Abstract
The composition of the active microbial (bacterial and fungal) soil community in an arable wheat field subjected to different management practices was examined at five times during a 1-year period. Field sections were fertilized either at good agricultural practice (GAP) levels or at reduced levels (0.5× GAP) and were inoculated with vesicular arbuscular mycorrhizae (VAM) at the same time. Field subsections were treated either with or without pesticides. Changes in the active microbial communities were investigated by denaturing gradient gel electrophoresis analysis of reverse transcription-PCR-amplified 16S and 18S rRNA. Microbial community structure was primarily determined by season, and the seasonal trends were similar for the fungal and bacterial components. Between-sample microbial heterogeneity decreased under a mature crop in the summer but increased following harvesting and plowing. Although similar overall trends were seen for the two microbial components, sample variability was greater for the fungal community than for the bacterial community. The greatest management effects were due to GAP fertilization, which caused increases in the bacterial numbers in the total and culturable communities. Microbial biomass similarly increased. GAP fertilization also caused large shifts in both the active bacterial community structure and the active fungal community structure and additionally resulted in a decrease in the heterogeneity of the active bacterial community. Pesticide addition did not significantly affect bacterial numbers or heterogeneity, but it led to major shifts in the active soil bacterial community structure. PCR primers specific for Glomales 25S rRNA genes were used to monitor the VAM population following inoculation. Glomales were detected initially only in VAM-inoculated field sections but were subsequently detected in noninoculated field sections as the season progressed. After plowing, the level of Glomales was reduced in noninoculated field sections but remained high in VAM-inoculated field sections. Inoculation of VAM correlated with elevated soil phosphate and carbon levels.
Intensification of land use practices has resulted in a widely documented reduction in soil quality and productivity (11, 15, 53, 60, 66, 73). The productivity and health of agricultural systems are, in part, dependent upon the functional processes of soil microbial communities (15, 38, 51, 54), and some agricultural practices may put these communities at risk. High inputs of inorganic fertilizers and pesticides not only are expensive and habitat damaging (56, 57) but can also cause changes in soil bacterial and fungal densities (6, 29), suppression or promotion of microbial growth and activity (7, 14, 31), and detectable shifts in microbial community structure (12, 13, 16, 18, 47). These changes may ultimately contribute to a loss of diversity and/or function within the soil microbial community.
With the movement towards more sustainable agricultural systems, recognition of the biological and economic importance of microorganisms has increased as microbially mediated processes, such as mineralization, nitrogen fixation, ammonification, and carbon storage, contribute to the maintenance of the overall dynamic equilibrium of soils (17, 51, 52). Mycorrhizal plant symbionts are an important group and are generally thought to increase plant and soil health by improving plant fitness and soil quality (2, 3, 36), resulting in improved nutrient (N and P) uptake (33, 35, 37) and soil stability (59, 75, 76). Thus, there may be an important role for vesicular arbuscular mycorrhizae (VAM) in enhancing agricultural sustainability in farm systems in which soil quality has been severely decreased.
Sustainable management may be enhanced by understanding the seasonal dynamics of microbial populations; therefore, seasonal molecular community profiling of agricultural soil communities has been undertaken by several researchers. Smit et al. (63) discovered a high degree of similarity (based on denaturing gradient gel electrophoresis [DGGE] profiles of 16S ribosomal DNA [rDNA]) between seasonal samples in a silt loam soil in which wheat was grown. Gelsomino et al. (25) investigated the reproducibility of community profiles and found remarkable similarity between communities at various depths, for different sample sizes, and over spatial and temporal distances (again determined by DGGE of 16S rDNA). The finding that similar soil types tend to have similar bacterial community profiles with regard to dominant components has been supported by subsequent microbial community studies (9, 28). Although many of the data suggest that soil bacterial communities are temporally and spatially dominated by a number of stable and ubiquitous organisms, profiles determined by 16S rDNA profiling are widely thought to represent a stable pool of organisms, many of which may be inactive (20, 67). Bacterial community profiles derived from reverse transcription (RT)-PCR of 16S rRNA, which are indicative of the active bacterial community, have provided higher resolution for differentiation and discrimination than rDNA-based studies of the same samples have provided (21, 28). Therefore, studying the bacterial community by rRNA-based approaches may reveal greater seasonal and management fluctuations. Both rDNA and rRNA approaches are, however, subject to the same biases that are observed with any PCR-based method (74). In the present study we aimed to examine seasonal trends for an active microbial community under winter wheat and to assess the community after treatment with potentially more sustainable agricultural practices (no pesticide and lower fertilization levels, as well as VAM inoculation) and after use of present recommended good agricultural practice (GAP) (48, 49). To address these aims, the active bacterial and fungal components of the community were monitored by RT-PCR amplification of small-subunit rRNA (16S rRNA and 18S rRNA, respectively) and DGGE profiling on five sampling dates throughout the year.
MATERIALS AND METHODS
Site description.
The field experiment was performed on a heavy clay-based soil (pH 7, Hanslope 411d soil series) in a 5.3-ha field at the Unilever test site at Colworth Farm in Sharnbrook, Bedfordshire, United Kingdom (OS grid reference 498430E 259960N). The field site was initiated, designed, and maintained by D. Pendlington and J. van Oostrum as part of the Unilever Sustainable Agriculture Initiative (Growing for the future II. Unilever and sustainable agriculture, Unilever, Rotterdam, The Netherlands). GAP fertilization and pesticide application levels were compared with lower-input fertilization (0.5× GAP amounts) and no pesticide addition. In addition, field sections with lower fertilizer inputs were inoculated with a VAM mixture containing Glomus brasillianum, Glomus clarum, Glomus deserticola, Glomus intraradices, Glomus mononsporus, Glomus mosseae, and Gigaspora margarita spores. Sampling began on November 2000 after one of the wettest autumns on record (Meteorological Office, Hydrological Summary, February 2001, Centre for Ecology and Hydrology, Wallingford, United Kingdom) and was completed in November 2001. Samples were taken in November 2000, April 2001, July 2001, September 2001, and November 2001; details are outlined below. This experiment was a large field experiment performed within the confines of practical large-scale commercial farming and as such was subject to some limitations on the experimental design and replication. Treatments were applied according to crop requirements; for example, pesticide addition was applied to maturing crops, and therefore no equivalent treatment was used for the initial, earlier samples. Randomized coring of each treatment area was performed rather than a randomized block treatment design, which, although statistically preferable, would in no way replicate real farm practices.
Field management.
