice. With regard to clinical factors, PTV significantly affects radiation pneumonitis. The radiation field is usually larger in patients with a higher PTV value. Therefore, radiation penetrates comparatively more pulmonary tissue. We believe that the actual effect of PTV is due to the amount of radiation to which the lung is exposed. Furthermore, all dosimetric parameters showed statistically significant differences between patients with radiation pneumonitis and patients without radiation pneumonitis. The ROC curve analysis showed that all lung dosimetric parameters were predictive of radiation pneumonitis. Many studies have suggested that adjuvant chemotherapy aggravates radiation pneumonitis.17,18 However, no statistically significant difference was found in this study. The explanation for our results might be due in part to the fact that erlotinib was administered orally to patients during radiotherapy, and the patients did not usually receive chemotherapy within 1 month after concurrent therapy. The patients who did receive chemotherapy were usually given the therapy after a relatively long period of time following radiotherapy, which was longer than the formative period of radiation pneumonitis.
It should be noted that this work was also a pilot study. At the beginning of the study, the specific population that benefited most from erlotinib therapy had not yet been identified. Moreover, the best indication for concurrent thoracic radiotherapy and erlotinib had not been fully identified. Concurrent thoracic radiotherapy and erlotinib remains at the exploratory stage of clinical applications. Therefore, we included clinical cases of patients with different pathological diagnoses and clinical stages, as well as different treatment plans (different radiation doses). Nevertheless, since this study used radiation pneumonitis as the endpoint, the information from this case group remains valuable. In addition, prior available studies describe mostly individual case reports.19–21
The existing studies reported radiation pneumonitis as a phenomenon in the patient population and did not further investigate its etiology. By contrast, our study was novel in that we studied and analyzed the incidence of radiation pneumonitis in patients who had received concurrent erlotinib and thoracic radiotherapy, the predictive factors of pneumonitis, the lung dosimetric parameters, and the clinical threshold value for radiation. Therefore, this study provides new insights for the field. Further, some previous studies showed that fewer cases of radiation pneumonitis occurred in patients treated with a combination of erlotinib and thoracic radiotherapy.22 Additionally, some previous studies showed that a high incidence of radiation pneumonitis occurred during this treatment,20,21 but all samples from the different studies were small and the results clearly need to be confirmed by further research.
In summary, we conducted an important investigation of concurrent erlotinib and thoracic radiotherapy as a cause of radiation pneumonitis and its impact on lung dosi metric parameters. This work provides preliminary data of the threshold values of lung radiation for concurrent erlotinib and thoracic radiotherapy in clinical practice. Although the case numbers in this study were relatively small, and additional clinical cases are needed, we believe that concurrent thoracic radiotherapy and erlotinib will have broader prospects for clinical application as additional clinical data are summarized and reported moving forward.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (81301925).
Footnotes
Disclosure
The authors do not have any conflicts of interest in this work.
