Abstract
Childhood adversity (CA) is strongly associated with youth psychopathology. Identifying factors that reduce vulnerability following CA is critical for developing preventive interventions. Vagal tone and vagal reactivity reactivity following psychosocial stressors might influence psychopathology among youths exposed to CA. We acquired heart period and impedance cardiography data to calculate respiratory sinus arrhythmia (RSA) and pre-ejection period (PEP) from 157 adolescents aged 13–17 years at rest and during the Trier Social Stress Test (TSST). Internalizing and externalizing symptoms and multiple forms of CA were assessed. Resting RSA and RSA reactivity interacted with CA in predicting internalizing but not externalizing psychopathology; CA was unassociated with internalizing problems in adolescents with high resting RSA and RSA reactivity. No interactions were observed with PEP. High resting RSA predicted greater vagal rebound and accelerated heart rate recovery following the TSST, highlighting one potential mechanism underlying low internalizing symptoms following CA among youths with high RSA.
Keywords: childhood adversity, adverse childhood experiences, trauma, respiratory sinus arrhythmia, RSA, vagal tone, vagal reactivity, internalizing psychopathology
Evidence from longitudinal and population-based studies consistently indicates that adverse childhood experiences, including child maltreatment, exposure to violence, and trauma, are among the most robust risk factors for the onset of psychopathology. Experiences of childhood adversity are associated with the onset of a wide range of both internalizing and externalizing disorders, including mood, anxiety, disruptive behavior, and substance use disorders (Cohen, Brown, & Smailes, 2001; Green et al., 2010; Kessler, Davis, & Kendler, 1997; McLaughlin et al., 2012; Scott, McLaughlin, Smith, & Ellis, 2012). This pattern suggests that a generalized diathesis for psychopathology might arise as a result of adverse environmental experiences early in development. Exposure to diverse forms of childhood adversity is associated with the onset of psychopathology not only in childhood and adolescence, but also in adulthood (Green, et al., 2010; Kessler, et al., 1997; Scott, Smith, & Ellis, 2010). Identifying factors that reduce vulnerability to psychopathology following exposure to childhood adversity is critical for informing the development and targeting of interventions to prevent the mental health sequelae of these experiences. In the current report, we investigate whether aspects of both resting autonomic nervous system function and reactivity to psychosocial stress protect youths from the mental health consequences of exposure to multiple forms of childhood adversity.
The autonomic nervous system (ANS) regulates an organism’s response to changes in the internal and external environment that require adaptation, including experiences of psychosocial stress (Berntson, Cacioppo, & Quigley, 1993a; Lucini, Di Fede, Parati, & Pagani, 2005; Lucini, Norbiato, Clerici, & Pagani, 2002; Porges, 1995a; Sloan et al., 1994). The ANS maintains homeostasis and regulates the functioning of multiple organs—including the heart, lungs, kidneys, salivary glands, and many others—through the dynamic interactions of its sympathetic and parasympathetic branches. Sympathetic nervous system activation occurs in response to stressors and other environmental challenges and coordinates a variety of physiological processes frequently described as the “fight or flight” response. This response serves the function of mobilizing physiological and cognitive resources to respond to environmental demands and results in heart rate acceleration, increased metabolic output, and catecholamine release from the adrenal glands (Cacioppa et al., 1998; Lane, Adcock, & Burnett, 1992; Lucini, et al., 2005; Lucini, et al., 2002). These changes result in increased cardiac output and availability of glucose, oxygenated blood, and other nutrients to skeletal muscles and the brain in order to permit enhanced motor activity and facilitate cognitive performance during stress. The parasympathetic nervous system serves an opposing set of functions that promote growth and restoration when the organism is at rest, and following exposure to stressors, parasympathetic nervous system serves to inhibit sympathetic activation, lower heart rate, and facilitate a return to homeostasis (Berntson et al., 1997; Porges, 1992, 1995a, 1995b, 2007). Although the sympathetic and parasympathetic nervous systems perform opposing sets of functions, they do not always operate in an antagonist fashion. Indeed, each branch of the autonomic nervous system may exhibit independent activation that is uncoupled with the opposing branch and patterns of co-activation and co-inhibition are also possible (Berntson, Cacioppo, & Quigley, 1991; Berntson, et al., 1993a; Berntson, Cacioppo, Quigley, & Fabro, 1994). Because of the central role that these systems play in regulating physiological responses to environmental change and psychosocial stress, individual differences in ANS function and reactivity may be associated with psychopathology among children and adolescents exposed to adverse environmental experiences.
Vagal tone and vagal reactivity are specific aspects of ANS function that are likely to play a role in conferring vulnerability to psychopathology following exposure to childhood adversity. Vagal tone refers to tonic parasympathetic nervous system control over heart rate and is mediated by the actions of the vagus nerve, which originates in the brain stem and terminates at the sino-atrial node of the heart (Allen, Chambers, & Towers, 2007; Berntson, et al., 1997; Porges, 1992, 1995a, 2007). The predominant theoretical conceptualization of the role that vagal tone plays in affective experience and social behavior is the polyvagal theory (Porges, 1995b, 2001, 2007). This theory argues that in mammals the “vagal brake” represents the primary mechanism involved in regulating rapid changes in heart rate in response to environmental demands in order to mobilize metabolic resources and facilitate social behavior (Porges, 2007). The vagal brake refers to the myelinated portion of the vagus nerve that originates in the nucleus ambiguus and which the polyvagal theory argues is present only in mammals (Porges, 1992, 1995a, 2007). At rest, the vagal brake has tonic inhibitory influences on cardiac chronotropy that results in resting heart rate that is lower than the basal firing rate of the sino-atrial node. The vagal brake is also highly responsive to changes in environmental circumstances. Withdrawal of the vagal break facilitates sympathetic activation and metabolic mobilization to support fight-or-flight behaviors in situations involving threat, whereas the vagal brake is increased or maintained in situations requiring social engagement and communication (Porges, 2007). The polyvagal theory thus argues that vagal regulation, via both tonic influences of the vagal brake and through responses to environmental demands, has downstream effects on emotional expression, emotion regulation, and social behavior.
Extensive evidence from developmental studies supports the basic tenets of the polyvagal theory in regards to social and emotional behavior. In children and adolescents, high resting vagal tone and vagal suppression in response to environmental changes are associated with greater expression of emotion, low levels of negative emotionality, and adaptive emotion regulation skills (Calkins & Keane, 2004; Cole, Zahn-Waxler, Fox, Usher, & Welsh, 1996; Eisenberg et al., 1995; Fabes, Eisenberg, & Eisenbud, 1993; Fabes, Eisenberg, Karbon, Troyer, & Switzer, 1994; Stifter, Fox, & Porges, 1989). High vagal tone is also associated with better performance on tests of sustained attention (Suess, Porges, & Blude, 1994). Multiple studies have observed negative associations of resting vagal tone with youth internalizing and externalizing problems (Calkins, Graziano, & Keane, 2007; Mezzacappa et al., 1997; Pine et al., 1998; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996; Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007). Environmental moderators of the association between vagal tone and internalizing problems, such as parental psychopathology, have also been reported (Forbes, Fox, Cohn, Galles, & Kovacs, 2006). With regard to vagal reactivity, a recent meta-analysis reported negative associations, albeit of small magnitude, between vagal reactivity and both internalizing and externalizing psychopathology as well as cognitive and academic problems (Graziano & Derefinko, 2013). No association was found between vagal reactivity and social functioning.
Taken together, these findings suggest that high vagal tone and vagal reactivity might serve a protective role for youths exposed to high levels of environmental adversity. Indeed, several prior studies have found that high resting vagal tone and greater vagal reactivity during challenge tasks is associated with improved functional outcomes among children living in families with high levels of marital conflict (El-Sheikh, Harger, & Whitson, 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995, 1997; Leary & Katz, 1997; Obradovic, Bush, & Boyce, 2011; Whitson & El-Sheikh, 2003). This pattern has been observed in predicting both mental health outcomes, including internalizing and externalizing psychopathology (El-Sheikh, et al., 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995, 1997), as well as physical health outcomes (Whitson & El-Sheikh, 2003) and peer relationships (Leary & Katz, 1997). Similarly, one study observed lower levels of aggressive problems among maltreated boys with high vagal tone compared to those with low vagal tone (Gordis, Feres, Olezeski, Rabkin, & Trickett, 2010). These studies provide preliminary evidence for a potential protective effect of high vagal tone and vagal suppression in the context of environmental adversity. However, we are unaware of studies examining the degree to which vagal tone and vagal reactivity buffer youths from psychopathology following a wider range of adverse childhood experiences.
