Abstract
Although nicotine accounts for a great deal of the neurodevelopmental damage associated with maternal smoking or second-hand exposure, tobacco smoke contains thousands of potentially neurotoxic compounds. We used PC12 cells, a standard in vitro model of neurodifferentiation, to compare tobacco smoke extract (TSE) to nicotine, matching TSE exposure (with its inherent nicotine content) to parallel concentrations of nicotine, or to benzo[a]pyrene, a tobacco combustion product. TSE promoted the transition from cell replication to differentiation, resulting in fewer, but larger cells with greater neurite extension. TSE also biased differentiation into the dopaminergic versus the cholinergic phenotype, evidenced by an increase in tyrosine hydroxylase activity but not choline acetyltransferase. Nicotine likewise promoted differentiation at the expense of cell numbers, but its effect on growth and neurite extension was smaller than that of TSE; furthermore, nicotine did not promote the dopaminergic phenotype. Benzo[a]pyrene had effects opposite to those of TSE, retarding neurodifferentiation, which resulted in higher cell numbers, smaller cells, reduced neurite information, and impaired emergence of both dopaminergic and cholinergic phenotypes. Our studies show that the complex mixture of compounds in tobacco smoke exerts direct effects on neural cell replication and differentiation that resemble those of nicotine in some ways but not others, and most importantly, that are greater in magnitude than can be accounted for from just the nicotine content of TSE. Thus, fetal tobacco smoke exposure, including lower levels associated with second-hand smoke, could be more injurious than would be anticipated from measured levels of nicotine or its metabolites.
Keywords: Benzo[a]pyrene, Neurodifferentiation, Nicotine, PC12 cells, Tobacco smoke extract
1. INTRODUCTION
Maternal cigarette smoking during pregnancy remains the single, most preventable cause of perinatal morbidity and mortality in developed countries (Abbott and Winzer-Serhan, 2012; DiFranza and Lew, 1995; Pauly and Slotkin, 2008). An even larger population of babies is impacted by second-hand tobacco smoke (DiFranza et al., 2004; Herrmann et al., 2008; Polanska et al., 2006). Aside from adverse effects in the immediate perinatal period, the long-term liability of prenatal tobacco exposure produces substantial increases in the risk of neurodevelopmental disorders, including learning disabilities, attention deficit/hyperactivity disorder and conduct disorders (Cornelius and Day, 2009; DiFranza and Lew, 1995; Gaysina et al., 2013; Pauly and Slotkin, 2008; Wakschlag et al., 1997). To a great extent, these outcomes reflect the adverse effect of nicotine itself on brain development (Pauly and Slotkin, 2008; Slikker et al., 2005; Slotkin, 2004, 2008). As a nicotinic acetylcholine receptor agonist, nicotine preempts normal cholinergic signals that are critical to the control of neuronal cell replication and differentiation, to the formation of axons and synapses, and to the development of neural circuits. A comparison of nicotine alone vs. tobacco smoke exposure in a variety of models shows similar effects on neurogenesis (Bruijnzeel et al., 2011; Gospe et al., 1996; Slotkin, 2004), oxidative stress (Lobo Torres et al., 2012; Qiao et al., 2005), neural plasticity (Heath and Picciotto, 2009; Sekizawa et al., 2008; Shingo and Kito, 2005), and on indices of cholinergic function, synaptic signaling, and neural cell differentiation into specific neurotransmitter phenotypes (Slotkin, 2004; Slotkin et al., 2000, 2001, 2002, 2006a, b).
