Abstract
Initial observations from as early as the mid-1800s suggested that patients suffering from hematological malignancies would transiently go into remission upon naturally contracting viral infections laid the foundation for the oncolytic virotherapy research field. Since then, research focusing on anticancer oncolytic virotherapy has rapidly evolved. Today, oncolytic viral vectors have been engineered to stimulate and manipulate the host immune system, selectively targeting tumor tissues while sparing non-neoplastic cells. Glioblastoma multiforme, the most common adult primary brain tumor, has a disasterous history. It is one of the most deadly cancers known to humankind. Over the last century our understanding of this disease has grown exponentially. However, the median survival of patients suffering from this disease has only been extended by a few months. Even with the best, most aggressive modern therapeutic approaches available, malignant gliomas are still virtually 100% fatal. Motivated by the desperate need to find effective treatment strategies, more investments have been applied to oncolytic virotherapy preclinical and clinical studies. In this review we will discuss the antiglioma oncolytic virotherapy research field. We will survey its history and the principles laid down to serve as basis for preclinical works. We will also debate the variety of viral vectors used, their clinical applications, the lessons learned from clinical trials and possible future directions.
Keywords: glioblastoma multiforme, malignant glioma, oncolytic virus, virotherapy
Viruses are very small infectious agents composed of two distinct parts: genetic material or viral genome (DNA or RNA) and a protein coat encasing the genetic material (sometimes covered in a lipid envelope). They are strictly intra-cellular organisms that replicate inside host cells, exploiting the host's cellular machinery. In the environment, they exist as inert natural nano-particles, a major agent of evolution by virtue of their capacity to operate as vehicles of horizontal gene transfer [1]. Viruses’ infectivity and tropism are cell- or target-specific. For example, HIV infects helper T lymphocytes, rabies viruses infect neurons and HBV infects hepatocytes, and so on. Moreover, some naturally occurring viruses have been shown to have tropism for tumor cells [2]. Due to the presence of some important and desirable features, such as very small size, ability to transfer genes and ability to specifically target different tissues as well as tumor cells, oncolytic viruses (OVs) emerged as a promising therapeutic arm for cancer treatment.
Malignant gliomas (anaplastic astrocytoma and glioblastoma multiforme [GBM], WHO grades III and IV) are glial-derived malignancies. They represent the most common primary intracranial brain tumors in adults. With a median survival of only 14.6 months following the best available therapeutic interventions, malignant gliomas are one of the most devastating and challenging illnesses of modern times [3]. The current standard of care for patients suffering from this terminal disease involves gross total resection when possible, followed by radiation and chemotherapy. However, the almost 100% failure rate of this regimen poses an urgent need for the development of novel and effective antiglioma therapies. Among many novel experimental therapeutic strategies that are currently being tested against malignant gliomas, OVs are emerging as one of the leading contenders.
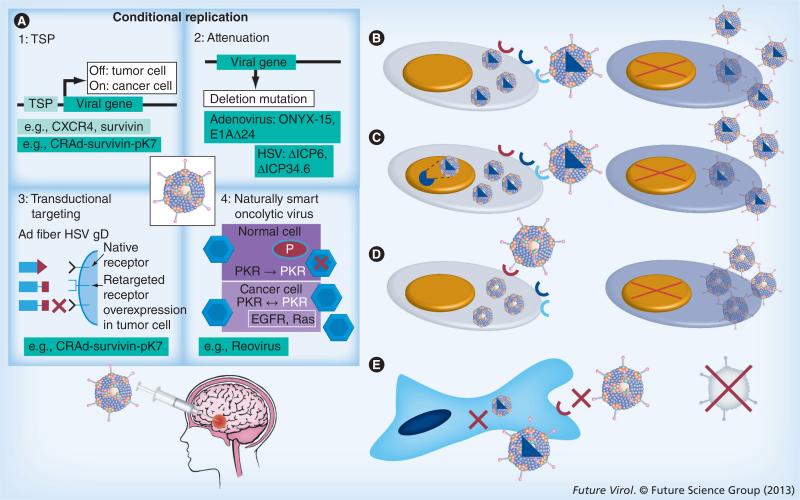
Sine qua non for oncolytic virotherapy is selectively targeting, infecting and destroying tumor cells via conditional replication, while sparing normal cells. Several ways in which OVs kill infected cancer cells have been described. They include rupture of infected cells upon replication and assembly of viral progeny, direct virus-initiated immune-mediated cytotoxicity, indirect killing of the uninfected surrounding cancer cells by initiating an antitumor immune response, direct destruction of tumor blood vessels and selective targeting via transgene-encoded proteins delivered by engineered viruses [4]. OVs can be divided into two categories: viruses with natural tropism for cancer cells, such as H1 autonomously replicating parvoviruses, reo-viruses, Newcastle disease virus (NDV) and mumps virus, among others; and viruses that are genetically engineered to specifically target cancer cells, such as measles, adenovirus, vesicular stomatitis virus (VSV), vaccinia and HSV. The ability to engineer a successful OV takes advantage of the basic principles of their life cycle. There are a number of genetically engineered OVs that are able to specifically target cancer cell through different mechanisms. First, by using the transductional targeting approach, adenoviruses and HSV OVs target tumor-specific surface markers (such as EGF receptor [EGFR], PDGF receptor, HER2/neu, folate receptor, PSA and CD20) for viral cell entry. Second, the transcriptional targeting approach uses tumor-specific nuclear transcription factors (e.g., estrogen receptor, androgen receptor, GATA factors and HIF-1) as essential cofactors for driving viral gene expression. The third tactic uses mutation/deletion-derived OVs, in which manipulation of the viral genome takes place, allowing mutant versions to escape immune-mediated viral clearance and interferon (IFN) sensitivity (Figure 1). Some examples include the deletion of some proteins important for virus replication, such as HSV γ34.5 protein [5], E1 and E3 regions of the adenovirus genome [6], M protein of VSV [7] and NS1 protein of influenza virus [8]. The last method integrates miRNAs into the viral genome to selectively block vector replication in normal tissues [9]. Overall, the antitumor effect of OVs is ultimately a delicate balance between antitumor and antiviral immune responses. Therefore, an ideal oncolytic vector should preferably be injected systemically, and be able to reach the tumor site in appropriate titer concentrations. It should then selectively target and destroy neoplastic cells (primary tumor or distant foci), preferably generating a robust antitumor immune response, which would lead to tumor clearance.
Figure 1. Different targeting strategies to develop tumor-selective oncolytic viruses.