Moreover, it is unknown whether these effects are specific to vagal regulation, or whether variation in sympathetic nervous system function might also buffer against the negative effects of environmental adversity. Lower sympathetic nervous system has been observed at rest in in response to tasks involving both reward and frustration in children with disruptive behavior disorders, particularly conduct disorder (Beauchaine, Hong, & Marsh, 2008; Crowell et al., 2006; Lorber, 2004; Shannon, et al., 2007). Few studies have examined the contribution of sympathetic nervous system activation, either at rest or in response to challenge, as a factor that moderates vulnerability to psychopathology among youths exposed to environmental adversity. One previous study using the pre-ejection period (PEP) as a measure of sympathetic activation found no association between marital conflict and internalizing problems among children with high PEP reactivity and a negative association between marital conflict and internalizing problems in children with low PEP reactivity (Obradovic, et al., 2011). In another study using skin conductance as a measure of sympathetic activation, marital conflict was associated with greater externalizing problems among girls with high sympathetic reactivity but not low reactivity (El-Sheikh, 2005). Together, these findings suggest that low sympathetic reactivity might serve as a protective factor against the development of psychopathology following childhood adversity. However, the extent to which this association extends to adverse childhood experiences other than marital conflict is unknown.
In the current study, we examined the associations of parasympathetic and sympathetic nervous system function—both at rest and in response to a psychosocial stressor—as moderators of the association between multiple forms of childhood adversity and adolescent internalizing and externalizing symptoms. Given the central role of vagal regulation in emotional functioning (Porges, 2007) and prior evidence for a moderating effect in response to marital conflict (e.g.,El-Sheikh & Erath, 2011), we expected to find protective effects specifically among adolescents with high resting vagal tone and high vagal reactivity. To examine this hypothesis, we measured respiratory sinus arrhythmia (RSA), a non-invasive measure of parasympathetic control of heart rate used to estimate vagal tone and vagal regulation (Berntson, Cacioppo, & Quigley, 1993b; Grossman & Taylor, 2007; Porges, 1992, 1995a). RSA reflects coupling of heart rate and respiration that leads to systematic variability in heart rate during inhalation as compared to exhalation (Allen, et al., 2007). Pharmacological blockade studies indicate that RSA is influenced solely by the vagus nerve and therefore reflects a pure measure of parasympathetic cardiac control (Cacioppo et al., 1994; Grossman, Stemmler, & Meinhardt, 1990; Kollai & Mizsei, 1990; McCabe, Yongue, Ackles, & Porges, 1985; Pagani et al., 1986). To assess sympathetic nervous system activation, we measured the pre-ejection period. Pharmacological blockades indicate that PEP becomes shorter solely with sympathetic activation and is uninfluenced by the vagus nerve (Cacioppo, et al., 1994). We expected that the association between childhood adversity exposure with internalizing and externalizing symptoms would be weaker in adolescents with low PEP reactivity, given some evidence for this type of association with regard to marital conflict (El-Sheikh, 2005; Obradovic, et al., 2011). We examined whether RSA and PEP at rest in response to a psychosocial stressor modified the associations of a broad range of childhood adversities—including child abuse, community violence, traumatic events, and low socioeconomic status—with internalizing and externalizing symptoms. Finally, we investigated a possible mechanism by which autonomic function is associated with lower psychopathology following childhood adversity. Specifically, we evaluated whether aspects of ANS function that buffered against psychopathology among adolescents exposed to adversity were associated with faster physiological recovery following a laboratory-based psychosocial stressor.
Methods
Sample
A community-based sample of 168 adolescents aged 13–17 was recruited for participation at schools, after-school programs, general medical clinics, and the general community in Boston and Cambridge, MA. Recruitment efforts were targeted at recruiting a sample with high racial/ethnic diversity as well as variability in exposure to childhood adversity. To do so, we recruited heavily from neighborhoods with high levels of violence and from clinics that served a predominantly low-SES catchment area. Adolescents taking medications known to influence cardiovascular functioning were excluded (n=4). The sample was 56.0% female (n=94) and had a mean age of 14.9 years (SD = 1.36). All females were post-menarchal. Racial/ethnic composition of the sample was as follows: 40.8% White (n=69), 18.34% Black (n=31), 17.8% Hispanic (n=30), 7.7% Asian (n=13), and 14.8% Biracial or Other (n=25). Approximately one-third of the sample (38.1%, n=64) was from single-parent households and a slightly smaller proportion (29.5%) was living below the poverty line (see below for greater detail). Equipment malfunctions resulted in loss of autonomic data from 8 participants. An additional 3 participants were excluded from analysis due to presence of a heart murmur (n=1), severe cognitive impairment (n=1), and presence of a pervasive developmental disorder (n=1). The final analytic sample included 157 participants.
Physiological measures
Continuous cardiac and hemodynamic measures were recorded noninvasively during a 10-minute baseline period in which participants were asked to sit quietly without moving as well as during a social-evaluative stress procedure (described in more detail below), and a 5-minute recovery period. Electrocardiogram (ECG) recordings were obtained with a Biopac ECG amplifier (Goleta, CA) using a modified Lead II configuration (right clavicle, left lower torso, and right leg ground). Cardiac impedance recordings were obtained with a Bio-Impedance Technology model HIC-2500 impedance cardiograph (Chapel Hill, NC). One pair of Mylar electrode tapes was placed on the neck and another pair was placed on the torso. A continuous 500 µA AC 95 kHz current was passed through the two outer electrodes, and basal thoracic impedance (z0) and the first derivative of basal impedance (dz/dt) was measured from the inner electrodes. Biopac MP150 hardware and Acqknoweldge software was used to integrate and acquire the ECG and impedance cardiography data, both of which were sampled at 1.0 kHz.
ECG and impedance cardiograph data were scored by raters blind to participant status on all variables of interest, including childhood adversity exposure and psychopathology. Signals were averaged into one-minute epochs using Mindware Software (Mindware Technologies, Gahanna, OH). Minutes with significant artifact in the ECG signal were not scored to prevent bias in RSA estimates (<3% of minutes recorded were discarded). The amount of missing data due to artifact did not vary by childhood adversity or by psychopathology. RSA was calculated from the inter-beat interval time series using spectral analysis implemented in Mindware HRV Software. RSA was calculated for the frequency band 0.12 – 0.40 Hz. Based on evidence suggesting that controlling for respiration rate is necessary in order for RSA to represent a measure of purely parasympathetic cardiac control (Grossman, Karemaker, & Wieling, 1991; Grossman & Taylor, 2007), we controlled for respiration rate in all analysis. Respiration rate was derived from the basal cardiac impedance signal. Impedance cardiography data scoring produced measures of pre-ejection period (PEP), a measure of sympathetic nervous system activation representing the amount of time that elapses from the beginning of ventricular depolarization to the moment the aortic valve opens and blood begins leaving the left ventricle (electrical systole). Because accurate scoring of impedance cardiography data requires manual placement of the B point (opening of the aortic valve),(Blascovich, Mendes, Vanman, & Dickerson, 2011) these data were scored by two independent raters. SV differences of more than 5% were reviewed and adjudicated by the first author (KM).
Childhood Adversity
Child abuse was assessed using the Childhood Trauma Questionnaire (CTQ) (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997; Bernstein et al., 2003). The CTQ is a 28-item scale that assesses the frequency of exposure to maltreatment during childhood and adolescence. Three types of abuse are assessed: physical, sexual, and emotional abuse. The CTQ is among the most commonly used measures of child maltreatment and has excellent internal consistency, test-retest reliability, and convergent and discriminant validity with both interview measures and clinician reports of maltreatment (Bernstein, et al., 1997; Bernstein, Fink, Hondelsman, Foote, & Lovejoy, 1994). We created an abuse composite by summing items from the physical, sexual, and emotional abuse sub-scales, which demonstrated good reliability in our sample (α=0.88).
Community Violence Exposure was assessed using the Screen for Adolescent Violence Exposure (SAVE) (Hastings & Kelley, 1997). The SAVE is a 32-item measure assessing violence exposure in school, home, and neighborhood contexts. Only items assessing school and neighborhood violence were considered to avoid overlap with the CTQ regarding experiences of child abuse. Respondents rate the frequency of exposure to indirect violence (e.g., “I have heard about someone getting shot”) as well as being the victim of violence (e.g., “Someone has pulled a knife on me”). The SAVE total violence exposure scale demonstrated excellent internal consistency in this sample (α=0.89).
Other Lifetime Traumatic Events were assessed using the trauma assessment included in the post-traumatic stress disorder section of the Composite International Diagnostic Interview (Kessler & Üstun, 2004). Traumatic events assessed within this module included interpersonal violence (e.g., physical assaults by a romantic partner; items querying child maltreatment were removed), accidents and injuries (e.g., natural disasters), and network events (e.g., unexpected death of a loved one). A trauma score was created by summing the total number of distinct lifetime traumatic events.