Notwithstanding the concordant effects of nicotine and tobacco smoke on brain development, smoke contains many more compounds that cross the placenta and that are potentially neurotoxic, few of which have been evaluated for their contributions to developmental neurotoxicity. Indeed, combustion products such as benzo[a]pyrene (BaP) and related polycyclic aromatic hydrocarbons, have also been shown to be associated with developmental deficits in children (Perera et al., 2005), and animal studies confirm that early-life exposure to BaP has adverse effects on neurodevelopment and synaptic function (Brown et al., 2007; Hood et al., 2000; Slotkin et al., 2013; Slotkin and Seidler, 2009). However, the effects of BaP on neuronal cell replication and differentiation are opposite to those seen with nicotine: whereas nicotine tends to promote neurodifferentiation at the expense of cell numbers, BaP slows neurodifferentiation and extends the period in which neural cells proliferate (Abreu-Villaça et al., 2005; Slotkin et al., 2013; Slotkin and Seidler, 2009). In a recent study using PC12 cells (Slotkin et al., 2013), a neuronotypic cell line commonly used to study neurodifferentiation (Costa, 1998; Teng and Greene, 1994), we found synergistic interactions between nicotine and BaP, diverting cell fate away from the cholinergic phenotype (and toward the dopaminergic phenotype. This raises the possibility that the complex mixture of compounds in tobacco smoke exposure could have effects on neurodevelopment distinct from those evoked by individual agents. In the current study, we show that the effects of tobacco smoke extract (TSE) on neurodifferentiation in PC12 cells cannot be explained solely by the effects of nicotine, and further, that TSE effects are totally unlike those obtained from exposure to BaP.
2. METHODS
TSE (Arista Laboratories, Richmond, VA) was prepared from Kentucky Reference cigarettes (KY3R4F) on a Rotary Smoke Machine under ISO smoke conditions. The smoke condensate was collected on 92 mm filter pads, which were then extracted by shaking for 20 min with dimethylsulfoxide, to obtain a solution of approximately 20 mg of condensate per ml. Condensate aliquots were stored in amber vials at −80°C until used. Two cigarettes were smoked to produce each ml of extract and the final product contained 0.8 mg/ml (5 mM) nicotine.
2.1 Cell cultures
Because of the clonal instability of the PC12 cell line (Fujita et al., 1989), the experiments were performed on cells that had undergone fewer than five passages. As described previously (Qiao et al., 2003; Song et al., 1998), PC12 cells (American Type Culture Collection CRL-1721, obtained from the Duke Comprehensive Cancer Center, Durham, NC) were seeded onto poly-D-lysine-coated plates in RPMI-1640 medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% horse serum (Sigma), 5% fetal bovine serum (Sigma), and 50 (^g/ml penicillin streptomycin (In vitro gen, Carlsbad, CA). Incubations were carried out with 5% CO2 at 37°C, standard conditions for PC12 cells. To initiate neurodifferentiation (Jameson et al., 2006b; Slotkin et al., 2007; Teng and Greene, 1994), the medium was changed to include 50 ng/ml of 2.5 S murine nerve growth factor (Promega Corporation, Madison, WI); each culture was examined under a microscope to verify the outgrowth of neurites.
Toxicant exposures were all commenced simultaneously with the addition of nerve growth factor, so as to be present throughout neurodifferentiation. As our positive controls for comparison with TSE, we used nicotine bitartrate or BaP (both from Sigma) at a final concentration of 10 µM, which is sufficient to produce significant effects of each agent on neurodifferentiation in the PC12 model (Abreu-Villaça et al., 2005; Slotkin et al., 2013; Slotkin and Seidler, 2009). The effects of TSE were evaluated at a low and high concentration, calculated to produce a final concentration of 1 µM or 10 µM nicotine in the culture medium, corresponding to a 1/1000 and 1/500 dilution of the condensate. All agents were dissolved in dimethylsulfoxide (Sigma; final concentration 0.2%), which was also added to all the samples regardless of treatment; this concentration of dimethylsulfoxide has no effect on PC12 cell growth or differentiation (Qiao et al., 2001; Song et al., 1998). The medium was changed every 48 hr with the continued inclusion of nerve growth factor and test substances; assays were carried out after six days of exposure.
