Abstract
The DNA damage response kinase ATR and its effector kinase CHEK1 are required for cancer cells to survive oncogene-induced replication stress. ATR inhibitors exhibit synthetic lethal interactions with deficiencies in the DNA damage response enzymes ATM and XRCC1 and with overexpression of the cell cycle kinase Cyclin E. Here we report a systematic screen to identify synthetic lethal interactions with ATR-pathway targeted drugs, rationalized by their predicted therapeutic utility in the oncology clinic. We found that reduced function in the ATR pathway itself provided the strongest synthetic lethal interaction. In addition, we found that loss of the structure specific-endonuclease ERCC1-XPF (ERCC4) is synthetic lethal with ATR pathway inhibitors. ERCC1-deficient cells exhibited elevated levels of DNA damage, which was increased further by ATR inhibition. When treated with ATR or CHEK1 inhibitors, ERCC1-deficient cells arrested in S phase and failed to complete cell cycle transit even after drug removal. Notably, triple-negative breast cancer cells and non-small cell lung cancer cells depleted of ERCC1 exhibited increased sensitivity to ATR-pathway targeted drugs. Overall, we concluded that ATR pathway-targeted drugs may offer particular utility in cancers with reduced ATR pathway function or reduced levels of ERCC4 activity.
Keywords: ATR inhibitor, ERCC1, XPF, synthetic lethal, CHK1 inhibitor
Introduction
Replicating DNA is sensitive to a wide array of endogenous and exogenous damaging agents, which can lead to replication fork stalling. To accomplish error free replication, stalled replication forks need to be stabilized to prevent their collapse into double strand breaks (DSBs), which can lead to genomic rearrangements and/or apoptosis(1, 2). The DNA damage response kinase ATR coordinates many of the activities at stalled forks including fork stabilization and restart(3). ATR activation also slows the cell cycle to allow for DNA repair through the phosphorylation of CHK1(3).
The loss of one or more DNA damage response or repair pathways drives tumorigenesis, but also forces cancer cells to be more reliant on other pathways, providing an opportunity for the development of targeted therapeutics(4). For example, cancers with mutations in BRCA1/2 are sensitive to PARP inhibitors since the replication-associated DSBs caused by PARP inhibition cannot be repaired when the BRCA-dependent homologous recombination system is non-functional(5, 6). PARP inhibitors can also trap PARP on DNA creating a toxic intermediate that requires a second DNA repair pathway such as homologous recombination or postreplicative repair to remove the PARP-DNA complexes(7). The synthetic lethality between PARP inhibitors and mutations in BRCA1/2 provides a paradigm for combining DNA repair inhibitors with specific mutations or in combination with chemotherapy agents.
Oncogene activation often initiates an ATR-dependent replication stress response that is needed for continued cell growth(8–10). Thus, ATR pathway inhibitors are being developed as cancer therapeutics. For example, CHK1 inhibitors have shown promise in pre-clinical models, and there are several ongoing and completed Phase I and II clinical trials(11–13). Recently, specific ATR inhibitors have been described by AstraZeneca(14), Vertex Pharmaceuticals(15, 16), and the Fernandez-Capetillo lab(17). These inhibitors function to inhibit the growth of cancer cell lines in vitro and synergize with DNA damaging agents such as cisplatin(15). Furthermore, ATR inhibition demonstrated efficacy in a xenograft mouse model of pancreatic cancer in combination with gemcitabine(18).
ATR inhibitors also exhibit synthetic lethal interactions with ATM and XRCC1 deficiency as well as with Cyclin E over-expression(15, 17, 19). To date no systematic approach to identify synthetic lethal interactions with ATR-pathway targeted drugs has been reported. This information could be used in the clinic to design better clinical trials with ATR-pathway targeted drugs and improve patient outcomes.
The current study was initiated to identify genes that, when lost, exhibit synthetic lethal relationships with ATR pathway inhibitors. We conducted a synthetic lethal screen with DNA repair proteins and identified reduced ATR pathway function and the ERCC1-XPF nuclease as synthetic lethal with ATR or CHK1 inhibition. ERCC1-XPF functions in the repair of bulky DNA adducts, double strand breaks, interstrand crosslinks (ICLs), and separation of sister chromatids at fragile sites(20, 21). Importantly, ERCC1 is being explored as a potential biomarker in lung and other kinds of cancer(22–25). Low levels of ERCC1 expression correlate with greater sensitivity to cisplatin and higher 5-year survival rates. Several phase II clinical trials are also underway using ERCC1 protein levels to determine whether to treat patients with platinum-based chemotherapy(22, 23). Our data demonstrate that both triple-negative breast cancer and non-small cell lung cancer cell lines depleted of ERCC1 exhibit increased sensitivity to ATR-pathway inhibition. Thus, ERCC1 status may be a useful indicator of sensitivity to ATR-pathway targeted drugs.
Materials and Methods
Cells and reagents
U2OS and 293T cells were obtained from the ATCC and maintained in DMEM supplemented with 7.5% FBS. Triple negative breast cancer cells lines BT549 and HCC1806 and non-small cell lung cancer cells lines A549 and H157 were obtained from ATCC and maintained in RPMI supplemented with 10% FBS. The HCT-116 derived ATR flox/+ and ATR flox/− were previously described (26). XPF-deficient fibroblasts XP2YO and XP2YO + XPF were provided by Orlando Scharer in August 2013 and maintained as described(27). The ERCC1-null (clone 216) and complemented A549 cells (clone 216 + 202) were provided by Jean Charles Soria, Ken Olaussen, and Luc Friboulet in December 2013 and maintained as described(25). All cells lines were thawed from early passage stocks and were passaged less than 30 times prior to use. No further cell line authentication was performed. The ATR inhibitor VE-821(15) was synthesized by the Vanderbilt Institute for Chemical Biology Chemical Synthesis Facility. The CHK1 inhibitor AZD7762 was previously described(28).
Synthetic lethal siRNA screen
The custom siRNA library targets 240 known DNA replication and DNA repair genes with four unique siRNAs per gene in individual wells. The siRNA was plated into 96-well plates in replicates and frozen at −80C. The plates were thawed and siRNA was resuspended in Optimem with Dharmafect1. U2OS cells were then added to each plate. The final siRNA concentration was 10nM. Cells were split into two 96-well dishes 72 hours after transfection and incubated in media with or without ATR inhibitor at a final concentration of 1 μM for an additional 72 hours. Cell viability was measured with alamar blue (Invitrogen). Non-fluorescent cell-permeable alamar blue dye is reduced to a molecule that fluoresces red in metabolically active cells which allows for a quantitative measure of viability. The percent viability of ATR inhibitor treated to untreated was determined for each siRNA to take into account siRNA specific effects on cell growth. The Z scores were calculated using the mean and standard deviation of the log10(Perecent Viability) values. The values presented in Figure 1 are the mean Z-scores from three independent transfections.