Low Socio-Economic Status (SES) was assessed with questions about family size and total household income completed by a parent/guardian. Income information was provided by 88.5% of parent/guardians in the final analytic sample (n=139). This information was used to create an income-to-needs ratio for the family, based on poverty thresholds created by the U.S. Census for the year 2011. The income-to-needs ratio is calculated by dividing total family income by the level of income that denotes the poverty threshold for a family of that size. Values less than one indicate that family income is below the poverty line. A total of 29.5% of families who provided income information (n=41) were living below the poverty line.
Psychopathology
Internalizing and externalizing psychopathology were assessed using the Youth Self Report form and the caregiver report version of the Child Behavior Checklist (CBCL) (Achenbach, 1991; Achenbach & Rescorla, 2001). The CBCL scales are among the most widely used measures of youth emotional and behavioral problems and use extensive normative data to generate age-standardized estimates of the severity of internalizing and externalizing psychopathology. The broad-scale internalizing and externalizing scales have demonstrated validity in discriminating between youths with and without psychiatric disorders (Achenbach, 1991; Chen, Faraone, Biederman, & Tsuang, 1994; Kendall et al., 2007; Seligman, Ollendick, Langley, & Baldacci, 2004). Because both parents and adolescents are known to contribute unique information in the assessment of adolescent psychopathology (Cantwell, Lewinsohn, Rohde, & Seeley, 1997), we examined internalizing and externalizing problems based both on youth and parent reports.
Procedure
Study procedures were completed between 1 and 7 pm for all participants. After completing the baseline resting period, all participants completed the Trier Social Stress Test (TSST) (Kirschbaum, Pirke, & Hellhammer, 1993), a widely used laboratory-based stress induction procedure that has been adapted for use with children and adolescents (Buske-Kirschbaum et al., 1997; Buske-Kirschbaum et al., 2003; Stroud et al., 2009). The TSST involves delivering a speech and completing an arithmetic task in front of two evaluators and reliably elicits an ANS response (Kirschbaum, et al., 1993; MacMillan et al., 2009; Stroud, et al., 2009). The TSST involves three discrete periods (speech preparation, speech, and math). After being told that they would be delivering a speech in front of trained evaluators who would judge their performance, participants were given five-minutes to prepare their speech. In the current study, participants were asked to talk about the qualities of a good friend and to describe which of those characteristics they did and did not possess. Next, participants delivered a five-minute speech in front of two evaluators. Evaluators were trained to provide neutral and mildly negative feedback (e.g., appearing bored) during the speech. Finally, participants completed a mental subtraction task out loud in front of the evaluators for five-minutes. Specifically, participants were asked to count backwards in steps of seven from a three-digit number and were stopped and asked to start again each time they made a mistake. ECG and cardiac impedance recordings were measured continuously across each period. Two participants declined to participate in the TSST. Following the TSST, participants engaged in a five-minute recovery period during which they were asked to sit quietly while their physiological activity was monitored.
Statistical Analysis
We evaluated the hypothesis that the association of childhood adversity with internalizing and externalizing symptoms would be weaker among adolescents with high vagal tone and high vagal reactivity. To do so, we created interaction terms between resting RSA and RSA reactivity with each of our four childhood adversity variables. RSA reactivity values were calculated by subtracting RSA during the first minute of speech preparation (i.e., anticipation) and speech (i.e., performance/evaluation) portions of the TSST from average baseline RSA (so that higher values indicate greater RSA suppression). Using the first minute of the TSST to calculate reactivity is standard practice (Jamieson, Mendes, Blackstock, & Schmader, 2010), as cardiovascular reactivity is greatest during the first minute of the task and habituates quickly. We used the speech portion of the TSST to calculate reactivity during the performance/evaluation component of the task rather than both the speech and math, both because RSA suppression was greatest during the speech portion of the TSST and in order to reduce the number of models being examined. The same procedures were used for examining interactions between PEP and childhood adversity in predicting psychopathology. All variables were standardized prior to creating interaction terms and conducting regression analysis, and main effect terms for RSA and childhood adversity were included in interaction models. Procedures outlined by Aiken and West (Aiken & West, 1991) were used to evaluate significant interactions. To evaluate whether vagal tone was associated with physiological recovery after psychosocial stress, we examined the associations of resting RSA with heart rate, PEP, and RSA recovery following the TSST. We controlled for age and gender in all analyses and for respiration rate in models including RSA.
Results
Descriptive Statistics and Bivariate Associations
Table 1 provides information on RSA at baseline and during each period of the TSST, childhood adversity variables, and psychopathology. Zero-order correlations among all study variables are provided in Table 2. Of note, resting RSA, RSA reactivity, resting PEP, and PEP reactivity were associated with internalizing or externalizing problems based on either parent or youth report. None of the childhood adversity variables were associated with resting RSA or PEP, and with the exception of two associations were not associated with RSA reactivity or PEP reactivity. In contrast, each of the domains of childhood adversity was positively and significantly associated with youth-reported externalizing problems and parent-reported externalizing problems. Youth-reported internalizing symptoms were positively associated with all domains of childhood adversity, with the exception of income-to-needs ratio. Childhood adversity exposure was unrelated to parent-reported internalizing problems.
Table 1.
Descriptive statistics of RSA, PEP, childhood adversity, and psychopathology variables (N=157)
| Mean | (SD) | Range | |
|---|---|---|---|
| Baseline RSA | 6.65 | (1.08) | 3.39 – 8.89 |
| RSA - Preparation | 6.55 | (1.12) | 2.49 – 8.95 |
| RSA – Speech | 6.45 | (1.09) | 3.53 – 9.81 |
| RSA – Math | 6.54 | (1.21) | 2.17 – 9.84 |
| RSA – Recovery | 6.58 | (1.12) | 1.94 – 8.63 |
| Baseline PEP | 103.88 | (14.98) | 89.78 – 144.10 |
| PEP - Preparation | 93.86 | (18.59) | 67.00 – 122.00 |
| PEP – Speech | 89.98 | (18.42) | 60.00 – 127.50 |
| PEP – Math | 92.94 | (17.21) | 69.50 – 129.50 |
| PEP – Recovery | 104.26 | (14.39) | 89.80 – 130.60 |
| Child Abuse | 4.65 | (7.03) | 0 – 37 |
| Community Violence | 42.17 | (10.39) | 26 – 78 |
| Peer Victimization | 8.09 | (7.11) | 0 – 33 |
| Other Trauma | 4.08 | (2.95) | 0 – 13 |
| Income-to-Needs Ratio | 4.50 | (3.15) | 0.05 – 13.95 |
| YSR Internalizing | 52.88 | (10.25) | 27 – 79 |
| CBCL Internalizing | 52.18 | (9.59) | 29 – 73 |
| YSR Externalizing | 50.99 | (10.59) | 33 – 81 |
| CBCL Externalizing | 48.32 | (10.35) | 34 – 82 |
Table 2.
Correlations among RSA, PEP, childhood adversity, and psychopathology variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline RSA | __ | |||||||||||||||||
| 2. RSA – Preparation | .68** | __ | ||||||||||||||||
| 3. RSA – Speech | .35** | .70** | __ | |||||||||||||||
| 4. RSA – Math | .44** | .76** | .82** | __ | ||||||||||||||
| 5. RSA – Recovery | .90** | .76** | .49** | .62** | __ | |||||||||||||
| 6. Baseline PEP | −.05 | .17 | .16 | .17 | .08 | __ | ||||||||||||
| 7. PEP – Preparation | .02 | .31** | .39** | .34** | .14 | .70** | __ | |||||||||||
| 8. PEP – Speech | −.04 | .27** | .41** | .35** | .09 | .68** | .94** | __ | ||||||||||
| 9. PEP – Math | −.08 | .20* | .30** | .33** | .04 | .73** | .91** | .94** | __ | |||||||||
| 10. PEP – Recovery | −.08 | .06 | .19* | .10 | −.05 | .82** | .81** | .79** | .85** | __ | ||||||||
| 11. Child Abuse | −.08 | .10 | .15 | .17** | −.04 | −.06 | .06 | .06 | .07 | .07 | __ | |||||||
| 12. Community Violence | .06 | .14 | .10 | .05 | .09 | −.01 | .08 | .02 | .05 | .02 | .24** | __ | ||||||
| 13. Other Trauma | .03 | .20* | .08 | .02 | .05 | −.09 | −.01 | −.04 | −.04 | −.03 | .57** | .61** | __ | |||||
| 14. Income-to-Needs Ratio | −.13 | .02 | −.04 | −.09 | −.17 | −.08 | −.08 | −.08 | −.05 | .01 | −.22** | −.38** | −.30** | __ | ||||
| 15. YSR Internalizing | −.02 | −.01 | .11 | .01 | −.02 | −.10 | −.04 | −.05 | −.05 | −.08 | .39** | .29** | .43** | −.03 | __ | |||
| 16. CBCL Internalizing | .08 | −.08 | −.05 | −.11 | .03 | −.10 | −.07 | −.12 | −.13 | −.14 | .10 | −.02 | .11 | −.05 | .36** | __ | ||
| 17. YSR Externalizing | .06 | .07 | .08 | .11 | .12 | −.01 | .06 | .05 | .09 | −.03 | .39** | .44** | .50** | −.20* | .55** | .17* | __ | |
| 18. CBCL Externalizing | .06 | .07 | .01 | .08 | .11 | −.07 | −.09 | −.12 | −.14 | −.17 | .20* | .14 | .21** | −.21* | .24** | .64** | .44** | __ |
Note: YSR=Youth Self-Report; CBCL=Child Behavior Checklist;
p < 0.05,
p < 0.01.