2.2 Assays
Cells were harvested, washed, and the DNA and protein fractions were isolated and analyzed as described previously (Slotkin et al., 2007). Measurements of DNA, total protein and membrane protein were used as biomarkers for cell number, cell growth and neurite growth (Qiao et al., 2003; Song et al., 1998). Since the DNA per cell is constant, cell growth entails an obligatory increase in the total protein per cell (protein/DNA ratio) as well as membrane protein per cell (membrane protein/DNA ratio). If cell growth represents simply an increase in the perikaryal area, then membrane protein decreases less than total protein because of the decline in the surface-to-volume ratio (volume increases with the cube of the perikaryal radius, whereas surface area increases with the square of the radius); however, when neurites are formed as a consequence of neurodifferentiation, this produces a increase in membrane protein larger than that predicted from this simple 2/3-power geometric relationship. Each of these biomarkers has been validated in prior studies by direct measurement of cell number (Powers et al., 2010; Roy et al., 2005), perikaryal area (Roy et al., 2005) and neurite formation (Das and Barone, 1999; Howard et al., 2005; Song et al., 1998). To assess neurodifferentiation into dopamine and acetylcholine phenotypes, we assayed the activities of tyrosine hydroxylase (TH) and choline acetyltransferase (ChAT), respectively, using established techniques (Jameson et al., 2006a, b).
2.3 Data analysis
Each study was performed using 2–5 separate batches of cells, with 3–4 independent cultures for each treatment in each batch; each batch of cells comprised a separately prepared, frozen and thawed passage. Results are presented as mean ± SE, with treatment comparisons carried out by analysis of variance (ANOVA) followed by Fisher's Protected Least Significant Difference Test for post-hoc comparisons of individual treatments. The initial comparison involved a two-factor ANOVA: factor 1 = treatment; factor 2 = cell batch. In each case, we found that the treatment effects were the same across the different batches of cells, although the absolute values differed from batch to batch. Accordingly, we normalized the results across batches prior to combining them for presentation. Significance was assumed at p < 0.05 (two-tailed).
3. RESULTS
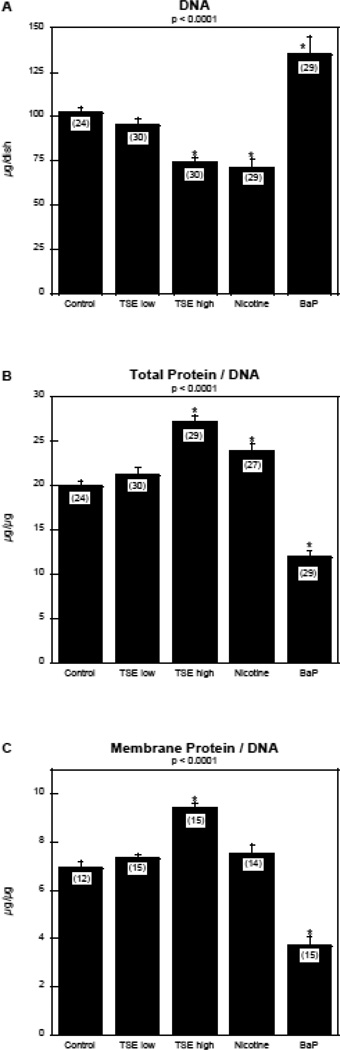
Exposure of differentiating PC12 cells to TSE produced a concentration-dependent reduction in the total number of cells, as monitored by DNA content (Figure 1A). At the high exposure level, TSE had an effect equivalent to that achieved with nicotine alone at the same final concentration (10 µM) as that achieved from the nicotine contained in TSE. In contrast, giving the same concentration of BaP produced a robust increase in DNA. Each agent had corresponding effects on cell growth, assessed by the total protein/DNA ratio (Figure 1B). TSE exposure produced a large increase that was significantly greater than that achieved by the equivalent concentration of nicotine alone (p < 0.003). Again, BaP alone had the opposite effect, reducing the ratio.
Figure 1.