Many oncolytic viruses belonging to several viral families have been engineered. These viruses can be categorized into four major groups on the basis of their oncolytic restriction. (A) Mutation/deletion-derived viruses: this is one of the earliest approaches that takes advantage of tumor-specific changes that allow preferential replication of the virus only in target cancer cells by introducing strategic mutations or deletions in the viral genome, thus inhibiting natural viral replication in the nontargeted normal cells. (B) Transductional-targeted oncolytic viruses: different viruses use different cellular receptors and distinct sequential steps to infect target cells. During tumorigenesis, various genetic and epigenetic events lead to the unique display or overexpression of so-called ‘tumor antigens’ on the surface of tumor cells. As recognition of specific cell surface receptors is the key initial step for virus entry and productive infection, researchers are able to engineer viruses that only recognize and use the antigen present on the tumor cell surface to enter the target cells, which then restricts replication of an oncolytic virus to malignant cells. (C) Transcriptional-targeted oncolytic viruses: achieving tumor-selective replication involves expressing viral genes under promoters that are only functional in tumor cells. (D) All these manipulations will allow oncolytic viruses to target malignant cells, but (E) prevent their ability to infect and replicate in normal cells.
Ad: Adenovirus; EGFR: EGF receptor; gD: Glycoprotein D; TSP: Tissue-specific promoter.
In this review, we will summarize important advances achieved in the field of antiglioma oncolytic virotherapy. We will also survey the main challenges faced by preclinical works that aim to be translated into clinical applications. Although nonreplicative viral vectors can also be used for successful gene therapy, summarizing such a vast literature is beyond the scope of this article. Thus, here we will only focus on replication-competent OVs used in antiglioma therapy.
History of oncolytic virotherapy
The oncolytic virotherapy research field emerged from the repeated astute observation as early as the mid-1800s that patients with hematological malignancies who also naturally acquired a variety of viral infections would undergo temporary remissions [10]. The most consistent message from all these reported observations was that in younger patients with compromised immune systems (suffering from leukemia or lymphoma), certain viruses could destroy tumors, leading to short-lived and incomplete remission without causing undue harm to the patient. The 1950s and 1960s marked an era of heightened interest in anticancer virotherapy due to the development of modern techniques for studying viruses in greater detail both in vivo and in vitro. Some of the earlier studies of anticancer OVs involved HBV, adenoidal–pharyngeal–conjunctival virus, mumps virus, and others. Hoster et al. inoculated 21 patients with Hodgkin's lymphoma with serum and tissue extract from patients suffering from viral hepatitis. Thirteen of the 21 patients developed hepatitis, and seven out of these 13 patients showed clinical remission of Hodgkin's disease [11]. Asada published one of the most optimistic earlier studies, where 90 patients with a variety of terminal cancers were treated with a nonattenuated mumps virus. They reported complete or more than 50% of remission in 30 patients. Forty-two patients experienced either suppression of tumor growth or less than 50% of regression, while 11 patients remained unresponsive [12]. Even though these earlier studies pointed towards a promising therapeutic modality for cancer, in the absence of a field-altering outcome, the oncolytic virotherapy research ground took a back seat at the time.
The 1990s marked the modern era of oncolytic virotherapy with the development of highly potent OVs. By then, viral genomes were engineered to enhance tumor specificity. Martuza et al. published the initial study that laid the foundation and provided the underlying inspiration to the field of engineered oncolytic virotherapy. In this report, the authors treated both glioma cell lines and nude mice bearing intracranial or sub-cutaneous xenografts of malignant glioma with a thymidine kinase-negative mutant of HSV-1 (dlsptk) that was attenuated for neurovirulence. As a result, they observed successful cell death in vitro and prolonged animal survival [13]. Thus, our understanding of virus biology together with the recent advancements in the field of cancer biology lay the foundation for the development of oncolytic virotherapy to target human malignancies. In this section we will briefly summarize the viral vectors used to target human glioblastoma.
Herpes virus
HSV-1 is an enveloped dsDNA virus that causes both lytic and latent infection. The capability of this virus to replicate in both dividing and nondividing cells leading to host cell lysis, its neurotropism and the ease of controlling the infection if necessary with antiherpetic agents make this vector an ideal candidate to target CNS malignancies. Lytic infection with HSV-1 enhances the expression of three distinct genes: α, which inhibits the transporter associated with antigen presentation, leading to decreased expression of MHC I in infected cells; β, which promotes viral DNA synthesis, includes viral DNA polymerase and incorporates both the large subunit of viral ribonucleotide reductase and HSV-tk; and γ, which creates an environment favorable for protein synthesis, contributes to encapsidation of viral DNA and promotes viral envelopment. Neurovirulence of HSV-1 is due to the presence of γ34.5 gene products. G207, a genetically engineered HSV with deletion of the γ34.5 gene at both loci and a disabled insertion of lacZ in UL39, replicates only in rapidly dividing cells but not in quiescent cells. These vectors show minimal virulence even after direct inoculation of high titers into the CNS [14,15]. Since G207 retains thymidine kinase activity, it remains susceptible to anti-herpetic agents like acyclovir. Decreasing the neurovirulence of HSV-1 by γ34.5 gene deletion leads to gross reduction in the ability of the virus to replicate and spread from cell to cell [14]. To overcome this issue, Kambara et al. designed the OV rQNestin34.5, a mutant HSV-1 in which a nestin promoter element drives the expression of γ134.5, leading to increased tumor specificity. This vector showed significantly more potent inhibition of tumor growth when compared with control virus [16]. Since its initial conception, several modifications have been made to the original vector, such as insertion of HSV-encoded cytokines (IL-4, IL-10 and IL-12) [17,18], induction of MHC I expression in infected cells [19] and development of immunostimulatory viruses [20].
Adenovirus
Adenovirus is a nonenveloped DNA virus that is highly immunogenic and causes benign upper respiratory tract infection. The viral genome consists of a 36-kb dsDNA with 10-kb transgene capability. Upon viral infection, the early gene E1A protein binds to pRB, p300 and other related proteins, and forces quiescent cells into S-phase. The E1B region of the viral genome encodes a 55-kDa protein (E1B 55K) that binds and inactivates p53 (a tumor-suppressor gene that is very frequently mutated or deleted in several human cancers). The inactivation of p53 is critical for a successful virus replication, because p53 can terminate virus infection by inducing apoptosis of the infected cells [21]. One of the earliest and most frequently used OVs in clinical trials is an engineered adenovirus, also known as ONYX-015. This virus has an E1B deletion, which restricts viral replication to cells lacking a wild-type p53 protein, such as different human cancer cells. When ONYX-015 infects normal cells containing wild-type p53, it fails to prevent p53-mediated apoptosis, which results in termination of virus replication. Initially, this viral vector received a great deal of attention because a large subset of human cancers was shown to carry the p53 mutation [22]. The therapeutic use of these viral vectors has evolved over time, and throughout the last 20 years, several conditionally replicative oncolytic adenoviral vectors have been designed and tested in clinical and preclinical trials [23–25].
Vaccinia
Vaccinia virus, a double-stranded enveloped lytic DNA poxvirus with a linear genome, is highly immunogenic and replicates within the cytoplasm of the host cell. Its life cycle is very short, lasting on average approximately 8 h. This virus also has a large transgene capacity (28 kb), which allows easy genetic manipulation. In addition, viral infection can be readily controlled by cidofovir or vaccinia immunoglobulin [26]. The major advantage of this virus over other OV vectors is its ability to infect a wide range of cells, which enhances viral targeting to different types of cancer. However, two main limitations for the wide use of this oncolytic vector are its high immunogenicity, as well as the pre-existing antivirus immune response present in the general population. In addition, the inability to manipulate viral replication with transcriptional targeting poses another obstacle to its extensive use in preclinical studies. Nevertheless, such a drawback offers the important advantage of eliminating any risk of integration of the viral genome into the host DNA. Regardless of the limitations described above, vaccinia viruses have proven to be effective oncolytic agents [27].