Interactions between Vagal Tone and Childhood Adversity
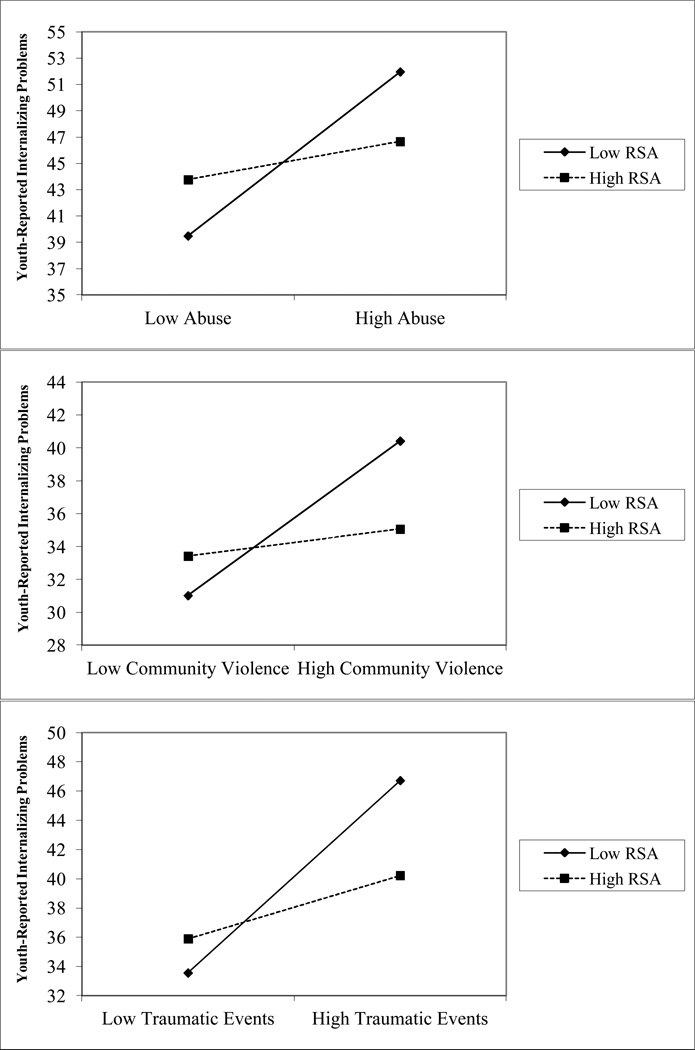
We next examined whether resting RSA moderated the association between childhood adversity exposure and adolescent symptomatology. We found significant interactions between resting RSA and all four domains of childhood adversity in predicting youth-reported internalizing problems (see Figure 1). Resting RSA interacted with child abuse, β=−0.19, p=.015, community violence exposure, β=−0.20, p=.012, other traumatic events, β=−0.22, p=.003, and income-to-needs ratio, β=0.19, p=.050, in predicting youth-reported internalizing problems.
Figure 1.
Interactions between resting RSA and childhood adversity in predicting youth-reported internalizing problems.
Note. Figures depict low RSA and childhood adversity as one standard deviation below the mean and high RSA and adversity as one standard deviation above the mean.
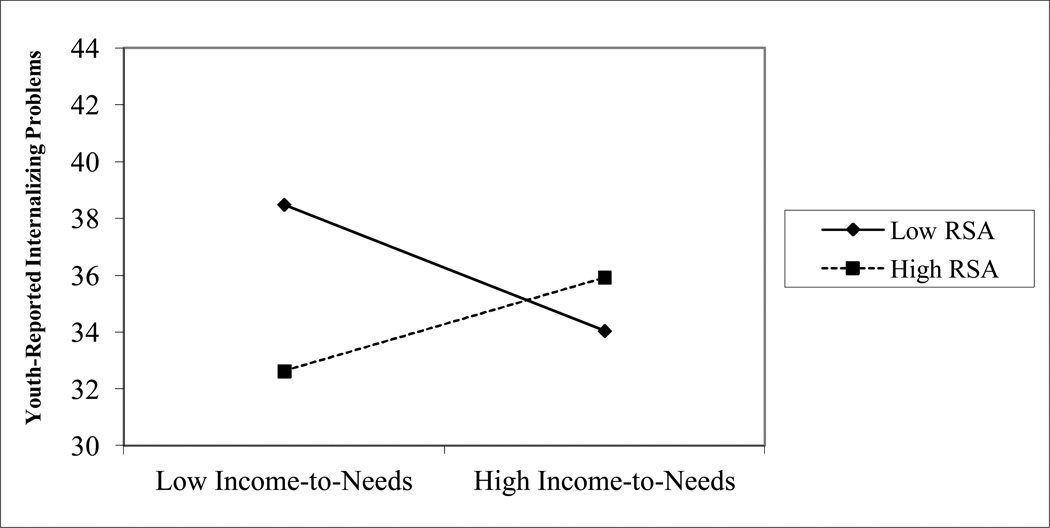
For significant interactions, we evaluated the simple slope of the association between childhood adversity and youth-reported internalizing problems at high and low levels of resting RSA (i.e., 1 standard deviation above and below the mean). The positive association between child abuse and internalizing problems was significant for adolescents with low resting RSA, β=6.24, t(145)=5.25, p<.001, but not for adolescents with high resting RSA, β=1.44, t(145)= 1.03, p=.31. A similar pattern was observed for community violence and other traumatic events. Community violence and internalizing problems were positively associated for adolescents with low resting RSA, β=4.70, t(144)=4.44, p<.001, but not high resting RSA, β=0.82, t(144)=0.68, p=.50. Similarly, the positive association between traumatic events and internalizing problems was stronger for adolescents with low resting RSA, β=6.57, t(145)=6.60, p< .001, than for those with high resting RSA, β=2.17, t(145)= 1.94, p=.055. Finally, income-to-needs ratio was negatively related to internalizing problems among adolescents with low resting RSA, β=−3.19, t(120)= −2.00, p=.047, and was unrelated to internalizing problems among adolescents with high resting RSA, β=2.61, t(120)=1.34, p=.18.
Resting RSA interacted with two of the four domains of CA in predicting parent-reported internalizing psychopathology including child abuse, β=−0.21, p=.012, and other traumatic events, β=−0.20, p=.016. The pattern of these interactions mirrored those for youth-reported internalizing problems. The positive association between CA and parent-reported internalizing problems was significant for adolescents with low resting RSA, β=3.72, t(138)=2.73, p=.007, but not high resting RSA, β=−1.81, t(138)= −1.13, p=.26. Similarly, traumatic events were positively associated with parent-reported internalizing problems among adolescents with low resting RSA, β=3.36, t(138)=2.81, p=.006, but not high resting RSA, β=−0.81, t(138)= −0.63, p=.53.
Resting RSA did not moderate the association between childhood adversity and externalizing problems based on either youth- or parent-report.