Effects of TSE, nicotine and BaP on indices of cell number and cell growth: (A) DNA, (B) total protein/DNA ratio, (C) membrane protein/DNA ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVAs for the main effects of treatment are shown at the top of each panel and asterisks denote groups that are statistically significant from the control group.
The pattern of effects on the membrane protein/DNA ratio resembled that seen for total protein/DNA (Figure 1C). TSE evoked a large increase, whereas nicotine evoked a smaller, nonsignificant increase, and BaP produced a decrease. Notably, the changes in membrane protein/DNA did not follow the 2/3-power rule that would pertain if the effects were restricted to the cell body. For TSE, the 2/3-power rule applied to the 35% increase in total protein/DNA ratio predicts an 11% increase in membrane protein/DNA, but the actual increase was 38% (p < 0.0001 vs. the predicted value, one-group t-test). Likewise, for BaP, the 40% decrease in total protein/DNA would predict a 12% decrease in membrane protein/DNA, but the actual reduction was 45% (p < 0.0001 vs. predicted value). These differences indicate that the changes in membrane protein reflect the formation of neuritic projections, not just the diameter of the cell body.
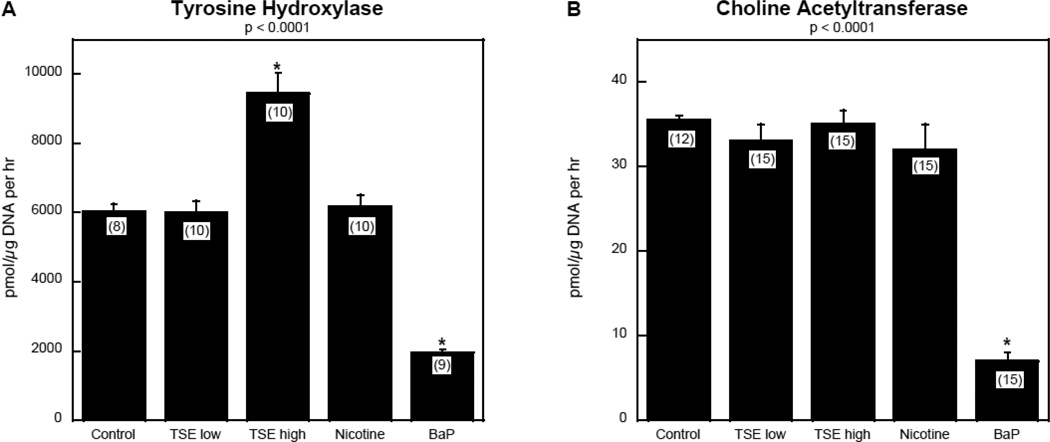
TSE exposure had a profound effect on differentiation into dopaminergic and cholinergic phenotypes. The high concentration of TSE evoked a large increase in TH activity, whereas nicotine produced only minor (nonsignificant) changes; in contrast, BaP evoked a large decrement (Figure 2A). For the cholinergic phenotype, neither TSE nor nicotine elicited significant effects on ChAT (Figure 2B); again, BaP was different, causing an even greater proportional decrease in ChAT (80%) compared to its effect on TH (65% decrease). Accordingly, both TSE and BaP shifted the endpoint of neurodifferentiation to favor the dopaminergic phenotype over the cholinergic phenotype, but by different mechanisms.
Figure 2.
Effects of TSE, nicotine and BaP on neurodifferentiation into dopaminergic and cholinergic phenotypes: (A) tyrosine hydroxylase, (B) choline acetyltransferase. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVAs for the main effects of treatment are shown at the top of each panel and asterisks denote groups that are statistically significant from the control group.