Reovirus
Reoviruses are RNA viruses with ten segments of dsRNA genome. They are known to cause only mild gastrointestinal or respiratory tract infection in humans. In normal cells, the presence of dsRNA leads to the death of infected cells by activation of PKR, a serine/threonine protein kinase that inhibits protein synthesis, stimulates the release of IFNs and promotes apoptosis. One of the most common oncogenic activations in human malignancies is the Ras–MAPK signaling cascade. Ras activation leads to inhibition of autophosphorylation and therefore the inactivation of PKR, thus allowing viral replication to proceed in Ras-transformed cells. However, in normal cells, viral infection triggers PKR phosphorylation, which in turn leads to inhibition of viral gene translation, cell apoptosis and termination of viral infection. Apart from the natural mechanism present in non-neoplastic cells, reovirus infection can be contained by mycophenolic acid, an immunosuppressive agent [28]. The major disadvantage of this oncolytic vector is its nonalterable ten segments of genome, which renders this virus impossible to engineer for specific mutations [29].
Newcastle disease virus
NDV is an enveloped, nonsegmented, negative-stranded RNA avian virus that is not pathological to humans. Its life cycle is approximately 18 h, and this vector is highly immunogenic. The antineoplastic activity of NDV is due to induction of TNF-α secreted by human peripheral blood mononuclear cells and enhancement of the sensitivity of neoplastic cells to the cytolytic effects of TNF-α [30].
Clinical trials
There are several clinical trials in the malignant glioma research field using a variety of OVs Table 1. The following four oncolytic vectors have been tested in clinical trials of patients with recurrent malignant glioma who have failed conventional therapy, and have shown promising safety profiles.
Table 1.
List of clinical trials of antiglioma oncolytic virotherapy.
| Oncolytic virus | Virus type | Genetic alteration | Total patients (n) | Mode of delivery | Adverse events | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| ONYX-015 (adenovirus) | Serotype 5 dsDNA | E1B gene deletion | 24 | RC: post-tumor resection | None | No significant efficacy observed | [34] |
| G207 (HSV) | ST5 dsDNA | Both copies of φ34.5 deleted, UL39 disrupted | 21 | it. | None | Decreased tumor volume as assessed by MRI in 6/21 patients | [36] |
| G207 (HSV) | ST5 dsDNA | Both copies of φ34.5 deleted, UL39 disrupted | 9 | it. and RC: post-tumor resection | None† | No significant efficacy observed | [38] |
| 1716 (HSV) | Glasgow ST17 dsDNA | Both copies of φ34.5 deleted | 21 | it. | None | 3/21 patients' diseases were stable for 12-15 months | [37] |
| 1716 (HSV) | Glasgow ST17 dsDNA | Both copies of φ34.5 deleted | 21 | it.: 4-9 days prior to resection | None | No significant efficacy observed | [137] |
| 1716 (HSV) | Glasgow ST17 dsDNA | Both copies of φ34.5 deleted | 21 | RC: post-tumor resection | None | 3 responders and 5 with stable disease | [35] |
| Reolysin (reovirus) | Reovirus dsDNA | None | 12 | it. | None | 1/12 long-term survivor (>6 months) | [39] |
| NDV-HUJ (Newcastle disease virus) | ssRNA virus | None | 14 | iv. | None‡ | 3/14 long-term survivors and 1/12 complete response | [45] |
One patient had headache, vomiting, anorexia and fever, possibly due to virus administration.
One patient had delirium, hemiparesis and transient fever after the second dose of oncolytic virus administration.
it: Intratumoral; iv: Intravenous; RC: Resection cavity; ST: Strain.
Adenovirus
Oncolytic adenovirus ONYX-015 is the first and the most studied OV in the field of cancer therapeutics. Its safety, efficacy, mode of delivery and potential for combination therapy has been tested in multiple solid tumors [31–33]. In the context of malignant glioma, Chiocca et al. conducted one of the earliest dose-escalation trials using intracerebral injections of OV ONYX-015 [34]. This study enrolled cohorts of six patients at each dose level, from 107 to 1010 PFU (in tenfold increments), into a total of ten sites within the glioma resection cavity. The authors reported no serious adverse events related to ONYX-015. The median time to progression after treatment was 46 days. The median survival time was 6.2 months. One patient had stable disease and the remaining patients’ diseases showed progression. One out of six recipients at a dose of 109 PFU and two out of six at a dose of 1010 PFU were alive at 19 months follow-up. Taken together, the above results show that injections with up to 1010 PFU of ONYX-015 into the glioma resection cavity are well tolerated by patients.
HSV
Due to their ability to replicate in neural tissues, HSVs are presented as a prime option for targeting malignant gliomas. In the year 2000, two independent Phase I clinical studies evaluated the safety and toxicity profile of HSV in patients with recurrent malignant glioma [35,36]. In the first study, Rampling et al. used the replication-competent mutant 1716 of HSV-1, which lacked both copies of the RL1 gene. This gene encodes the protein ICP34.5, a specific determinant of viral virulence. They treated nine patients with intratumoral inoculation of 105 PFU. They did not observe any induction of encephalitis, adverse clinical symptoms or reactivation of latent HSV. Of the nine patients treated with this regimen, four were reported to be alive 14–24 months after treatment [35]. A subsequent study published by the same group in 2002 demonstrated the efficacy of this virus in a group of 12 patients. They demonstrated that the virus survives, replicates and kills tumor cells by cytolysis when injected into the tumors. Patients in this study received an intratumoral injection of 105 PFU of HSV-1716. At 4–9 days after inoculation all patients underwent tumor resection, which was then examined for evidence of viral replication. In two patients, viruses injected in excess of the input dose were collected from the injection site. HSV DNA was detected by PCR at the sites of inoculation in ten patients. Viral DNA was also identified at distant tumor areas in four individuals. Five patients had changes in levels of IgG and IgM, demonstrating an immunological response to HSV1716 [37]. In the second independent report, Markert et al. conducted a Phase I dose-escalation study, in which G207 was used to treat malignant glioma patients [36]. G207 is a conditionally replicating HSV-1 virus containing mutations in both copies of the γ134.5 genes, as well as a disabling lacZ insertion in UL39, the gene encoding the large subunit of the viral ribonucleotide reductase. The study recruited 21 patients, and dose-escalation treatment started at 106 PFU inoculated at a single enhancing site. The study was completed when the 21st patient was inoculated with 3 × 109 PFU in five different tumor areas. The authors reported no incidence of encephalitis, and four out of 21 patients were alive at the time results were published (mean survival of 12.8 months following inoculation). Six out of 21 patients had a decrease in enhancement at the 1-month follow-up MRI. Specimens from two out of four patients who underwent re-resection on days 56 and 157 after viral inoculation were positive for both HSV-1 and lacZ sequences by PCR. This was a strong indicator that G207 DNA was present in the tumor sample [36]. The follow-up Phase Ib trial by the same group evaluated the safety of multiple injections of this virus in patients with recurrent glioma. They showed that the multiple-dose delivery regimen was safe. This included direct virus inoculation into the brain area surrounding the tumor resection cavity. In this study, six patients received two doses of G207 totaling 1.15 × 109 PFU. The first dose consisted of 13% of the total volume administered via a catheter placed stereotactically in the tumor. The second one enclosed 87% of the total dose, which was injected into the brain area surrounding the resection cavity after en bloc resection of the tumor. This study also reported no incidence of encephalitis. The median survival was approximately 6.6 months from initial treatment. PCR analysis confirmed infection of the neoplastic tissues with G207 in all samples from all patients. Viral replication was noted in the last three patients [38].