Interactions between Vagal Reactivity and Childhood Adversity
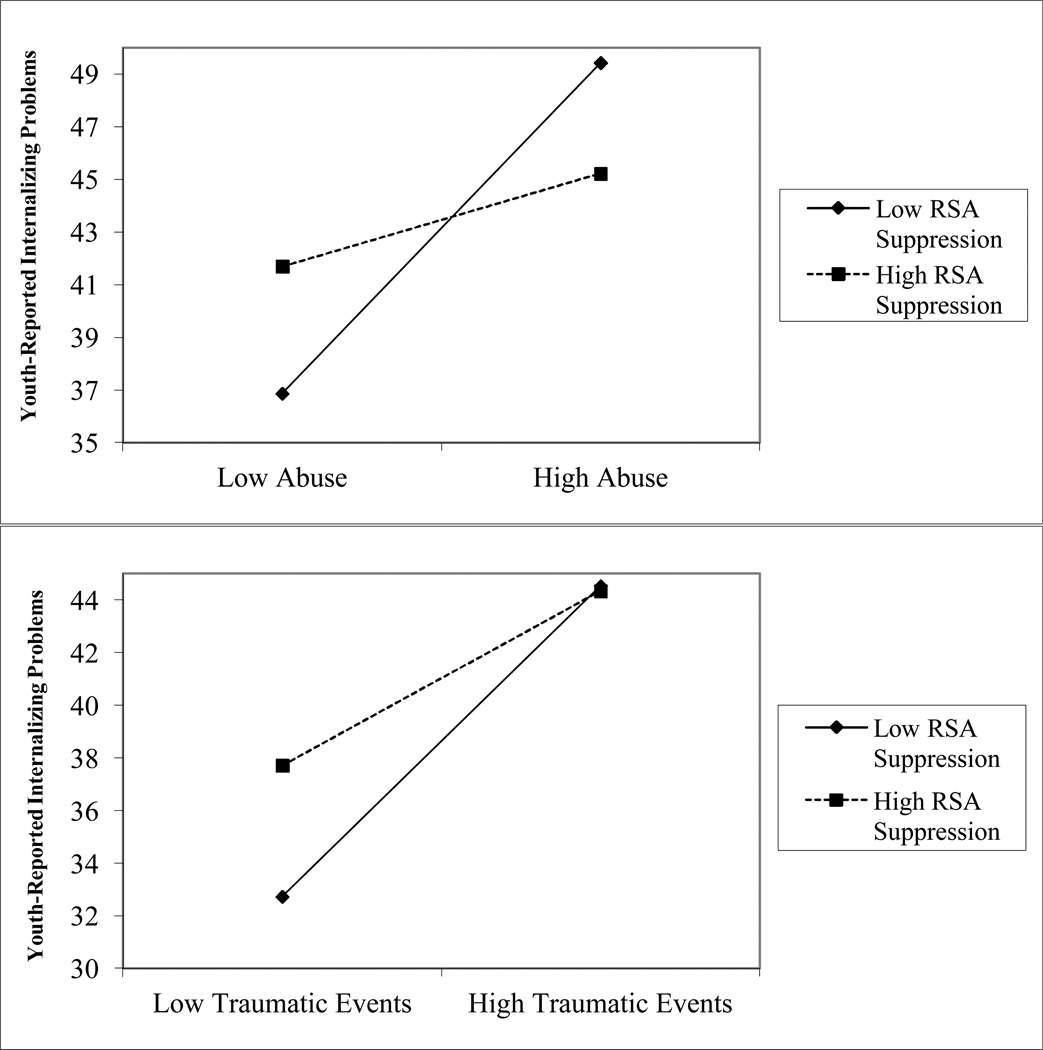
Speech Preparation
We next examined whether RSA reactivity (i.e., changes in RSA during the TSST relative to baseline) moderated the association between childhood adversity exposure and adolescent symptoms. We found interactions between RSA reactivity during the preparation period and two of the four domains of childhood adversity in predicting youth-reported internalizing problems (seeFigure 2). RSA reactivity interacted marginally with child abuse, β=−0.15, p=.059, and significantly with other traumatic events, β=−0.17, p=.029, in predicting youth-reported internalizing problems. When we evaluated the simple slopes, the positive association between child abuse and internalizing problems was significant for adolescents with low RSA suppression, β=6.28, t(144)=4.35, p<.001, but not for adolescents with high RSA suppression, β=1.76, t(144)= 1.18, p=.24. The positive association between traumatic events and internalizing problems was stronger for adolescents with low RSA suppression, β=5.90, t(144)=6.12, p< .001, than for adolescents with high RSA suppression, β=3.32, t(144)= 3.35, p=.001.
Figure 2.
Interactions between RSA suppression during the speech preparation and childhood adversity in predicting youth-reported internalizing problems.
Note. Figures depict low RSA suppression and childhood adversity as one standard deviation below the mean and high RSA suppression and adversity as one standard deviation above the mean.
RSA suppression during the preparation period interacted with child abuse, β=−0.18, p=.033, and marginally with other traumatic events, β=−0.16, p=.073, in predicting parent-reported internalizing psychopathology. The positive association between child abuse and parent-reported internalizing problems was significant for adolescents with low RSA suppression, β=4.36, t(137)=2.56, p=.012, but not high RSA suppression, β=−1.69, t(137)= −0.96, p=.34. Similarly, traumatic events were positively associated with parent-reported internalizing problems among adolescents with low RSA suppression, β=2.78, t(137)=2.46, p=.015, but not high RSA suppression, β=0.32, t(137)=0.28, p=.78.
RSA suppression during the preparation period did not moderate the association between CA and externalizing problems based on either youth- or parent-report.
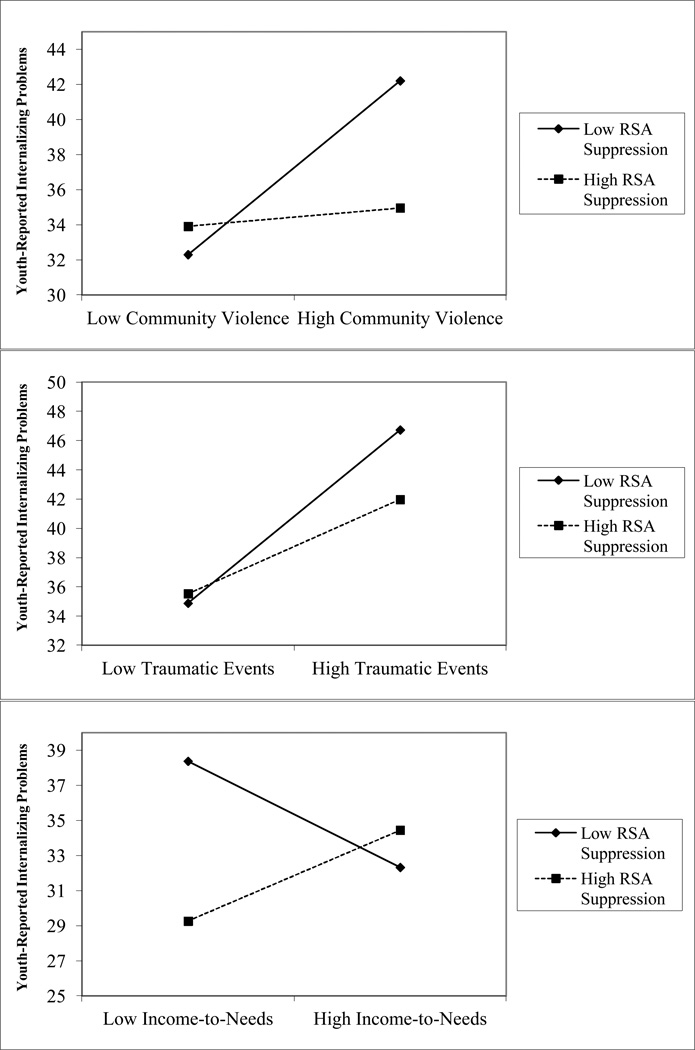
Speech
We next examined RSA suppression during the speech portion of the TSST. Interactions between RSA reactivity during the speech were observed for three of the four domains of childhood adversity in predicting youth-reported internalizing problems (seeFigure 3). RSA reactivity interacted with community violence exposure, β=−0.19, p=.030, other traumatic events, β=−0.15, p=.042, and income-to-needs ratio, β=0.27, p=.005, in predicting youth-reported internalizing problems. When we evaluated the simple slopes, the positive association between child abuse and internalizing problems was significant for adolescents with low RSA suppression, β=4.95, t(142)=4.08, p<.001, but not for adolescents with high RSA suppression, β=0.52, t(142)= 0.37, p=.72. A similar pattern was observed for other traumatic events and income-to-needs ratio. The positive association between other traumatic events and youth-reported internalizing problems was larger for adolescents with low RSA suppression, β=5.93, t(142)=5.97, p<.001, than for adolescents with high RSA suppression, β=3.23, t(142)= 3.14, p=.002. Finally, the negative association between income-to-needs ratio and internalizing problems was significant only among adolescents with low RSA suppression, β=−3.03, t(142)= −2.41, p=.017, and not among those with high RSA suppression, β=2.59, t(142)=1.77, p=.08.
Figure 3.
Interactions between RSA suppression during the speech and childhood adversity in predicting youth-reported internalizing problems.
Note. Figures depict low RSA suppression and childhood adversity as one standard deviation below the mean and high RSA suppression and adversity as one standard deviation above the mean.
One significant interaction was observed between child abuse and RSA suppression in predicting youth-reported externalizing problems, β=0.24, p=.004. The simple slope pattern did not follow those previously reported, however. Child abuse was more strongly associated with youth-reported externalizing problems among those with high RSA suppression, β=8.00, t(142)=4.82, p<.001, than those with low RSA suppression, β=1.96, t(142)=1.91, p=.058. Given the large number of models tested, however, it is likely that a single significant association in the opposite direction is the result of chance.
No interactions were observed between RSA suppression during the speech and childhood adversity in predicting parent-reported internalizing problems or externalizing problems.
Interactions between PEP and Childhood Adversity
To determine whether these buffering effects were specific to RSA, we tested a similar set of models examining resting PEP and PEP reactivity. Resting PEP did not interact with childhood adversity in any of these models to predict either youth- or parent-reported psychopathology, nor did PEP reactivity.
