Abstract
Five genes essential for folate biosynthesis in Lactococcus lactis were cloned on a broad-host-range lactococcal vector and were transferred to the folate auxotroph Lactobacillus gasseri. As a result L. gasseri changed from a folate consumer to a folate producer. This principle can be used to increase folate levels in many fermented food products.
Folate is an essential component in the human diet, and adequate intake of folate may prevent the occurrence of diseases and syndromes like neural tube defect, coronary heart disease, anemia, and certain types of cancer (9). Food products like green vegetables, meat, and fermented dairy products contain significant folate levels. Despite this, folate deficiency occurs throughout the world, including several well-developed countries. Recently, it has been shown that metabolic engineering can be used to increase folate levels in fermented foods (12).
Lactic acid bacteria such as Lactococcus lactis and Lactobacillus plantarum have the ability to synthesize folate, which is a biological cofactor involved in their amino acid and nucleotide metabolism (7, 11). The genes for folate biosynthesis have been identified (6, 12). The biosynthetic pathway includes seven consecutive steps, in which the precursor guanosine triphosphate is converted into tetrahydrofolate (10). However, some lactic acid bacteria, such as Lactobacillus gasseri strain ATCC 33323, cannot synthesize folate, because the genes involved in folate biosynthesis are lacking in the genome except for the two genes, folA and folC, involved in regeneration and retention of reduced folates taken up from the medium (http://genome.jgi-psf.org/draft_microbes/lacga/lacga.draft.html).
The folate biosynthetic genes of L. lactis MG1363 are organized in a folate gene cluster, consisting of six genes (folA, folB, folKE, folP, ylgG, and folC) (Fig. 1) (12). In the present work we describe the transformation of the folate-consuming L. gasseri into a folate producer by the transfer of a broad-host-range plasmid containing the folate gene cluster from L. lactis.
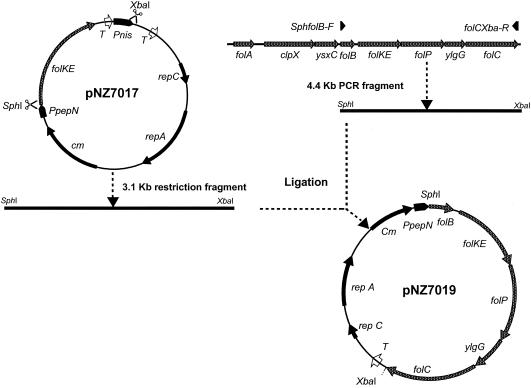
FIG. 1.
Construction of pNZ7019 by restriction of pNZ7017 and the amplification of the folate gene cluster from chromosomal DNA of L. lactis MG1363.
Cloning and transformation of the folate gene cluster of L. lactis into L. gasseri.
The plasmid pNZ7017 (12) was digested by using XbaI and SphI (both Invitrogen, Paisley, United Kingdom) as restriction enzymes. The 3.1-kb DNA fragment that was obtained from the digestion consisted of the constitutive pepN promoter (14), a part of the multiple cloning site, chloroamphenicol resistance marker, and replication genes that originated from pNZ12 (1). The folate gene cluster (folB, folKE, folP, ylgG, and folC) of L. lactis was amplified by PCR by using high-fidelity Pwo polymerase (Invitrogen). The forward primer SphfolB-F (5′-AGGAAGCATGCCTTACAAAATAAAACTTAATAATATG-3′) was extended at the 5′ end, creating an SphI restriction site overlapping the start codon of folB. The reverse primer folCXba-R (5′-TCTCTAGACTACTTTTCTTTTTTCAAAAATTCACG-3′) was extended at the 5′ end, creating an XbaI restriction site that overlapped the stop codon of folC (Fig. 1). The amplified PCR fragment was restricted with XbaI and SphI. Subsequently, the two fragments were ligated by using T4 ligase (Invitrogen), generating a translational fusion between the constitutive promoter of the pepN gene (14) and the folate gene cluster (Fig. 1). The resulting plasmid was designated pNZ7019. After transformation to L. lactis NZ9000 and subsequent cultivation of the strain, the plasmid was harvested as described previously (2).
L. gasseri (ATCC 33323) was transformed with purified pNZ7019 by using an established procedure (8) and was plated on MRS medium (Merck, Darmstadt, Germany) containing 10 μg of chloramphenicol/ml. After incubation for 40 h at 37°C, chloramphenicol-resistant colonies were examined for the presence of pNZ7019 by using restriction analyses. An L. gasseri colony harboring pNZ7019 was used for renewed cultivation by using the same growth conditions as previously described. Random amplified polymorphic DNA fingerprint analysis was used to confirm the identity of the transformant harboring pNZ7019 as L. gasseri ATCC 33323 (results not shown).
Conversion of folate consumer into folate producer.
A modified Folic Acid Casei Medium (FACM) (Difco, Becton Dickinson and Co., Sparks, Md.) was developed for growth and subsequent folate analysis of the L. gasseri wild-type strain and the L. gasseri strain harboring pNZ7019. The FACM was enriched with 1 mg of vitamin B12 (Sigma-Aldrich Chemie, Gmbh, Steinheim, Germany)/liter and 1 ml of Tween 80 (Merck, Darmstadt, Germany)/liter. The wild-type strain could not grow at 37°C unless folate was added (1.0 mg/liter), whereas the strain harboring pNZ7019 showed folate-independent growth.