4. DISCUSSION
Results obtained in this study provide some of the first evidence showing that the cellular effects of tobacco smoke on neurodevelopment are distinct from those of prominent individual components such as nicotine or BaP. The net outcome of TSE exposure was promotion of cell growth and neurite extension, achieved at the cost of suppressing cell numbers, and with the additional effect of diverting neurodifferentiation toward the dopaminergic phenotype. The reduction in cell numbers, characterized by a deficit in total DNA, does not reflect cytotoxicity, which also would have suppressed cell growth. Instead, we saw an increase in the protein/DNA ratio, connoting larger cells. The augmented cell growth was accompanied by an increase in membrane protein that exceeded the values predicted from simple enlargement of the cell body, thus pointing to growth associated specifically with increased membrane complexity; in the case of differentiating PC12 cells, this represents neurite extension (Song et al., 1998; Teng and Greene, 1994), as confirmed here by qualitative microscopic observation. It is important to note that, in this study, the toxicants were added simultaneously with nerve growth factor, a trophin which triggers a gradual transition from cell replication to neurodifferentiation (Teng and Greene, 1994). Accordingly, TSE-induced promotion of cell growth and neurite formation, along with reduction of cell numbers, indicates accelerated neurodifferentiation. That conclusion is reinforced by the observation that TSE promoted the emergence of the dopaminergic phenotype, a highly specific effect in that we did not observe any change for differentiation into the cholinergic phenotype.
The effects of TSE on neurodifferentiation stand out clearly from those of BaP, a known toxic component of tobacco smoke. In agreement with earlier observations (Slotkin et al., 2013; Slotkin and Seidler, 2009), BaP retarded neurodifferentiation, increasing cell numbers while slowing cell growth and neurite formation, and reducing expression of both neurotransmitter phenotypes; these effects are entirely opposite to those for TSE. Although BaP, like TSE, resulted in a preponderance of dopaminergic cells vs. cholinergic cells, it did so by an different underlying mechanism, suppressing the cholinergic phenotype to a greater extent than the dopaminergic phenotype.
The comparison with nicotine is more pertinent, given the prominent role of nicotine in neurodevelopmental deficits associated with maternal smoking. The effects of TSE on cell numbers, cell growth and neurite formation were in the same direction as those of nicotine alone, differing primarily in the larger magnitude of TSE effects on growth and neurite extension than for nicotine. Thus, TSE (which contains nicotine) and nicotine by itself share the important characteristic of accelerating neurodifferentiation at the expense of cell numbers, but TSE is more effective than would be expected simply from its nicotine content. This points to clear contributions from other components of TSE besides nicotine, a conclusion that is strengthened by our finding of differences between TSE and nicotine in their effects on neurotransmitter phenotypes. Nicotine had relatively little effect, whereas TSE strongly promoted the dopaminergic phenotype.
The limitations of an in vitro approach to developmental neurotoxicity have been delineated previously (Coecke et al., 2007; Qiao et al., 2001; Song et al., 1998) but it is worth repeating the major points. An in vitro model enables us to show that TSE is a directly-acting developmental neurotoxicant, allowing for dissection of cause-and-effect relationships that cannot readily be studied in vivo. However, cell cultures are incapable of detecting the complex events involved in brain assembly, such as cell-to-cell interactions and architectural modeling of brain regions; effects in animal models are thus likely to be considerably more sensitive than seen here in vitro. Further, with in vitro exposures, adverse effects have to be detected within a period of hours or days, as compared to weeks of in vivo exposure, necessitating higher concentrations (Coecke et al., 2007). This is exacerbated in the case of transformed cell lines, such as PC12 cells, which are generally less responsive to toxicants than are primary neurons. On the other hand, primary neurons would be inappropriate to study the effects seen here, which involve terminating the cell cycle and affecting specific neurodifferentiation endpoints. Primary neurons do not divide in culture and are in heterogeneous states of neurodifferentiation, whereas PC12 cells undergo mitosis and differentiate uniformly upon addition of nerve growth factor. Thus, primary neurons are problematic for these assessments, whereas the PC12 line is especially useful (Coecke et al., 2007; Radio et al., 2008). PC12 cells are also problematic for quantitative morphologic investigation of neurite formation because they grow in clumps, with only a minority of cells separated sufficiently to permit accurate measurement. Although quantitative morphology has been published using PC12 cells, these studies typically exclude cells that are in clumps or in contact with each other, or use nonstandard PC12 subclones that do not clump, but have not been widely used for toxicologic evaluations (Das et al., 1998; Leach et al., 2007; Obin et al., 1999; Radio et al., 2008). Even so, where quantitative morphology has been carried out, the results replicate the conclusions reached with biochemical approaches as used here (Das and Barone,1999; Howard et al., 2005; Song et al., 1998). Finally, in the case of TSE, pharmacokinetic considerations, which also cannot be modeled in cell cultures, are also likely to be important. Whereas we studied TSE as obtained directly from tobacco smoke condensate, placental transfer and metabolism will undoubtedly change the concentration that actually reaches the fetus, and there is little or no information for most of the thousands of components of TSE. In that light, our studies point to the potential neurodevelopmental outcomes of TSE exposure but do not obviate the need for parallel in vivo studies, which are currently underway in our laboratory.