Vagal Tone and Physiological Recovery from Psychosocial Stress
In a final set of analyses, we examined whether resting RSA was associated with physiological recovery following the TSST. Higher baseline RSA was associated with lower heart rate at baseline, β=−0.64, p<.001, but was not associated with heart rate reactivity during the preparation, speech, or math portions of the TSST. Resting RSA was related to heart rate recovery following the TSST, however. We examined the association between baseline RSA and heart rate during the recovery period in a model that controlled for heart rate during the last component of the TSST, the math task. Individuals with higher baseline RSA experienced greater reductions in heart rate from the end of the TSST to the recovery period, β=−0.39, p<.001. Resting RSA was associated with heart rate recovery during each of the five minutes in the recovery period relative to the end of the TSST (βs=−0.37 to −0.42, p<.001).
To explore this relationship further, we examined the associations of resting RSA with PEP and RSA reactivity and recovery following the TSST. A more complicated pattern emerged from this analysis. Resting RSA was unrelated to PEP reactivity during the TSST, but was associated with PEP recovery after the task. Higher resting RSA was associated with lower PEP (i.e., greater sympathetic activation) during the recovery period (Table 3). This pattern was observed when examining the association of resting RSA with PEP values during the recovery period relative to baseline, β=−0.19, p=.051, and during minutes 1–4 of the recovery period relative to PEP values during the last component of the TSST (βs=−0.23 to −0.30, p=.011 to .051).
Table 3.
Pre-ejection period Reactivity during the Trier Social Stress Test (TSST) and Recovery Period according to Resting Baseline RSA (N=157)
| High RSA1 | Low RSA1 | RSA and PEP Reactivity2 |
||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | β | p-value | |
| Preparation Reactivity3 | −11.86 | (9.53) | −15.70 | (13.43) | .12 | .162 |
| Speech Reactivity3 | −17.40 | (12.01) | −17.31 | (14.71) | .05 | .545 |
| Math Reactivity3 | −12.94 | (11.39) | −13.82 | (11.64) | −.08 | .365 |
| Recovery3 | 0.90 | (4.07) | 1.85 | (8.64) | −.19 | .051 |
| Minute 14 | 8.99 | (8.83) | 12.45 | (13.90) | −.23 | .051 |
| Minute 24 | 10.67 | (9.04) | 14.20 | (12.63) | −.30* | .011 |
| Minute 34 | 10.91 | (8.17) | 13.15 | (13.48) | −.29* | .013 |
| Minute 44 | 10.09 | (9.30) | 13.25 | (14.21) | −.27* | .020 |
| Minute 54 | 9.81 | (8.49) | 12.05 | (10.28) | −.14 | .243 |
p < .05, 2-sided test
Abbreviations: PEP, pre-ejection period; RSA, respiratory sinus arrhythmia
High RSA defined as ≥ 1 standard deviation above the mean; low RSA defined as ≤ 1 standard deviation below the mean
Association of baseline resting RSA with PEP reactivity (i.e., PEP during the task minus PEP at baseline) during each portion of the TSST and recovery period
Values represent PEP relative to baseline value; regression controls for PEP at baseline
Values represent PEP relative to the last minute of the TSST; regression controls for PEP during the last minute of the TSST
A different pattern emerged when we examined resting RSA and RSA reactivity and recovery. First, adolescents with high resting RSA also exhibited greater RSA reactivity during all three components of the TSST (βs=−0.29 to −0.48, p<.001) (Table 4). Second, the association of resting RSA with RSA recovery varied depending on whether the comparison was RSA at baseline or RSA during the TSST. Specifically, high resting RSA was associated with greater RSA rebound during the recovery period relative to the end of the TSST; this was observed for 4 of the 5 minutes of the recovery period (βs=0.21 to 0.25, p=.006 to .018). This suggests that RSA rebound following the TSST occurred more quickly for adolescents with high resting RSA. However, when we examined RSA during the recovery period relative to baseline values, the opposite pattern was observed. Specifically, average RSA during the recovery period relative to baseline was lower for adolescents with high resting RSA compared to adolescents with low resting RSA. However, this pattern likely reflects the fact that adolescents with high resting RSA also exhibited markedly greater vagal suppression during the TSST, and thus were starting the recovery period at a much lower point compared to baseline than adolescents with low resting RSA.
Table 4.
RSA Reactivity during the Trier Social Stress Test (TSST) and Recovery Period according to Resting Baseline RSA (N=157)
| High RSA1 | Low RSA1 | RSA and PEP Reactivity2 |
||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | β | p-value | |
| Preparation Reactivity3 | −0.67 | (0.99) | 0.45 | (1.01) | −.29* | <.001 |
| Speech Reactivity3 | −1.32 | (1.47) | 0.98 | (0.87) | −.48* | <.001 |
| Math Reactivity3 | −1.02 | (1.53) | 0.86 | (0.92) | −.43* | <.001 |
| Recovery3 | −0.23 | (0.39) | 0.18 | (0.53) | −.21* | .026 |
| Minute 14 | 0.80 | (1.51) | −0.45 | (1.22) | .25* | .006 |
| Minute 24 | 0.54 | (1.40) | −0.66 | (1.39) | .21* | .018 |
| Minute 34 | 0.35 | (1.38) | −0.56 | (1.24) | .13 | .155 |
| Minute 44 | 0.38 | (1.44) | −0.74 | (1.29) | .22* | .014 |
| Minute 54 | 0.52 | (1.65) | −0.82 | (1.38) | .22* | .012 |
p < .05, 2-sided test
Abbreviations: RSA, respiratory sinus arrhythmia
High RSA defined as ≥ 1 standard deviation above the mean; low RSA defined as ≤ 1 standard deviation below the mean
Association of baseline resting RSA with RSA reactivity (i.e., RSA during the task minus RSA at baseline) during each portion of the TSST and recovery period
Values represent RSA relative to baseline value; regression controls for RSA at baseline
Values represent RSA relative to the last minute of the TSST; regression controls for PEP during the last minute of the TSST
Discussion
Exposure to childhood adversity is remarkably common, with population-based studies estimating that nearly half of U.S. adolescents and adults have experienced at least one form of such adversity in childhood or adolescence (Green, et al., 2010; Kessler, et al., 1997; McLaughlin, et al., 2012). Childhood adversity is associated strongly with the onset of mental disorders and is responsible for a substantial proportion of psychiatric morbidity in the population (Green, et al., 2010; McLaughlin et al., 2010a, 2010b; McLaughlin, et al., 2012). Identifying factors that reduce vulnerability to psychopathology following childhood adversity exposure is critical in order to identify youths most likely to develop mental health problems following experiences of environmental adversity and to develop effective preventive intervention strategies. We provide novel evidence indicating that multiple aspects of vagal regulation, including both resting vagal tone and vagal suppression during a psychosocial stressor, are associated with low levels of internalizing symptoms among youths exposed to childhood adversity. Specifically, we found that childhood adversity was associated strongly with internalizing symptoms among adolescents with low vagal tone and low vagal reactivity but not among those with high vagal tone and reactivity. This pattern was observed across numerous types of environmental adversity including child abuse, community violence exposure, traumatic events, and low SES. No evidence was found for a buffering role of sympathetic nervous system activity, either at rest or in response to a psychosocial stressor.
High vagal tone and vagal suppression have been found to protect against the development of internalizing and externalizing problems among children living in families with high levels of marital conflict (El-Sheikh & Erath, 2011; El-Sheikh, et al., 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995, 1997). We extended this literature in several ways. First, we documented that resting vagal tone and vagal regulation in response to a psychosocial stressor buffered against internalizing symptoms following exposure to multiple forms of childhood adversity. Indeed, we examined a diverse range of adverse childhood experiences in this study, ranging from maltreatment and traumatic events involving serious threat of harm to markers of resource deprivation associated with poverty. Interactions between resting vagal tone and vagal reactivity were observed between each of these forms of childhood adversity in predicting adolescent internalizing symptoms, suggesting that vagal regulation might serve a generalized protective function for youths living in a wide range of adverse environments. This pattern can be understood within the context of the polyvagal theory. Specifically, this theory argues that the myelinated vagal brake has evolved to facilitate rapid regulation of the heart in response to environmental demands (Porges, 2001, 2007). Rapid vagal regulation facilitates attentional deployment and the use of appropriate social and communicative behaviors in response to changes in the environment. High levels of vagal regulation, both at rest and in response to stress, may confer protection against internalizing problems through several mechanisms. High vagal tone may prepare youths to respond appropriately to environmental adversity through downstream influences on attention and social behavior (El-Sheikh & Erath, 2011). In situations of relative safety, the vagal brake inhibits both the sympathetic nervous system and the HPA axis and promotes social engagement behaviors through inter-connections with the striated muscles that regulate facial expression and eye gaze (Porges, 1997, 2001). High resting vagal tone may thus promote positive social behavior and communication, which in turn facilitates better adaptation in situations of stress and adversity. In addition, vagal reactivity may influence sensitivity to stressors and psychological responses to childhood adversity (El-Sheikh & Erath, 2011). During a stressor, vagal withdrawal permits rapid mobilization of metabolic resources in response to environmental threats through parasympathetic disengagement (Porges, 1995a, 2007; Porges, et al., 1996). High vagal reactivity is argued to not only facilitate active coping in response to stress but also to promote rapid recovery and reductions in arousal when the stressor has ended (Porges, 1995a). Together, high vagal tone and vagal reactivity act in concert to promote effective attentional, emotional, and behavioral regulation in response to stress (Appelhans & Luecken, 2006; Calkins, et al., 2007; Calkins & Keane, 2004; Fabes, et al., 1993; Fabes, et al., 1994; Suess, et al., 1994), which may ultimately confer protection against the development of internalizing psychopathology.
