Abstract
Testosterone is essential for maintaining spermatogenesis and male fertility. However, the molecular mechanisms by which testosterone acts have not begun to be revealed until recently. With the advances obtained from the use of transgenic mice lacking or overexpressing the androgen receptor, the cell specific targets of testosterone action as well as the genes and signaling pathways that are regulated by testosterone are being identified. In this review, the critical steps of spermatogenesis that are regulated by testosterone are discussed as well as the intracellular signaling pathways by which testosterone acts. We also review the functional information that has been obtained from the knock out of the androgen receptor from specific cell types in the testis and the genes found to be regulated after altering testosterone levels or androgen receptor expression.
Keywords: Testosterone, testis, fertility, Sertoli cell, blood testis barrier, meiosis
1.1 Introduction
1.1.1 Spermatogenesis
Male fertility is dependent upon the successful perpetuation of spermatogenesis, the multi-step process of male germ cell expansion and development that occurs within the seminiferous tubules of the testes. Although other hormones facilitate the process of spermatogenesis, only the steroid hormone testosterone is essential to maintain spermatogenesis. Testosterone actions in the testis in relation to the regulation of spermatogenesis have been discussed in recent reviews [1–6]. Here, we summarize the spermatogenesis processes regulated by testosterone, cell specific actions of testosterone as well as the intracellular pathways and genes that are controlled by testosterone signaling in the testis.
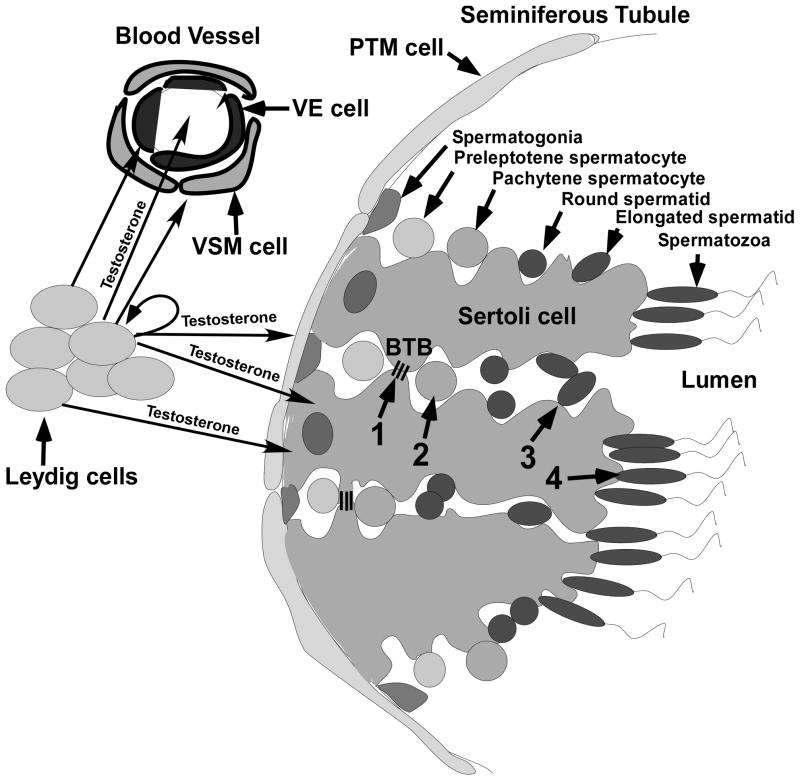
Spermatogenesis occurs in the seminiferous tubules of the testis. The seminiferous tubules are composed of three major cell types: peritubular myoid (PTM) cells, Sertoli cells and germ cells. PTM cells surround the external wall of the tubule and contract to force sperm down the tubule. Sertoli cells relay external signals and provide factors required for the proliferation and differentiation of germ cells. PTM cells cooperate with Sertoli cells to produce the basement membrane of the seminiferous tubule and provide the niche for spermatogonial stem cells (SSCs) that produce the germ cells that will develop into sperm [7, 8]. The cytoplasm of Sertoli cells extends from the basement membrane to the lumen of the tubule surrounding the developing germ cells. Leydig cells are present in the interstitial space between the tubules and produce testosterone, which diffuses into the seminiferous tubules, as well as blood vessels in the interstitial space (Fig. 1).
Figure 1.
The anatomy of the testis and the process of spermatogenesis. Leydig cells and blood vessels that are lined by vascular endothelial (VE) and vascular smooth muscle (VSM) cells are localized to the interstitial space between seminiferous tubules. Peritubular myoid (PTM) cells line the outside of the seminiferous tubule. Sertoli cells extend from the PTM cells to the lumen and surround the developing germ cells. The process of spermatogenesis is outlined at the top of the figure where germ cell development is shown progressing toward the lumen from spermatogonia on the basement membrane to the production of spermatozoa. Testosterone produced by the Leydig cells diffuses into the VE and VSM cells as well as into the blood vessels. Testosterone also diffuses from the Leydig cells into PTM, Sertoli cells and Leydig cells. The four critical processes regulated by testosterone (1–4 in the middle of the figure) are indicated: 1) the maintenance of the BTB (represented by 3 lines extending between 2 Sertoli cells), 2) completion of meiosis by spermatocytes, 3) adherence of elongated spermatids to Sertoli cells and 4) the release of mature spermatozoa.
The division of SSCs along the basement membrane of the seminiferous tubule initiates the spermatogenesis process. The proliferation of SSCs results in either the production of two new stem cells to retain the stem cell pool or undifferentiated spermatogonia that are destined to develop into sperm. The undifferentiated spermatogonia undergo a series of mitotic divisions with incomplete cytokinesis to form chains of spermatogonia. Once the chains reach a length of 16 or 32 cells, they undergo differentiation en mass to become differentiated spermatogonia that are committed to becoming sperm. The differentiated spermatogonia undergo a series of divisions with a final mitosis resulting in the production of preleptotene spermatocytes that initiate the process of meiosis. At the conclusion of meiosis, haploid round spermatids are produced that undergo differentiation into elongated spermatids and then finally spermatozoa (Fig. 1).
Spermatogenesis is supported by somatic Sertoli cells that surround and nurture the developing germ cells. Sertoli cells contribute to the niche required to maintain the renewal of spermatogonial stem cells so that developing germ cells can be produced continuously. Sertoli cells also provide growth factors and nutrients for the developing germ cells. Specialized adhesion junctions are formed between adjacent Sertoli cells that in total form the blood testis barrier (BTB) near the basement membrane of the seminiferous tubules. The BTB divides the seminiferous tubule into basal and adluminal compartments. During the initial preleptotene stage of meiosis, spermatocytes “pass through” the BTB moving from the basement membrane to adluminal compartment. Once through the BTB, the germ cells continue to develop into spermatozoa in a defined, protected microenvironment. However, because the BTB denies germ cells in the adluminal compartment access to factors supplied by the circulatory system, the Sertoli cell must provide for the needs of the more mature germ cells [9, 10]
1.1.2 Testosterone production and bioavailability
Testosterone is the major androgen in the testis that regulates spermatogenesis. Testosterone is produced by the Leydig cell in response to stimulation with luteinizing hormone (LH) and acts as a paracrine factor that diffuses into the seminiferous tubules. Androgen effects are mediated by the androgen receptor (AR, also denoted NR3C4), which is a 110 kD protein localized to the nucleus and cytoplasm. There are no functional receptors for androgen in germ cells [11–14]. Instead the testosterone that diffuses into Sertoli cells binds to the AR present in the cytoplasm and nucleus to initiate the functional responses required to support spermatogenesis. In the testis, testosterone also interacts with AR expressed in Leydig, PTM cells, arteriole smooth muscle and vascular endothelial cells.
Because of the localized production of testosterone from Leydig cells, testosterone levels in the testes of men and rodents are 25- to 125-fold higher than that present in serum [15–19]. The physiological importance of high testosterone levels in the testis is not fully understood. However, it has been established that sperm production decreases exponentially once testosterone levels in the testis fall below 70 mM [20]. The high levels of testosterone in the testis cannot be explained by a sequestration mechanism to “deactivate” the hormone because at least two thirds of testicular testosterone is free or weakly bound to albumen and is bioavailable. Only one third of testosterone is tightly bound by sex hormone binding globulin (SHBG) or androgen binding protein (ABP) [21, 22]. Thus, the bioavailable testosterone in the testis greatly exceeds the Kd for AR binding of approximately 1 mM [23].
2.1 AR expression
2.1.1. Developmental patterns of AR expression
In humans and rodents, AR is expressed in PTM cells at high levels from the fetal period throughout adulthood [24–27]. Adult Leydig cells also express AR at a constant level [28]. Sertoli cells do not express AR in fetal life [29]. In humans, AR is first detectable in the nuclei of a few Sertoli cells at the age of 5 months. Labeling is weak until 4 years of age and increases thereafter [24–27]. In monkeys, androgen binding activity of Sertoli cells cultured from infants is at least 4-fold lower than that for cells cultured at puberty [30]. The lack of AR in the infant human and monkey likely explains the lack of Sertoli sensitivity to the testosterone that is present during the first few months after birth. In mice and rats, AR in the Sertoli cell is first expressed 3 to 5 days after birth after which AR levels increase up to 35 or 60 days of age [29, 31–33]. Because testosterone levels are elevated early during testis development but spermatogenesis only initiates after AR is expressed in Sertoli cells, AR in the Sertoli cells is believed to be the rheostat for testosterone signaling.