In summary, we found that TSE has direct effects on neuronal cell replication and differentiation that resemble those of nicotine in some ways but not others, and most importantly, that are greater in magnitude than can be accounted for from the nicotine content of TSE. The exacerbation of nicotine's effects could represent direct actions of the other TSE components on neurodifferentiation, or alternatively, cross-talk of these agents with nicotinic receptor activation. Although it might seem appropriate to then pursue which TSE component(s) contribute the additional effects, there are thousands of compounds in TSE that would need to be examined; furthermore, we have already shown that a combination of just two chemicals, nicotine and BaP, produces effects that could not have been predicted from either agent alone or from simple summation of effects (Slotkin et al., 2013). It is therefore likely that deconvoluting individual contributors in TSE may prove fruitless, and that comparative analysis of complex TSE fractions may be a more efficient way to identify the critical components underlying the developmental neurotoxicity of tobacco smoke. The same constraints operate for delineation of specific mechanisms underlying TSE's effects on neurodifferentiation: there are likely to be many different contributory pathways to the outcome, involving complex interactions of the effects of multiple compounds. Indeed, even for nicotine alone, although nicotinic receptor antagonists block many of the effects on PC12 cells (Jonnala and Buccafusco, 2001; Qiao et al., 2003; Reuben et al., 1998), there are additional contributions from oxidative stress (Guan et al., 2003; Qiao et al., 2005). Notwithstanding these issues, the implications of our findings are clear. If TSE elicits corresponding effects in the developing fetal brain, then we would expect to see the same kinds of neural cell deficits and miswiring that are already attributable to nicotine (Pauly and Slotkin, 2008; Slikker et al., 2005; Slotkin, 2004, 2008), but to a greater extent and perhaps more directed toward specific neurotransmitter systems, especially dopamine. In turn, a greater sensitivity to neurodevelopmental damage implies that fetal tobacco smoke exposure, including the lower levels associated with second-hand smoke, could be more injurious than would be anticipated from measured levels of nicotine or its metabolites (DeLorenze et al., 2002; Koren et al., 1998; Luck et al., 1985).
Supplementary Material
Highlights.
We compared effects of tobacco smoke extract (TSE) to nicotine or benzo[a]pyrene on neurodifferentiation in PC12 cells
TSE promoted the transition from cell replication to neurodifferentiation and specifically enhanced emergence of the dopamine phenotype
TSE effects on replication and neurodifferentiation were greater than those of nicotine, nor did nicotine promote the dopaminergic phenotype
Benzo[a]pyrene had opposite effects from TSE, retarding neurodifferentiation
TSE effects on neurodifferentiation are distinct those of nicotine or benzo[a]pyrene
Acknowledgment
Research was supported by NIH ES022831 and EPA 83543701. EPA support does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Abbreviations
- ANOVA
analysis of variance
- BaP
benzo[a]pyrene
- ChAT
choline acetyltransferase
- TH
tyrosine hydroxylase
- TSE
tobacco smoke extract
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: TAS has received consultant income in the past three years from the following firms: The Calwell Practice (Charleston WV), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA), Alexander Hawes (San Jose, CA), Pardieck Law (Seymour, IN), Tummel & Casso (Edinburg, TX), Shanahan Law Group (Raleigh NC), and Chaperone Therapeutics (Research Triangle Park, NC).