In early September 2000 the field was plowed, and Glyphosate 360 (2 liters ha−1 [within GAP recommended levels]) was applied to the entire field in October 2000; then in January 2001 the field was harrowed and drilled with winter wheat (cv. Consort). Initially, the field was divided in half, and an additional treatment consisting of inoculation of a dehydrated VAM spore complex was applied to one half of the field. In April 2001 inorganic fertilizer was applied only to the half of the field that was not inoculated with VAM (GAPF) at a rate of 13 kg of N ha−1 and 57 kg of P ha−1; this was followed by 48 kg of N ha−1 in May 2001 and 123 kg of N ha−1 in June 2001. The VAM-inoculated field section served as the 0.5× GAP fertilization treatment (LOWF+V) and was not fertilized in April but was fertilized with 24 kg of N ha−1 in early May 2001 and 70 kg of N ha−1 in early June 2001; the latter treatment comprised approximately half as much mineral N as was applied to the GAPF field, and no phosphate was applied to the VAM-inoculated half of the field. GAP-recommended levels of the pesticides Ally (active ingredient, metsulfuron; applied at a rate of 34.11 g ha−1 [0.34 μg kg−1]) and Marathon (active ingredient, imidacloprid; applied at a rate of 143.41 ml ha−1 [1.43 μl g−1]) were applied to half of the field in May 2001, and the fungicides Folicur (active ingredient, tebucconazol; applied at a rate of 470 ml ha−1 [4.70 μl g−1]) and Amistar (active ingredient, azoxystrobin; applied at a rate of 660 ml ha−1 [6.60 μl g−1]) were applied in early July 2001 (GAPF+P and LOWF+P+V field sections). No pesticides were applied to the other half of the field (GAPF and LOWF+V), so there was a total of four treatments. Harvesting was in late August 2001, and plowing for the subsequent crop occurred in late September 2001.
Sampling procedure.
Twenty soil cores (diameter, 2 cm; depth, 15 cm) were taken from throughout the field in November 2000 to generate baseline data. These cores were pooled, stones and roots were removed aseptically, and triplicate samples were then taken. Subsequently, as each treatment was implemented, the same procedure was followed; 20 soil cores were taken from each subdivided field section. Samples to be processed for determination of culturable numbers were processed directly; substrate induced respiration (SIR) measurements were obtained after appropriate equilibration (1). Values for labile nutrients were obtained with soil stored at 4°C for <1 month. Soil for nucleic acid extraction was frozen and stored at −20°C on the day of sampling.
Soil physical, chemical, and biological analyses.
Labile phosphate and nitrate concentrations in the soil were measured by ion chromatography by using a Dionex chromatograph (Dionex Corporation, Sunnyvale, Calif.). Total C and N concentrations were determined with a CHN analyzer (model 2400; Perkin-Elmer, Beaconsfield, Buckinghamshire, United Kingdom). Total soil bacterial counts were determined by acridine orange staining (34), and viable counts were determined following plating on 0.1× tryptone soya agar.
Biomass and DNA concentration measurements.
Relative microbial biomass was measured by two methods. The SIR analysis was performed as described by Anderson and Domsch (1) to obtain values for microbial biomass carbon. In addition, the relative amounts of crude soil DNA extract were determined by gel electrophoresis (78). To do this, DNA mass ladders (Bioline, London, United Kingdom) were electrophoresed in lanes adjacent to the lanes containing the environmental DNA. Following analysis with the one-dimensional advanced Phoretix software (Nonlinear Dynamics Ltd., Newcastle, United Kingdom), calibration was performed with the known standards, and concentrations of the environmental DNA were calculated. Plant roots were removed manually aseptically to prevent plant respiration or DNA content from being included in the measurements.
Microbial community RT-PCR and DGGE analysis.
Extraction, quantification, and purification of nucleic acids were performed as described by Girvan et al. (28). RT-PCR amplification was performed with total RNA as described by Girvan et al. (28). Bacterial 16S rRNA was amplified by using universal bacterial DGGE primers p2 and p3 (synthesized by Invitrogen Custom Primers, Paisley, United Kingdom) (Table 1) with a GC clamp attached to the 5′ end of p3 (50). Fungal 18S rRNA was amplified by using 18S rRNA fungal primers FR1 and FF390 (69). In this case a GC tail was added to FR1 (5′-CCC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GCC3-′). The PCR mixtures and reaction conditions were the same as those described in the original study. DGGE gels were formed, electrophoresed, stained, and analyzed as described by Girvan et al. (28). The denaturant range for the fungal gels was 45 to 60%, and the gels were electrophoresed at a constant voltage of 50 V at 58°C for 20 h.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence | Orientation | Target | Reference |
|---|---|---|---|---|
| Muyzer (p3) | 5′-CTA CGG GAG GCA GCA G-3′ | Forward | Bacterial 16S rRNA | 50 |
| Muyzer (p2) | 5′-ATT ACC GCG GCT GCT GG-3′ | Reverse | Bacterial 16S rRNA | 50 |
| FRI | 5′-AIC CAT TCA ATC GGT AIT-3′ | Reverse | Fungal 18S rRNA | 69 |
| FF390 | 5′-CGA TAA CGA ACG AGA CCT-3′ | Forward | Fungal 18S rRNA | 69 |
| LSU0061 | 5′-AGC ATA TCA ATA AGC GGA GGA-3′ | Forward | Eukaryote 25S rRNA | 39 |
| LSU0599 | 5′-TGG TCC GTG TTT CAA GAC G-3′ | Reverse | Eukaryote 25S rRNA | 39 |
| LSU4RKf | 5′-GGG AGG TAA ATT TCT CCT CCG GC-3′ | Forward | Glomales 25S rRNA | 39 |
| LSU7RKr | 5′-ATC GAA GCT ACA TTC CTC C-3′ | Reverse | Glomales 25S rRNA | 39 |
Glomales-specific PCR.
Glomales-specific primers (Table 1) (39) were used for PCR amplification. The first-round PCR was performed by amplifying fragments of the large ribosomal subunit with eukaryote-specific forward primer LSU0061, which corresponds to LR1 (71), and reverse primer LSU0599 (nomenclature from reference 24), which corresponds to NDL22 (71), to produce a 700-bp product. This procedure was combined with a nested PCR performed with primers LSURK4f and LSURK7r (39) to produce a 300-bp product. The PCR mixtures and reaction conditions were the same as those described by Kj¢ller and Rosendahl (39). As a positive control, nucleic acid was extracted from spores of G. clarum BEG 142 produced on Tephrosia in an acid soil and from spores of G. intraradices BEG 144 produced on Trifolium in a neutral soil (supplied by V. Gianinazzi-Pearson at the Banque Européenne des Glomales). Penicillium sp., Fusarium sp., and Pseudomonas fluorescens DNA were used as negative controls.
General statistical analysis.