2.1.2. AR expression in adult Sertoli cells is cyclical
In the adult male, testosterone levels in the testis are maintained at relatively constant, high levels in the testis and AR levels in Leydig and peritubular cells are constant suggesting that testosterone signaling is constitutively activated in these cells (reviewed in [28]). In contrast, AR expression in Sertoli cells changes dramatically in a cyclical fashion related to the stages of the cycle of the seminiferous epithelium. In men, AR levels are highest in stage III of the six stages, although AR was easily detected in all other stages [34]. In the adult rat, the expression of AR in the Sertoli cell is similarly cyclical. AR mRNA and protein expression progressively increases during stages II-VII and then decline sharply after stage VII to become barely detectable in stages IX-XIII [32, 35, 36]. Because AR expression is greatest during stage VII, this stage is thought to be the most regulated and sensitive to testosterone [37]. Various models of testosterone withdrawal from rats confirm that progressive germ cell loss begins during stage VII in the absence of testosterone [38].
3.1. Testosterone regulation of essential spermatogenesis processes
Testosterone is required for at least four critical processes during spermatogenesis: maintenance of the BTB, meiosis, Sertoli-spermatid adhesion and sperm release (indicated by the numbers 1, 2, 3, and 4 in Fig. 1).
3.1.1. Maintenance of the BTB
During stages VI-VII of the cycle of the seminiferous epithelium, preleptotene spermatocytes move off of the basement membrane and tight junctions making up the BTB are formed on the basal side of the spermatocyte by adjacent Sertoli cells while the original BTB is dissolved above the cell. It is this mechanism by which preleptotene spermatocytes transit through the BTB [39]. The elevated levels of AR present during stages VI-VII may facilitate testosterone-dependent transport of BTB proteins from the original apical side to the basal side of transiting germ cells. The expression of at least three tight junction protein components of the BTB (occludin, claudin 11 and claudin 3) are decreased in the absence of AR [40], suggesting that testosterone signaling through AR is required for the remodeling of the BTB. Testosterone signaling accelerates the kinetics of internalization of BTB proteins from the cell surface and induces the expression of proteins such as caveolin-1 and Rab11 that regulate protein transcytosis and recycling, respectively as well as their association with occludin and N-cadherin internalized from the cell surface [41, 42]. Thus, testosterone acts to maintain the dynamic BTB by facilitating reassembly of BTB components on the basal side of the transiting spermatocyte after the dismantling of old BTB structures.
3.1.2. Meiosis
In the absence of testosterone signaling, spermatogenesis is halted during meiosis such that few germ cells develop to the haploid spermatid stage and elongated spermatids are not formed [43]. Elimination of AR expression specifically in Sertoli cells causes spermatogenesis to be halted at the pachytene or diplotene stage of meiosis [44, 45]. Information regarding direct testosterone effects on genes and proteins required for meiosis has been lacking. However, proteomics analysis of rat models having various levels of testosterone depletion and replenishment has identified proteins and processes that are regulated in meiotic cells by testosterone [46]. Specifically, testosterone deprivation was found to alter the expression and post-translational modification of almost 25 proteins involved in oxidative metabolism, DNA repair, RNA processing, apoptosis and meiotic division. In total, the protein expression survey indicated that upon the loss of testosterone signaling from somatic cells, meiotic cells undergo cellular stresses including the unfolded protein response and oxidative damage, DNA damage and apoptosis plus proteins with roles in meiotic division are affected. In addition, proteins contributing to RNA splicing and processing as well as posttranslational processing and DNA repair were identified. Further studies are required to confirm the testosterone-mediated expression of the proteins identified and their functional importance.
3.1.3. Sertoli-spermatid adhesion
In testosterone suppressed rats, elongated spermatids are absent because round spermatids are prematurely detached from Sertoli cells [47]. Studies of AR hypomorph mice having reduced AR activity revealed that Sertoli cell attachments to elongated spermatids could not be maintained and germ cells were released prematurely [48]. During stages VII-VIII, adhesive connections between Sertoli cells and spermatids are remodeled as the spermatids begin to elongate. At this stage of development, desmosome based connections between Sertoli cells and the less mature germ cells are replaced by the stronger ectoplasmic specialization (ES) adhesion complex that is specific for attachments to elongated spermatids (reviewed in [49]. Two of the protein complexes that form the ES connections between Sertoli cells and elongated spermatids (cadherin/cadherin and α6β1-integrin/lamininγ3) are targets of androgen suppression [50, 51]. After testosterone suppression, there are changes in the degree of protein phosphorylation of several ES-associated proteins including focal adhesion kinase (FAK) and β-catenin that leads to detachment [50, 52]. Tyrosine phosphorylation of β-catenin is associated with increased dissociation of the cadherin/cadherin complex. Increased association with the c-Src and FAK kinases after testosterone depletion also decreases the integrity of the α6β1-integrin/lamininγ3 connection [50]. The adhesion proteins associated with the ES are cyclically induced to form new connections between Sertoli cells and elongating spermatids as they are formed but thus far, the expression only one of eight genes encoding proteins associated with the ES is repressed in the absence of AR [53].
3.1.4. Sperm release
In the absence of testosterone signaling, mature sperm that are normally released during stage VIII are retained and phagocytized by Sertoli cells [48]. Src activation has been shown to be required for the release of sperm. Src phosphorylation is transiently induced during stages VII-VIII and Src is structurally associated with proteins at the ES. Also, Src phosphorylates the β-catenin and N-cadherin proteins in Sertoli cells that contribute to the formation of the ES adhesion sites with maturing elongated spermatids [52, 54, 55]. After Src-mediated phosphorylation of β-catenin and N-cadherin, the two proteins diffuse away from each other, the cell linkage is lost and mature sperm can be released [54–56]. Analysis of gene expression after suppression of both testosterone and FSH in rats identified genes expressed by Sertoli cells that are associated with adhesion. These genes included Sparc (osteonectin) that modules focal adhesions, Ctgf that modulates adhesion and interacts with integrins that form contacts with elongated spermatids, Lgals1 (galectin 1) that surrounds spermatids during spermiation and can modulate integrin-mediated adhesion and signaling [57]. Presently, it is not known to what extent testosterone versus FSH regulates these adhesion-associated genes.
4.1. Testosterone signaling pathways
4.1.1. Classical testosterone signaling
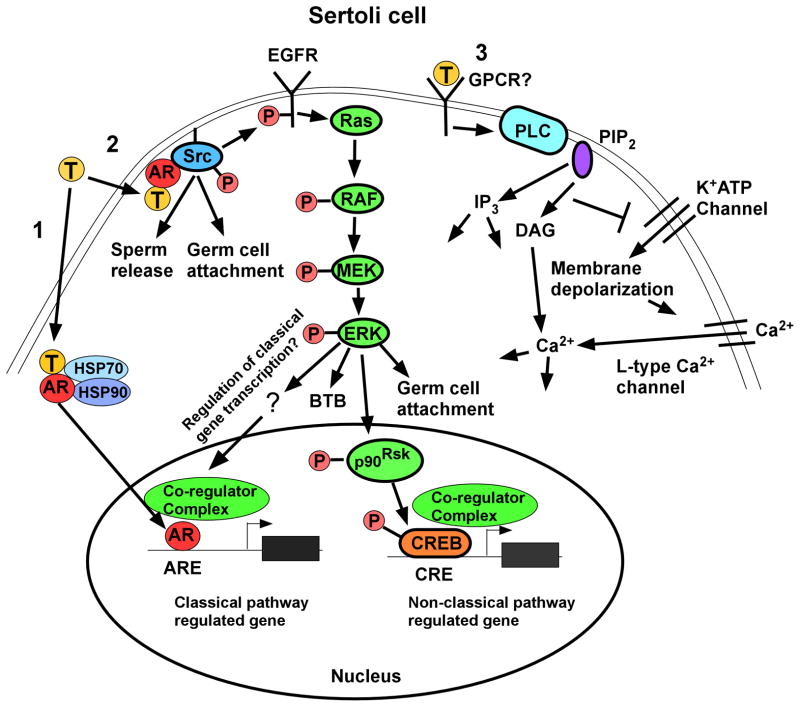
AR is the only specific receptor for androgens that has been identified. However, AR is capable of transmitting testosterone signals by at least 2 mechanisms, the classical and non-classical pathways. In the classical signaling pathway, testosterone that diffuses through the cell membrane interacts with AR that is often sequestered in the cytoplasm by heat shock proteins (Fig. 2, pathway 1). After binding androgen, a conformational change in AR allows the receptor to disengage from the heat shock proteins, dimerize and translocate to the nucleus. AR bound by androgen in the nucleus binds as a dimer to specific DNA sequences called androgen response elements (AREs) in gene regulatory regions and recruits co-activator or co-repressor proteins to regulate gene expression. The classical pathway is distinguished by the time required to produce a functional response. At least 30 to 45 minutes is required for productive transcription after testosterone stimulation with additional time required to alter protein levels in the cell [58].