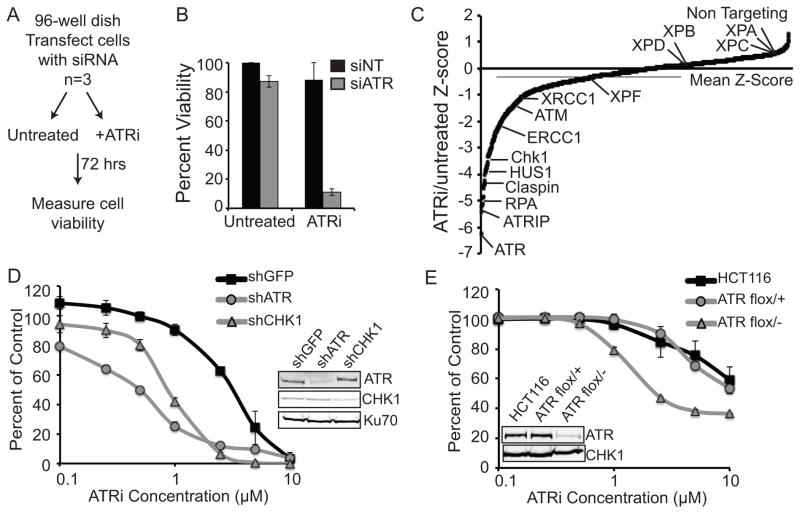
Figure 1. siRNA screen identifies synthetic lethal interactions with ATR inhibition.
(A) Schematic of the siRNA screen. U2OS cells were transfected with siRNAs and then were left untreated or treated with 1μM ATRi. Cell viability was determined with alamar blue. (B) Percent viability of the non-targeting (NT) and ATR siRNA controls from the screen. Values represent the mean ± standard deviation of 3 independent replicates. (C) The Z-scores of treated compared with untreated cell viability for each gene is shown. Each data point represents the mean of 3 independent replicates and calculation of the Z-scores is described in the Materials and Methods. (D) U2OS cells were infected with lentiviruses expressing the indicated shRNA and selected with puromycin and then treated with increasing doses of ATRi for 72 hours. (E) HCT-116 wild type, ATR flox/+, and ATR flox/− cells were treated with increasing doses of ATRi for 72 hours. For D and E, cell viability was determined with alamar blue and reported as a percent of the untreated control cells.
RNAi transfection and sequences
All siRNA transfections were done with 10nM siRNA and Dharmafect 1 (Invitrogen). Cells were transfected with individual siRNAs 72 hours before the start of an experiment. The following siRNA sequences were used siERCC1-1 GAGAAGAUCUGGCCUUAUG, siERCC1-2 CGACGUAAUUCCCGACUAU, siERCC1-4 CAGCGGACCUCCUGAUGGA, siXPF-2 GUAGGAUACUUGUGGUUGA, siXPF-3 ACAAGACAAUCCGCCAUUA, siXPC-2 GGAGGGCGAUGAAACGUUU, siXPC-3 GAGGUGGACUCUCUUCUGA, siXPA-1 GAAAGACUGUGAUUUAGAA, siXPA-2 GCCAUGAACUGACAUAUGA. The non-targeting siRNA was Qiagen All Star Negative control. Construction and use of lentiviruses expressing shRNA targeting GFP, ATR, and CHK1 has been previously described(29).
Cell Viability Assays
Cells were plated in 96-well plates 72 hours after siRNA transfection, the ATR or CHK1 inhibitor was added to the media for an additional 72 hours and cell viability was measured with alamar blue. Values represent the mean (n=6) and error bars represent the standard deviation. For statistical analysis, normalized data were fitted to nonlinear regression curves and used to determine IC50 values. These IC50 values were compared using an F test in Prism version 6.
Western blot analysis
Performed as previously described(30). Primary antibodies ERCC1 (D-10) and PCNA (FL-261) were purchased from Santa Cruz; H2B (Ab1790) and Ku70 (Ab3114) were purchased from Abcam; XPF (Bethyl Labs A301-315); XPC (Novus NB100-477); XPA (NeoMarkers MS-650-P0); Cleabed-PARP (Cell Signaling 9541); and GAPDH (Millipore MAB374).
Immunofluorescence analysis
Performed and quantified as previously described(30). The γH2AX (JBW301) antibody was purchased from Millipore. Nuclei were stained with DAPI.
Cell cycle analysis and isolation of proteins on nascent DNA (iPOND)
Propidium iodide staining and iPOND were performed as previously described(30–32).
Results
Identification of synthetic lethal interactions with ATR inhibition
We performed a siRNA synthetic lethal screen with an ATR inhibitor (ATRi) to determine cancer contexts that might provide a therapeutic window for ATR pathway drugs. Since ATR is required for the cellular response to many forms of DNA damage during S-phase we reasoned that ATR pathway inhibition might be most useful in cancer cells lacking other genome maintenance pathways. Therefore, we generated a custom library targeting 240 DNA replication and repair genes with 4 individual siRNAs per gene. The library was transfected into U2OS cells, then cells were either left untreated or treated with ATRi for 72 hours and cell viability was measured with alamar blue dye (Fig 1A). Cell viability of each ATRi treated sample was scored and compared to the same untreated siRNA to control for any siRNA specific effects. The dose of ATRi was optimized such that it had only minimal cell killing on cells expressing a control siRNA and had maximal killing of cells depleted of ATR (Fig. 1B). Lower doses of ATRi are sufficient to kill cells depleted of ATR presumably because less ATRi is needed to completely inhibit all of the remaining ATR protein. This result also suggests that the ATR inhibitor does not work by trapping an inactive protein at the site of damage like PARP inhibitors. The percent viability was calculated for all genes in the library and converted to a Z-score as described in the Materials and Methods. Smaller Z-scores indicate more confident synthetic lethal relationships to ATRi.
Genes in the ATR pathway were the largest family of DNA repair proteins to yield strong synthetic lethal relationships with ATR inhibition (Fig. 1C and Table S1). While not a comprehensive library, every known ATR pathway gene in the library was synthetic lethal (at least 3 out of 4 siRNAs) with ATRi including ATR, ATRIP, RPA, CHEK1, CLSPN, HUS1, RAD1, RAD17, TIMELESS, and TIPIN. ATR pathway genes are mutated or deleted in up to 25% of some cancer types(33), suggesting that reduced functionality of the ATR pathway itself could provide a therapeutic window for ATR-targeted therapies. To confirm that reduced expression of ATR pathway genes is synthetic lethal with ATRi we depleted cells of ATR or CHK1 using shRNA and performed dose response curves with ATRi. Silencing of both ATR and CHK1 sensitized cells to ATR inhibition as compared to a control shRNA (Fig. 1D). Lastly, we confirmed that even heterozygous mutation in ATR is sufficient to hypersensitize cancer cells to ATR inhibitors using HCT116 cells lacking one ATR allele (Fig. 1E)(26). The known synthetic lethal interactions with ATM and XRCC1 were also identified in the screen further validating the screening procedure.