The Shannon index (H′) (61) (H′ = −Σ pi ln pi) and equitability (J) (55) (J = H′/H′max) were calculated from the microbial community profiles, where pi is the proportion of individuals that a species contributes to the total individuals in the sample. Data were produced by normalization of the volume data derived from the DGGE gels as described by Girvan et al. (28). Statistical significance was determined by analysis of triplicate samples by analysis of variance and Tukey tests (Microsoft Excel 7a); a P value of <0.05 was required to establish a significant difference between samples.
RESULTS
Changes in the soil nutrient status in response to management treatments.
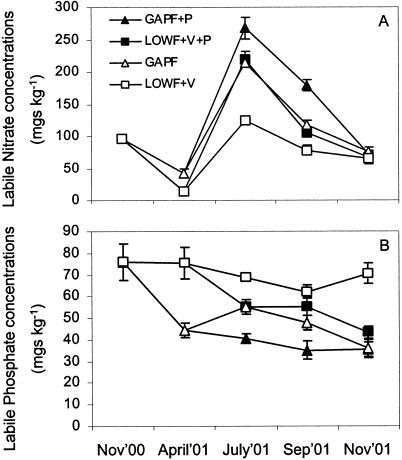
Seasonally, labile nitrate levels peaked in all soils in July (Fig. 1A). Immediately following the first fertilizer application in late April 2001, the labile nitrate concentrations in the soil were higher in the GAPF field sections (P < 0.05) (Fig. 1A). After further fertilizer and pesticide addition in July, the GAPF+P field section had the highest labile nitrate concentrations, and the LOWF+V field section had the lowest. There was, however, no significant difference in the labile nitrate concentrations in July between the LOWF+V+P field section and the GAPF field section despite the fact that much lower levels of mineral N were added to the LOWF+V+P field section. The field sections treated with pesticides had greater labile nitrate concentrations than their counterparts to which no pesticide was added (P < 0.05). By November 2001, after harvest, no significant differences were observed between field sections. Conversely, major seasonal fluctuations were not observed for labile phosphate concentrations. By April 2001 the labile phosphate concentrations were greater in the soils that were inoculated with VAM without added fertilizer (LOWF+V) than in the soils that were GAP fertilized without VAM addition (GAPF) (P < 0.05) (Fig. 1B). By July the value for the LOWF+V field section was significantly higher than the values for both the GAPF field sections (P < 0.05), and the LOWF+V+P field section had higher levels of labile soil phosphate than the non-VAM-inoculated pesticide-treated counterpart (GAPF+P) (P < 0.05). This trend continued after harvest, and by November the section that received the LOWF+V treatment had greater phosphate concentrations than all of the other field sections (Fig. 1B). Pesticide addition reduced the labile phosphate concentrations in this case. The LOWF+V treatment resulted in small (10%) but significant (P < 0.05) increases in the total carbon concentrations compared to the concentrations in the GAPF-treated soils (data not shown), and no great seasonal fluctuations were observed. The values for total nitrogen concentrations ranged from 0.25 to 0.37% and exhibited a pattern similar to that observed for total carbon concentrations, although the differences were small and not significant (data not shown).
FIG. 1.
Labile soil concentrations of nitrate (A) and phosphate (B), as determined by ion chromatography. All values are expressed as soil dry weight equivalents. The bars indicate standard errors for three replicates.
Crop yields.
The grain yield per hectare of wheat was recorded after harvest in August; the greatest grain yield (4.00 metric tons ha−1) was obtained for the GAPF+P field section, and the lowest yield (2.70 metric tons ha −1) was obtained for the LOWF+V field section. The LOWF+V+P field section, however, produced higher yields of wheat than the GAPF field section (3.31 and 3.04 metric tons ha −1, respectively).
Bacterial numbers.
The numbers of culturable bacteria (cultured on 0.1× tryptone soya agar) showed a large peak in April 2001 for the GAPF soil (7 × 108 ± 2.3 × 107 CFU g of soil−1) and smaller peaks in September 2001 for the GAPF+P and GAPF soils (1.8 × 107 ± 4.2 × 106 and 3.53 × 107 ± 2.4 × 106 CFU g of soil−1, respectively). The data for the LOWF+V+P and LOWF+V soils showed that there were single peaks for culturable numbers in September (2.12 ×107 ± 4.6 × 106 and 3.77 × 107 ± 4.8 × 106 CFU g of soil−1, respectively) (data not shown). The numbers of culturable bacteria sampled 4 days after N and P fertilizer application in April (GAPF field section) were significantly greater than the numbers of culturable bacteria obtained for the LOWF+V field section (2 orders of magnitude greater for the GAPF soil; 7 × 108 ± 2.3 × 107 CFU g of soil−1 compared to 7.5 × 106 ± 8.1 × 106 CFU g of soil−1) (data not shown) (P < 0.05). However, by July (1 month after the final N fertilizer application) there was no significant difference in the numbers of culturable bacteria between fertilizer treatments. However, by September and November the numbers of culturable bacteria for both the GAPF+P and LOWF+V+P field sections were significantly lower than the numbers of culturable bacteria for their non-pesticide-treated counterparts (>40% and >55% lower for both pesticide-treated field sections for September and November, respectively) (P < 0.05). Similar to the culturable bacterial results, the total bacterial numbers peaked for the GAPF soil in April (1.65 × 1010 ± 5.3 × 108 cells g of soil−1), and the total bacterial numbers peaked for the LOWF+V+P and LOWF+V soils in September (1.31 × 1010 ± 1.3 × 109 and 1.23 × 1010 ± 9.4 × 108 cells g of soil−1, respectively). The total bacterial numbers showed significant (P < 0.05) increases after GAPF treatment compared to the values for the LOWF+V field section in both April and July (60 and 50% higher, respectively) (data not shown), but as seen for the numbers of culturable bacteria, the increase was greatest immediately after fertilizer addition in April. Pesticide addition had no deleterious effects on the total bacterial numbers, and by September there were again no differences in the numbers of bacteria among the four treatments.
Microbial biomass and DNA concentrations.
Microbial biomass measured by SIR (Fig. 2A) peaked in July for all treatments (P < 0.05); there was a small significant initial increase in biomass in April 2001 in the GAPF soil, but subsequently no clear treatment differences were observed when SIR was used (P < 0.05). The relative nucleic acid concentrations (Fig. 2B) also showed peaks in July (P < 0.05). The DNA concentrations were lowest overall in November 2000, and this was followed initially in April 2001 by a significant increase (30% increase) in the DNA concentration in the GAPF soil (P < 0.05) compared to the concentration in the LOWF+V soil. Larger increases (>45%) in the DNA concentrations in both the GAPF and GAPF+P samples were observed by July (P < 0.05) compared to the concentrations in both of the LOWF samples. No subsequent management differences were observed.
FIG. 2.
Estimation of SIR (A) and concentrations of total extractable DNA (B), as determined by agarose gel electrophoresis. All measurements are expressed as soil dry weight equivalents. The bars indicate standard errors for three replicates.