Figure 2.
Testosterone signaling pathways in Sertoli cells. Left (Pathway 1): The classical testosterone signaling pathway: Testosterone diffuses through the plasma membrane and binds with the AR that then undergoes an alteration in conformation allowing it to be released from heat shock proteins (HSP70, HSP90) in the cytoplasm. AR then translocates to the nucleus where it binds to specific DNA sequences called androgen response elements (AREs). AR binding to an ARE allows the recruitment of co-regulators (co-activator or co-repressor proteins) that alter the expression of genes and eventually cellular function. Middle (Pathway 2): The non-classical kinase activation pathway: Testosterone interacts with AR that is then able to bind with and activate Src, which is required for sperm release and germ cell attachment. Src also causes the activation of the EGF receptor that then activates the MAP kinase cascade most likely through Ras resulting in the sequential phosphorylation and activation of RAF and MEK and then ERK, which regulates BTB integrity and germ cell attachment. ERK may also regulate classical pathway-mediated gene expression through pathways that are not yet known for Sertoli cells. ERK also can facilitate non-classical pathway mediated gene expression. For example, ERK activates p90RSK kinase, which is known to phosphorylate CREB on serine 133. As a result, CREB-regulated genes can be induced by testosterone. Right (Pathway 3): The non-classical Ca2+ influx pathway: Testosterone interacts with a receptor in the plasma membrane that has characteristics of a Gq coupled G-protein coupled receptor (GPCR). Phospholipase C (PLC) is activated to cleave PIP2 into IP3 and DAG. Decreased PIP2 concentrations inhibit K+ATP channels causing membrane depolarization and Ca2+ entry via L-type Ca2+ channels.
The first AREs found to bind AR were inverted repeats separated by three base pairs with the consensus sequence 5′-TGTTCT-3′. These “general” (also known as classical) AREs can be occupied by AR as well as other steroid hormone receptors. A second class of AREs (selective AREs) bind AR more selectively and have a direct rather than inverted repeat consensus sequence 5′-TGTTCT-3′. Mice engineered to express an AR mutant that can bind to general but not selective AREs (Specificity affecting AR knock in (SPARKI) mice) were found to have a 35% decrease in the size of the testis and a 50% reduction in litter size. Spermatogenesis was normal in SPARKI mice through the completion of meiosis but the number of round and elongated spermatids are reduced by 53% and 56%, respectively. The number of Sertoli cells was also decreased by 32% in SPARKI mice. These results suggest that reduced spermatogenesis efficiency is due to reduced classical testosterone signaling through selective AREs in Sertoli cells as well as the decrease in number of Sertoli cells [59]. A subset of known testosterone-regulated genes in the testis including Rhox5, Tsx and Drd4 were down-regulated in SPARKI mice indicating that some genes in the testis are regulated via selective AREs and others via general AREs or another mechanism.
In studies of adult AR hypomorph mice and AR hypomorphs having Sertoli cell specific ablation of AR, 46 and 57 testis-expressed genes were found to be regulated by testosterone. About twice as many genes were down-regulated by testosterone than up-regulated. It was found that 65% of AR regulated genes were associated with a conserved ARE within 6 kb of their transcription start sites and 23% of the testosterone regulated genes were previously shown to be regulated by validated AREs. These studies suggest that a substantial percentage of testosterone-mediated gene expression is regulated via the classical pathway. However, some gene expression is mediated by events downstream of AR-regulated gene expression, via post-transcriptional processes or by other mechanisms such as non-classical testosterone signaling.
4.1.2. Non-classical testosterone signaling
In contrast to classical signaling, non-classical testosterone signaling translates signals into alterations in cellular function within second to minutes. Two non-classical pathways have been defined in Sertoli cells. In the first pathway, stimulation of Sertoli cells with levels of testosterone (10–250 nM) that are similar to or lower than that found in the testis causes a population of AR to localize near the plasma membrane (Fig. 2 pathway 2). Binding of testosterone to AR allows the receptor to interact with and activate Src tyrosine kinase. Specifically, a proline rich region of AR (amino acids 352–359) associates with the SH3 domain of Src. Once activated, Src causes the phosphorylation of the epidermal growth factor receptor (EGFR) via an intracellular pathway. The stimulation of EGFR results in the activation of the MAP kinase cascade including the kinases RAF, MEK and ERK followed by the activation of the p90Rsk kinase, the subsequent phosphorylation of the CREB transcription factor and activation of CREB-mediated transcription. Examples of the rapid actions of the non-classical pathway include the increased phosphorylation of ERK and CREB that occurs within 1 minute. The non-classical testosterone pathway also provides sustained cellular signals as ERK and CREB phosphorylation can be maintained for at least 12 hours [60, 61].
The activation of Src and Erk kinase by non-classical signaling alters processes that are critical for maintaining spermatogenesis. In cell culture studies, the number of germ cells attached to Sertoli cells was increased after stimulation with testosterone. However, the addition of Src or Erk kinase reduced the numbers of attached germ cells to basal levels [62]. Using cultured AR-defective Sertoli cells, it was found that germ cell attachment could be increased by testosterone stimulation after infection with adenoviruses expressing wild type AR or an AR mutant that can activate only the non-classical pathway. Adenovirus constructs expressing an AR mutant that could only activate the classical pathway had no effect [62]. These results suggest that testosterone stimulation of Src and ERK kinases can contribute to maintaining Sertoli-germ cell attachment. Also, it is possible that the increase in AR expression levels during stages VI-VII of the cycle of the seminiferous epithelium in rodents may facilitate the non-classical pathway-mediated remodeling of adhesion complexes that results in the formation of the ES between Sertoli cells and elongated spermatids.
The release of mature sperm from Sertoli cells is regulated by Src kinase family members [52, 63, 64] that can be activated by non-classical signaling [62]. Culturing seminiferous tubule fragments in the presence of a Src kinase inhibitor decreased the relative numbers of sperm released. These results are consistent with earlier studies showing that during stages VII-VIII when sperm are released, activated Src levels increase at the ES near the site of the Sertoli-elongated spermatid adhesion complex [52, 63, 64]. Src also is known to phosphorylate FAK, β-catenin and N-cadherin proteins that contribute to the formation of the adhesion complexes between Sertoli cells and the mature elongated spermatids [54–56]. Redistribution of proteins required for the formation, dissolution and reformation of the BTB may be dependent upon non-classical testosterone signaling as testosterone stimulation of hpg mice caused the rapid redistribution of claudin 11 to the area of the forming BTB prior to activating the transcription of genes encoding BTB components [65]. Also kinases that are known to regulate the dynamics of tight and adherens junctions [66–68] are regulated by non-classical testosterone signaling [61, 62].
It is possible that the activation of kinases by non-classical signaling in Sertoli cells may result in the regulation of AR-mediated nuclear (classical) gene transcription. One such mechanism has been described in androgen-sensitive prostate cells. Testosterone binding to membrane localized AR results in the ERK-mediated activation of paxillin, which previously had been considered to be a scaffold protein thought to regulate cytoskeletal remodeling and focal adhesion function. After being phosphorylated, paxillin is localized to the nucleus and complexes with AR to retain the receptor in the nucleus and facilitate AR regulation of transcription [69, 70]. Further studies are required to determine whether similar pathways are initiated by testosterone signaling in Sertoli cells.
The second non-classical pathway causes the influx of [Ca2+] into Sertoli cells via L-type [Ca2+] within 20 to 40 seconds (Fig. 2 pathway 3). The [Ca2+] influx occurs due to the activation of an unidentified Gq type G protein coupled receptor and the subsequent activation of phospholipase C that hydrolyzes PIP2 in the plasma membrane to produce IP3 and diacylglycerol (DAG). The closing of ATP-mediated K+ATP channels is promoted by decreased levels of PIP2, causing an increase in membrane resistance and depolarization of the cell. As a result, voltage dependent L-type [Ca2+] channels open allowing the influx of [Ca2+], which can alter many cellular processes. Thus far, the physiological functions in Sertoli cells that are regulated by the testosterone-mediated influx of [Ca2+] have not been identified [71].
It has been proposed that only classical testosterone signaling is required for spermatogenesis because germ cell development is halted during meiosis in transgenic mice in which exon 3 of the AR containing a portion of the DNA binding domain was removed [72]. However, the non-classical activity of the exon 3-deleted AR mutation was not reported in the study. Analysis of non-classical testosterone signaling in a Sertoli cell line lacking endogenous AR expression but expressing a recreated AR mutant lacking exon 3 showed that ERK phosphorylation did not increase in response to testosterone stimulation (WW, unpublished data). This result indicates that the removal of 39 amino acids of AR in this model may alter the structure of AR to inhibit both non-classical and classical activity.