Reovirus
In a Phase I trial, reovirus was administered intratumorally in patients with malignant glioma at 107, 108 or 109 50% tissue culture infectious doses. The virus was well tolerated in all 12 treated patients, with no adverse events. Ten patients had tumor progression, one had stabilization and one was not evaluable for response. The median survival was 21 weeks (range: 6–234 weeks). The median time to progression was 4.3 weeks (range: 2.6–39 weeks) [39].
Newcastle disease virus
Multiple strains of NDV have been evaluated in different human cancers [40–43]. In the setting of gliomas, Csatary et al. published a case series of four patients with recurrent GBM treated with MTH-68/H, a live-attenuated strain of NDV. They reported survival rates of 5–9 years [44]. A Phase I/II clinical trial using intravenous administration of 1010 IU/injection of NDV-HUJ found it to be safe in 11 patients with recurrent malignant glioma. One patient achieved complete response. Anti-NDV hemagglutinin antibodies appeared within 5–29 days post-initial treatment. Analysis of blood, saliva and urine showed the presence of NDV-HUJ in all samples. However, only one tumor biopsy sample was positive for the presence of the virus [45].
Preclinical–clinical dichotomy
Preclinical work in the field of oncolytic viro-therapy proposed a very lofty goal of designing highly targeted and potent natural pathogens (i.e., OVs) that could selectively kill all tumor cells in an organism, without damaging normal tissues. Such a particle should also activate the host immune memory, which would offer protection against any future recurrence. However, the results from clinical trials both in the field of GBM and all other malignancies fell short of this expectation. Even though all OVs so far tested in clinical trials have proven to be safe, the efficacy of these vectors remained elusive. In the context of GBM, none of these OVs clearly demonstrated therapeutic efficacy. On the other hand, in the context of other solid tumors, two viral vectors, OncoVEX (metastatic melanoma) and JX-594 (hepatocellular carcinoma), tested in recent Phase I/II studies showed initial evidence of viral efficacy [46,47]. Why did all of the clinical trials fall short of their goal? There are several factors that contribute to this preclinical–clinical dichotomy.
First, although well validated in animal studies [48], direct lysis of infected cells as a mechanism of successful tumor destruction is an observation that still needs to be proven in humans. Based on this discrepancy, it has become clear that more reliably predictive preclinical models are needed. The majority of preclinical works in the field of oncolytic virotherapy have been performed in immunodeficient nude mice implanted with human-derived malignant xenografts. Such a model completely ignores one of the major players for the development of an effective anti-cancer virotherapy: the patient immune system. Therefore, the oncolytic virotherapy research field needs novel orthotopic cancer models in immunocompetent animals, in which species-specific OVs could be employed in order to mirror the immune response of the viral infection. The use of OVs such as VSV or vaccinia, which can infect both human and mouse cells with similar efficiency, represents one of the possible solutions. Recombinant vaccinia virus JX-594 expressing GM-CSF significantly inhibited tumor growth and prolonged survival in rats bearing RG2 intracranial tumors and mice bearing GL261 brain tumors. This effect was further enhanced by the combination therapy of JX-594 with rapamycin [49]. Naturally developed glioma in canines is another attractive model to evaluate experimental oncoytic virotherapy [50]. Preclinical testing of adenoviral vectors encoding immunostimulatory cytokines, such as hsFlt3L, and suicide gene systems, such as HSV-tk, are conducted in order to prime the canine APCs to generate antiglioma immunity. These studies provided a fair justification for the evaluation of this strategy in canine GBM before translating to human clinical trials [51].
Second, for designing a successful OV regimen, it is very important to monitor and follow intratumoral viral spread. This can be easily accomplished in animal models by post-mortem analysis of the biodistribution of virus-infected cells at different time points. However, it is a challenging endeavor in the clinical setting. Failure of clinical trials has been partially attributed to nonspecificity of the viral vector [45] and failure of the OV to efficiently replicate and infect surrounding tissues [38]. Such outcomes could be conclusively monitored by the presence of a noninvasive virus tracking system. This observation led to the development of OVs with reporter genes implanted in their genome, which would facilitate the repetitive and noninvasive tracking of virus-infected cells in the host body [52]. The study of a number of OVs with reporter genes in several solid tumors has demonstrated very optimistic preclinical results. However, this approach still needs to be validated in the clinical setting [53–56]. In the context of GBM, in a Phase I/II trial, Jacobs et al. used PET with I-124-labeled 2’-fluoro-2’-deoxy-1β-D-arabinofuranosyl-5-iodo-uracil, a specific marker substrate for gene expression of HSV-1-tk, to identify the location, magnitude and extent of the vector-mediated HSV-1-tk gene. This report was important to illustrate that an exogenous gene introduced by gene therapy could be monitored noninvasively by imaging devices [57]. The art of designing OVs with reporter genes is still in its infancy. It needs better fine-tuning in order to increase the sensitivity and specificity of viral tracking. This would finally allow a reliable distinction between virus-infected versus noninfected cells.