Active bacterial community structure.
The relative equitability of the bacterial community (Fig. 3A) was assessed by DGGE profiling of RT-PCR-amplified 16S rRNA. The relative equitability was initially lower in the GAPF field sections in April (P < 0.05). By July all field sections exhibited similar equitability, which was due particularly to the dominance of one ribotype (10 to 15% of the total population). After harvest, the GAPF+P field section had the lowest equitability (P < 0.05). By November no significant treatment trends were observed. The relative diversity of the bacterial community (Fig. 3B) was initially greater in the LOWF+V soils (P < 0.05), and by July a small but significant increase was still observed, and the LOWF+V+P field section had greater bacterial diversity than both of the fertilized field sections (GAPF and GAPF+P). By September the GAPF+P soil had relatively lower bacterial diversity than the GAPF soil (P < 0.05). Again, by November no differences were found.
FIG. 3.
Heterogeneity of the community as measured by equitability (55) (A) and diversity (Shannon index) (61) (B), derived from a DGGE analysis of RT-PCR-amplified bacterial 16S rRNA.
Bacterial community structure as monitored by DGGE profile comparisons of RT-PCR-amplified 16S rRNA showed that although differences due to management were large, overall the greatest differences between samples were caused by seasonal fluctuations (Fig. 4). The similarity between replicates was high in November 2000 (92%). The overall community structure in April showed that the GAPF soil community was more similar to the November 2000 baseline control than the LOWF+V soil community was, but all nine samples formed a discrete cluster (Fig. 4, cluster I). The active bacterial communities in July (12 samples) formed a discrete cluster at 82% similarity, and the replicate similarity was high, with all replicates exhibiting >90% similarity (Fig. 4, cluster II). The variability between sample replicates increased by September, ranging from 73 to 88%, and no distinct seasonal cluster was formed (Fig. 4, clusters III and IV). By November 2001 the replicate variability increased further to between 68 and 78%, with the exception of one GAPF treatment (92% similarity) (Fig. 4, clusters V and VI). The greatest separation due to management was observed for the GAPF and LOWF+V regimens. After application of fertilizer in April, the active bacterial communities were clearly separated from each other (Fig. 4, cluster I) based on band distribution and formed discrete clusters determined by management (87% similarity for LOWF+V samples and 90% similarity for GAPF samples). Despite the high degrees of similarity for all of the communities in July 2001, discrete separations due to management practices were again caused by the LOWF+V treatments (91% similarity) separating from GAPF treatments (84% similarity). However, additional community separation was observed between pesticide and nonpesticide treatments for both the GAPF and LOWF+V field sections (Fig. 4, cluster II). In September 2001, despite increasing variability, separation was maintained between the LOWF+V field section (Fig. 4, cluster IV [68% similarity]) and the GAPF field section (Fig. 4, cluster III [68.5% similarity]). Separation was also maintained between the pesticide and nonpesticide treatments (Fig. 4, clusters III and IV). By November 2001 the LOWF+V and GAPF field sections were still separated but no longer formed distinct clusters, although distinct replicate clusters for pesticide and no-pesticide treatments remained (Fig. 4, clusters V and VI).
FIG. 4.
Unweighted pair group with mathematical average dendrogram constructed from the similarity matching data (Dice/Sorensen's index) produced by using the DGGE profile of RT-PCR-amplified 16S rRNA from soil and generated by using MVSP (version 3.12 h). B, baseline samples obtained in November 2000; A, April 2001 samples; J, July 2001 samples; S, September 2001 samples; N, November 2001 samples. The scale bar indicates levels of similarity, expressed as percentages. Triplicate samples were used.
Active fungal community structure.
Initially, fertilization appeared to have a small (but not significant) positive effect on fungal community equitability as determined by DGGE analysis of RT-PCR-amplified 18S rRNA when samples were obtained 4 days after fertilizer application, but this effect was not observed subsequently. No other significant differences in equitability or diversity between treatments were observed. Fungicide application did not reduce the equitability or diversity of the fungal community as determined by DGGE analysis of RT-PCR-amplified 18S rRNA. Overall, the variability in community structure between replicates for the fungal community was high, despite the fact that replicates were obtained from pooled cores. The November 2000 baseline samples were only 81% similar, compared to the 92% similarity value obtained for the bacterial population. The variability increased towards the end of the season. As seen for the bacterial community in April 2001, the active fungal community in the GAPF field section was more similar to the baseline November 2000 sample than the LOWF+V field section was (Fig. 5, cluster I). The active fungal communities also formed one seasonal cluster in July (Fig. 5, cluster II) (70% similarity), but the similarity was lower than that of the bacterial communities (Fig. 4, cluster II) (82%). After herbicides and fungicides were applied in July 2001, the strongest treatment differences were still maintained between GAPF field sections (Fig. 5, cluster II) (73% similar to each other) and LOWF+V field sections (Fig. 5, cluster II) (71% similar to each other), with all but 1 of the 12 samples exhibiting fertilizer-specific clustering. There were no consistent discrete separations between the pesticide and nonpesticide treatments. Again, as observed with the bacterial community profiles, there was no discrete clustering of the active fungal communities in September, and increased variability was observed in all replicates. GAPF field sections were still separated from LOWF+V field sections but with lower confidence as replicate samples did not form discrete groups (Fig. 5, clusters III, IV, and V). By November the replicate variability was great, and no replicates formed discrete groups (Fig. 5, cluster VI). No reproducible difference in community structure between pesticide-treated and non-pesticide-treated field sections was observed.
FIG. 5.
Unweighted pair group with mathematical average dendrogram constructed from the similarity matching data (Dice/Sorensen's index) produced by using the DGGE profile of RT-PCR-amplified fungal small-subunit 18S rRNA from soil and generated by using MVSP (version 3.12 h). B, baseline samples obtained in November 2000; A, April 2001 samples; J, July 2001 samples; S, September 2001 samples; N, November 2001 samples. The scale bar indicates levels of similarity, expressed as percentages. Triplicate samples were used.
Glomales-specific PCR.
Glomales 25S rRNA genes could not be detected by PCR in the soil in November 2000. These genes were detected in April 2001 after VAM inoculation in January in the LOWF+V field sections but not in the GAPF non-VAM-inoculated field sections. By July Glomales were detected in four of the six samples from the GAPF soils and in all six of the LOWF+V samples. In September Glomales were detected in all six of the GAP fertilized field sections and in five of six of the VAM-inoculated samples. By November, however, Glomales were detected in only two of the six GAPF field sections and in five of the six LOWF+V field sections. Fungicide addition had no effect on the detection of Glomales.