ERCC1-XPF-decificent cells are sensitive to ATR pathway inhibition
One of the high scoring genes identified in the synthetic lethal screen was ERCC1. As described in the introduction, ERCC1 has recently been suggested as an important biomarker in lung and other kinds of cancer(22). To validate ERCC1, and its binding partner XPF, deficiencies as synthetic lethal with ATR inhibition we knocked down ERCC1 and XPF with two individual siRNAs and performed dose response curves with ATRi. Both siRNAs for each gene sensitized cells to ATRi by as much as one order of magnitude (Fig. 2A–B). To test if the synthetic lethality was specific to ATR or more generalizable to the ATR pathway, we also treated knockdown cells with a CHK1 inhibitor (CHK1i). Both ERCC1 and XPF knockdown also sensitized cells to CHK1i, suggesting that inhibition of the ATR pathway and not only ATR is synthetic lethal in ERCC1-XPF deficient cells. ERCC1 knockdown always yielded greater synthetic lethality with ATRi than XPF, although knockdown of either significantly reduced the IC50 values of ATRi and CHK1i as compared to control cells. This difference may derive from the differences in viability caused by the ERCC1 or XPF knockdown on their own. In all siRNA experiments presented, knockdown of XPF by itself reduces the viability of cells more than knockdown of ERCC1. For example, in U2OS cells knockdown of ERCC1 or XPF results in 80% and 60% viability respectively. Thus, it is harder to observe large losses in viability with the addition of ATR pathway inhibitors on top of the lethality caused by XPF knockdown alone. To control for this we tested synthetic lethality by colony forming assays, which are not influenced by different rates of cell growth. We observe that both ERCC1 and XPF knockdown significantly sensitize cells to ATRi and CHK1i treatment (Fig. 2C). As expected, knockdown of ERCC1 and XPF also sensitized cells to cisplatin.
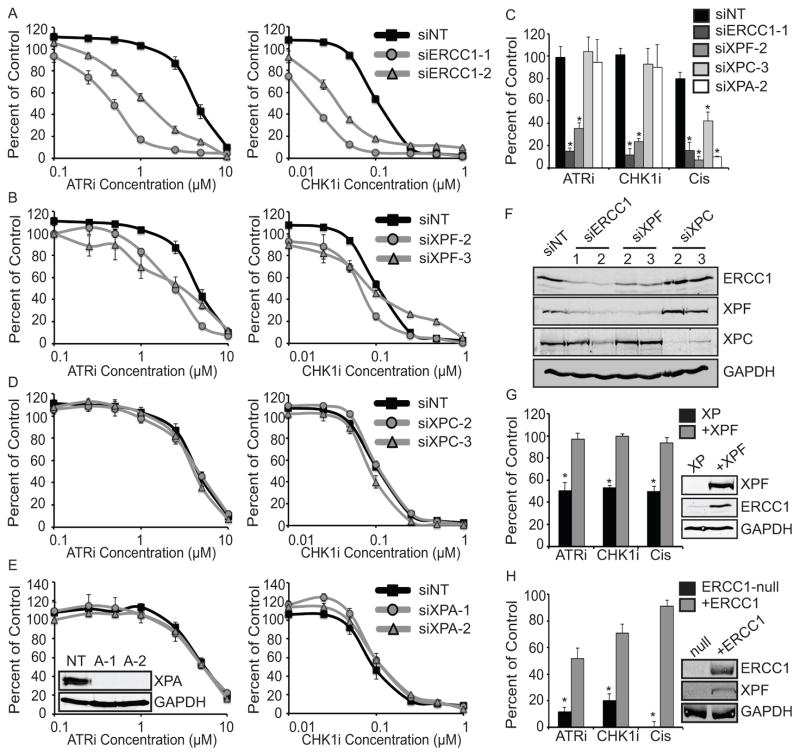
Figure 2. ATR pathway inhibition is synthetic lethal with ERCC1/XPF deficiency.
(A–D) U2OS cells were transfected with the indicated siRNAs and then treated with the ATR or CHK1 inhibitor. NT represents the non-targeting siRNA and the numbers after specific siRNAs refer to the sequences described in the Materials and Methods. Cell viability was determined with alamar blue and reported as a percent of the untreated control cells for (A) ERCC1, (B) XPF, (C) XPC, (D) XPA. All knockdowns were done simultaneously and separated into individual graphs for ease in comparison, and the control siRNA values are identical in each graph. Knockdown of ERCC1 or XPF yielded significant reductions in IC50 values as compared to control cells (p<0.001) while knockdown of XPC or XPA did not. Statistics are described in the materials and methods. (E) U2OS cells transfected with the indicated siRNAs were treated with 1μM ATRI, 0.05μM CHK1i, or 1μM cisplatin for 24 hours and surviving colonies were scored 14 days later. * p<0.001, as compared to the control siRNA for each treatment. (D and F) Western blots of the cells used in A–E. (G) XPF-deficient patient fibroblast (XP) and cells complemented with XPF (+XPF) and (H) ERCC1-null A549 cells and cells complemented with ERCC1 (+ERCC1) were either left untreated or treated with 1μM ATRI, 0.05μM CHK1i, or 1μM cisplatin for 24 hours and surviving colonies were scored 14 days later. * p<0.001, as compared to the complemented cell line for each treatment. Western blots show ERCC1 and XPF levels in the null and complemented cell lines.
Since ERCC1-XPF regulates sensitivity to cisplatin via the nucleotide excision repair (NER) and interstrand crosslink (ICL) repair pathways we wished to determine if loss of NER sensitized cells to ATR pathway inhibition. Neither the core NER genes XPC and XPA nor the TFIIH components XPB and XPD were synthetic lethal with ATRi in our screen (Fig. 1C). When tested individually, XPC and XPA depletion sensitized cells to cisplatin but did not sensitize cells to ATRi or CHK1i in either short term growth/viability or colony forming assays (Fig. 2C–E) validating the screen results and suggesting that NER deficiency does not sensitize cells to ATR pathway inhibition. Protein knockdown for all siRNAs was confirmed by western blotting (Fig. 2E and F). As expected, knockdown of ERCC1 also knocked down XPF and vice versa with siRNA targeting ERCC1 more efficiently reducing the levels of both proteins as compared to siRNA targeting XPF (34, 35). One of the siRNAs targeting ERCC1 also mildly reduced the level of XPC, but since the other siRNA did not knock down XPC and since XPC knockdown had no effect on synthetic lethality, this potential off-target effect cannot explain the observed synthetic lethality. The dose response curves are presented as percent of untreated controls and values above 100 percent for low doses of ATRi and CHK1i are likely due to increased rates of cell growth associated with partial ATR pathway inhibition.
