Abstract
Objective
Executive dysfunction may play a key role in the pathophysiology of late-life depression. Executive dysfunction can be assessed with cognitive tests and subjective report of difficulties with executive skills. The present study investigated the association between subjective report of executive functioning complaints and time to escitalopram treatment response in older adults with Major Depressive Disorder (MDD).
Methods
100 older adults with MDD (58 with executive functioning complaints and 42 without executive functioning complaints) completed a 12-week trial of escitalopram. Treatment response over 12 weeks, as measured by repeated Hamilton Depression Rating Scale scores, was compared for adults with and without executive complaints using mixed-effects modeling.
Results
Mixed effects analysis revealed a significant group by time interaction, F (1, 523.34) = 6.00, p = .01. Depressed older adults who reported executive functioning complaints at baseline demonstrated a slower response to escitalopram treatment than those without executive functioning complaints.
Conclusion
Self-report of executive functioning difficulties may be a useful prognostic indicator for subsequent speed of response to antidepressant medication.
Keywords: Depression, Executive Functioning, Aging
Objective
Executive dysfunction is often present in late-life depression (1, 2). Clinically, examples of executive dysfunction include disorganization, mental inflexibility, disinhibition, and poor problem solving (3). Assessments of executive functioning include neuropsychological tests and subjective report of difficulties with executive skills. Poor performances on select measures of executive functioning (i.e., susceptibility to interference and use of semantic strategies) are associated with poor response to antidepressants in older adults (4, 5). Thus, executive dysfunction may play a key role in the pathophysiology of late-life depression.
While the relationship between executive dysfunction and antidepressant response has been investigated across a number of performance-based neuropsychological measures (4-7), to our knowledge there have been no studies examining the relationship of executive functioning complaints to antidepressant response. The use of validated self-report measures to identify the relationship of executive functioning complaints to antidepressant response serves the following purposes: 1. To provide converging support for the role of executive dysfunction, using an alternative method of measurement, in the antidepressant response of late-life depression. 2. To identify individuals who may be at risk for poor antidepressant response using a measurement of executive dysfunction that is feasible across a wide range of clinical settings.
This study investigated the association between self-reported executive functioning deficits and response to antidepressant treatment in late-life depression. We hypothesized that older adults with Major Depressive Disorder and executive functioning complaints would respond more slowly to treatment with escitalopram and be less likely to achieve remission compared to older adults with Major Depressive Disorder but no executive functioning complaints.
Methods
Participants
Participants were 100 depressed adults 60 years or older from a university geriatric psychiatry outpatient clinic who were recruited for one of two escitalopram treatment trials. Participants were recruited into the clinic via community advertisements and clinician referrals. Participants met DSM-IV (8) criteria for Major Depressive Disorder and had a score ≥ 18 on the 24-item Hamilton Depression Rating Scale (HDRS)(9) after a two-week placebo lead-in. Exclusion criteria were: 1) other psychiatric disorders (except generalized anxiety disorder) or psychotic symptoms; 2) severe medical illness (i.e., metastatic cancer; unstable cardiac, hepatic, or renal disease; myocardial infarction) within the three months preceding the study; 3) neurological disorders (i.e., delirium, history of stroke, head trauma, multiple sclerosis, and brain degenerative diseases, including Parkinson's disease); 4) drugs causing depression (i.e., steroids, ∝-methyl-dopa, clonidine, reserpine, tamoxifen, or cimetidine); and 5) cognitive impairment (i.e., Mini-Mental State Examination (10) score < 25). All subjects signed consent approved by the Weill Cornell Medical College Institutional Review Board.
Treatment
Following a two week drug washout and single-blind placebo lead-in, participants who still met DSM-IV criteria for major depression and had an HDRS score ≥ 18 received controlled treatment with fixed-dose escitalopram of either 10 mg daily or 20 mg daily for 12 weeks. Participants were not randomized to treatment trials as the 10 mg trial preceded the 20 mg in time. In the second trial we administered 20 mg to avoid under treatment. If patients could not tolerate the 20 mg, they were offered a lower dosage of 15 or 10 mg. The treatment phase consisted of weekly follow up sessions for all participants until the 4th week of treatment; at that time, participants in the 20 mg trial were followed biweekly until study completion, while those in the 10 mg trial continued to be followed weekly. During each follow-up meeting, a research assistant administered the HDRS, questioned the participants about medication adherence, and counted the remaining pill tablets. The meeting with the research assistant was followed by a session with a research psychiatrist to assess the participant's clinical status.