Second, we found that only vagal regulation, and not sympathetic tone or sympathetic nervous system reactivity, served to buffer youths from internalizing symptoms following exposure to high levels of childhood adversity. In other words, this relationship was specific to vagal regulation rather than being a function of high levels of ANS reactivity more broadly. These results are at least partially consistent with theoretical conceptualizations of the specialization of each branch of the ANS, with emotional and attentional regulation functions subsumed within the parasympathetic branch and motivational functions related to approach and withdrawal behaviors governed by the sympathetic branch (Beauchaine, 2001). Our findings suggest that it is the regulatory functions of the parasympathetic branch that confer protection against internalizing problems in the context of environmental adversity. However, two previous studies of younger children found that marital conflict was associated with lower levels of externalizing symptoms among children with low sympathetic reactivity (El-Sheikh, 2005; Obradovic, et al., 2011), highlighting the importance of replicating our findings in future studies of younger children. It is also important to note that previous studies in younger children have found more complex interactions between marital conflict and reactivity in both branches of the ANS (El-Sheikh et al., 2009), using skin conductance to measure sympathetic reactivity. These findings point to the importance of examining higher-level interactions of childhood adversity with both sympathetic and parasympathetic functions in future studies of adolescents.
In addition, we also observed specificity with regards to the type of psychopathology that was predicted by the interplay of vagal regulation and childhood adversity. This relationship was associated with reductions in internalizing, but not externalizing, symptomatology. These findings were not expected, as previous research has found that vagal regulation predicts lower levels of externalizing psychopathology among children living in families with high levels of marital conflict (El-Sheikh, et al., 2001; Katz & Gottman, 1995, 1997), and vagal tone has been inversely associated with externalizing psychopathology in numerous prior studies of children and adolescents (Calkins, et al., 2007; Calkins & Keane, 2004; Mezzacappa, et al., 1997; Pine, et al., 1998), including a recent meta-analysis (Graziano & Derefinko, 2013). The specificity with regards to internalizing psychopathology suggests the possibility of developmental variation in the outcomes predicted by the interplay of vagal regulation and childhood adversity, as the current study focused on adolescents and previous studies observing a moderating effect on externalizing psychopathology were based on samples of younger children. Future research is needed to determine whether the specificity with regard to internalizing problems is replicated in other samples of adolescents.
Finally, we provide preliminary evidence for a potential physiological mechanism explaining the buffering role of high vagal tone and vagal regulation following childhood adversity exposure. The parasympathetic nervous system promotes physiological recovery following stressors through antagonistic influences on sympathetic arousal (Beauchaine, 2001; Berntson, et al., 1997; Porges, 1992, 1995a). Adaptive physiological recovery following psychosocial stressors is one potential mechanism through which high vagal tone might be associated with lower internalizing symptoms among youths exposed to childhood adversity. Our findings provide partial support for this explanation. Although resting vagal tone was unrelated to heart rate reactivity to a social/evaluative stressor, adolescents with high vagal tone exhibited greater heart rate deceleration in the immediate aftermath of the stressor. This greater reduction in heart rate was observable not only during the early stages of recovery, but was maintained across a five-minute recovery period. When we decomposed this effect to examine the contribution of sympathetic and parasympathetic influences on heart rate deceleration, the findings indicated that parasympathetic rebound was driving this effect more strongly than sympathetic withdrawal. In fact, although adolescents exhibited higher PEP—suggesting lower sympathetic activation—during the recovery period compared to baseline and to levels during the TSST, this rebound effect was stronger in adolescents with low resting vagal tone. In contrast, RSA rebound during the recovery period relative to the end of the stressor was larger among adolescents with high vagal tone. This complex pattern of recovery across the sympathetic and parasympathetic branches suggests that although adolescents with high vagal tone experience rapid vagal rebound following a stressor, inhibition of sympathetic activation occurs more slowly over time. This pattern is consistent with assertions of the polyvagal theory that the actions of the vagus nerve mediate rapid changes in heart rate in response to environmental demands (Porges, 2007). The functional implications of this pattern remain to be determined, however. The overall heart rate findings are consistent with a prior report documenting faster heart rate adaptation to psychosocial stress among adults with high RSA (Lane, et al., 1992) as well as findings that high RSA is associated with accelerated heart rate adaptation following trauma reminders among adults with PTSD (Sack, Hopper, & Lamprecht, 2004). Although we examined physiological recovery only as a function of resting vagal tone and not vagal regulation, high resting vagal tone was associated with enhanced vagal suppression during a social/evaluative stressor in the current study, suggesting overlap in these dimensions of adaptive vagal regulation. Greater vagal rebound following stress exposure therefore might be one explanation for reduced levels of internalizing symptoms following exposure to childhood adversity among youths with high vagal tone. Identifying additional mechanisms that explain this pattern is an important goal for future research.
Vagal tone and regulation are potentially promising markers of reduced vulnerability to the negative mental health consequences of exposure to childhood adversity. Identifying neurobiological markers of vulnerability provides the advantage of not having to rely on self-reported information about emotion, personality, and other dispositional characteristics, for which self-awareness may be poor and reporting biases are prominent (Robinson & Clore, 2002; Thomas & Deiner, 1990). Interventions designed to increase vagal tone and regulation might reduce stress sensitivity and vulnerability to internalizing psychopathology among youths experiencing high degrees of childhood adversity. Vagal tone therefore represents a potentially useful target for intervention. Studies with adults suggest that relaxation training and mindfulness are associated with increased vagal tone (Ditto, Eclache, & Goldman, 2006; Sarang & Telles, 2006; Wu & Lo, 2008). Determining whether these intervention strategies lead to improvements in vagal tone among youths is an important goal for future research. Finally, vagal tone has potential utility as a process measure of improvement during the course of intervention. Because of the ease with which it is acquired, vagal tone can feasibly be collected at multiple points during an intervention to determine whether changes in vagal tone are a mechanism underlying ameliorative effects on psychopathology.
Study findings should be interpreted in light of several limitations. The most notable limitation is our use of cross-sectional data that does not allow us to establish the temporal ordering of childhood adversity exposure and psychopathology. As a result, it is possible that internalizing symptoms in some youths predated the occurrence of childhood adversity experiences. Replication of these findings in prospective studies is therefore warranted. In addition, the interactions of vagal tone and reactivity with childhood adversity in predicting internalizing problems were stronger for youth-reported as compared to parent-reported symptoms. This finding is not surprising, however, as information provided by parents regarding internalizing problem in their children is only weakly related to information provided directly by youths (Achenbach, McConaughy, & Howell, 1987; Cantwell, et al., 1997). Indeed, adolescents are considered to be more valid reporters of internalizing problems than their parents (Cantwell, et al., 1997). In our data, the robust and frequently documented association between childhood adversity and internalizing problems was not observed for parent-reported symptoms, which lends greater credibility to the pattern observed for youth-reported internalizing problems. Finally, symptoms of psychopathology were assessed using the CBCL scales rather than a diagnostic interview. Determining whether the patterns of interaction between childhood adversity and both vagal tone and vagal regulation in predicting internalizing symptoms observed here are replicated in studies that measure internalizing disorders is another important goal for future research.
The relationship between exposure to childhood adversity and internalizing problems is attenuated in adolescents with high resting vagal tone and high vagal reactivity to a social/evaluative stressor, indicating that vagal regulation might be a promising a neurobiological marker of vulnerability to internalizing psychopathology following exposure to childhood adversity. Interventions that improve vagal tone and regulation among children and adolescents at high risk of exposure to adversity might confer protection against the deleterious mental health consequences of these experiences.
Acknowledgements
This research was funded by the National Institutes of Mental Health (K01-MH092625 to McLaughlin and K01-MH092555 to Sheridan).
Footnotes
The authors have no financial disclosures or conflicts of interest to report.