To further confirm the specificity of the synthetic lethality with ERCC1/XPF we tested it in more well-defined genetic systems to eliminate the possibility of any off target effects of siRNA knockdowns. XPF mutant patient cells were more sensitive to ATRi, CHK1i, and cisplatin as compared to the complemented cells (Fig. 2G). In addition, A549 lung cancer cells in which ERCC1 was specifically disrupted were more sensitive to ATRi, CHK1i, and cisplatin as compared to the complemented cells (Fig. 2H). These two isogenic cell lines with deficiencies in XPF or ERCC1 further validate the specificity of this genetic interaction and demonstrate that loss of ERCC1 and XPF are required for the observed synthetic lethality.
ERCC1-deficiency causes elevated DNA damage that is further increased when the ATR pathway is inhibited
In a previous screen, knockdown of ERCC1 and XPF both caused significant increases in the amount of γH2AX suggesting the induction of DNA damage in their absence(36). We confirmed this observation by immunofluorescence of control and ERCC1-depleted cells either untreated or treated with ATRi. The dose of ATRi used does not cause a noticeable increase in the amount of γH2AX in control cells; however ERCC1 knockdown causes a significant increase in the amount of γH2AX in untreated cells, which is further increased in ATRi treated cells (Fig. 3A and B). Of note, in untreated cells ERCC1 knockdown results in γH2AX foci but when ATRi is added the staining changes to a combination of foci and pan-nuclear γH2AX, with the pan-nuclear staining being much brighter than the staining of γH2AX in foci (Fig. 3A), Pan-nuclear γH2AX is often associated with high levels of replication stress(30, 37).
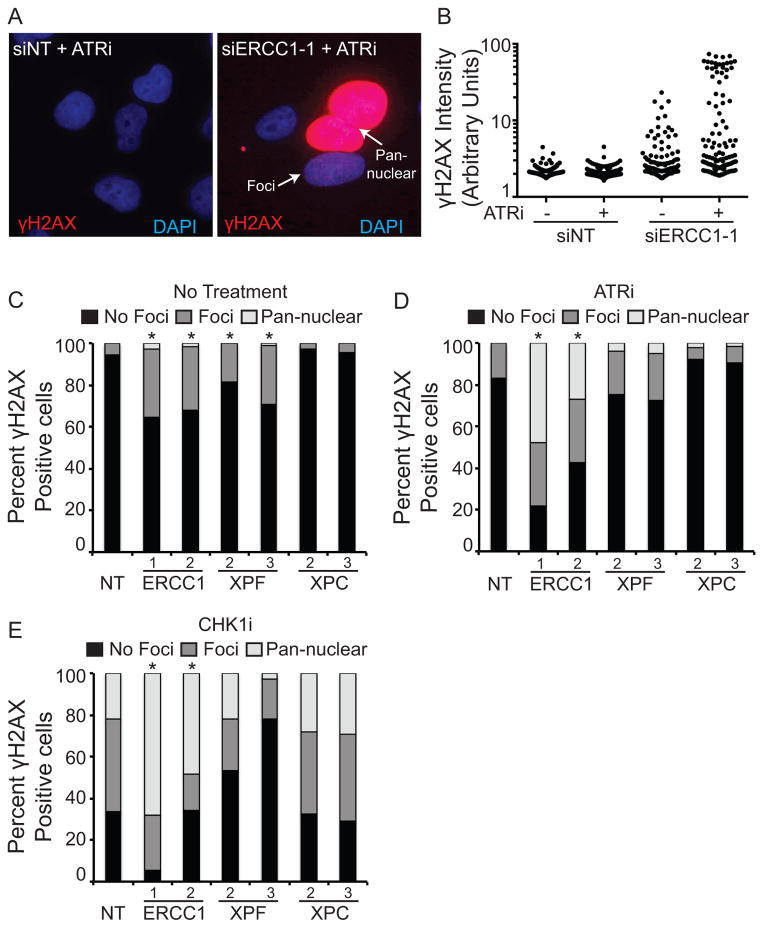
Figure 3. ERCC1/XPF knockdown causes γH2AX elevation, which is further increased in the presence of ATR and CHK1 inhibitors.
U2OS cells were transfected with the indicated siRNAs and then treated with 1μM ATRi or 0.05μM CHK1i for 24 hours. Cells were then fixed and stained for γH2AX. (A) Representative images of Non-Targeting (NT) or ERCC1 siRNA transfected cells treated with the ATR inhibitor. Both cells with γH2AX foci and pan-nuclear γH2AX are indicated with arrows. (B) Quantification of γH2AX intensity of cells shown in panel A. (C–E) The percentage of γH2AX in foci (>10 foci/cell) or in a pan-nuclear staining pattern was scored for (C) untreated, (D) ATRi treated, and (E) CHK1i treated cells. At least 200 cells were scored for each condition between two independent experiments. * p<0.05 for each siRNA sample compared to the control cells for each condition.
To further quantify this data we scored the percentages of cells with no γH2AX, γH2AX in foci, and γH2AX in a pan-nuclear staining pattern in untreated, ATRi, and CHK1i treated cells. ERCC1 and XPF knockdown both caused a statistically significant increase in the amount of γH2AX foci in untreated cells (Fig 3C). The amount of these foci was increased, as where the percentage of cells exhibiting pan-nuclear γH2AX with the addition of ATRi (Fig. 3D). As expected, CHK1i treatment of control cells caused a large increase in the amount of γH2AX foci and a small percent of cells with pan-nuclear γH2AX (Fig. 3E)(38). ERCC1 knockdown further increased these phenotypes with as many as 50–70 percent of cells exhibiting pan-nuclear γH2AX (Fig. 3E). As observed previously, the increases in γH2AX were larger in ERCC1-depleted cells than in XPF-depleted cells, likely due to the slower growth rate of XPF-depleted cells. XPC knockdown exhibited the same phenotypes as control knockdown cells, further confirming our observation that the synthetic lethality is independent of NER. Together, these experiments suggest that the ATR pathway is needed to repair DNA damage caused by loss of ERCC1-XPF.