Measures
Subjective complaints were defined using the executive functioning subscale of the Frontal Systems Behavior Scale (FrSBe)(11), a self-report assessment of difficulties in working memory, planning, sequencing, organizing, and abstracting. We chose to use the FrSBe as it has demonstrated validity for the assessment of behavioral disturbances associated with damage to frontal brain systems and allows for normative comparisons (for a review of validity evidence see Malloy & Grace 12). The FrSBe contains 17 questions regarding behavioral manifestations of executive dysfunction that are self-rated on a Likert scale from 1 (almost never) to 5 (almost always). Whereas the FrSBe allows for ratings of different time points (i.e., both before and after illness), the current study only used ratings of behavior at the present time (i.e., the time of study entry). Participants' raw scores on the FrSBe were transformed into T scores based upon normative data adjusted for age, education, and gender(11). Using clinical recommendations, participants were considered to have significant executive functioning complaints if their T score was ≥ 60. Escitalopram treatment response was defined as HDRS scores collected every two weeks over the 12 week trial.
In addition to the FrSBe and HDRS, participants also completed assessments of disability, anxiety, and medical burden. Disability was quantified using the composite score from the World Health Organization Disability Assessment Schedule II (WHODAS II)(13). Higher WHODAS scores are indicative of greater difficulty in areas of daily functioning such as transportation, household and work activities, and self-care. Symptoms of anxiety were measured with the Clinical Anxiety Scale(14), where higher scores represent increased psychic anxiety, tension, and worry. Finally, medical comorbidity was quantified using the Charlson Comorbidity Index (15), a weighted sum of medical comorbidities.
Statistical Analyses
Analyses used the intent-to-treat sample. This included all individuals who met inclusion/exclusion criteria and completed a baseline assessment. Baseline demographic and clinical characteristics were compared for the two groups (presence or absence of executive functioning complaints) using the Mann-Whitney U test or Pearson's Chi-Squared test. Biweekly HDRS scores over 12 weeks were compared for the two groups using mixed-effects models. These models included main fixed effects for time and group, their interaction, and random intercepts using maximum likelihood estimates. We considered both linear and quadratic trends, and the selection of the best model was based on the likelihood ratio test. Potential group differences in HDRS over time were of primary interest. Diagnostics (e.g., influence statistics) were computed after selection of the best fitting model to ensure assumptions were upheld.
Results
Of the 100 participants, 58 had executive functioning complaints and 42 had no executive functioning complaints. Dosage of escitalopram treatment was evenly distributed among the groups (45% of adults with executive complaints received 20mg compared to 55% without complaints; χ2 (1, n = 100) = 0.96, p = 0.33). Twenty-one patients exited the treatment trial before completion (15 (26%) patients with and six (14%) patients without executive functioning complaints). Of the 21 patients who exited early, nine (42%) were no longer interested in participating in research or were lost to follow-up, eight (38%) found the treatment ineffective or had worsening depression, and four (19%) had significant side effects. Compared to those who completed the trial, participants who exited early were younger (dropouts M = 68.73, SD = 5.68; completers M = 72.32, SD = 7.26, Mann-Whitney z = 2.17, p = 0.03) and reported greater disability on the WHODAS II (dropouts M = 43.40, SD = 15.13; completers M = 36.62, SD = 9.84, Mann-Whitney z = 2.01, p = 0.04). Dropouts and participants who completed the trial were evenly distributed among patients with and without executive functioning complaints and did not significantly vary by baseline Hamilton Depression Rating Scale scores, age of depression onset, number of prior depressive episodes, duration of current depressive episode, gender, cognitive functioning, and comorbid medical illnesses.
Overall, patients had an average age of 71.57 (SD = 7.09), 16.16 years of education (SD = 3.04), and averaged 28.23 points out of 30 on the MMSE (SD = 1.58). Women comprised the majority (54%) of the sample. On average, patients reported moderate symptoms of depression at baseline (Baseline HDRS M = 22.62, SD = 3.46) that responded well to treatment with escitalopram (Week 12 HDRS M = 10.77, SD = 6.81). Patients reported an average of 2.93 prior episodes of depression (SD = 2.59) with onset in middle age (M = 51.31, SD = 21.85). Patients acknowledged mild anxiety (M = 4.32, SD = 3.49) and a low level of medical comorbidity (68% scored 0-2 on the Charlson Comorbidity Index(15)). No statistically significant differences in the above mentioned demographic and clinical variables were observed between patients with and without executive functioning complaints at baseline (see Table 1). Compared to patients without executive functioning complaints, those with complaints reported greater symptoms of disability on the WHODAS II (Mann-Whitney z = 2.51, p = 0.01).