Third, when it comes to oncolytic virotherapy it is very important to account for the immune system. This is particularly true because, during the course of evolution, mammalian cells have evolved to resist viral infections. One of the key factors for a successful viral infection is the presence of virulence proteins, which are encoded by the viral genome in order to suppress the host immune defense, facilitate virus spread between cells and seize the host cell's metabolic activities. Usually, this process triggers a counterattack by the host cell via expression of antiviral proteins. The virus, in turn, maintains a continued retaliation until either the virus wins or gets cleared by the host immune system. The antiviral response of normal cells involves jump-starting apoptotic programs in the infected cells [58], which blocks the production of the viral progeny and thus spread to adjacent cells [59], also triggering the production of proteins that counteract virus infection. One of the most important participants in the antiviral immune response is type I IFN, which reprograms the physiological properties of infected and surrounding cells, induces cell cycle arrest, provides antiangiogenic signals, promotes apoptosis, inhibits protein synthesis and activates the immune response [60]. Virulence genes are specifically deleted or mutated in conditionally replicative OVs to decrease their off-target toxicity, and spare normal tissues from viral infection [13,61]. Just like wild-type viruses, OVs entering a normal cell trigger the cellular antiviral response. However, due to their absence, virulence proteins cannot counterattack, leading to quick elimination of the infection. Type I IFNs are a group of proteins that are essential for a normal cell's first line of defense against viral infection [62]. When infection does occur, most cells induce expression and secretion of type I IFNs. These secreted cytokines bind to IFN receptors to activate downstream transcription factors STAT1 and STAT2 to form a heterotrimeric complex that translocates to the infected cell nucleus and binds to IFN-stimulated responsive elements to induce hundreds of IFN-stimulating genes, including some antiviral genes. Oncolytic vectors such as reovirus can lead to the activation of dsRNA-activated PKR, as well as the subsequent activation of the IFN pathway. However, transformed cells have inactivated gene products that control both cell growth and death programs and are often unresponsive to type I IFNs. Tumor cells harboring an activated Ras oncogene or hyperactive EGFR fail to activate PKR upon viral infection, thus becoming susceptible to OV infection. These IFN-nonresponsive cancer cells may have acquired a growth/survival advantage over their normal counterparts, but they simultaneously compromised their antiviral response, making them good targets for OVs, despite the presence of defective virulence genes [63]. Current research has been extremely successful in containing the aspect of virulence associated with oncolytic virotherapy. Every OV that has been tested in clinical trials has shown an excellent safety profile. Nevertheless, in our quest for safety we may have compromised on viral potency, since deleting or manipulating virulence genes also decreases the virus's ability to replicate and infect. At this point, as researchers have sufficient understanding of viral infection and immune system interaction, they have started to incorporate previously attenuated or deleted virulence genes under strict control in order to fine-tune and enhance the therapeutic efficacy of OVs [64].
Lessons learned
OVs & tumor selectivity
Four ways of increasing OV specificity include translational targeting, transcriptional targeting, transductional targeting and miRNA targeting (Figure 1). Replication-competent dlsptk, the first engineered OV of the modern era, uses translational targeting. In this case, deletion of thymidine kinase attenuated neurovirulence of this OV, without significantly altering its replication ability in the context of glioma [13]. One way of controlling viral protein translation is by altering the internal ribosome entry site element of the OV [65]. For example, replacing the poliovirus internal ribosome entry site with an internal ribosome entry site element from human rhinovirus type 2 has proven to attenuate the neurotoxicity of PVS-RIPO [66]. Oncolytic vectors can transcriptionally target tumor cells by taking advantage of tumor-specific promoter elements (several of which have been identified) [67]. HSV, by using albumin promoter enhancer in hepatoma cell lines, or adenovirus, by using prostate-specific antigen promoter in prostate cancer cell lines, are examples of oncolytic vectors driven by tumor-specific promoters [68,69]. Nestin, hTERT, E2F1, CXCR4, midkine, survivin and others are some of the glioma-specific promoters that can be used for the transcriptional control of OVs [70]. CRAd-Survivin-pk7, a chimeric vector containing a pk7 fiber modification and a surviving promoter driving E1A replication in neoplastic cells, was shown to inhibit tumor growth by more than 300%. In addition, 67% of the mice bearing U87MG glioma xenografts exhibited long-term survival (>120 days) [71]. Recently, researchers successfully produced modified viral vectors by combining the two approaches described above. They were able to engineer OVs that utilize both transcriptional and translational targeting in order to increase vector specificity [72,73]. Transductional targeting was shown to be a viable option for viruses that replicate entirely in the cytoplasm of host cells. Since these vectors regulate their transcription independently of the host cell nuclear machinery, they are not amenable to transcriptional targeting. For instance, VSV pseudotyped with the non-neurotropic envelope glycoprotein of the lymphocytic choriomeningitis virus showed enhanced infectivity for brain cancer cells in vitro, while sparing primary human and rat neurons both in vitro and in vivo [74]. Even though transcriptional and transductional targeting was shown to increase the specificity of oncolytic virotherapy, they have also been associated with potential off-target effects. miRNA targeting to OVs, in turn, has been described as an emerging strategy for increasing viral specificity [75,76]. It takes advantage of the differential expression of certain miRNA species in tumors and normal tissues. Kelly et al. demonstrated that neurotoxicity of the wild-type VSV could be ameliorated in VSV clones carrying four tandem copies of a neuronal miR-125 target sequence inserted in the 3′-untranslated region (UTR) of the viral polymerase (L) gene. Such a strategy has been shown to not compromise antitumor efficacy [77]. In addition, the inclusion of binding sites for the hepatocyte-selective miRNA miR-122 within the 3′-UTR of the E1A transcription cassette in Ad5, which is naturally toxic to hepatocytes, eliminated its hepatotoxicity without destroying the tumor cell-killing activity [78]. Similar results were seen with the coxsackievirus A21, where inclusion of muscle-specific miRNAs into the 3′-UTR of the virus eliminated muscle toxicity without compromising its anticancer activity [79]. A potential issue with this approach is that some miRNA targets can mutate during viral replication. Therefore, it may be prudent to use a second strategy to minimize the chances of toxic escape variants that might arise during therapy. Telomerase-specific replication-competent adenovirus, which possesses an E1 gene expression cassette (also expressed in some normal cells) driven by the human telomerase reverse transcriptase promoter, has shown promising results in human clinical trials, with some associated toxicity in normal cells. The incorporation of binding sites for miR-143, −145 or −199a into the 3′-UTR of the E1 gene expression cassette resulted in efficient oncolytic activity comparable to the wild-type telomerase-specific replication-competent adenovirus in tumor cells. Such an approach has also shown significantly improved safety profiles [80].
OVs & potency
Creating a perfect marriage between viruses and a complementary antitumor mechanism could enhance the potency of oncolytic vectors. In this relationship, each partner would have their own unique arsenal of weapons and attenuating mutations, but the combination of both would be able to complement each one's deficits. Following this rationale, Le Boeuf et al. combined a double-deleted vaccinia virus with VSV, a rapidly replicating RNA virus that is exquisitely sensitive to type I IFN. The results showed efficient replication in refractory tumor cells when they were coinfected [81]. Double-deleted vaccinia virus is an interesting genetically modified vaccinia virus. It consists of a DNA virus with deletions in the virally encoded tk and VGF genes, which restricts the viral replication only to tumor cells that overexpress E2F and have activated EGFR pathways. This mutated virus expresses the gene product B18R, which locally antagonizes the effects of type I IFNs. In this study, the combination of both viruses showed itself to be a safe option, since VSV is an RNA virus and vaccinia is a DNA virus. Moreover, neurotoxicity of the VSV can be attenuated by miRNA-targeting strategies, as described in the previous section [77]. Therefore, an exchange of virulence genes leading to a pathogenic ‘supervirus’ would seem improbable. This will increase the repertoire of available viruses that can be used for such a complementary strategy [82–84]. However, the safety of such therapeutic approaches must be evaluated thoroughly in the orthotopic glioma xenograft model.