DISCUSSION
Application of inorganic fertilizer (GAP levels) resulted in higher soil labile nitrate concentrations in GAPF field sections than in 0.5× GAP field sections. However, subsequent pesticide addition raised the nitrate levels in the LOWF+V+P field section to levels similar to those in the GAPF non-pesticide-treated field sections. Although no inorganic phosphate was added, the labile phosphate concentrations were greatest in the LOWF+V field sections, and pesticide addition correlated with reduced phosphate levels. Labile phosphate supplied to the plants from the Glomales symbionts by utilization of organic P (37) may account for this finding. Additionally, the higher concentrations of total and particularly culturable bacterial cells in the GAPF soil after fertilization may have led to more rapid phosphate utilization and a decrease in the soil phosphate concentrations. VAM inoculation in conjunction with lower levels of fertilization may improve phosphate concentrations in soil and thus may be useful under phosphate-limiting conditions or when organic sources of phosphate are applied (42, 43). Similarly, the increased carbon content in the LOWF+V soils compared to the carbon content in the GAPF soils during April and July may have been due to beneficial mycorrhizal transfer of plant carbon to the soil (36, 64).
Fertilization with inorganic N and P was followed by immediate increases in bacterial numbers in April (4 days after application) and to a lesser extent in July (1 month after application). The proximity of the sampling time to fertilizer application rather than the overall fertilizer concentration seemed to be the cause of the increases in the total bacterial numbers and particularly the culturable bacterial numbers. This suggests that the bacterial community responds quickly to nutrient addition but that the response decreases over time, perhaps due to rapid utilization of the readily available inorganic N and P, followed by predation. Readily culturable bacteria are thought to be r strategists; i.e., they have high maximal growth rates, are capable of responding rapidly to readily available nutrients, and therefore become numerically dominant at high nutrient concentrations (38, 62, 72). In contrast, K strategists grow best under oligotrophic conditions and do not compete well under high-nutrient conditions. This appears to explain the increase in total bacterial numbers and especially culturable bacterial numbers immediately following nutrient addition.
Pesticide addition had a negative effect on the culturable bacterial numbers in the samples, but there was no such effect for the total bacterial community. Decreases in soil bacterial numbers have been observed with sulfonurea herbicides, such as Ally, but only at high concentrations of the herbicide (>10 μg kg−1) that are not within the GAP-recommended levels of application (0.34 μg kg−1 was applied in this study) (26, 27). The values for SIR and total DNA concentrations were in broad agreement, and both peaked in July. As both culturable and total bacterial numbers were highest in April, the fungal community may have contributed to the increase in July. SIR measurements showed that there was only a small significant treatment difference in April alone. In contrast, the DNA concentrations showed that there were large significant differences between the GAPF and LOWF+V soils in both April and July, when the concentrations in the GAPF soil were greatest.
Considerable temporal variation in soil bacterial communities has previously been reported for agricultural soils (10, 62). In the present study, the greatest community variation was also found to be temporal. The bacterial communities in the July samples showed the greatest temporal clustering and the greatest replicate similarity. The high level of similarity of the rRNA profiles may have been due in part to sampling under a mature crop and the physical stability of the soil, as there had been no physical disturbance since drilling in January. Smit et al. (63) observed a similar phenomenon when they examined an organically farmed wheat field; they found that the bacterial communities in July were most similar to each other (although only two samples were taken per season). This phenomenon was not, however, observed by Buckley and Schmidt (10) when they examined temporal variation between bacterial groups in arable soil in which wheat was growing. In the present study, seasonal separation became less distinct as variability increased. The variability may have been due in part to the physical disturbance caused by harvesting in late autumn and particularly by plowing in late October, soon after which the early November 2001 samples were obtained. In the previous year plowing had been performed in early September, 2 months prior to the November 2000 sampling date; this, in addition to the severity of the autumn rains, may account for the greater similarity between replicates in the November 2000 samples. These findings are very relevant to the timing of sampling when land use management is investigated. Single-point sampling, especially under a mature crop, may result in finding only small differences between management treatments (9, 10, 28). Unlike these previous studies, the fertilization regimen used in this study resulted in large community differences and was the primary management determinant acting upon the bacterial communities from November 2000 to September 2001. Fertilization increased the microbial biomass and bacterial numbers, yet community equitability declined. The fertilized (GAPF) field sections exhibited large decreases in equitability and increases in dominance in the active community compared with the baseline sample obtained in November 2000 and with the nonfertilized field section sample obtained in April. Heterogeneity, as measured by the Shannon index, was also significantly lower for the GAPF soils in April and July. These findings further support the hypothesis that there is r-strategist dominance under high-nutrient conditions (38, 70) and are in agreement with competitive exclusion principles of macroecologists who believe that that high levels of readily available nutrients can decrease overall community heterogeneity (4, 30, 46, 65).
In this study, addition of pesticide did not result in major differences in total bacterial numbers or in community heterogeneity; it did, however, significantly alter the community structure (Fig. 4). In a 20-year experiment at Rothamsted workers have studied, in detail, the effects of a range of pesticides on large-scale soil processes, such as crop yield (8), and have shown that pesticides have no deleterious effects. Similarly, GAP level pesticides have not been reported to affect broad-scale microbial process measurements (such as C and N mineralization and SIR) that are often used for ecotoxicological testing (19).
Little research has been performed thus far to ascertain the effects of pesticide addition on the structure and diversity of soil microbial communities (16), and this is generally the case for the pesticides used in this present study. These pesticides have been shown to adversely affect the numbers of organisms in the microbial community only when they are applied at rates that far exceed the recommended rates (26, 27, 58, 68). As neither bacterial numbers nor community heterogeneity of the bacterial community was severely affected by pesticide application, it may be that microbial degradation and utilization of these products caused community differences. Degradation of metsulfuron by microbial consortia, specifically pseudomonads, has been observed both in enriched soil cultures (77) and in situ (26), but with the exception of the sulfonureas most of the research has been carried out on photodegradation, physical degradation, and chemical degradation of these chemicals (5, 41, 45). Seasonal variation again resulted in the greatest differentiation between the active fungal communities, with clustering patterns similar to those observed for bacterial communities (Fig. 5). Again, in July the fungal community formed one discrete cluster, and the variation increased throughout the season, particularly after harvesting and after plowing. The variability was especially high in November 2001, following plowing, which is known to affect the distribution of arbuscular mycorrhizal fungi (6). In general, the replicate variability was much greater in the fungal populations than in the bacterial populations. Homogeneity in active agricultural bacterial communities, described by 16S rRNA and rDNA fingerprinting (temperature gradient gel electrophoresis), has been reported previously (20). However, there have been few equivalent studies of fungal community responses and no studies of the active (18S rRNA) fungal component in soil. Klamer et al. (40) investigated the effect of elevated CO2 levels on the fungal community by terminal-restriction fragment length polymorphism analysis of amplified DNA from internal transcribed spacer regions but found that any treatment effects were obscured by the large variation between samples. Despite increased variability, fertilizer application again produced the greatest management effect, maintaining fungal community structure discrimination until November 2001 (Fig. 5).