The ATR pathway is required for S-phase progression in ERCC1-deficient cells
To determine the mechanism for synthetic lethality with ATRi in ERCC1-deficient cells we knocked down ERCC1, treated cells with either ATRi or CHK1i and analyzed their progression through the cell cycle. Control cells left untreated or treated with either inhibitor completed DNA replication and proceeded to M phase of the cell cycle where they arrested at the mitotic checkpoint in the presence of nocodazole (Fig. 4A and B). Conversely, ERCC1-deficient cells treated with ATRi accumulated in S-phase and did not complete DNA synthesis (Fig. 4B). The ERCC1-deficient cell population exhibits a large enrichment in S-phase and a reduction in cells with 4N DNA content (Fig. 4C). Furthermore, ATRi-treated, ERCC1-deficient cells exhibit higher levels of cleaved PARP at both 24 and 48 hours after addition of ATRi as compared to control ATRi treated control cells (Fig. 4D). This is also consistent with the increase in cells with less than 2N DNA content in ERCC1-deficient cells observed by flow cytometry (Fig. 4B and C). Thus, ERCC1-deficient cells undergo apoptosis after addition of ATR pathway targeted drugs.
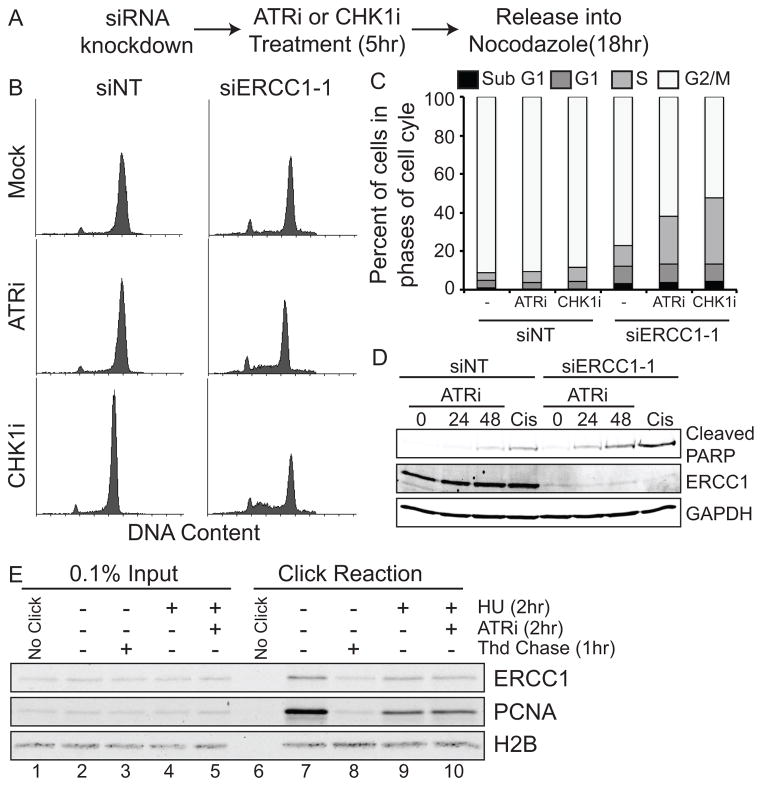
Figure 4. ERCC1 knockdown causes an S-phase arrest in ATRi and CHK1i treated cells.
U2OS cells were transfected with the indicated siRNAs, treated with 1μM ATRi or 0.05μM CHK1i for 5 hours, and released into nocodazole for 18 hours. Cells were then fixed and stained with PI and analyzed by flow cytometry. (A) Diagram of experimental design. (B) Histograms of DNA content. (C) Quantification of the percent of cells in each phase of the cell cycle. At least 10,000 live cells were analyzed for each condition. (D) U2OS cells were treated as in A with the addition of EdU prior to addition of the ATR inhibitor. Cells were fixed and stained for EdU and P-Histone H3 (pH3). At least 100 cells were scored for each condition. (E) 293T cells labeled for 10 minutes with EdU were chased into low dose thymidine, HU, or HU and ATRi as described in the materials and methods. Western blots were done for ERCC1, PCNA, and H2B.
Since ERCC1-deficient cells accumulate in S-phase, we reasoned that ERCC1 may be necessary for DNA replication in cancer cells. To test this we purified replication forks using iPOND (isolation of proteins on nascent DNA)(31, 32) and identified ERCC1 at replication forks (lane 7, Fig. 4E). Briefly, cells were labeled with the thymidine analog EdU for a short amount of time to label active replication forks. The EdU was then purified and proteins co-purifying with it were analyzed by Western blot analysis. ERCC1 was also present at stalled (HU treated) and collapsed forks (HU and ATRi treated) (lanes 9 and 10, Fig. 4E). Its association with replication forks is not due to a general interaction with chromatin since ERCC1 is not associated with bulk chromatin that is not adjacent to a replication fork, as it can be chased away with thymidine (lane 8, Fig. 4E). These results are also consistent with the prior identification of XPF at replication forks using iPOND(39).
ATR pathway inhibition does not increase micronuclei formation in ERCC1-deficient cells
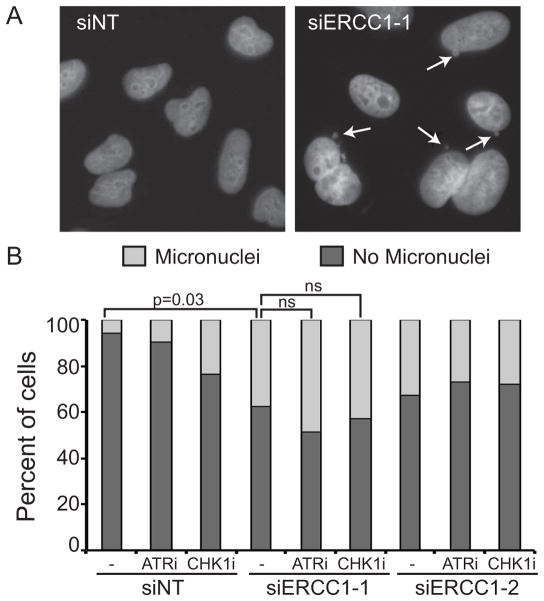
After DNA replication, ERCC1 plays a role in proper segregation of chromosomes and knockdown of ERCC1 causes an increase in micronuclei formation as chromosomes fail to segregate properly(40, 41). As expected, we observed a significant increase in the amount of micronuclei formation when we knocked down ERCC1 (Fig. 5A–B). This induction was not increased further with the addition of ATRi or CHK1i. These data suggest that ATR pathway inhibition does not affect the post-replicative functions of ERCC1, such as chromosome segregation.
Figure 5. ERCC1 knockdown-induced micronuclei formation is not increased in ATRi or CHK1i treated cells.
U2OS cells were transfected with non-targeting or ERCC1 siRNA and then treated with 1μM ATRi or 0.05μM CHK1i for 24 hours. Cells were then fixed and stained with DAPI to score micronuclei formation. (A) Representative images of micronuclei formation in ERCC1 depleted cells. The arrows indicate micronuclei. (B) Quantification of micronuclei formation. At least 200 cells were scored for each condition between two independent experiments. p values are indicated on the graph, ns = not significant.