Table 1.
Baseline Demographic and Clinical Data of 100 Major Depression Patients With and Without Executive Functioning Complaints.
| No Executive Functioning Complaints (N = 42) | Executive Functioning Complaints (N = 58) | p | |||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | |
| Age in years | 72.02 | 7.33 | 71.23 | 6.95 | 0.60 |
| Education in years | 15.93 | 3.10 | 16.33 | 3.02 | 0.32 |
| Gender, n (percent women) | 21 (50%) | - | 33 (57%) | - | 0.49 |
| Mini-Mental State Exam (/30) | 28.31 | 1.61 | 28.17 | 1.58 | 0.55 |
| HDRS | 22.83 | 3.72 | 22.47 | 3.28 | 0.60 |
| Age of depression onset | 53.93 | 20.88 | 49.47 | 22.50 | 0.40 |
| Current episode duration in months | 27.10 | 25.17 | 46.49 | 116.18 | 0.93 |
| Number of previous major depressive episodes | 2.68 | 2.50 | 3.11 | 2.66 | 0.31 |
| Clinical Anxiety Scale (/28) | 4.40 | 3.97 | 4.26 | 3.13 | 0.84 |
| WHODAS II (total score) | 34.67 | 8.25 | 40.45 | 12.75 | < .05 |
| Charlson Comorbidty Index, n (percent scoring 0-2) | 28 (72%) | - | 38 (69%) | - | 0.59 |
HDRS: Hamilton Depression Rating Scale; WHODAS II: World Heath Organization Disability Assessment Schedule II
Comparison of mixed models with linear and quadratic terms revealed the quadratic model best fit the data (model with quadratic terms -2 Log Likelihood = 3608.73, model with linear terms -2 Log Likelihood = 3695.29, χ2 (2) = 88.18, p < 0.001). Therefore, the final model predictors were time, group, the interaction of time and group, time squared, and the interaction of time squared and group. Analyses revealed a significant main effect of time F (1, 523.34) = 255.72, p < 0.001, but not group F (1, 184.86) = 0.05, p = 0.82. Consistent with our hypothesis, the group by time interaction was significant F (1, 523.34) = 6.00, p = 0.01, as was the time by time interaction F (1, 520.57) = 93.45, p < 0.001. Group by time squared was also significant F (1, 520.57) = 4.12, p = 0.04. Relative to patients who did not report executive functioning complaints at baseline, depressed older adults who reported executive functioning complaints at baseline demonstrated a slower response (reduction of HDRS) to escitalopram treatment (see Figure 1).
Figure 1.
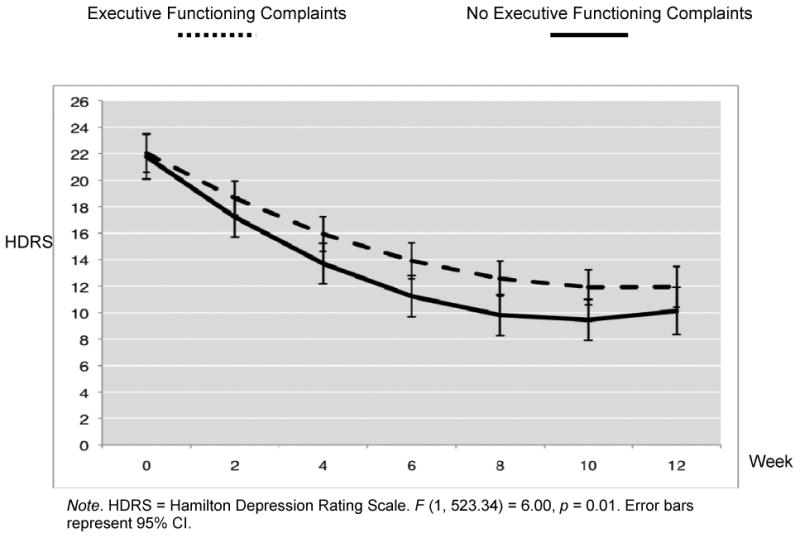
Treatment Response Over 12 Weeks of Escitalopram Treatment in 100 Older Adult Major Depression Patients With and Without Executive Functioning Complaints.