The potency of OVs can be increased without compromising their specificity. This can be achieved by combining small-molecule inhibitors with OVs in order to eliminate any residual IFN responsiveness of tumor cells. One such group of small-molecule inhibitors is the histone deacetylase inhibitors (HDIs), known to influence the epigenetic modifications of chromatin. HDIs were shown to effectively blunt the cellular antiviral response. When combined with VSV, HDIs showed marked enhancement of viral spread in a variety of cancer cells in vitro, in primary tumor tissue explants and in multiple animal models [85]. Valproic acid, a commonly used antiepileptic agent with HDI activity, when used as a pretreatment for glioma cell lines, was shown to inhibit the induction of several IFN-responsive antiviral genes. It was also shown to augment the transcriptional level of viral genes and improve viral propagation, even in the presence of type I IFNs. Moreover, valproic acid pretreatment improved the propagation and therapeutic efficacy of oncolytic HSV in a human glioma xenograft model in vivo [86]. Several other small molecules that are able to enhance OV growth in refractory tumor cells have been identified via high-throughput screening. However, one has to keep in mind that the use of HDIs to dampen immune responses during OV infection can be risky due to the possibility of increased susceptibility to infection and spread in the absence of an intact host immune system. Available experimental evidence would argue that the immunosuppressive effects of HDIs on OV-mediated oncolysis are specific to tumor cells and that they do not reduce replication selectivity of the OV against the normal tissue. Moreover, such effects on the host immune system are reported to be reversible: the removal of HDI treatment halted OV replication, while reinitiating the HDI treatment resulted in the resumption of OV replication within the tumor mass [85]. Thus, HDIs can be utilized as a chemical switch to regulate antiviral innate immunity during oncolytic virotherapy, which will allow the therapeutic virus to propagate more effectively within the tumor mass and provide a powerful means to enhance the therapeutic efficacy.
OVs & intratumoral spread
In order to achieve a robust clinical response, it is absolutely essential for a viral vector to homogeneously spread within the tumor. One way of accomplishing this is by using OVs that are naturally well equipped to spread within cells. Examples include extracellular enveloped poxviruses, in which the extracellular envelope facilitates widespread viral dissemination and helps it to avoid neutralization by circulating antibodies [87], the actin tail of the vaccinia virus, which helps to propel the virus into adjacent tumor cells [88], and the NV1020 mutant of HSV-1, which increases virus dissemination by promoting the fusion of cancer cells [89]. Another strategy to improve intratumoral OV spread is by decreasing the intratumoral connective tissue network, which can be achieved by cytotoxic agents and radiation [90]. Following this rationale, the dissociation of tumor extracellular matrix by intratumoral coadministration of hyaluronidase in mice bearing malignant xenografts has been shown to improve the antitumor activity of an oncolytic adenovirus. In addition, the application of this same approach in mice with melanoma xenografts treated with OV AdwtRGD-PH20, a replication-competent adenovirus expressing a soluble form of the human sperm hyaluronidase (PH20) under the control of the major late promoter, revealed enhanced viral distribution and tumor regression in all treated animals [91]. Losartan, an angiotensin II receptor antagonist, inhibits collagen I production by carcinoma-associated fibroblasts, which leads to a dose-dependent reduction in stromal collagen. Such a treatment was shown to improve the distribution and therapeutic efficacy of intratumorally injected oncolytic HSVs. It was also shown to enhance the efficacy of intravenously injected pegylated liposomal doxorubicin [92].
Hypoxia is an integral component of solid tumors, and the ability of OVs to replicate in hypoxic conditions is one of the critical determinant factors for the successful translation of the antiglioma oncolytic virotherapy in the clinic. Histologically, GBM is characterized by aberrant vasculature and a rapidly dividing tumor mass that initiates the establishment of a hypoxic microenvironment (PO2 = 5 mmHg) [93]. Hypoxia has been thought, at least in part, to contribute to increased tumor invasion, loss of apoptosis and resistance to conventional radiotherapy and chemotherapy [93]. The presence of hypoxia in gliomas is associated with recurrence of the disease and decreases in patient survival. Thus, it is critical to understand how the therapeutic efficacy of the oncolytic virotherapy might be affected by such a tumor microenvironment as compared with the natural milieu of the virus. It has been reported that hypoxia inhibits adenoviral replication by reducing the translation of the replication-essential adenoviral gene E1A and fiber, a structural gene [94,95]. However, another study reported contradictory results with regards to the ability of oncolytic adenovectors to replicate in hypoxic conditions [95]. Similar conflicting results also exist for the VSV vectors [96,97]. In the glioma model, oncolytic HSV G207 exhibited enhanced replication in hypoxic environments, which is mediated by a GADD34-dependent pathway [98]. A recent report also showed that another oncolytic vector, G47Δ, could effectively target conventional therapy-resistant hypoxia-induced glioma stem cells [99]. This suggests that HSV-based oncolytic vectors represent a promising therapeutic strategy to target hypoxic tumor-like glioblastoma.
OVs & delivery
There are two major ways of delivering OVs to neoplastic cells in vivo: intratumoral delivery or systemic delivery. Most oncolytic vectors that are being tested in clinical trials are delivered intratumorally. However, since in most solid tumors the natural progression of the disease leads to metastatic spread, systemic delivery has been the mainstay for the treatment of metastatic cancers. There are several drawbacks that are specific to systemic delivery, such as sequestration of the OV in the liver and spleen, and off-target toxicity due to systemic viral circulation. On the other hand, there are also some important benefits, such as the ability of the OV to evade neutralization by serum factors, which facilitates viral extravasations into the tumor tissue, and selectively enhances tumor vessel permeability. Malignant gliomas are an exception in this category, in the sense that systemic metastasis is very infrequent. In this case, most distant neoplastic foci are within the CNS. Here, the blood–brain barrier represents an added layer of protection against OVs that try to reach the brain from the systemic route. Therefore, for gliomas, intratumoral delivery is the most logical and effective delivery method. Even though there is a tremendous amount of research going on in the field of systemic delivery of OVs, for the purposes of this review, we will focus on intratumoral delivery.
One of the main reasons why oncolytic virotherapy failed to deliver its promised therapeutic efficacy, even when it was delivered locally into the tumor cavity, was the in situ premature viral inactivation by the host immune system. One way to overcome this issue is by hiding OVs inside cell carriers. In the context of multiple myeloma, a hematological malignancy, lethally irradiated myeloma cells infected with an oncolytic measles virus were tested as cellular carriers. Researchers observed significantly increased animal survival when these loaded vehicles were administered intravenously to myeloma-bearing mice together with protective titers of antimeasles antibodies [100]. Other cells that have been successfully evaluated as systemic carriers for OVs include dendritic cells and T cells [101–103]. Two cell types that have shown promising preclinical results as carriers for antiglioma OVs are mesenchymal stem cells (MSCs) [104] and neural stem cells (NSCs) [105]. MSCs possess a natural tropism for primary tumors and their metastases, and are also considered immune privileged. NSCs hold immunosuppressive properties as well as specific tropism for tumor cells. Given the very robust preclinical safety profile of MSCs [104,106], they were evaluated in a small clinical trial of children with metastatic neuroblastomas. Four children with metastatic neuroblastoma refractory to frontline therapies received several doses of autologous MSCs carrying ICOVIR-5 (oncolytic adenovirus). The treatment was well tolerated, and no significant systemic toxicity was observed. One out of four patients showed satisfactory clinical response [107].