Pesticides had no significant effect on community diversity or structure. Less research has been conducted on the effects of pesticides on soil fungal communities than on the effects of pesticides on bacterial communities by using molecular approaches. Neither tebucconazol nor azoxystrobin is a contact fungicide, but these compounds are absorbed into the leaf, where the infecting fungi can then absorb the active ingredient. The effects of such fungicides on soil microorganisms may be limited, due to their general immobility in soil (18). Soil organic matter and carbon have also previously been shown to protect against many pollutants by immobilization (22, 32, 41, 44) and have been proven to reduce imidacloprid sorption by competing with the pesticide molecules for sorption sites on the soil surface (23). These factors may explain the apparent lack of pesticide effects on the active fungal community in this study.
Glomales were not initially detected by PCR in the soil in November 2000, and their absence may have been due to previous GAP management, as conventional management practices, such as high levels of inorganic P addition, are known to inhibit VAM populations (64). Three months after VAM inoculation (April), Glomales were detected only in LOWF+V field sections. However, by July and continuing through September, the VAM had spread throughout the field, indicating that there was spore germination and vegetative dispersal to the non-VAM-inoculated field sections. After plowing in November 2001, Glomales were absent from most of the GAPF samples but present in most of the LOWF samples. Arbuscular mycorrhizal fungi are prone to damage by physical disturbance (6), and the failure to detect Glomales in most samples of GAPF soil inoculated with VAM suggests that the level of Glomales may have been lower in these samples.
The present study highlighted the importance of temporal variability of the structure of the active bacterial and fungal communities and the variability of the fungal community between samples, which soil scientists must consider when they investigate management differences and establish sampling strategies. From a management perspective, the different fertilization regimens had the greatest effects on bacterial and fungal community structure, causing short-term increases in total culturable bacterial counts. Additionally a decrease in the overall diversity and heterogeneity of the bacterial community, but not the fungal community, was observed. Pesticide application did not reduce the total bacterial numbers or the active bacterial diversity, but it did affect the community structure, possibly due to bacterial pesticide degradation. Smaller management effects were observed in the fungal population, and although the different fertilization regimens resulted in variability in community structure, pesticide addition had no such effect. Finally, although the lowest grain yield was obtained for the low-input LOWF+V treatment, it appears to be possible to reduce the levels of inorganic nitrogen fertilizer, as the LOWF+V+P field sections produced a higher yield than the GAPF field section. Moreover, the GAPF treatment had the greatest impact on bacterial community diversity and warrants further investigation.
Acknowledgments
We thank D. Pendlington and J. van Oostrum for their contribution as part of the Unilever Sustainable Agriculture Initiative, and we particularly thank Steve Parry and Mike Lane for continuing advice and help. We also thank V. Gianinazzi-Pearson at the Banque Européenne des Glomales for supplying Glomales spores.
We are grateful for the financial support of Unilever and Syngenta.
REFERENCES
- 1.Anderson, J. P. E., and K. H. Domsch. 1978. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 10:215-221. [Google Scholar]
- 2.Augé, R. M. 2000. Stomatal behavior of arbuscular mycorrhizal plants, p. 201-238. In Y. Kapulnick and D. D. Douds, Jr. (ed.), Arbuscular mycorrhizas: physiology and function. Kluwer Academic Press, Dordrecht, The Netherlands.
- 3.Barea, J. M., M. Toro, M. O. Orozco, E. Campos, and R. Azcon. 2002. The application of isotopic (32P and 15N) dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate for legume crops. Nutr. Cycling Agroecosyst. 63:35-42. [Google Scholar]
- 4.Begon, M., J. L. Harper, and C. R. Townsend. 1996. Ecology. Blackwell Science, Oxford, United Kingdom.
- 5.Berger, B. M., M. Muller, and A. Eing. 2002. Quantitative structure-transformation relationships of sulfonylurea herbicides. Pestic. Manag. Sci. 58:724-735. [DOI] [PubMed] [Google Scholar]
- 6.Boddington, C. L., and J. C. Dodd. 2000. The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. I. Field studies in an Indonesian Ultisol. Plant Soil 218:137-144. [Google Scholar]
- 7.Boldt, T. S., and C. S. Jacobsen. 1998. Different toxic effects of the sulfonylurea herbicides metsulfuron methyl, chlorsulfuron and thifensulfuron methyl on fluorescent pseudomonads isolated from an agricultural soil. FEMS Microbiol. Lett. 161:29-35. [Google Scholar]
- 8.Bromilow, R. H., A. A. Evans, P. H. Nicholls, A. D. Todd, and G. G. Briggs. 1996. The effect on soil fertility of repeated applications of pesticides over 20 years. Pestic. Sci. 48:63-72. [Google Scholar]
- 9.Buckley, D. H., and T. M. Schmidt. 2001. The structure of microbial communities in soil and the lasting impact of cultivation. Microb. Ecol. 42:11-21. [DOI] [PubMed] [Google Scholar]
- 10.Buckley, D. H., and T. M. Schmidt. 2003. Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ. Microbiol. 5:441-452. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, C. A., and R. P. Zentner. 1993. Soil organic matter as influenced by crop rotations and fertilization. Soil Sci. Soc. Am. J. 57:1034-1040. [Google Scholar]
- 12.Clegg, C. D., K. Ritz, and B. S. Griffiths. 1998. Broad-scale analysis of soil microbial community DNA from upland grasslands. Antonie Leeuwenhoek 73:9-14. [DOI] [PubMed] [Google Scholar]
- 13.Clegg, C. D., K. Ritz, and B. S. Griffiths. 2000. %G+C profiling and cross hybridisation of microbial DNA reveals great variation in below-ground community structure in UK upland grasslands. Appl. Soil Ecol. 14:125-134. [Google Scholar]
- 14.Crecchio, C., M. Curci, M. D. R. Pizzigallo, P. Ricciuti, and P. Ruggiero. 2001. Molecular approaches to investigate herbicide-induced bacterial community changes in soil microcosms. Biol. Fertil. Soils 33:460-466. [Google Scholar]
- 15.Doran, J. W., and M. R. Zeiss. 2000. Soil health and sustainability: managing the biotic component of soil quality. Appl. Soil Ecol. 15:3-11. [Google Scholar]
- 16.El Fantroussi, S., L. Verschuere, W. Verstraete, and E. M. Top. 1999. Effect of phenylurea herbicides on soil microbial communities estimated by analysis of 16S rRNA gene fingerprints and community-level physiological profiles. Appl. Environ. Microbiol. 65:982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Embley, M. T., R. P. Hirt, and D. M. Williams. 1994. Biodiversity at the molecular level: the domains, kingdoms and phyla of life. Philos. Trans. R. Soc. London B Biol. Sci. 345:21-33. [DOI] [PubMed] [Google Scholar]
- 18.Engelen, B., K. Meinken, F. von Wintzingerode, H. Heuer, H.-P. Malkomes, and H. Backhaus. 1998. Monitoring impact of a pesticide treatment on bacterial soil communities by metabolic and genetic fingerprinting in addition to conventional testing procedures. Appl. Environ. Microbiol. 64:2814-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Environmental Protection Agency. 7 February 1997, posting date. Pesticide fact sheet for azoxystrobin. [Online.] Environmental Protection Agency, Washington, D.C. http://www.epa.gov/opprd001/factsheets/azoxystr.pdf.