REFERENCES
- Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit. Rev. Toxicol. 2012;42:279–303. doi: 10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Qiao D, Slotkin TA. Modeling the developmental neurotoxicity of nicotine in vitro: cell acquisition, growth and viability in PC12 cells. Dev. Brain Res. 2005;154:239–246. doi: 10.1016/j.devbrainres.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson CV, Ramesh A, Sheng L, et al. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28:965–978. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bauzo RM, Munikoti V, Rodrick GB, Yamada H, Fornal CA, et al. Tobacco smoke diminishes neurogenesis and promotes gliogenesis in the dentate gyrus of adolescent rats. Brain Res. 2011;1413:32–42. doi: 10.1016/j.brainres.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Coecke S, Goldberg AM, Allen S, Buzanska L, Calamandrei G, Crofton K, et al. Workshop report: incorporating in vitro alternative methods for developmental neurotoxicity into international hazard and risk assessment strategies. Environ. Health Perspect. 2007;115:924–931. doi: 10.1289/ehp.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Curr. Opin. Neurol. 2009;22:121–125. doi: 10.1097/WCO.0b013e328326f6dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG. Neurotoxicity testing: a discussion of in vitro alternatives. Environ. Health Perspect. 1998;106(Suppl. 2):505–510. doi: 10.1289/ehp.98106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol. Appl. Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- DeLorenze GN, Kharrazi M, Kaufman FL, Eskenazi B, Bernert JT. Exposure to environmental tobacco smoke in pregnant women: the association between self-report and serum cotinine. Environ. Res. 2002;90:21–32. doi: 10.1006/enrs.2001.4380. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and Sudden Infant Death Syndrome. J. Family Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, et al. Maternal smoking during pregnancy and offspring conduct problems: evidence from three independent genetically-sensitive research designs. JAMA Psychiat. 2013;70:956–963. doi: 10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospe SM, Zhou SS, Pinkerton KE. Effects of environmental tobacco smoke exposure in utero and/or postnatally on brain development. Pediatr. Res. 1996;39:494–498. doi: 10.1203/00006450-199603000-00018. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Yu WF, Nordberg A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem. Intl. 2003;43:243–249. doi: 10.1016/s0197-0186(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(Suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr. Opinion Pediatr. 2008;20:184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of Spl subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal. Toxicol. 2000;12:511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang DR. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol. Appl. Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Adverse neurodevelopmental effects of dexamethasone modeled in PC12 cells: identifying the critical stages and concentration thresholds for the targeting of cell acquisition, differentiation and viability. Neuropsychopharmacology. 2006a;31:1647–1658. doi: 10.1038/sj.npp.1300967. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ. Health Perspect. 2006b;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnala RR, Buccafusco JJ. Relationship between the increased cell surface α 7 nicotinic receptor expression and neuroprotection induced by several nicotinic receptor agonists. J. Neurosci. Res. 2001;66:565–572. doi: 10.1002/jnr.10022. [DOI] [PubMed] [Google Scholar]
- Koren G, Eliopoulos C, Klein J. Measuring fetal exposure to nicotine. In: Benowitz NL, editor. Nicotine Safety and Toxicity. New York: Oxford University Press; 1998. pp. 99–106. [Google Scholar]
- Leach JB, Brown XQ, Jacot JG, DiMilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J. Neural Eng. 2007;4:26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- Lobo Torres LH, Moreira WL, Tamborelli Garcia RC, Annoni R, Nicoletti Carvalho AL, Teixeira SA, et al. Environmental tobacco smoke induces oxidative stress in distinct brain regions of infant mice. J. Toxicol. Environ. Health A. 2012;75:971–980. doi: 10.1080/15287394.2012.695985. [DOI] [PubMed] [Google Scholar]
- Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev. Pharmacol. Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- Obin M, Mesco E, Gong X, Haas AL, Joseph J, Taylor A. Neurite outgrowth in PC12 cells: distinguishing the roles of ubiquitylation and ubiquitin-dependent proteolysis. J. Biol. Chem. 1999;274:11789–11795. doi: 10.1074/jbc.274.17.11789. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Psediatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Polanska K, Hanke W, Ronchetti R, van den Hazel P, Zuurbier M, Koppe JG, et al. Environmental tobacco smoke exposure and children's health. Acta Pædiatr. Supp. 2006;95:86–92. doi: 10.1080/08035320600886562. [DOI] [PubMed] [Google Scholar]
- Powers CM, Wrench N, Ryde IT, Smith AM, Seidler FJ, Slotkin TA. Silver impairs neurodevelopment: studies in PC12 cells. Environ. Health Perspect. 2010;118:73–79. doi: 10.1289/ehp.0901149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ. Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev. Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Radio NM, Breier JM, Shafer TJ, Mundy WR. Assessment of chemical effects on neurite outgrowth in PC12 cells using high content screening. Toxicol. Sci. 2008;105:106–118. doi: 10.1093/toxsci/kfn114. [DOI] [PubMed] [Google Scholar]
- Reuben M, Louis M, Clarke PBS. Persistent nicotinic blockade by chlorisondamine of noradrenergic neurons in rat brain and cultured PC12 cells. Br. J. Pharmacol. 1998;125:1218–1227. doi: 10.1038/sj.bjp.0702215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TS, Sharma V, Seidler FJ, Slotkin TA. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Dev. Brain Res. 2005;155:71–80. doi: 10.1016/j.devbrainres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Chen CY, Bechtold AG, Tabor JM, Brie JM, Pinkerton KE, et al. Extended second-hand tobacco smoke exposure induces plasticity in nucleus tractus solitarius second-order lung afferent neurons in young guinea pigs. Eur. J. Neurosci. 2008;28:771–781. doi: 10.1111/j.1460-9568.2008.06378.x. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Effects of nicotine on neurogenesis and plasticity of hippocampal neurons. J. Neural Transmission. 2005;112:1475–1478. doi: 10.1007/s00702-005-0370-2. [DOI] [PubMed] [Google Scholar]
- Slikker W, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction — developmental neurotoxicity of nicotine. Crit. Rev. Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol. Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Adverse benzo[a]pyrene effects on neurodifferentiation are altered by other neurotoxicant coexposures: interactions with dexamethasone, chlorpyrifos, or nicotine in PC12 cells. Environ. Health Perspect. 2013;121:825–831. doi: 10.1289/ehp.1306528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ. Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Dev. Brain Res. 2002;133:175–179. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Garofolo MC, Auman JT, McCook EC, Seidler FJ. Perinatal exposure to environmental tobacco smoke induces adenylyl cyclase and alters receptor-mediated signaling in brain and heart of neonatal rats. Brain Res. 2001;898:73–81. doi: 10.1016/s0006-8993(01)02145-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal exposure to environmental tobacco smoke alters cell signaling in a primate model: autonomic receptors and the control of adenylyl cyclase activity in heart and lung. Dev. Brain Res. 2000;124:53–58. doi: 10.1016/s0165-3806(00)00105-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal environmental tobacco smoke exposure in Rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ. Health Perspect. 2006a;114:34–39. doi: 10.1289/ehp.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Tate CA, Seidler FJ. Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke. Brain Res. 2006b;1111:30–35. doi: 10.1016/j.brainres.2006.06.094. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Benzo[a]pyrene impairs neurodifferentiation in PC12 cells. Brain Res. Bull. 2009;80:17–21. doi: 10.1016/j.brainresbull.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol. Appl. Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. San Diego: Academic Press; 1994. pp. 218–224. [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch. Gen. Psychiat. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.