Our group has performed extensive preclinical studies with the goal of evaluating cellular carriers for the effective delivery of OVs to malignant gliomas. To enhance and prolong the infectivity of OVs delivered intratumorally, Sonabend et al. tested CRAd-CXCR4-5/3 viruses loaded into human MSCs (hMSCs) in the setting of malignant glioma. The authors showed that virus-loaded hMSCs effectively migrated in vitro, and released CRAds that infected U87MG glioma cells. When injected away from the primary tumor site in vivo, hMSCs migrated to the neoplastic area and delivered 46-fold more viral copies than injection of CRAd-CXCR4-5/3 alone [108]. Using the semipermissive cotton rat model, Ahmed et al. studied the immune response to adenovirus-loaded MSCs. They showed that cotton rat MSCs enhanced the dissemination and persistence of adenoviruses as compared with virus injection alone [109]. NSC-based carriers have the potential to improve the clinical efficacy of antiglioma virotherapy by not only protecting therapeutic viruses from the host immune system, but also by amplifying the therapeutic payload selectively at tumor sites. A study by Tyler et al. published at a similar time evaluated the role of NSCs as cellular carriers of OVs. Using in vitro and in vivo migration studies, they showed that NSCs infected with CRAd-S-pk7 viruses migrate and preferentially deliver CRAds to U87MG gliomas [110]. Intratumoral delivery of NSCs loaded with the CRAd-S-pk7 in an orthotopic xenograft model of human glioma not only inhibited tumor growth, but, more importantly, also increased median survival by approximately 50%. In addition, OV infection significantly enhanced the migratory capacity of NSCs both in vitro and in vivo [111]. A subsequent study comparing MSCs and NSCs as cellular carriers for delivering OVs to malignant gliomas found that, despite possessing a comparable migratory capacity, NSCs display superior therapeutic efficacy in the context of intracranial tumors [112]. Based on these promising preclinical findings, the US FDA recently approved the NSC HB1.F3-CD as a cell carrier in clinical trials. Our preclinical studies using this vehicle loaded with CRAd-S-pk7 show that NSCs migrate and release infectious CRAd-S-pk7 progeny capable of lysing different glioma cell lines. Ex vivo-loaded NSCs intracranially injected in nude mice bearing human-derived glioma xenografts retained their tumor tropism [8], continued to replicate CRAd-S-pk7 for more than 1 week after reaching the tumor site and successfully handed off CRAd-S-pk7 to glioma cells in vivo [113]. In patients with recurrent malignant gliomas, the first human Phase I study of genetically modified NSC-based therapy carrying a suicide gene, cytosine deaminase, is currently underway (NCT01172964).
OVs & the immune system
In general, replicative viruses and the immune system are very intricately related, since the host immune response is primed and equipped to fight viral infections. One of the important lessons that can be presumed by looking at the history of oncolytic virotherapy is that systemically immunosuppressed patients generally respond better to OVs than those with an intact immune response. On the other hand, these immunosuppressed patients also experience higher viral toxicity due to unrestrained infection [10]. This can be attributed to the fact that, in the presence of an impaired adaptive immune system, the virus has ample time to replicate and spread without being attacked by an immune response. In this setting, one of the ways of decreasing viral toxicity without compromising the beneficial effect of the impaired adaptive antiviral immune response is to increase viral specificity to tumors. Thus, it is a reasonable strategy to combine immunosuppressive drugs with the new generation of tumor-specific OVs. To our advantage, immunosuppression is the most common side effect of most chemotherapeutic drugs like temozolomide (TMZ), methotrexate and cyclophosphamide (CPA) [114]. One chemotherapeutic agent that has shown promising preclinical results when combined with OVs is CPA. These exciting results are mostly due to the drug's toxic effect on both T and B lymphocytes [115]. In the context of malignant gliomas, a recent study showed that animals pretreated with CPA, and then treated with HSV-derived OVs, lived significantly longer as compared with the ones that received HSV OV alone. This increased survival was correlated with enhanced HSV replication, virus-related oncolysis, reduced HSV-mediated expansion of CD68+ and CD163+ cells and decreased intratumoral expression of IFN-γ in mice that received the combined therapy [116]. Another major player in the interplay between OVs and the immune system is the tumor itself. Tumors in general, but more specifically GBM, actively manipulate the local microenvironment by producing immunosuppressive cytokines (e.g., TGF-β), and recruiting immune-inhibitory cells (e.g., Tregs) in order to paralyze the antitumor immune response [117]. This local immune suppression can be overcome by selective infection of the tumor with OVs, which leads to a localized inflammatory response and robust antitumor immunity [118]. In addition, virus-mediated delivery of genes that encode immune-stimulating cytokines, like GMCSF and IL-2, has shown a good safety profile in clinical trials [119,120]. One of the oncolytic viral vectors that combines oncolysis with immune activation is OncoVEX, a HSV vector encoding GM-CSF that was tested in a Phase II study of patients with metastatic melanoma. This Phase II study showed that direct injection of OncoVEX led to a 28% objective response rate. Responding patients demonstrated regression of both injected and noninjected lesions. This finding highlights the dual mechanism of action that includes both a direct oncolytic effect in injected tumors and a secondary immune-mediated antitumor effect on noninjected tumors. In this report, the antitumor immune response was associated with a decrease in the number of both Tregs and myeloid-derived suppressive cells in patients exhibiting therapeutic responses [121]. Based on these results, a Phase III trial using this OV recently reached its primary end point on 20 March 2013, and the final results will most likely be published by the end of the year or early next year [122]. Vaccinia virus JX-594 is another oncolytic vector engineered to express GM-CSF. When tested in the setting of hepatocellular carcinoma, the treatment showed an objective response in three out of ten patients [47]. A study that very creatively combined several antitumor strategies was published by Kottke et al. In this report, the authors adoptively transferred OT-1 antigen-specific T cells loaded with oncolytic VSV into immune-competent mice bearing B16 melanoma. These mice were preconditioned with Treg depletion and IL-2. As a result, the combination therapy led to a highly significant increase in antitumor efficacy, with no detectable toxicity [123]. The delicate interplay of tumor, virus and immune response requires a careful orchestration of the following events. First, initial immunosuppression needs to be achieved so that the viral vector can have enough time to replicate and infect the entire tumor mass. The second step encloses the activation of the immune system in order to achieve virus-mediated tumor destruction, with immune recognition of tumor-specific antigens. However, in the context of glioma, the situation is slightly different, since the emphasis is on local delivery (due to the lack of systemic metastases and the presence of a highly restrictive blood–brain barrier) and local viral spread with tumor-specific immune responses. Therefore, an ideal vector in this setting should be delivered locally, should take advantage of the immunosuppressive microenvironment of glioma to replicate, should infect cells without being detected by the host immune system and should be able to generate a potent antitumor immune response upon cytolysis.