5.1. Mouse cell specific AR knock out and over expression models
To examine the functions of testosterone signaling in various cells in the testis, several groups have developed mouse models in which AR expression is ablated in specific cell types in the testis. Studies of the cell specific AR knock out mice plus gain of function mouse models have been the subject of recent reviews [1, 4, 73] and are summarized below as well as in Table 1.
Table 1.
Phenotypes of mice having cell-specific alterations in AR expression.
| Cell specific AR alteration | Phenotype |
|---|---|
| Germ cell KO | None |
| Peritubular cell KO | Progressive germ cell loss in adults, incomplete Leydig cell development. |
| Leydig cell KO | Decreased steroidogenic enzyme activity, effect on germ cells not conclusive. |
| Vascular endothelial KO | None |
| Vascular smooth muscle KO | Normal spermatogenesis, impaired testicular vasomotion, increased interstitial fluid. |
| Sertoli cell KO | Spermatogenesis halted during meiosis, disruption of the BTB, fewer Leydig cells. |
| AR overexpression in Sertoli cells | Accelerated Sertoli cell maturation, fewer Sertoli cells and post meiotic germ cells, decreased Leydig cell proliferation. |
5.1.1. Germ cell AR knock out mouse
Male germ cell specific AR knock out (GC-ARKO) mice have been generated using Cre recombinase driven by the Sycp1 gene promoter that is active in leptotene and zygotene spermatocytes. These mice display normal testes, spermatogenesis and fertility indicating that AR expression in germ cells is not essential for spermatogenesis, at least after the initiation of meiosis [14]. This conclusion is in agreement with the commonly accepted idea that AR is not expressed in germ cells [74].
5.1.2. Peritubular myoid cell AR knock out mice
The loss of PTM cells results in abnormal spermatogenesis [75]. However, the function of testosterone in PTM cells has only recently begun to be revealed. Two models of AR ablation in PTM cells (PTM-ARKO mice) have been published but poor targeting of the AR knockout to PTM cells [76] in one model has led to the acceptance of the second model, as a more robust characterization of AR ablation from PTM cells [77]. For mice lacking AR in PTM cells, there are no detectable differences in the testes up to postnatal day 12, indicating that AR signaling in PTM cells is dispensable for the initial development of the testis. However, at day 15 and later there is a reduction in testis weight [77]. In adult male PTM-ARKO mice, the number of germ cells decrease progressively through their developmental program until there are nearly no elongated spermatids present and the mice are infertile. In addition, there are changes in expression and localization of several PTM proteins such as the intermediate filament desmin and basement membrane protein laminin. Seminiferous tubule lumen diameter was reduced in PTM-ARKO mice reflecting an impact on the secretion of fluid by Sertoli cells. In addition, there were significant changes in gene expression of several key genes, including those previously identified as responsive to AR signaling within Sertoli cells [76, 77].
Mice with a targeted disruption of AR in PTM cells experienced a progressive loss of spermatogonia [77], suggesting that PTM cells require testosterone (T) action to produce factors influencing SSC renewal in the niche. Recent studies indicate that PTM cells produce GDNF (glial cell line-derived neurotrophic factor), which is required for the maintenance of SSCs. Testosterone was found to induce GDNF expression at the mRNA and protein levels in cultured PTM cells. Furthermore, spermatogonial stem cells co-cultured with PTM cells in the presence of testosterone were more efficient at restoring spermatogenesis after transplantation into germ cell deficient testes (Liang-Yu Chen and Mitch Eddy, personal communication). These studies suggest that PTM cells provide important support for SSCs in response to testosterone signaling.
The elimination of AR expression in PTM cells also causes adult Leydig cell development to be incomplete. PTM-ARKO mice have normal numbers of adult Leydig cells but, there are also significant numbers of precursor Leydig cells present in adult mice [77, 78]. Circulating testosterone concentrations in PTM-ARKO mice are within the normal range, but only because of increased LH input. However, there is a four-fold increase in testicular testosterone in these mice suggesting that testosterone secretion by Leydig cells or testosterone escape from the testis via the vascular system may be altered after ablation of AR in PTM cells [77, 78].
5.1.3. Leydig cell AR knock out mouse
Although testosterone produced by Leydig cells is essential for spermatogenesis, testosterone actions on Leydig cells that might regulate spermatogenesis have been more difficult to interpret. A Leydig cell-specific AR knock out (LC-ARKO) model has been reported [14, 79]. The LC-ARKO mice were infertile with spermatogenesis arrested predominately at the round spermatid stage. Spermatozoa were not found in the epididymis. In LC-ARKO mice, the numbers of Leydig cells did not decrease, but expression of several key steroidogenic enzymes required for the synthesis of testosterone was reduced [79]. Unfortunately, the use of the Anti-Mullerian Hormone receptor 2 AmhR2-Cre to eliminate AR expression has made analysis of the resulting phenotype complex because the AmhR2-Cre mouse has previously been utilized to target genes in both Sertoli cells and Leydig cells [80, 81]. In agreement with this idea, AR was not eliminated in all Leydig cells and AR was lost in some Sertoli cells of the LC-ARKO mice. Thus, the functional role of testosterone signaling within Leydig cells remains to be established.
5.1.4. Testis vasculature AR knock out mice
Studies of vascular endothelial (VE) AR knock out (VEARKO) mice did not cause any altered phenotypes indicating that AR actions in VE cells are not required for spermatogenesis [82]. Elimination of AR in vascular smooth muscle (VSM) cells (SMARKO mice) also resulted in normal reproductive development but adult testis weight is reduced [83]. SMARKO mice are fully fertile but, ablation of AR from VSM increases interstitial fluid volume within the testis, due to impairment of testicular vasomotion [83], which is the androgen-dependent rhythmical contraction/relaxation mechanism that regulates fluid and nutrient exchange between the vascular system and peripheral tissues [84, 85]. The disruption of testicular vasomotion is associated with diminished Leydig cell function, possibly due to changes in testicular fluid exchange and microvascular blood flow within the testis.
5.1.5. Sertoli cell AR knock out mice
In three initial Sertoli cell specific AR knock out mice (SCARKO) models [44, 45, 48], the number of Sertoli cells was normal and the cells underwent appropriate maturation with the correct timing during puberty. This finding demonstrates that the development and final number of Sertoli cells is not determined by AR. However, testis weight was reduced and the mice were infertile. The numbers of spermatogonia were not affected and spermatocytes were slightly reduced, but post-meiotic spermatids were significantly reduced or absent. These findings were similar to that found after suppression of testosterone production [86] and are consistent with the loss of AR causing a block in spermatogenesis at the completion of meiosis.
Analyses of various SCARKO models confirmed that testosterone signaling contributes to maintaining the BTB. However, some studies argue that AR is essential for BTB formation [53] and that the BTB is open in the absence of AR [40]; whereas, others provide evidence that the formation of the BTB is delayed during testis development but is only partially disrupted in the adult mouse [87]. Numerous genes encoding proteins contributing to the integrity of the BTB have been found to be mis-regulated in SCARKO mice including Claudin 3, Claudin-11, Occludin, Gelsolin, Cadherin 2, Espin and Jam 3 [40, 87],
One limitation of the cell specific AR ablation studies performed thus far is that AR expression was eliminated early in development so that studies of the effect on ongoing spermatogenesis in adults could not be performed. To address this situation, a ubiquitously expressed tamoxifen-inducible Cre recombinase was used to knock out AR in all cells of adult mice [88] In these inducible AR knock out (iARKO) mice, spermatogenesis was arrested in adults after treatment with tamoxifen plus there was impaired Sertoli cell function and decreased expression of AR regulated genes in Sertoli and Leydig cells. Inducible knock out of AR has the potential to provide important information regarding the mechanisms by which testosterone regulates spermatogenesis, especially if inducible cell specific knockouts can be developed for Sertoli, Leydig and PTM cells. However, the use of tamoxifen to induce the knockout of AR has limitations. Specifically, in the iARKO mouse, treatment with tamoxifen alone, a potent estrogen receptor (ER)-alpha agonist, was found to alter expression of genes associated with steroid hormone synthesis, and reduce the levels of FSH, LH, circulating testosterone as well as intra-testicular testosterone [88].