ERCC1 deficiency sensitizes cancer cell lines to ATR pathway targeted drugs
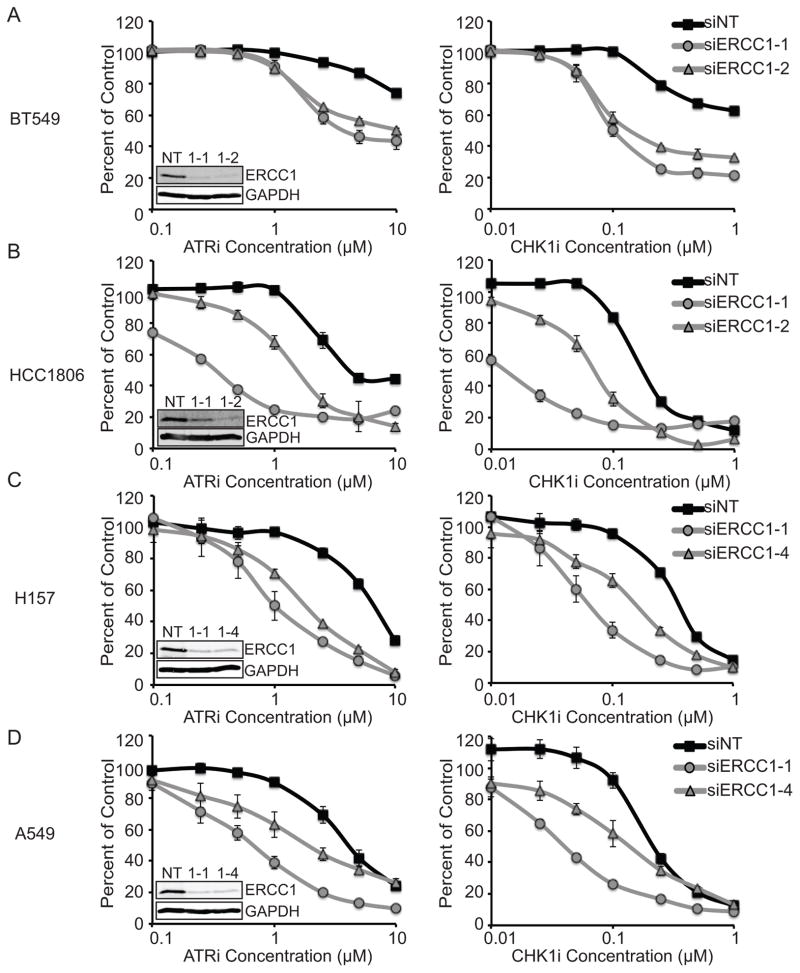
To test how generalizable our observations were we extended our study to include two triple negative breast cancer cell lines and two non-small cell lung cancer cell lines. Dose response curves of ATRi and CHK1i were completed on cancer cell lines depleted of ERCC1 with two specific siRNAs (Fig. 6). In all cases, cells depleted of ERCC1 were more sensitive to ATRi and CHK1i treatment than control cells. We did not observe a strict correlation between the degree of ERCC1 knockdown and the observed synthetic lethality perhaps due to changes in cell growth rates or unknown off-target effects. Nonetheless, these data indicate that ERCC1 protects cancer cells from ATR pathway targeted drugs in multiple cancer types.
Figure 6. ERCC1 depletion sensitizes Triple Negative Breast Cancer and Non-Small Cell Lung Cancer cell lines to ATRi and CHK1i.
(A) BT549, (B) HCC1806, (C) H157, and (D) A549 were transfected with the indicated control or ERCC1 siRNA and then treated with the ATR or CHK1 inhibitor. Cell viability was determined with alamar blue and reported as a percent of the untreated control cells. Knockdown of ERCC1 yielded significant reductions in IC50 values for both siERCC1 siRNAs as compared to control cells (p<0.001). Statistics are described in the materials and methods. Western blots of cells after knockdown are shown in the insets.
Discussion
ATR pathway inhibitors are currently in clinical trials and have shown promise in combination with platinum drugs and gemcitabine(11, 15, 18). The current study was initiated to determine if any synthetic lethal interactions exist between DNA repair proteins and ATR pathway targeted drugs. Our siRNA screen identified ERCC1-XPF deficiency as a potent sensitizer to ATR-pathway inhibition in cancer cells. Loss of ERCC1 or XPF causes an increase in the number of γH2AX foci in untreated cells and addition of the ATR or CHK1 inhibitors results in pan-nuclear γH2AX, suggestive of large amounts of replication stress. ATR and CHK1 inhibitors cause an S-phase arrest in ERCC1-deficient cells and these cells fail to enter mitosis even when the inhibitors are removed. ERCC1-XPF are often lost in many types of cancers(22, 23, 42), and we report that depletion of ERCC1-XPF in both triple negative breast cancer and non-small cell lung cancer sensitize these cancer cells to killing with ATR pathway targeted drugs. Together, our data indicated that ERCC1 status in cancer may help guide the choice of an ATR pathway targeted drug for treatment.
Impaired ATR pathway function sensitizes to ATR inhibitors
In addition to ERCC1-XPF, our screen also identified the known synthetic lethal interactions between ATM(15) and XRCC1(19) suggesting the validity of our approach and screen design. In addition, we also observed strong synthetic lethality with all of the known ATR pathway genes that were present in the siRNA library. ATR pathway genes are mutated or deleted in up to 15–25% of samples from some cancer types suggesting that reduced ATR pathway functionality may be a useful criteria for selecting patients for ATR targeted drugs(33). Furthermore, the screening strategy is also a useful approach to identify novel ATR-pathway proteins, some of which may prove to be druggable targets, such as the recently reported Replication Protein A inhibitors(43, 44).
Notably, not all DNA repair pathways are synthetic lethal with ATR inhibition. For example, neither nucleotide excision repair nor mismatch repair deficiencies cause hypersensitization to ATR inhibitors. This study represents the first systematic search for synthetic lethal interactions with ATR pathway targeted drugs. Performing this screen with the CHK1 inhibitor or in a whole genome siRNA library may reveal additional genetic relationships that can be exploited for cancer therapy.
ERCC1-XPF synthetic lethality with ATR inhibition
We present two possible explanations for the observed synthetic lethality between ERCC1-XPF deficiency and ATR inhibition. First, we and others have observed ERCC1-XPF at replication forks and it is possible that in their absence, replication forks become unstable and require ATR to maintain them. Second, defects in the repair of interstrand crosslinks and replication of genomic fragile sites when ERCC1-XPF are inactivated (20, 21) may create an increased need for ATR signaling. The first possible explanation is unlikely, as it does not appear that ERCC1 loss affects the inherent stability of stalled replication forks. ERCC1 depleted cells are not sensitive to hydroxyurea, a fork stalling agent, and are able to resume DNA synthesis quickly after HU removal ((45) and data not shown).