- 20.Felske, A., and A. D. L. Akkermans. 1998. Spatial homogeneity of abundant bacterial 16S rRNA molecules in grassland soils. Microb. Ecol. 36:31-36. [DOI] [PubMed] [Google Scholar]
- 21.Felske, A., A. Wolterink, R. van Lis, W. M. De Vos, and A. D. L. Akkermans. 2000. Response of a soil bacterial community to grassland succession as monitored by 16S rRNA levels of the predominant ribotypes. Appl. Environ. Microb. 66:3998-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez, S., S. Seone, and A. Merino. 1999. Plant heavy metal concentrations and biological soil properties in agricultural serpentine soils. Commun. Soil Sci. Plant Anal. 30:1867-1884. [Google Scholar]
- 23.Flores-Cespedes, F., E. Gonzalez-Pradas, M. Fernandez-Perez, M. Villafranca-Sanchez, M. Socias-Viciana, and M. D. Urena-Amate. 2002. Effects of dissolved organic carbon on sorption and mobility of imidacloprid in soil. J. Environ. Qual. 31:880-888. [DOI] [PubMed] [Google Scholar]
- 24.Gargas, A., and P. T. De Priest. 1996. A nomenclature for fungal PCR primers with examples from intron-containing SSU rDNA. Mycologia 88:745-748. [Google Scholar]
- 25.Gelsomino, A., A. C. Keijzer-Wolters, G. Cacco, and J. D. van Elsas. 1999. Assessment of bacterial community structure in soil by polymerase chain reaction and denaturing gradient gel electrophoresis. J. Microbiol. Methods 38:1-15. [DOI] [PubMed] [Google Scholar]
- 26.Ghani, A., and D. A. Wardle. 2001. Fate of C-14 from glucose and the herbicide metsulfuron-methyl in a plant-soil microcosm system. Soil Biol. Biochem. 33:777-785. [Google Scholar]
- 27.Gigliotti, C, L. Allievi, C. Salardi, F. Ferrari, and A. Farini. 1998. Microbial ecotoxicity and persistence in soil of the herbicide bensulfuron-methyl. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 33:381-398. [Google Scholar]
- 28.Girvan, M. S., J. Bullimore, J. N. Pretty, A. M. Osborn, and A. S. Ball. 2003. Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl. Environ. Microbiol. 69:1800-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorlach-Lira, K., O. Stefaniak, W. Slizak, and I. Owedyk. 1997. The response of forest soil microflora to the herbicide formulations Fusilade and Roundup. Microbiol. Res. 152:319-329. [Google Scholar]
- 30.Grime, J. P. 1973. Competitive exclusion in herbaceous vegetation. Nature 242:344-347. [Google Scholar]
- 31.Haney, R. L., S. A. Senseman, F. M. Hons, and D. A. Zuberer. 2000. Effect of glyphosate on soil microbial activity and biomass. Weed Sci. 48:89-93. [Google Scholar]
- 32.Hassen, A., N. Saidi, M. Cherif, and A. Boudabous. 1998. Resistance of bacteria to heavy metals. Bioresource Technol. 64:7-15. [Google Scholar]
- 33.Hawking, H. J., A. Johansen, and E. George. 2000. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 226:275-285. [Google Scholar]
- 34.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodge, A., C. D. Campbell, and A. H. Fitter. 2001. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297-299. [DOI] [PubMed] [Google Scholar]
- 36.Jeffries, P., S. Gianinazzi, S. Perotto, K. Turnau, and J. M. Barea. 2003. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 37:1-16. [Google Scholar]
- 37.Kahiluoto, H., and M. Vestberg. 1998. The effect of arbuscular mycorrhiza on biomass production and phosphorus uptake from sparingly soluble sources by leek (Allium porrum L.) in Finnish field soils. Biol. Agric. Hortic. 16:65-85. [Google Scholar]
- 38.Killham, K. 1994. Soil ecology. Cambridge University Press, Cambridge, United Kingdom.
- 39.Kjøller, R., and S. Rosendahl. 2000. Detection of glomalean fungi (Glomales) in roots by nested PCR and SSCP (single stranded conformation polymorphism). Plant Soil 226:189-196. [Google Scholar]
- 40.Klamer, M., M. S. Roberts, L. H. Levine, B. G. Drake, and J. L. Garland. 2002. Influence of elevated CO2 on the fungal community in a coastal scrub oak forest soil investigated with terminal-restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 68:4370-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koskinen, W. C., L. Cox, and P. Y. Yen. 2001. Changes in sorption/bioavailability of imidacloprid metabolites in soil with incubation time. Biol. Fertil. Soils 33:546-550. [Google Scholar]
- 42.Linderman, R. G. 1992. Vesicular-arbuscular mycorrhizae and soil microbial interactions, p. 45-70. In G. J. Bethlenfalvay and R. G. Linderman (ed.), Mycorrhizae in sustainable agriculture. ASA, Madison, Wis.
- 43.Linderman, R. G. 1994. Role of VAM fungi in biocontrol, p. 1-26. In F. L. Pfleger and R. G. Linderman (ed.), Mycorrhizae and plant health. APS Press, St. Paul, Minn.
- 44.Luthy, R. G., G. R. Aiken, M. L. Brusseau, S. D. Cunningham, P. M. Gschwend, J. Pignatello, M. Reinhard, S. J. Traina, W. J. Weber, and J. C. Westall. 1997. Sequestration of hydrophobic organic contaminants by geosolids. Environ. Sci. Technol. 31:3341-3347. [Google Scholar]
- 45.Malato, S., J. Blanco, J. Caceres, A. R. Fernandez-Alba, A. Aguera, and A. Rodriguez. 2002. Photocatalytic treatment of water-soluble pesticides by photo-fenton and TiO2 using solar energy. Catal. Today 21:4359-4366. [Google Scholar]
- 46.Marilley, L., and M. Aragno. 1999. Phylogenetic diversity of bacteria communities from different proximity to Lolium perenne and Trifolium repens roots. Appl. Soil Ecol. 13:127-136. [Google Scholar]
- 47.McCaig, A. E., L. A. Glover, and J. I. Prosser. 1999. Molecular analysis of bacterial community structure and diversity in unimproved and improved upland grass pastures. Appl. Environ. Microbiol. 65:1721-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ministry of Agriculture and Fisheries. 1993. Code of good agricultural practice for the protection of soil. Ministry of Agriculture and Fisheries, London, United Kingdom.