5.1.6. Sertoli cell AR gain of function mice
In contrast to the AR loss of function studies performed in SCARKO mice, a gain of function AR mouse model was created in which additional AR is expressed in Sertoli cells from an AR transgene driven by the promoter of the gene encoding androgen binding protein (tgSCAR). In the tgSCAR model, testis size was reduced but formation of the seminiferous tubule lumen occurred earlier and mRNA transcripts associated with Sertoli cell maturation were induced prematurely. These results confirm previous findings that testosterone signaling and AR regulate the formation of the BTB and fluid production required to form the lumen. The increased AR expression caused Sertoli cells to accelerate their maturation and halt their proliferation more rapidly with the result that fewer Sertoli cells were present in the adult at every step of development [89]. The number of Sertoli cells also was decreased in tfm and ARKO mice further implicating AR in regulation of Sertoli cell numbers. However, AR expression in other cell types may be more important to establish Sertoli cell numbers in normal mice because AR in Sertoli cells is dispensible for final Sertoli cell number and maturation as shown by the normal numbers of Sertoli cells that matured at the correct developmental time point in SCARKO mice [90]. Germ cell development from the meiotic pachytene spermatocyte stage onward was accelerated in the TgSCAR in agreement with the idea that the meiosis is regulated by testosterone signaling in Sertoli cells. However, the absolute numbers of post meiotic germ cells were decreased, which likely occurs in response to the decreased number of Sertoli cells. In addition, there was a proportional decrease in Leydig cell proliferation and absolute numbers in response to the fewer Sertoli cells in the tgSCAR mouse [91] that is consistent with Sertoli-Leydig cell paracrine interactions that are dependent upon Sertoli AR activity for optimal Leydig cell development [44, 48].
6.1. Testosterone regulation of gene expression in the testis
6.1.1 Animal models of testosterone depletion and enhancement
Until the employment of RiboTag mouse + RNA seq strategies (see section 6.2.1) studies of AR regulated gene expression have provided some important but somewhat limited information regarding testosterone-mediated gene expression that is required for spermatogenesis. Mice in which testosterone levels were depleted or enhanced in all cells were the first models used to assay gene expression in the testis. These models included normal 8 day-old neonatal mice injected with testosterone propionate (TP) [92] and hypogonadal (hpg) mice injected with TP [93] or DHT [65]. These models revealed that few genes were regulated by more than two-fold, there was relatively little overlap between models, a large percentage of the genes were down-regulated by testosterone and few identified genes had known fertility phenotypes. Using the tfm (testicular feminized) mouse model that is defective for AR function, half of the testosterone-regulated genes expressed in somatic cells were associated with vitamin A metabolism, solute transport and tight junction formation or cytoskeleton and endocytosis function were identified [94]. These results were interpreted as evidence that testosterone regulates spermatogenesis in part by modulating the tubular environment and controlling retinoic acid metabolism, which is required for spermatogonia differentiation and the entry into meiosis. Unfortunately, most of the transcripts identified in the tfm model were expressed in germ cells and were not directly related to testosterone signaling due to the early arrest of germ cell development because testes from tfm animals do not descend into the scrotum.
6.1.2. Testosterone regulation of genes identified with Sertoli AR knock out models
Although specific knock out of AR has been studied in every cell in the testis, nearly all testosterone-mediated gene expression information related to spermatogenesis has been limited to Sertoli cells. Until recently, all survey studies employing mouse models in which AR is absent or inactive specifically in Sertoli cells assayed mRNA levels in extracts from total testis, which may have limited the detection of differences in cell specific gene expression. A comprehensive review of these transcription profiling studies performed using SCARKO and global AR defective mouse models was published recently by Verhoeven and colleagues [4]. Here we discuss four observations from the gene survey studies employing SCARKO mice.
First, in these studies, large sets of genes were found to be regulated by testosterone, but relatively few were found to be consistently regulated by more than 2-fold. For example, only the four highly regulated genes Rhox5, Lrp4, Drd4 and Fhod3 are quantitatively regulated in the same direction in studies employing the SCARKO, SCARKO-jsd and tfm models [95]. In the SCARKO and tfm models, the additional Tsx, and three serine endopeptidase inhibitors: Eppin, Serpina 3N and PCI are similarly regulated. Of the differentially expressed genes, a large percentage appears to be down-regulated by testosterone signaling. Down-regulation of gene expression after testosterone stimulation may occur due to indirect actions such as the regulation of a second gene downstream of the initial testosterone-mediated transcriptional regulatory event, post-transcriptional regulation, induction of inhibitory factors including microRNAs, or in response to the activation of kinases by non-classical testosterone signaling.
Second, some families of transcripts are regulated similarly by testosterone in various models including proteases, protease inhibitors, cell adhesion and cytoskeletal proteins [76, 94, 96, 97]. These data support the idea that testosterone signaling targets cell-cell junctional dynamics and restructuring of the seminiferous tubules required for germ cell adhesion and migration [4]. Genes encoding solute carriers and transport proteins (transferrin, fatty acid binding protein and ABP) as well as modifiers of metabolism are also common to some AR-deficient models suggesting that testosterone contributes to creating a specific unique environment needed for germ cell development [53, 94, 96].
The third observation is that the tfm and AR hypomorph model studies identified genes involved in vitamin A metabolism including alcohol dehydrogenase I that is the rate limiting step in the conversion of vitamin A to the potent signaling factor, retinoic acid, suggesting that testosterone signaling contributes to retinoic acid-dependent actions in the testis [94, 96]. Because analysis of SCARKO models found less of a retinoic acid connection, it is possible that other cell types may be responsible for translating testosterone signals into retinoic acid-mediated spermatogonia differentiation and entry into meiosis [4, 98–100].
A final observation from the gene survey studies analyzed thus far is that the deletion of AR regulated genes expressed in Sertoli cells rarely results in the lack of sperm production although there are examples of reduced fertility. For example, the testosterone regulated Galgt1, PCI, Eppin and Lrp8 genes identified in SCARKO mice are required for fertility but may not directly affect spermatogenesis or may not affect fertility due to AR actions in the Sertoli cell [101–104]. Galgt1 (β1,4-N-acetylgalactosaminyltransferase) encodes an enzyme required for the formation of complex gangliosides. Mice lacking Galgt1 in all cells cannot complete spermatogenesis due to the inability of germ cells to produce a specific class of fucoylated glycosphingolipids that may be required for the formation of the unique plasma membrane of haploid germ cells [104, 105]. However, there is no evidence that AR-regulated Galgt1 derived from Sertoli cells is required for germ cells to produce glycosphingolipids. The transport of testosterone out of Leydig cells and into the seminiferous tubules and the blood stream is also dependent on glycoshongolipids but, again this process is not directly regulated by Galgt1 in Sertoli cells [103]. PCI encodes a serine protease inhibitor and PCI knockout mice have spermatogenesis defects including detached germ cells, disruption of the BTB and damage to Sertoli cells. However, sperm are produced by the PCI deficient mice but, they are incapable of fertilization [102]. Eppin has not been shown to be required for spermatogenesis but it is required for fertilization of the egg by sperm and has been the target of contraceptive development [101]. Also, the lack of Lrp8 causes sperm defects that develop in the epididymis [106, 107].
Rhox5, is an example of a highly induced gene by testosterone (up to 50-fold) that supports cellular metabolism and insulin signaling plus produces factors required by germ cells including Ins2 [108]. Rhox5 encodes one of a family of 12 Rhox homeobox proteins. Expression of Rhox5 mRNA is repressed in every AR defective model, but mice lacking the Rhox5 gene are still capable of completing meiosis and retain some fertility. In total, analysis of the findings from the SCARKO models reinforce the idea that in the absence of testosterone signaling spermatogenesis is disrupted due to the combined effects of testosterone on multiple genes. Furthermore, testosterone support of fertility can occur via the regulation of protein expression in the testis that may not have effects until well downstream.
6.2.1 RiboTag-SCARKO models
RiboTag mouse models are a promising strategy for the identification of cell-specific mRNA transcripts within tissues consisting of multiple cell types. RiboTag mice express an HA-epitope tagged Rpl22 ribosomal protein as a transgene. Conditional expression of the transgene using a cell-specific Cre recombinase allows the purification of mRNAs associated with ribosomes by immunoprecipitation of the tagged Rpl22 protein from extracts of cells having activated Cre [109]. In microarray analysis of mRNAs in RiboTag mice expressing a Sertoli cell specific CRE, the use of gonadotropin releasing hormone (GnRH) antagonists to deplete testosterone and FSH followed by testosterone restoration for 4 h resulted in the identification of only 11 testosterone regulated translated transcripts (7 up-regulated, all regulated 3.6-fold or less) in Sertoli cells in vivo [110]. Together, these data agree with previous studies suggesting that testosterone may maintain spermatogenesis via altering the expression of a broad spectrum of genes and that the sum of many small changes may be required to support fertility [4].
The results of a new study using a more sensitive model system to detect AR regulated genes in vivo challenges the previous conclusions that testosterone is not a dynamic regulator of gene expression in Sertoli cells. In this model system, the RiboTag mouse was used in conjunction with RNA seq analysis of mRNAs expressed specifically in Sertoli cells of SCARKO versus control mice [97]. As found with most other models, the percentage of AR regulated genes that were down regulated by AR was 50% or more. In contrast to earlier survey studies of AR regulated genes, this strategy identified hundreds of AR regulated Sertoli cell expressed transcripts that were not identified previously including at least 100 genes being regulated by more than 2-fold. It is likely that the enrichment of Sertoli cell specific genes using the RiboTag strategy combined with the more sensitive and more quantitative RNA-seq assay allowed for the unmasking of AR regulated genes. The long term inhibition of testosterone signaling in the RiboTag-SCARKO mouse as opposed to short term testosterone stimulation after testosterone deprivation also likely contributed to the identification of more AR-regulated genes than the first RiboTag model.