Conclusions
The principle finding of this study is that older adults with major depression and executive functioning complaints responded slower to escitalopram treatment than older adults with major depression and no executive functioning complaints. To our knowledge, this is the first study to identify a relationship between subjective report of executive dysfunction and antidepressant response.
The present findings replicate and extend existing evidence for the role of executive dysfunction in antidepressant response (4, 5). The use of a validated self-report measure to examine risk for nonresponse has the advantage of the ease of administration and interpretation, as the use of neuropsychological measures is not feasible in many clinical settings where elderly depressed patients are treated. For example, executive functioning complaint questionnaires could be administered to elderly depressed patients in psychiatry waiting rooms. This information could then be used to alert clinicians in real-time to potential poor treatment response. Formal assessment of executive functioning complaints may be especially important, as older adults are unlikely to spontaneously report subjective cognitive difficulties (16).
These findings need to be interpreted within the context of some limitations. Limitations include the lack of a placebo control group, the lack of randomization to the escitalopram trials and no long-term follow-up. It is possible that some participants would have demonstrated a better response to escitalopram had longer treatment been offered. Dosage of escitalopram, however, does not account for the present findings as participants with and without executive functioning complaints were evenly distributed among the medication trials. Furthermore, while groups were selected post-hoc, there were no significant clinical differences at baseline between participants with and without executive functioning complaints on variables associated with prediction of treatment resistance. Further, the Executive Function subscale of the FrSBe was the only subscale of the FrSBe administered in this study, limiting data regarding the specificity of the finding. Finally, the measures included in the analyses were self-report (i.e., the HDRS, the FrSBe).
In conclusion, although our results are preliminary, self-report of executive functioning difficulties may be a useful prognostic indicator for subsequent speed of response to antidepressant medication. Use of the FrSBe to measure executive functioning complaints may offer a feasible way to identify individuals who are at risk for poor response to traditional antidepressant treatment.
Acknowledgments
This paper was supported by National Institute of Mental Health grants P30 MH085943 (GSA), R01 MH065653 (GSA), R01 MH079414 (GSA), and T32 MH019132 (GSA) and by the Sanchez Foundation. Escitalopram and placebo were provided free of cost by Forest Pharmaceuticals, Inc.
Footnotes
Conflicts of Interest: Dr. Alexopoulos had a grant from Forest Pharmaceuticals; consulted to Pfizer and Otsuka; and serves on the speaker's bureaus of Astra Zeneca, Avanir, Novartis, and Sunovion. All other authors report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- 2.Elderkin-Thompson V, Kumar A, Bilker WB, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18:529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 3.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morimoto SS, Gunning FM, Kanellopoulos D, et al. Semantic organizational strategy predicts verbal memory and remission rate of geriatric depression. Int J Geriatr Psychiatry. 2012;27:506–512. doi: 10.1002/gps.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sneed JR, Roose SP, Keilp JG, et al. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 6.Potter GG, Kittinger JD, Wagner HR, et al. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29:2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 7.Story TJ, Potter GG, Attix DK, et al. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16:752–759. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV - patient version (SCID-P) Washington: American Psychiatric Press; 1995. [Google Scholar]
- 9.Hamilton M. A rating scale for depression. Journal of Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Grace J, Malloy P. Frontal Systems Behavior Scale (FrSBe): Professional Manual, Lutz, FL. Psychological Assessment Resources; 2001. [Google Scholar]
- 12.Malloy P, Grace J. A review of rating scales for measuring behavior change due to frontal systems damage. Cogn Behav Neurol. 2005;18:18–27. doi: 10.1097/01.wnn.0000152232.47901.88. [DOI] [PubMed] [Google Scholar]
- 13.Epping-Jordan J, Ustun T. The WHODAS-II: Leveling the playing field for all disorders. WHO Mental Health Bulletin. 2000;6:5–6. [Google Scholar]
- 14.Snaith R, Baugh S, Clayden A, et al. The Clinical Anxiety Scale: An instrument derived from the Hamilton Anxiety Scale. Brit J Psychiat. 1982;141:518–523. doi: 10.1192/bjp.141.5.518. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski T, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 16.Begum A, Morgan C, Chiu CC, et al. Subjective memory impairment in older adults: aetiology, salience and help seeking. Int J Geriatr Psychiatry. 2012;27:612–620. doi: 10.1002/gps.2760. [DOI] [PubMed] [Google Scholar]