We favor the second explanation that incomplete repair and replication processes induced by loss of ERCC1-XPF are the source of ATR dependency. The amount of endogenous DNA damage is increased in the absence of ERCC1-XPF as indicated by the increase in γH2AX. This γH2AX is likely caused as incomplete repair intermediates collapse into double strand breaks. Repair of these breaks and completion of DNA replication require ATR, explaining the observed synthetic lethality. This explanation is also consistent with our observation that ERCC1-XPF deficient cells do not complete S-phase when ATR is inhibited. Importantly, the repair requirement for ERCC1-XPF that is synthetic lethal with ATR inhibition is not standard nucleotide excision repair since other NER genes are not synthetic lethal with ATRi.
We did observe some differences between knockdown of ERCC1 and XPF in some assays. We suspect this difference is largely due to differences in knockdown efficiencies and resulting cell growth rates. The similar results seen in the colony forming assays, which are less influenced by cell growth rates than the alamar blue assay are consistent with this explanation. However, it is also possible that some differences are due to XPF-independent functions of ERCC1(46).
Loss of ERCC1-XPF is also synthetic lethal with PARP inhibition although the mechanism is likely to be different than the synthetic lethality observed with ATR. ERCC1 deficient cells treated with PARP inhibitors undergo a prolonged G2/M arrest accompanied by activated checkpoint signaling(45, 47). The authors of these studies suggest that in the context of PARP inhibition, the essential role of ERCC1 is in homologous recombination, as ERCC1 loss yields the same phenotype as BRCA2 loss. This contrasts with the strong S-phase arrest seen in ERCC1-deficient cells treated with ATRi. Our data suggest that ERCC1 is also functioning during DNA replication, presumably to repair endogenous sources of DNA damage that stall the replication fork and in the resolution of replication intermediates at fragile sites.
ERCC1 as a predictive biomarker for ATR pathway targeted therapies
ERCC1 is a recognized modulator of the response to platinum-based chemotherapies(22, 25). As such, much effort has been devoted to evaluating ERCC1 levels in tumor samples to guide treatment. While there has been controversy in the best methods to detect ERCC1 in tumors due to the inability of PCR and antibody based detection methods to discriminate between the functional and non-functional ERCC1 isoforms(22, 25), several laboratories are working to develop appropriate tumor screening methods. Thus clinical evaluation of ERCC1 levels in tumors is a viable diagnostic option prior to the initiation of chemotherapy. Depletion of ERCC1 in every cancer cell line we have tested sensitizes the cells to ATR pathway inhibition. Therefore, tumors with low levels of ERCC1 may prove more sensitive to ATR pathway inhibitors and provide a patient selection strategy. We are also excited about the recent disclosure of ERCC1-XPF small molecule inhibitors(48). These inhibitors, combined with ATR pathway targeted drugs, could be used in combination therapies to treat cancer cells and improve outcomes.
Supplementary Material
Acknowledgments
This work was supported by a Breast Cancer Research Foundation and NIH CA102792 grants to DC, T32CA093240 to KNM, and F32GM103254 to GMK.
We thank Orlando Scharer for providing the XPF mutant patient cells and Jean Charles Soria, Ken Olaussen, and Luc Friboulet for providing the ERCC1-null cells.
Footnotes
The authors report no conflict of interest.
References
- 1.Aguilera A, Gomez-Gonzalez B. Genome instability: a mechanistic view of its causes and consequences. Nature reviews Genetics. 2008 Mar;9(3):204–17. doi: 10.1038/nrg2268. [DOI] [PubMed] [Google Scholar]
- 2.Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010 Mar;11(3):208–19. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- 3.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008 Aug;9(8):616–27. doi: 10.1038/nrm2450. Epub 2008/07/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nature reviews Cancer. 2012 Dec;12(12):801–17. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- 5.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005 Apr 14;434(7035):913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 6.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005 Apr 14;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 7.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012 Nov 1;72(21):5588–99. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005 Apr 14;434(7035):864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 9.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005 Apr 14;434(7035):907–13. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 10.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007 Dec 10;26(56):7773–9. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 11.Carrassa L, Damia G. Unleashing Chk1 in cancer therapy. Cell Cycle. 2011 Jul 1;10(13):2121–8. doi: 10.4161/cc.10.13.16398. [DOI] [PubMed] [Google Scholar]
- 12.Garrett MD, Collins I. Anticancer therapy with checkpoint inhibitors: what, where and when? Trends in pharmacological sciences. 2011 May;32(5):308–16. doi: 10.1016/j.tips.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Ma CX, Janetka JW, Piwnica-Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends in molecular medicine. 2011 Feb;17(2):88–96. doi: 10.1016/j.molmed.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foote KM, Blades K, Cronin A, Fillery S, Guichard SS, Hassall L, et al. Discovery of 4-{4-[(3R)-3-Methylmorpholin-4-yl]-6-[1- (methylsulfonyl)cyclopropyl]pyrimidin-2-y l}-1H-indole (AZ20): a potent and selective inhibitor of ATR protein kinase with monotherapy in vivo antitumor activity. J Med Chem. 2013 Mar 14;56(5):2125–38. doi: 10.1021/jm301859s. [DOI] [PubMed] [Google Scholar]
- 15.Reaper PM, Griffiths MR, Long JM, Charrier JD, Maccormick S, Charlton PA, et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nature chemical biology. 2011 Jul;7(7):428–30. doi: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- 16.Charrier JD, Durrant SJ, Golec JM, Kay DP, Knegtel RM, MacCormick S, et al. Discovery of potent and selective inhibitors of ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase as potential anticancer agents. J Med Chem. 2011 Apr 14;54(7):2320–30. doi: 10.1021/jm101488z. [DOI] [PubMed] [Google Scholar]