The AR regulated genes identified by RiboTag-RNA-seq strategy included those encoding cytoskeletal protein binding and actin binding proteins plus NTPase and GTPase regulators that alter the actin and microtubular cytoskeleton. This result is consistent with the idea that testosterone signaling regulates Sertoli cell cytoskeletal dynamics. There was also an over representation of genes encoding proteins involved in cellular adhesion, including proteins located at the cell-matrix, cell-cell and anchoring junctions plus cellular projections. These findings support the idea that testosterone signaling regulates Sertoli cell interactions with the basement membrane and at the BTB as well as Sertoli-germ cell interactions at the apical ectoplasmic specialization. The RiboTag-RNA-seq strategy also permitted the identification of genes expressed in other testicular cells that are responsive to the activity of AR in Sertoli cells. Many of these genes encode proteins involved in binding to adenyl ribonucleotides, nucleosides and ATP suggesting that testosterone signaling in Sertoli cells instructs other cells to alter their nucleic acid and energy metabolism [97]. It is anticipated that additional studies of gene expression using the RiboTag-RNA-seq strategy will reveal additional testosterone regulated genes in Sertoli and other testis cells that are essential to maintain spermatogenesis.
6.3.1. Testosterone induces the expression of microRNAs in Sertoli cells
Testosterone regulates the expression of several microRNAs (miRNAs) in Sertoli cells that can decrease the half-life or translation of mRNAs that are highly expressed in Sertoli and germ cells. Using a testosterone-deprivation model in which mice were treated with the anti-androgen, flutamide, and the GnRH antagonist, acyline, the expression of 28 miRNAs were found to be highly altered [111]. In the same study, many of the 28 miRNAs were similarly regulated in hypoandrogenic mice lacking LHβ. At least 9 of the miRNAs were developmentally expressed such that their expression peaked on postnatal day 13 or 21 during the androgen dependent steps of spermatocyte progression though meiosis I or the initiation of spermatid differentiation. Pathway analysis suggested that testosterone-regulated miRNAs targeted genes are required for cell junction restructuring and cell signaling, two androgen dependent processes required for germ cell development. Further analysis showed that the miR-471 miRNA binds to the 3′UTRs and down-regulates the Sertoli cell genes Foxd1, a forkhead/winged-helix transcription factor that is important in Sertoli metabolism, and desmocollin 1 (Dsc1) a demosomal cadherin that is required for Sertoli-germ cell adhesion [111]. The down-regulation of gene expression by testosterone-regulated miRNAs may be one explanation for the relatively high percentage of genes that are inhibited by testosterone signaling in Sertoli cells.
7.1 Summary and Conclusions
Testosterone is required for processes that are critical for spermatogenesis including maintaining the BTB, supporting the completion of meiosis, the adhesion of elongated spermatids to Sertoli cells and the release of sperm. Previously, studies of testosterone and AR actions have be centered on the Sertoli cell but increasingly testosterone actions in other cell types including PTM and vascular smooth muscle cells are being found to affect processes that occur in the seminiferous tubules. The results from cell-specific AR knock out mice have greatly advanced the understanding of testosterone effects in all cell types within the testes. Gene expression analysis of SCARKO mice have identified a number testosterone regulated genes but this model was somewhat mystifying in the inability to detect large changes in gene expression that would be expected to result in response to a hormone that is essential for spermatogenesis. However, taken in total the results from the SCARKO mice do support the idea that testosterone regulates many genes and that it may be the sum of all the alterations in transcription that supports the complex development of germ cells. The use of the RiboTag-RNA-seq strategy has now allowed the detection of more dramatic gene expression changes in response to the loss of AR and provides promise that additional genes regulated by testosterone will be detected in other testis cells expressing AR. The refinement of employing inducible cell specific knock out mice also would provide added information regarding the ongoing functions of testosterone signaling in the adult without the complexity of the animals lacking testosterone signals since prior to birth.
One consistent finding from the use of any model regulating testosterone activity is the high percentage of genes in Sertoli cells that appear to be down-regulated in response to testosterone signaling. It does not seem likely that AR would directly decrease the expression of a large percentage of genes. Instead it is expected that increased AR-mediated gene expression will result in the production of proteins that in turn down-regulate other genes or that testosterone signaling may alter the metabolism of the Sertoli cell resulting in decreased gene expression. Kinase pathways regulated by non-classical testosterone signaling may also contribute to decreasing gene expression. It is also possible that testosterone-induced miRNAs will be found to inhibit the transcription and translation of numerous transcripts.
Although testosterone deprivation studies and AR knock out mice had provided lists of testosterone regulated genes and collections of proteins contributing to various cellular processes, many of the molecular and cellular events that are regulated by testosterone remain to be characterized. One question that requires further investigation is how meiosis is supported by testosterone. Although new proteomics studies have identified proteins in meiotic germ cells that are responsive to testosterone signaling in other cells, it is not yet known how the testosterone signals are relayed to the meiotic germ cells. Further study is also required to better characterize the germ cell proteins that are actually regulated by testosterone-initiated signals derived from other cells versus those simply responding to the germ cell failing to thrive. Another focus of needed investigation is the identification of the critical testosterone regulated factors that are produced in Sertoli and other cells that support the completion of meiosis.
The extent of the contribution of non-classical testosterone toward the support of spermatogenesis also remains to be determined. There is evidence for testosterone-mediated rapid changes in Sertoli cells such as phosphorylation events to alter the activity of regulatory proteins, transcytosis of adhesion factors and exocytosis of signaling factors destined for germ cells. Although some genes encoding proteins required for cellular adhesion are regulated by testosterone, the formation and disruption of Sertoli-Sertoli and Sertoli–germ adhesion junctions is associated with phosphorylation of critical proteins in the adhesion complexes. Formation and disruption of cell adhesion processes are essential to maintain the BTB and Sertoli-elongated spermatid connections as well as to permit spermiation. Further investigation is required better characterize the relative contributions of non-classical and classical testosterone signaling to the regulation of cellular adhesions complexes that are needed to maintain spermatogenesis.
With the genetic models in hand and additional models that are likely to be created, a better foundation will be available for the investigations of the molecular and cellular processes regulated by testosterone. The results of these studies will hopefully be translatable to men to address the challenges of treating infertility that is due to defects in testosterone signaling.
Testosterone maintains BTB, meiosis, Sertoli-spermatid adhesion and spermiation.
Testosterone acts via classical and non-classical pathways.
Testosterone regulates PTM, Leydig, and Sertoli cells via the androgen receptor.
The combined regulation of many genes by testosterone supports spermatogenesis.
Acknowledgments
This work was supported by the UK Medical Research Council (L.B.S.) and the Magee Womens Research Institute (W.H.W.).
Abbreviations
- PTM
peritubular myoid cell
- SSC
spermatogonia stem cell
- DHT
dihydrotestosterone
- BTB
blood testis barrier
- AR
androgen receptor
- SHBG
sex hormone binding globulin
- ABP
androgen binding protein
- FAK
focal adhesion kinase
- ES
ectoplasmic specialization
- ARE
androgen response element
- RGFR
epidermal growth factor receptor
- GDNF
glial derived neurotrophic factor
- jsd
juvenile spermatogonial depletion mutant
- TP
testosterone propionate
- tfm
testicular feminization
- GnRH
gonadotropin releasing hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lee B. Smith, Email: Lee.Smith@ed.ac.uk.
William H. Walker, Email: walkerw@pitt.edu.