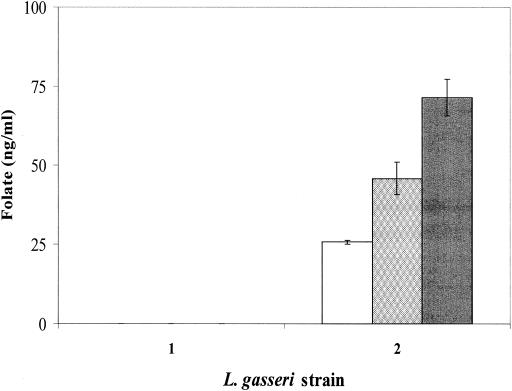
Folate levels were quantified by using the Lactobacillus casei microbiological assay, including enzymatic deconjugation of the polyglutamate tail (5, 11, 12). The L. gasseri strain harboring pNZ7019 produced significant intracellular and extracellular folate levels (Fig. 2). As expected, the wild-type strain consumed folate from the medium and intracellular folate concentrations remained below the detection limit (Fig. 2).
FIG. 2.
Folate production of the L. gasseri wild-type strain (side marked 1) and the L. gasseri strain harboring pNZ7019 (side marked 2), grown on modified FACM. White bar, extracellular folate levels; gray bar, intracellular folate levels; and black bar, total folate levels. The L. gasseri wild-type strain could not grow without supplementation of folate. Therefore, folate levels depicted in the figure are corrected for folate added to the medium. Error bars indicate the standard deviation of the folate microbiological assay over two independent measurements.
Stability of the folate production in the L. gasseri strain.
L. gasseri strain ATCC 33323 harboring pNZ7019 was cultivated for approximately 30 generations on MRS medium supplemented with 10 μg of chloramphenicol/ml at 37°C. The culture was plated on MRS agar plates supplemented with 10 μg of chloramphenicol/ml. Subsequently, 100 colonies were transferred to folate-free FACM plates containing 10 μg of chloramphenicol/ml. Since all colonies grew on these plates, it appears that the folate biosynthesis is stably maintained in the pNZ7019 vector for more than 30 generations of growth in the presence of folate. Sequential cultivation in folate-rich medium resulted in decreased folate production by the transformant (data not shown). This is presumably a result of instability of the folate gene cluster.
Conclusion.
The five genes, i.e., folB, folKE, folP, ylgG, and folC, directing folate biosynthesis in L. lactis were transferred to L. gasseri by using a derivative of the broad-host-range vector pNZ12 (1). These genes are sufficient to introduce a folate biosynthesis pathway in this folate auxotroph lactic acid bacterium, thereby transforming a folate consumer into a folate producer. L. gasseri is currently marketed as a probiotic (4), and when the described strategy is used, this lactic acid bacterium can be used to enrich (fermented) foods with the essential B vitamin, folate, in addition to conferring its health-promoting effect on the consumer (3, 13).
REFERENCES
- 1.De Vos, W. M. 1987. Gene cloning and expression in lactic streptococci. FEMS Microbiol. Rev. 46:281-295. [Google Scholar]
- 2.De Vos, W. M., P. Vos, H. de Haard, and I. Boerrigter. 1989. Cloning and expression of the Lactococcus lactis SK11 gene encoding an extracellular serine proteinase. Gene 85:169-176. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez, M. F., S. Boris, and C. Barbes. 2003. Probiotic properties of human lactobacilli strains, to be used in the gastrointestinal tract. J. Appl. Microbiol. 94:449-455. [DOI] [PubMed] [Google Scholar]
- 4.Holzapfel, W. H., P. Haberer, J. Snel, U. Schillinger, and J. H. J. Huis in't Veld. 1998. Overview of gut flora and probiotics. Int. J. Food Microbiol. 41:85-101. [DOI] [PubMed] [Google Scholar]
- 5.Horne, D. W., and D. Patterson. 1988. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin. Chem. 34:2357-2359. [PubMed] [Google Scholar]
- 6.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA. 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin, M. Y., and C. M. Young. 2000. Folate levels in cultures of lactic acid bacteria. Int. Dairy J. 10:409-414. [Google Scholar]
- 8.Luchansky, J. B., P. M. Muriana, and T. R. Klaenhammer. 1988. Application of electroporation for transfer of plasmid DNA to Lactobacillus, Lactococcus, Leuconostoc, Listeria, Pediococcus, Bacillus, Staphylococcus, Enterococcus, and Propionibacterium. Mol. Microbiol. 2:637-646. [DOI] [PubMed] [Google Scholar]
- 9.Lucock, M. 2000. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol. Genet. Metab. 71:121-138. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki, Y., and G. M. Brown. 1974. The biosynthesis of folic acid. XII. Purification and properties of dihydroneopterin triphosphate pyrophosphohydrolase. J. Biol. Chem. 249:2405-2410. [PubMed] [Google Scholar]
- 11.Sybesma, W., M. Starrenburg, L. Tijsseling, M. H. N. Hoefnagel, and J. Hugenholtz. 2003. Effects of growth conditions on folate production by lactic acid bacteria. Appl. Environ. Microbiol. 69:4542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sybesma, W., M. Starrenburg, M. Kleerebezem, I. Mierau, W. M. de Vos, and J. Hugenholtz. 2003. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69:3069-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usman and A. Hosono. 2001. Hypocholesterolemic effect of Lactobacillus gasseri SBT0270 in rats fed a cholesterol-enriched diet. J. Dairy Res. 68:617-624. [DOI] [PubMed] [Google Scholar]
- 14.Van Alen-Boerrigter, I. J., R. Baankreis, and W. M. de Vos. 1991. Characterization and overexpression of the Lactococcus lactis pepN gene and localization of its product, aminopeptidase N. Appl. Environ. Microbiol. 57:2555-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]