References
- 1.Schneider CP, Heigener D, Schott-von-Römer K, et al. Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: an analysis of patients from german centers in the TRUST study. J Thorac Oncol. 2008;3(12):1446–1453. doi: 10.1097/JTO.0b013e31818ddcaa. [DOI] [PubMed] [Google Scholar]
- 2.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multi-centre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 3.Reguart N, Cardona AF, Rosell R. Role of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancer. Cancer Manag Res. 2010;2:143–156. doi: 10.2147/cmar.s5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NSCLC Meta-analyses Collaborative Group. Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375(9722):1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 6.Lind JS, Smit EF, Grünberg K, Senan S, Lagerwaard FJ. Fatal interstitial lung disease after erlotinib for non-small cell lung cancer. J Thorac Oncol. 2008;3(9):1050–1053. doi: 10.1097/JTO.0b013e318183a9f5. [DOI] [PubMed] [Google Scholar]
- 7.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro DL, Finkelstein JN, Rubin P, Penney DP, Siemann DW. Radiation induced secretion of surfactant from cell cultures of type II pneumocytes: an in vitro model of radiation toxicity. Int J Radiat Oncol Biol Phys. 1984;10(3):375–378. doi: 10.1016/0360-3016(84)90057-9. [DOI] [PubMed] [Google Scholar]
- 9.Vasić L, Durdević P. Radiation-induced lung damage – etiopathogenesis, clinical features, imaging findings and treatment. Med Pregl. 2012;65:7–8. 319–325. doi: 10.2298/mpns1208319v. Serbian [with English abstract] [DOI] [PubMed] [Google Scholar]
- 10.Kong FM, Ten Haken R, Eisbruch A, Lawrence TS. Non-small cell lung cancer therapy-related pulmonary toxicity: an update on radiation pneumonitis and fibrosis. Semin Oncol. 2005;32(2 Suppl 3):S42–S54. doi: 10.1053/j.seminoncol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Ren S, Li Y, Li W, Zhao Z, Jin C, Zhang D. Fatal asymmetric interstitial lung disease after erlotinib for lung cancer. Respiration. 2012;84(5):431–435. doi: 10.1159/000339508. [DOI] [PubMed] [Google Scholar]
- 12.Tsubata Y, Hamada A, Sutani A, Isobe T. Erlotinib-induced acute interstitial lung disease associated with extreme elevation of the plasma concentration in an elderly non-small-cell lung cancer patient. J Cancer Res Ther. 2012;8(1):154–156. doi: 10.4103/0973-1482.95201. [DOI] [PubMed] [Google Scholar]
- 13.Barber NA, Ganti AK. Pulmonary toxicities from targeted therapies: a review. Target Oncol. 2011;6(4):235–243. doi: 10.1007/s11523-011-0199-0. [DOI] [PubMed] [Google Scholar]
- 14.Stinchcombe TE, Bogart JA. Novel approaches of chemoradiotherapy in unresectable stage IIIA and stage IIIB non-small cell lung cancer. Oncologist. 2012;17(5):682–693. doi: 10.1634/theoncologist.2012-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Rourke N, Roqué I, Figuls M, Farré Bernadó N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010;(6):CD002140. doi: 10.1002/14651858.CD002140.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baas P, Belderbos JS, van den Heuvel M. Chemoradiation therapy in nonsmall cell lung cancer. Curr Opin Oncol. 2011;23(2):140–149. doi: 10.1097/CCO.0b013e328341eed6. [DOI] [PubMed] [Google Scholar]
- 17.Solomon B, Bunn PA., Jr Adjuvant chemotherapy for non-small cell lung cancer. Cancer Invest. 2007;25(4):217–225. doi: 10.1080/07357900701206281. [DOI] [PubMed] [Google Scholar]
- 18.Barriger RB, Fakiris AJ, Hanna N, Yu M, Mantravadi P, McGarry RC. Dose-volume analysis of radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent cisplatinum and etoposide with or without consolidation docetaxel. Int J Radiat Oncol Biol Phys. 2010;78(5):1381–1386. doi: 10.1016/j.ijrobp.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Togashi Y, Masago K, Mishima M, Fukudo M, Inui K. A case of radiation recall pneumonitis induced by erlotinib, which can be related to high plasma concentration. J Thorac Oncol. 2010;5(6):924–925. doi: 10.1097/JTO.0b013e3181dab0dd. [DOI] [PubMed] [Google Scholar]
- 20.Nanda A, Dias-Santagata DC, Stubbs H, et al. Unusual tumor response and toxicity from radiation and concurrent erlotinib for non-small-cell lung cancer. Clin Lung Cancer. 2008;9(5):285–287. doi: 10.3816/CLC.2008.n.044. [DOI] [PubMed] [Google Scholar]
- 21.Chang CC, Chi KH, Kao SJ, et al. Upfront gefitinib/erlotinib treatment followed by concomitant radiotherapy for advanced lung cancer: a mono-institutional experience. Lung Cancer. 2011;73(2):189–194. doi: 10.1016/j.lungcan.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Xia TY, Wang YJ, et al. Prospective study of epidermal growth factor receptor tyrosine kinase inhibitors concurrent with individualized radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(3):e59–e65. doi: 10.1016/j.ijrobp.2010.12.035. [DOI] [PubMed] [Google Scholar]