References
- 1.Chang C, Lee SO, Wang RS, Yeh S, Chang TM. Androgen receptor (AR) physiological roles in male and female reproductive systems: lessons learned from AR-knockout mice lacking AR in selective cells. Biol Reprod. 2013;89:21. doi: 10.1095/biolreprod.113.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1557–69. doi: 10.1098/rstb.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1:116–120. doi: 10.4161/spmg.1.2.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K. Androgens and spermatogenesis: lessons from transgenic mouse models. Philos Trans R Soc Lond B Biol Sci. 2010;365:1537–56. doi: 10.1098/rstb.2009.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves MG, Rato L, Carvalho RA, Moreira PI, Socorro S, Oliveira PF. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell Mol Life Sci. 2013;70:777–93. doi: 10.1007/s00018-012-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205:117–31. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- 7.Richardson LL, Kleinman HK, Dym M. Basement membrane gene expression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod. 1995;52:320–30. doi: 10.1095/biolreprod52.2.320. [DOI] [PubMed] [Google Scholar]
- 8.Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100:1941–7. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 10.Skinner MK. Sertoli Cell Secreted Regulatory Factors. In: Skinner MK, Griswold MD, editors. Sertoli Cell Biology. Elsevier Science; San Diego: 2005. pp. 107–120. [Google Scholar]
- 11.Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30:119–32. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon MF, Glenister PH, Lamoreux ML. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature. 1975;258:620–2. doi: 10.1038/258620a0. [DOI] [PubMed] [Google Scholar]
- 13.Johnston DS, Russell LD, Friel PJ, Griswold MD. Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology. 2001;142:2405–8. doi: 10.1210/endo.142.6.8317. [DOI] [PubMed] [Google Scholar]
- 14.Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, et al. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci U S A. 2006;103:18975–80. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comhaire FH, Vermeulen A. Testosterone concentration in the fluids of seminiferous tubules, the interstitium and the rete testis of the rat. J Endocrinol. 1976;70:229–235. doi: 10.1677/joe.0.0700229. [DOI] [PubMed] [Google Scholar]
- 16.Turner TT, Jones CE, Howards SS, Ewing LL, Zegeye B, Gunsalus GL. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- 17.Awoniyi CA, Santulli R, Sprando RL, Ewing LL, Zirkin BR. Restoration of advanced spermatogenic cells in the experimentally regressed rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:1217–23. doi: 10.1210/endo-124-3-1217. [DOI] [PubMed] [Google Scholar]
- 18.Maddocks S, Hargreave TB, Reddie K, Fraser HM, Kerr JB, Sharpe RM. Intratesticular hormone levels and the route of secretion of hormones from the testis of the rat, guinea pig, monkey and human. Int J Androl. 1993;16:272–8. doi: 10.1111/j.1365-2605.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 19.Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkint BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J Androl. 2001;22:640–5. [PubMed] [Google Scholar]
- 20.Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–9. doi: 10.1210/endo-124-6-3043. [DOI] [PubMed] [Google Scholar]
- 21.Hammond GL, Ruokonen A, Kontturi M, Koskela E, Vihko R. The simultaneous radioimmunoassay of seven steroids in human spermatic and peripheral venous blood. J Clin Endocrinol Metab. 1977;45:16–24. doi: 10.1210/jcem-45-1-16. [DOI] [PubMed] [Google Scholar]
- 22.Jarow JP, Wright WW, Brown TR, Yan X, Zirkin BR. Bioactivity of androgens within the testes and serum of normal men. J Androl. 2005;26:343–8. doi: 10.2164/jandrol.04100. [DOI] [PubMed] [Google Scholar]
- 23.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro E, Huang H, Masch RJ, McFadden DE, Wu XR, Ostrer H. Immunolocalization of androgen receptor and estrogen receptors alpha and beta in human fetal testis and epididymis. J Urol. 2005;174:1695–8. doi: 10.1097/01.ju.0000179540.28209.de. [DOI] [PubMed] [Google Scholar]
- 25.Berensztein EB, Baquedano MS, Gonzalez CR, Saraco NI, Rodriguez J, Ponzio R, et al. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr Res. 2006;60:740–4. doi: 10.1203/01.pdr.0000246072.04663.bb. [DOI] [PubMed] [Google Scholar]
- 26.Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, Gonzalez-Peramato P, et al. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab. 2008;93:4408–12. doi: 10.1210/jc.2008-0915. [DOI] [PubMed] [Google Scholar]
- 27.Boukari K, Meduri G, Brailly-Tabard S, Guibourdenche J, Ciampi ML, Massin N, et al. Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J Clin Endocrinol Metab. 2009;94:1818–25. doi: 10.1210/jc.2008-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rey RA, Musse M, Venara M, Chemes HE. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc Res Tech. 2009;72:787–95. doi: 10.1002/jemt.20754. [DOI] [PubMed] [Google Scholar]
- 29.You L, Sar M. Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine. 1998;9:253–61. doi: 10.1385/ENDO:9:3:253. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar SS, Sarda K, Bhattacharya I, Plant TM. Insufficient androgen and FSH signaling may be responsible for the azoospermia of the infantile primate testes despite exposure to an adult-like hormonal milieu. Hum Reprod. 2012;27:2515–25. doi: 10.1093/humrep/des184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buzek SW, Sanborn BM. Increase in testicular androgen receptor during sexual maturation in the rat. Biol Reprod. 1988;39:39–49. doi: 10.1095/biolreprod39.1.39. [DOI] [PubMed] [Google Scholar]
- 32.Bremner WJ, Millar MR, Sharpe RM, Saunders PTK. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135:1227–1234. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Kudo A, Kawakami H, Hirano H. Immunohistochemical localization of androgen receptor in mouse testicular germ cells during fetal and postnatal development. Anat Rec. 1996;245:509–518. doi: 10.1002/(SICI)1097-0185(199607)245:3<509::AID-AR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Suarez-Quian CA, Martinez-Garcia F, Nistal M, Regadera J. Androgen receptor distribution in adult human testis. J Clin Endocrin Metab. 1999;84:350–358. doi: 10.1210/jcem.84.1.5410. [DOI] [PubMed] [Google Scholar]
- 35.Vornberger W, Prins G, Musto NA, Suarez-Quian CA. Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology. 1994;134:2307–2316. doi: 10.1210/endo.134.5.8156934. [DOI] [PubMed] [Google Scholar]
- 36.Shan L-X, Zhu K, L-J, Bardin CW, Hardy MP. Quantitative analysis of androgen receptor messenger ribonucleic acid in developing Leydig cells and Sertoli cells by in situ hybridization. Endocrinology. 1995;136:3856–3862. doi: 10.1210/endo.136.9.7649092. [DOI] [PubMed] [Google Scholar]
- 37.Kerr JB, Millar M, Maddocks S, Sharpe RM. Stage-dependent changes in spermatogenesis and Sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat Rec. 1993;235:547–59. doi: 10.1002/ar.1092350407. [DOI] [PubMed] [Google Scholar]
- 38.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neil JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 1363–1434. [Google Scholar]
- 39.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan HH, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. Faseb J. 2008;22:1945–59. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGF-beta3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–60. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–32. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, et al. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–81. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanton PG, Sluka P, Foo CF, Stephens AN, Smith AI, McLachlan RI, et al. Proteomic changes in rat spermatogenesis in response to in vivo androgen manipulation; impact on meiotic cells. PLoS One. 2012;7:e41718. doi: 10.1371/journal.pone.0041718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55:895–901. doi: 10.1095/biolreprod55.4.895. [DOI] [PubMed] [Google Scholar]
- 48.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–67. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 49.Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci. 2010;365:1593–605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong CH, Xia W, Lee NP, Mruk DD, Lee WM, Cheng CY. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Mruk DD, Cheng CY. Myotubularin phosphoinositide phosphatases, protein phosphatases, and kinases: their roles in junction dynamics and spermatogenesis. J Cell Physiol. 2005;204:470–83. doi: 10.1002/jcp.20303. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Wong CH, Xia W, Mruk DD, Lee NP, Lee WM, et al. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-beta-catenin protein complex which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology. 2005;146:1268–84. doi: 10.1210/en.2004-1194. [DOI] [PubMed] [Google Scholar]
- 53.Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–33. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 54.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 55.Xia W, Cheng CY. TGF-beta3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: An in vivo study. Dev Biol. 2005;280:321–43. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 56.Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995;130:461–71. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Donnell L, Pratis K, Wagenfeld A, Gottwald U, Muller J, Leder G, et al. Transcriptional profiling of the hormone-responsive stages of spermatogenesis reveals cell-, stage-, and hormone-specific events. Endocrinology. 2009;150:5074–84. doi: 10.1210/en.2009-0755. [DOI] [PubMed] [Google Scholar]
- 58.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 59.Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, et al. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci U S A. 2007;104:4961–6. doi: 10.1073/pnas.0610814104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng J, Watkins SC, Walker WH. Testosterone Activates MAP Kinase Via Src Kinase and the EGF Receptor in Sertoli Cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 62.Shupe J, Cheng J, Puri P, Kostereva N, Walker WH. Regulation of Sertoli-Germ Cell Adhesion and Sperm Release by FSH and Nonclassical Testosterone Signaling. Mol Endocrinol. 2011;25:238–52. doi: 10.1210/me.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W, WRN, Chapin RE. Rat testicular src: Normal distribution and involvement in ethylene glycol monomethyl ether-induced apoptosis. Toxicology and Applied Pharmacology. 2000;163:125–134. doi: 10.1006/taap.1999.8870. [DOI] [PubMed] [Google Scholar]