- 17.Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, et al. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat Struct Mol Biol. 2011 Jun;18(6):721–7. doi: 10.1038/nsmb.2076. Epub 2011/05/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell death & disease. 2012;3:e441. doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultana R, Abdel-Fatah T, Perry C, Moseley P, Albarakti N, Mohan V, et al. Ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase inhibition is synthetically lethal in XRCC1 deficient ovarian cancer cells. PLoS One. 2013;8(2):e57098. doi: 10.1371/journal.pone.0057098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedberg EC, Friedberg EC. DNA repair and mutagenesis. 2. Washington, D.C: ASM Press; 2006. p. xxix.p. 1118. [Google Scholar]
- 21.Naim V, Wilhelm T, Debatisse M, Rosselli F. ERCC1 and MUS81-EME1 promote sister chromatid separation by processing late replication intermediates at common fragile sites during mitosis. Nat Cell Biol. 2013 Aug;15(8):1008–15. doi: 10.1038/ncb2793. [DOI] [PubMed] [Google Scholar]
- 22.Besse B, Olaussen KA, Soria JC. ERCC1 and RRM1: ready for prime time? J Clin Oncol. 2013 Mar 10;31(8):1050–60. doi: 10.1200/JCO.2012.43.0900. [DOI] [PubMed] [Google Scholar]
- 23.Kirschner K, Melton DW. Multiple roles of the ERCC1-XPF endonuclease in DNA repair and resistance to anticancer drugs. Anticancer Res. 2010 Sep;30(9):3223–32. [PubMed] [Google Scholar]
- 24.McNeil EM, Melton DW. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 2012 Nov 1;40(20):9990–10004. doi: 10.1093/nar/gks818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013 Mar 21;368(12):1101–10. doi: 10.1056/NEJMoa1214271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001 Nov 23;294(5547):1713–6. doi: 10.1126/science.1065521. Epub 2001/11/27. eng. [DOI] [PubMed] [Google Scholar]
- 27.Orelli B, McClendon TB, Tsodikov OV, Ellenberger T, Niedernhofer LJ, Scharer OD. The XPA-binding domain of ERCC1 is required for nucleotide excision repair but not other DNA repair pathways. J Biol Chem. 2010 Feb 5;285(6):3705–12. doi: 10.1074/jbc.M109.067538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zabludoff SD, Deng C, Grondine MR, Sheehy AM, Ashwell S, Caleb BL, et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Molecular cancer therapeutics. 2008 Sep;7(9):2955–66. doi: 10.1158/1535-7163.MCT-08-0492. [DOI] [PubMed] [Google Scholar]
- 29.Mohni KN, Dee AR, Smith S, Schumacher AJ, Weller SK. Efficient Herpes Simplex Virus 1 Replication Requires Cellular ATR Pathway Proteins. J Virol. 2013 Jan;87(1):531–42. doi: 10.1128/JVI.02504-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couch FB, Bansbach CE, Driscoll R, Luzwick JW, Glick GG, Betous R, et al. ATR phosphorylates SMARCAL1 to prevent replication fork collapse. Genes Dev. 2013 Jul 15;27(14):1610–23. doi: 10.1101/gad.214080.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirbu BM, Couch FB, Feigerle JT, Bhaskara S, Hiebert SW, Cortez D. Analysis of protein dynamics at active, stalled, and collapsed replication forks. Genes Dev. 2011 Jun 15;25(12):1320–7. doi: 10.1101/gad.2053211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirbu BM, Couch FB, Cortez D. Monitoring the spatiotemporal dynamics of proteins at replication forks and in assembled chromatin using isolation of proteins on nascent DNA. Nature protocols. 2012 Mar;7(3):594–605. doi: 10.1038/nprot.2012.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012 May;2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaillard PH, Wood RD. Activity of individual ERCC1 and XPF subunits in DNA nucleotide excision repair. Nucleic Acids Res. 2001 Feb 15;29(4):872–9. doi: 10.1093/nar/29.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arora S, Kothandapani A, Tillison K, Kalman-Maltese V, Patrick SM. Downregulation of XPF-ERCC1 enhances cisplatin efficacy in cancer cells. DNA Repair (Amst) 2010 Jul 1;9(7):745–53. doi: 10.1016/j.dnarep.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulsen RD, Soni DV, Wollman R, Hahn AT, Yee MC, Guan A, et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell. 2009 Jul 31;35(2):228–39. doi: 10.1016/j.molcel.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bansbach CE, Betous R, Lovejoy CA, Glick GG, Cortez D. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 2009 Oct 15;23(20):2405–14. doi: 10.1101/gad.1839909. Epub 2009/10/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005 May;25(9):3553–62. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilljam KM, Muller R, Liabakk NB, Otterlei M. Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA. PLoS One. 2012;7(11):e49199. doi: 10.1371/journal.pone.0049199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melton DW, Ketchen AM, Nunez F, Bonatti-Abbondandolo S, Abbondandolo A, Squires S, et al. Cells from ERCC1-deficient mice show increased genome instability and a reduced frequency of S-phase-dependent illegitimate chromosome exchange but a normal frequency of homologous recombination. J Cell Sci. 1998 Feb;111( Pt 3):395–404. doi: 10.1242/jcs.111.3.395. [DOI] [PubMed] [Google Scholar]
- 41.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004 Jul;24(13):5776–87. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozkan C, Gumuskaya B, Yaman S, Aksoy S, Guler G, Altundag K. ERCC1 expression in triple negative breast cancer. Journal of BUON: official journal of the Balkan Union of Oncology. 2012 Apr-Jun;17(2):271–6. [PubMed] [Google Scholar]
- 43.Glanzer JG, Carnes KA, Soto P, Liu S, Parkhurst LJ, Oakley GG. A small molecule directly inhibits the p53 transactivation domain from binding to replication protein A. Nucleic Acids Res. 2013 Feb 1;41(3):2047–59. doi: 10.1093/nar/gks1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patrone JD, Kennedy JP, Frank AO, Feldkamp MD, Vangamudi B, Pelz NF, et al. Discovery of Protein-Protein Interaction Inhibitors of Replication Protein A. ACS medicinal chemistry letters. 2013 Jul 11;4(7):601–5. doi: 10.1021/ml400032y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Postel-Vinay S, Bajrami I, Friboulet L, Elliott R, Fontebasso Y, Dorvault N, et al. A high-throughput screen identifies PARP1/2 inhibitors as a potential therapy for ERCC1-deficient non-small cell lung cancer. Oncogene. 2013 Aug 12; doi: 10.1038/onc.2013.311. [DOI] [PubMed] [Google Scholar]
- 46.Rageul J, Fremin C, Ezan F, Baffet G, Langouet S. The knock-down of ERCC1 but not of XPF causes multinucleation. DNA Repair (Amst) 2011 Sep 5;10(9):978–90. doi: 10.1016/j.dnarep.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Cheng H, Zhang Z, Borczuk A, Powell CA, Balajee AS, Lieberman HB, et al. PARP inhibition selectively increases sensitivity to cisplatin in ERCC1-low non-small cell lung cancer cells. Carcinogenesis. 2013 Apr;34(4):739–49. doi: 10.1093/carcin/bgs393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordheim LP, Barakat KH, Heinrich-Balard L, Matera EL, Cros-Perrial E, Bouledrak K, et al. Small molecule inhibitors of ERCC1-XPF protein-protein interaction synergize alkylating agents in cancer cells. Mol Pharmacol. 2013 Jul;84(1):12–24. doi: 10.1124/mol.112.082347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.