References
- Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR and TRF Profiles. Burlington, VT: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. [PubMed] [Google Scholar]
- Achenbach TM, Rescorla L. The Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, London: Sage; 1991. [Google Scholar]
- Allen JB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10:229–240. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Hondelsman L, Foote J, Lovejoy M. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin. 1993a;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993b;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, Fabro VT. Autonomic space and physiological response. Psychophysiology. 1994;31:44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB, Vanman E, Dickerson S. Social psychophysiology for social and personality psychology. Los Angeles: Sage; 2011. [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: A general feature of atopic disease? Psychosomatic Medicine. 2003;64:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Cacioppa JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: The reactivity hypothesis. Annals of the New York Academy of Sciences. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavioral problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Cantwell D, Lewinsohn PM, Rohde P, Seeley JR. Correspondance between adolescent report and parent report of psychiatric diagnostic data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:610–619. doi: 10.1097/00004583-199705000-00011. [DOI] [PubMed] [Google Scholar]
- Chen W, Faraone SV, Biederman J, Tsuang MT. Diagnostic accuracy of the Children Behavior Checklist scales for attention-deficit hyperactivity disorder. Journal of Consulting and Clinical Psychology. 1994;62:1017–1025. doi: 10.1037/0022-006X.62.5.1017. [DOI] [PubMed] [Google Scholar]
- Cohen P, Brown J, Smailes E. Child abuse and neglect and the development of mental disorders in the general population. Development and Psychopathology. 2001;13:981–999. [PubMed] [Google Scholar]
- Cole PM, Zahn-Waxler C, Fox NA, Usher BA, Welsh JD. Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology. 1996;105:518–529. [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead HK, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Annals of Behavioral Medicine. 2006;32:277–234. doi: 10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy BC, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children's social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- El-Sheikh M. The role of emotional responses and physiological reactivity in the marital conflict-child functioning link. Journal of Child Psychology and Psychiatry. 2005;46:1191–1199. doi: 10.1111/j.1469-7610.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children's adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children's externalizing behavior: interactions between parasympathetic and sympathetic nervous system activity. Monographs for the Society for Research in Child Development. 2009;74(1):1–79. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Eisenbud L. Behavioral and physiological correlates of children's reactions to others in distress. Developmental Psychology. 1993;29:655–663. [Google Scholar]
- Fabes RA, Eisenberg N, Karbon M, Troyer D, Switzer G. The relations of children's emotion regulation to their vicarious emotional responses and comforting behaviors. Child Development. 1994;65:1678–1693. doi: 10.1111/j.1467-8624.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Fox NA, Cohn JF, Galles SF, Kovacs M. Children's affect regulation during a disappointment: Psychophysiological responses and relation to parent history of depression. Biological Psychology. 2006;71:264–277. doi: 10.1016/j.biopsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Feres N, Olezeski CL, Rabkin AN, Trickett PK. Skin conductance reactivity and respiratory sinus arrhythmia among maltreated and comparison youth: Relations with aggressive behavior. Journal of Pediatric Psychology. 2010;35:547–558. doi: 10.1093/jpepsy/jsp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Derefinko K. Cardiac vagal control and children's adaptive functioning: A meta-analysis. Biological Psychology. 2013;94:22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund P, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;62:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Stemmler G, Meinhardt E. Paced respiratory sinus arrhythmia as an index of cardiac parasympathetic tone during varying behavioral tasks. Psychophysiology. 1990;27:404–416. doi: 10.1111/j.1469-8986.1990.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Hastings TL, Kelley ML. Development and validation of the Screen for Adolescent Violence Exposure (SAVE) Journal of Abnormal Child Psychology. 1997;25:511–520. doi: 10.1023/a:1022641916705. [DOI] [PubMed] [Google Scholar]
- Jamieson JP, Mendes WB, Blackstock E, Schmader T. Turning the knots in your stomach into bows: Reappraising arousal improves performance on the GRE. Journal of Experimental Social Psychology. 2010;46:208–212. doi: 10.1016/j.jesp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26:157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Puliafico AC, Barmish AJ, Choudhury MS, Henin A, Treadwell KS. Assessing anxiety with the Child Behavior Checklist and the Teacher Report Form. Journal of Anxiety Disorders. 2007;21:1004–1015. doi: 10.1016/j.janxdis.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Üstun TB. The World Mental Health (WHM) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) International Journal of Methods in Psychiatric Research. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer D. The 'Trier Social Stress Test' - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kollai M, Mizsei G. Respiratory sinus arrhythmia is a limited measure of cardiac parasympathetic control in man. Journal of Physiology. 1990;424:329–342. doi: 10.1113/jphysiol.1990.sp018070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JD, Adcock RA, Burnett RE. Respiratory sinus arrhythmia and cardiovascular responses to stress. Psychophysiology. 1992;29:461–470. doi: 10.1111/j.1469-8986.1992.tb01720.x. [DOI] [PubMed] [Google Scholar]
- Leary A, Katz LF. Coparenting, family-level processes, and peer outcomes: The moderating role of vagal tone. Development and Psychopathology. 1997;16:593–608. doi: 10.1017/s0954579404004687. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. 2005;46:1201–1206. doi: 10.1161/01.HYP.0000185147.32385.4b. [DOI] [PubMed] [Google Scholar]
- Lucini D, Norbiato G, Clerici M, Pagani M. Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension. 2002;39:184–188. doi: 10.1161/hy0102.100784. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the Youth Mood Project. Biological Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe PM, Yongue BG, Ackles PK, Porges SW. Changes in heart period, heart-period variability, and a spectral analysis estimate of respiratory sinus arrhythmia in response to pharmacological manipulations of the baroreceptor reflex in cats. Psychophysiology. 1985;22:195–203. doi: 10.1111/j.1469-8986.1985.tb01585.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber M, Sampson NA, Zaslavsky A, Kessler RC. Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R): II. Associations with persistence of DSM-IV disorders. Archives of General Psychiatry. 2010a;62:124–132. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber M, Sampson NA, Zaslavsky A, Kessler RC. Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R): III. Associations with severity of DSM-IV disorders. Psychological Medicine. 2010b;40:847–859. doi: 10.1017/S0033291709991115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky A, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of adolescents. Archives of General Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay RW, Kindlon D, Saul JP, Arseneault L, Seguin J, Earls F. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal of Child Psychology and Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Boyce WT. The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology. 2011;23:101–114. doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Malliani A. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pine DS, Wasserman MS, Miller L, Coplan JD, Bagiella E, Kovelenku P, Sloan RP. Heart period variability and psychopathology in urban boys at risk for delinquency. Psychophysiology. 1998;35:521–529. doi: 10.1017/s0048577298970846. [DOI] [PubMed] [Google Scholar]
- Porges SW. Vagal tone: A physiologic marker of stress vulnerability. Pediatrics. 1992;90:498–504. [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995a;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995b;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of the New York Academy of Sciences. 1997;807:62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal "brake" predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: Heart rate dynamics and individual differences in arousal regulation. Biological Psychiatry. 2004;55:284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- Sarang P, Telles S. Effects of two yoga based relaxation techniques on heart rate variability (HRV) International Journal of Stress Management. 2006;13:460–475. [Google Scholar]
- Scott KM, McLaughlin KA, Smith DAR, Ellis PM. Childhood maltreatment and DSM-IV adult mental disorders: comparison of prospective and retrospective findings. British Journal of Psychiatry. 2012;200:469–475. doi: 10.1192/bjp.bp.111.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Smith DR, Ellis PM. Prospectively ascertained childhood maltreatment and its associations with DSM-IV mental disorders in young adults. Archives of General Psychiatry. 2010;67:712–719. doi: 10.1001/archgenpsychiatry.2010.71. [DOI] [PubMed] [Google Scholar]
- Seligman L, Ollendick T, Langley AK, Baldacci B. The utility of measures of child and adolescent anxiety: a meta-analytic review of the Revised Children's Manifest Anxiety Scale, the State-Trait Anxiety Inventory for Children, and the Child Behavior Checklist. Journal of Clinical Child and Adolescent Psychology. 2004;33:557–565. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Boni SM, Paik M, Bigger JT, Jr, Gorman JM. Effect of mental stress throughout the day on cardiac autonomic control. Biological Psychology. 1994;37:89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Fox NA, Porges SW. Facial expressivity and vagal tone in 5- and 10-month-old infants. Infant Behavior and Development. 1989;12:127–137. [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Blude DL. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Deiner E. Memory accuracy in the recall of emotions. Journal of Personality and Social Psychology. 1990;59:291–297. [Google Scholar]
- Whitson SA, El-Sheikh M. Marital conflict and health: Processes and protective factors. Aggression and Violent Behavior. 2003;8:283–312. [Google Scholar]
- Wu S, Lo P. Inward-attention meditation increases parasympathetic activity: a study based on heart rate variability. Biomedical Research. 2008;29:245–250. doi: 10.2220/biomedres.29.245. [DOI] [PubMed] [Google Scholar]