- 64.Chapin RE, Wine RN, Harris MW, Borchers CH, Haseman JK. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl. 2001;22:1030–52. doi: 10.1002/j.1939-4640.2001.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 65.McCabe MJ, Allan CM, Foo CF, Nicholls PK, McTavish KJ, Stanton PG. Androgen initiates Sertoli cell tight junction formation in the hypogonadal (hpg) mouse. Biol Reprod. 2012;87:38. doi: 10.1095/biolreprod.111.094318. [DOI] [PubMed] [Google Scholar]
- 66.Li JC, Mruk D, Cheng CY. The inter-Sertoli tight junction permeability barrier is regulated by the interplay of protein phosphatases and kinases: an in vitro study. J Androl. 2001;22:847–56. [PubMed] [Google Scholar]
- 67.Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev Biol. 2005;286:1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 69.Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P, et al. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012;122:2469–81. doi: 10.1172/JCI62044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sen A, O’Malley K, Wang Z, Raj GV, Defranco DB, Hammes SR. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem. 2010;285:28787–95. doi: 10.1074/jbc.M110.134064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18:371–8. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Lim P, Robson M, Spaliviero J, McTavish KJ, Jimenez M, Zajac JD, et al. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology. 2009;150:4755–65. doi: 10.1210/en.2009-0416. [DOI] [PubMed] [Google Scholar]
- 73.De Gendt K, Verhoeven G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol. 2012;352:13–25. doi: 10.1016/j.mce.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–81. [PubMed] [Google Scholar]
- 75.Franca LR, Leal MC, Sasso-Cerri E, Vasconcelos A, Debeljuk L, Russell LD. Cimetidine (Tagamet) is a reproductive toxicant in male rats affecting peritubular cells. Biol Reprod. 2000;63:1403–12. doi: 10.1095/biolreprod63.5.1403. [DOI] [PubMed] [Google Scholar]
- 76.Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, et al. The effect of a Sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol. 2006;20:321–34. doi: 10.1210/me.2005-0113. [DOI] [PubMed] [Google Scholar]
- 77.Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23:4218–30. doi: 10.1096/fj.09-138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welsh M, Moffat L, Belling K, de Franca LR, Segatelli TM, Saunders PT, et al. Androgen receptor signalling in peritubular myoid cells is essential for normal differentiation and function of adult Leydig cells. Int J Androl. 2012;35:25–40. doi: 10.1111/j.1365-2605.2011.01150.x. [DOI] [PubMed] [Google Scholar]
- 79.Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, et al. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine. 2007;32:96–106. doi: 10.1007/s12020-007-9015-0. [DOI] [PubMed] [Google Scholar]
- 80.Smith L. Good planning and serendipity: exploiting the Cre/Lox system in the testis. Reproduction. 2011;141:151–61. doi: 10.1530/REP-10-0404. [DOI] [PubMed] [Google Scholar]
- 81.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–10. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 82.O’Hara L, Smith LB. Androgen receptor signalling in Vascular Endothelial cells is dispensable for spermatogenesis and male fertility. BMC Res Notes. 2012;5:16. doi: 10.1186/1756-0500-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Welsh M, Sharpe RM, Moffat L, Atanassova N, Saunders PT, Kilter S, et al. Androgen action via testicular arteriole smooth muscle cells is important for Leydig cell function, vasomotion and testicular fluid dynamics. PLoS One. 2010;5:e13632. doi: 10.1371/journal.pone.0013632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collin O, Bergh A, Damber JE, Widmark A. Control of testicular vasomotion by testosterone and tubular factors in rats. J Reprod Fertil. 1993;97:115–21. doi: 10.1530/jrf.0.0970115. [DOI] [PubMed] [Google Scholar]
- 85.Damber JE, Maddocks S, Widmark A, Bergh A. Testicular blood flow and vasomotion can be maintained by testosterone in Leydig cell-depleted rats. Int J Androl. 1992;15:385–93. doi: 10.1111/j.1365-2605.1992.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 86.Franca LR, Parreira GG, Gates RJ, Russell LD. Hormonal regulation of spermatogenesis in the hypophysectomized rat: quantitation of germ-cell population and effect of elimination of residual testosterone after long-term hypophysectomy. J Androl. 1998;19:335–40. [PubMed] [Google Scholar]
- 87.Willems A, Batlouni SR, Esnal A, Swinnen JV, Saunders PT, Sharpe RM, et al. Selective ablation of the androgen receptor in mouse sertoli cells affects sertoli cell maturation, barrier formation and cytoskeletal development. PLoS One. 2010;5:e14168. doi: 10.1371/journal.pone.0014168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willems A, De Gendt K, Deboel L, Swinnen JV, Verhoeven G. The development of an inducible androgen receptor knockout model in mouse to study the postmeiotic effects of androgens on germ cell development. Spermatogenesis. 2011;1:341–353. doi: 10.4161/spmg.1.4.18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hazra R, Corcoran L, Robson M, McTavish KJ, Upton D, Handelsman DJ, et al. Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol Endocrinol. 2013;27:12–24. doi: 10.1210/me.2012-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, et al. The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology. 2005;146:2674–83. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- 91.Hazra R, Jimenez M, Desai R, Handelsman DJ, Allan CM. Sertoli cell androgen receptor expression regulates temporal fetal and adult Leydig cell differentiation, function, and population size. Endocrinology. 2013;154:3410–22. doi: 10.1210/en.2012-2273. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD. Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod. 2005;72:1010–9. doi: 10.1095/biolreprod.104.035915. [DOI] [PubMed] [Google Scholar]
- 93.Sadate-Ngatchou PI, Pouchnik DJ, Griswold MD. Identification of testosterone-regulated genes in testes of hypogonadal mice using oligonucleotide microarray. Mol Endocrinol. 2004;18:422–33. doi: 10.1210/me.2003-0188. [DOI] [PubMed] [Google Scholar]
- 94.O’Shaughnessy PJ, Abel M, Charlton HM, Hu B, Johnston H, Baker PJ. Altered expression of genes involved in regulation of vitamin A metabolism, solute transportation, and cytoskeletal function in the androgen-insensitive tfm mouse testis. Endocrinology. 2007;148:2914–24. doi: 10.1210/en.2006-1412. [DOI] [PubMed] [Google Scholar]
- 95.Zhou W, Wang G, Small CL, Liu Z, Weng CC, Yang L, et al. Gene expression alterations by conditional knockout of androgen receptor in adult Sertoli cells of Utp14b jsd/jsd (jsd) mice. Biol Reprod. 2011;84:400–8. doi: 10.1095/biolreprod.110.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eacker SM, Shima JE, Connolly CM, Sharma M, Holdcraft RW, Griswold MD, et al. Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol. 2007;21:895–907. doi: 10.1210/me.2006-0113. [DOI] [PubMed] [Google Scholar]
- 97.De Gendt K, Verhoeven G, Amieux PS, Wilkinson MF. The AR-regulated Translome in Sertoli Cells Defined Using the RiboTag Approach. Mol Endocrinol. 2014 doi: 10.1210/me.2013-1391. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morales C, Griswold MD. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology. 1987;121:432–4. doi: 10.1210/endo-121-1-432. [DOI] [PubMed] [Google Scholar]
- 99.Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–37. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 100.Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128:610–24. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 101.O’Rand GM, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, et al. Reversible immunocontraception in male monkeys immunized with eppin. Science. 2004;306:1189–90. doi: 10.1126/science.1099743. [DOI] [PubMed] [Google Scholar]
- 102.Uhrin P, Dewerchin M, Hilpert M, Chrenek P, Schofer C, Zechmeister-Machhart M, et al. Disruption of the protein C inhibitor gene results in impaired spermatogenesis and male infertility. J Clin Invest. 2000;106:1531–9. doi: 10.1172/JCI10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takamiya K, Yamamoto A, Furukawa K, Zhao J, Fukumoto S, Yamashiro S, et al. Complex gangliosides are essential in spermatogenesis of mice: possible roles in the transport of testosterone. Proc Natl Acad Sci U S A. 1998;95:12147–52. doi: 10.1073/pnas.95.21.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sandhoff R, Geyer R, Jennemann R, Paret C, Kiss E, Yamashita T, et al. Novel class of glycosphingolipids involved in male fertility. J Biol Chem. 2005;280:27310–8. doi: 10.1074/jbc.M502775200. [DOI] [PubMed] [Google Scholar]
- 105.Rabionet M, van der Spoel AC, Chuang CC, von Tumpling-Radosta B, Litjens M, Bouwmeester D, et al. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J Biol Chem. 2008;283:13357–69. doi: 10.1074/jbc.M800870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, Erdmann B, et al. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem. 2003;278:23989–95. doi: 10.1074/jbc.M302157200. [DOI] [PubMed] [Google Scholar]
- 107.Maclean JA, 2nd, Chen MA, Wayne CM, Bruce SR, Rao M, Meistrich ML, et al. Rhox: a new homeobox gene cluster. Cell. 2005;120:369–82. doi: 10.1016/j.cell.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 108.Maclean JA, 2nd, Hu Z, Welborn JP, Song HW, Rao MK, Wayne CM, et al. The RHOX Homeodomain Proteins Regulate the Expression of Insulin and Other Metabolic Regulators in the Testis. J Biol Chem. 2013;288:34809–25. doi: 10.1074/jbc.M113.486340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–44. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanz E, Evanoff R, Quintana A, Evans E, Miller JA, Ko C, et al. RiboTag analysis of actively translated mRNAs in Sertoli and Leydig cells in vivo. PLoS One. 2013;8:e66179. doi: 10.1371/journal.pone.0066179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Panneerdoss S, Chang YF, Buddavarapu KC, Chen HI, Shetty G, Wang H, et al. Androgen-responsive microRNAs in mouse Sertoli cells. PLoS One. 2012;7:e41146. doi: 10.1371/journal.pone.0041146. [DOI] [PMC free article] [PubMed] [Google Scholar]