- 49.Ministry of Agriculture and Fisheries. 2001. Fertiliser recommendations for agricultural and horticultural crops (RB209), 7th ed. The Stationary Office, London, United Kingdom.
- 50.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Donnell, A. G., M. Goodfellow, and D. L. Hawksworth. 1994. Theoretical practical aspects of the quantification of biodiversity among microorganism. Philos. Trans. R. Soc. London B Biol. Sci. 345:65-73. [DOI] [PubMed] [Google Scholar]
- 52.O'Donnell, A. G., and H. E. Görres. 1999. 16S rDNA methods in soil microbiology. Curr. Opin. Biotechnol. 10:225-229. [DOI] [PubMed] [Google Scholar]
- 53.Oldeman, L. R. 1994. The global extent of soil degradation, p. 99-118. In D. J. Greenland and I. Szabolcs (ed.), Soil resilience and sustainable land use. CAB International, Wallingford, Oxon, United Kingdom.
- 54.Pankhurst, C. E., B. M. Doube, and V. V. S. R. Gupta (ed.). 1997. Biological indicators of soil health. CAB International, Wallingford, United Kingdom.
- 55.Pielou, E. C. 1966. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13:131-134. [Google Scholar]
- 56.Pretty, J., C. Brett, D. Gee, R. Hine, C. F. Mason, J. I. L. Morison, H. Raven, M. Rayment, and G. van der Bijl. 2000. An assessment of the total external costs of UK agriculture. Agric. Syst. 65:113-136. [Google Scholar]
- 57.Pretty, J. N., C. F. Mason, D. B. Nedwell, and R. E. Hine. 2003. Environmental costs of freshwater eutrophication in England and Wales. Environ. Sci. Technol. 37:201-208. [DOI] [PubMed] [Google Scholar]
- 58.Rebecchi, L., M. A. Sabatini, C. Cappi, P. Grazioso, A. Vicari, G. Dinelli, and R. Bertolani. 2000. Effects of a sulfonylurea herbicide on soil microarthropods. Biol. Fertil. Soils 30:312-317. [Google Scholar]
- 59.Rillig, M. C., S. F. Wright, and V. T. Eviner. 2002. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species. Plant Soil 238:325-333. [Google Scholar]
- 60.Royal Commission on Environmental Pollution. 1996. Sustainable use of soil. 19th report of the Royal Commission on Environmental Pollution. Publication Cmnd 3165. HMSO, London, United Kingdom.
- 61.Shannon, C. E., and W. Weaver. 1949. The mathematical theory of communication. University of Illinois Press, Urbana.
- 62.Smalla, K., U. Wachtendorf, H. Heuer, W.-T. Liu, and L. Forney. 1998. Analysis of BIOLOG GN substrate utilization patterns by microbial communities. Appl. Environ. Microbiol. 64:1220-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smit, E., P. Leeflang, S. Gommans, J. van den Broek, S. van Mil, and K. Wernars. 2001. Diversity and seasonal fluctuations of the dominant members of the bacterial soil community in a wheat field as determined by cultivation and molecular methods. Appl. Environ. Microbiol. 67:2284-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith, S. E., and D. J. Read. 1997. Mycorrhizal symbiosis. Academic Press, London, United Kingdom.
- 65.Tilman, D., and J. A. Downing. 1994. Biodiversity and stability in grasslands. Nature 367:363-365. [Google Scholar]
- 66.Tilman, D., K. G. Cassman, P. A. Matson, R. Naylor, and S. Poolasky. 2002. Agricultural sustainability and intensive production practices. Nature 418:671-677. [DOI] [PubMed] [Google Scholar]
- 67.Torsvik, V., R. Sørheim, and J. Goksøyr. 1996. Total bacterial diversity in soil and sediment communities—a review. J. Ind. Microbiol. 17:170-178. [Google Scholar]
- 68.Tu, C. M., C. F. Marks, P. W. Johnson, S. K. Gayed, and J. M Elliot. 1995. Effects of pesticides on soil enzymatic-activities, Pratylenchus penetrans populations, black root-rot, and growth of flue-cured tobacco. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 30:141-162. [Google Scholar]
- 69.Vainio, E. J., and J. Hantula. 2000. Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol. Res. 104:927-936. [Google Scholar]
- 70.van Overbeek, L. S., and J. D. van Elsas. 1997. Adaptation of bacteria to soil conditions: applications of molecular physiology in soil microbiology, p. 441-447. In J. D. van Elsas, E. M. H. Wellington, and J. T. Trevors (ed.), Modern soil microbiology. Marcel Dekker, Inc., New York, N.Y.
- 71.van Tuinen, D., E. Jacquot, B. Zhao, A. Gollotte, and V. Gianinazzi-Pearson. 1998. Characterization of root colonization profiles by a microcosm community of glomalean fungi using 25S rDNA-targeted nested PCR. Mol. Ecol. 7:879-887. [DOI] [PubMed] [Google Scholar]
- 72.van Veen, J. A., L. S. van Overbeek, and J. D. van Elsas. 1997. Fate and activity of microorganisms following release into soil. Microbiol. Mol. Biol. Rev. 61:121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wander, M. M., and L. E. Drinkwater. 2000. Fostering soil stewardship in the U. S. through soil quality assessment. Appl. Soil Ecol. 15:61-73. [Google Scholar]
- 74.Wintzingerode, F. V., U. B. Göbel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 75.Wright, S. F., M. Franke-Snyder, J. B. Morton, and A. Upadhyaya. 1996. Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 181:193-203. [Google Scholar]
- 76.Wright, S. F., A. Upadhyaya, and J. S. Buyer. 1998. Comparison of n-linked oligosaccharides of glomalin from arbuscular mycorrhizal fungi and soils by capillary action electrophoresis. Soil Biol. Biochem. 30:1853-1857. [Google Scholar]
- 77.Zanardini, E., A. Arnoldi, G. Boschin, A. D'agostina, M. Negri, and C. Sorlini. 2002. Degradation pathways of chlorsulfuron and metsulfuron-methyl by a Pseudomonas fluorescens strain. Ann. Microbiol. 52:25-37. [Google Scholar]
- 78.Zhou, J., Z., M. A. Bruns, and J. M. Tiedje. 1996. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62:316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]