OVs & combination therapy
The wide variety of currently available anticancer therapies offers endless possibilities for the use of one or more therapeutic combinations. However, the shortest route to the clinic for this approach seems to be combining an OV with a pre-existing standard-of-care chemotherapy or radiation treatment. Radiation and TMZ are the current standard of care for patients with malignant glioma. In subcutaneous xenograft models of glioma, both adenoviral vectors AdΔ24-p53 and AdΔ24 showed increased tumor regression and extended animal survival when combined with radiation [124]. Ad5-Δ24RGD, when combined with radiation, showed enhanced tumor destruction both in vitro and in vivo [125]. In addition, ONYX-015, when also combined with radiation, substantially increased radiation-mediated toxicity to tumor cells [126]. Taken together, these results demonstrate the synergistic effect of OVs with radiation therapy. Nonetheless, in a more recent study, when Ad5-Δ24RGD was tested in an orthotopic model of glioma in combination with radiation, the results were disappointing [127]. This brings us back to the importance of having more accurate preclinical models for testing the efficacy of OVs. Our group has shown that radiation increases survivin mRNA activity several-fold. Moreover, survivin-driven adenovirus CRAd-survivin-pk7, when combined with radiation, has demonstrated a markedly enhanced antitumor activity [128]. The strategy of combining OVs with radiation is currently being tested in a clinical trial of glioma involving the OV G207 with LacZ+ modification.
Chemotherapeutic drugs are the frontline treatment for all malignancies. Therefore, it is very important to verify the compatibility of this strategy with oncolytic virotherapy. In an ideal situation, these two modalities should have a synergistic effect. CPA, an antineoplastic alkylating agent in clinical use since 1959, is known to dampen antiviral innate immune response and decrease the levels of Tregs [129]. The combination of CPA with reovirus in a melanoma model showed high levels of intratumoral viral access and replication [130]. When this combination was further enhanced with the addition of IL-2, the antitumor effect of reovirus was further potentiated [131]. In both syngeneic and athymic rat models of intracranial malignant glioma treated with HSV-1, CPA administration has led to a time-dependent increase in infection of tumor cells. CPA-mediated toxicity was not due to a direct enhancement of viral replication in tumor cells. It was instead associated with drug-related immunosuppressive effects. As a result, the combination treatment increased the survival of OVs not only within infected tumor cells, but also in normal brain tissues surrounding the tumor [132]. However, the replicative alphaviral vector VA7, which has been shown to be effective against orthotopic human U87-glioma xenografts in an athymic mouse model, when tested in immuno-competent orthotopic GL261 and CT-2A glioma models of C57BL/6 mice in vivo, was found to be ineffective even when pretreated by the administration of CPA and rapamycin [133]. TMZ, another DNA-alkylating agent, is the standard of care for patients with malignant glioma. Its efficiency against glioma depends on the status of the DNA repair enzyme MGMT. In a study by Aghi et al., U87 human glioma cells transfected with or without a gene enhancing MGMT expression were treated with HSV vector G207 and/or TMZ. Treated cells showed variation in TMZ-induced DNA repair pathways with MGMT expression. The authors also observed an enhanced HSV-mediated oncolysis in glioma cells with the combination therapy. These results unveiled the potential of HSV to target cells surviving TMZ treatment [134]. In addition, the oncolytic AdΔ24-RGD, when combined with TMZ, was shown to overcome the reported MGMT-mediated resistance, and induce a profound therapeutic synergy in glioma cells [135]. Athymic mice bearing glioma xenografts treated with the combination of ICOVIR-5, radiation and TMZ showed a potent antiglioma effect, resulting in a dramatic extension of median survival. Approximately 20–40% of the animals were disease free at 90 days post-initial therapy [136]. As demonstrated by some of the preclinical trials, there is a potential risk of increased toxicity in combining OVs with chemotherapeutic agents [131]. However, the possible risks and benefits of this strategy still need to be carefully assessed in the clinical setting. There are numerous ongoing clinical trials using this combined strategy to treat a variety of malignancies. Regarding malignant gliomas, clinicals trial remain to be conducted for oncolytic adenovirus treated with combinations of conventional radiotherapy and chemotherapy.
Conclusion & future perspective
The field of oncolytic virotherapy has come a long way since its inception. Clinicians were treating cancer patients with OVs long before the discovery of the virus [10]. New-generation OVs are highly specific and safe, and incorporate multiple tumoricidal mechanisms in one vector. For example, a single viral vector can, simultaneously, cause oncolysis and stimulate antitumor immune responses. GBM is unique in the sense that most of the time it is confined within the CNS. It hides behind an ultraselective blood–brain barrier and actively modulates antitumor immune responses to its advantage. In the arena of malignant glioma, several viral vectors have shown very robust preclinical safety and efficacy, as well as an excellent safety profiles in the clinical setting. However, preclinical efficacy has so far not been translated into clinical practice. As previously discussed, the outcome of a successful oncolytic virotherapy is contingent on a complex interplay of several interconnected factors, such as the virus itself, antiviral and antitumor host immune responses and the tumor microenvironment.
An ideal oncolytic vector for the treatment of malignant glioma should be delivered locally. When intratumorally injected, it should be able to specifically target all tumor cells, in both local and distant intracranial foci. It must effectively travel within the CNS to reach distant invasive areas, mount an effective antitumor immune response and then be effectively cleared by the host immune system. Moreover, it should also have a synergistic effect with conventional treatment modalities, such as chemotherapy and radiation therapy. In an ideal situation, an antiglioma OV should be loaded into stem cell carriers, which would enhance viral tropism towards glioma cells while hiding viruses from the host immune response. Such a therapeutic package should be delivered locally after surgery or stereotactic biopsy. After being released from the cell carrier, OVs would effectively find and infect tumor cells. Viral replication would be driven by a tumor-specific promoter, which would also encode for immune-stimulatory cytokines, being sensitive to an antiviral agent. This may sound like a daunting task for one single vector. However, this limitation could be overcome by designing customized cocktails including several vectors, which would possess complementary features depending on specific glioma subtypes.
Several of the new-generation OVs with multimodal oncolytic features are currently undergoing clinical testing in a wide variety of malignancies. Previous clinical trials have already proven that oncolytic virotherapy is a safe option for anticancer treatment. Hopefully, the results of most of the ongoing trials may prove the efficacy of these viral vectors in the clinical setting. Today, approximately 20 years since the first engineered OV showed promising results for the treatment of GBM, neither the field of oncolytic virotherapy nor antiglioma therapy has reached the lofty goals they set for themselves. However, research from the past two decades has clearly set the stage for a successful story in the making.
Executive summary.
Glioblastoma multiforme
■ Glioblastoma multiforme (GBM) is unique malignancy in the sense that it is confined within the CNS. It hides behind an ultraselective blood–brain barrier and actively modulates the antitumor immune response to its advantage.
■ In the arena of GBM, several viral vectors have shown very robust preclinical safety and efficacy, as well as an excellent safety profile in the clinical setting.
■ However, preclinical efficacy has so far not been translated into clinical practice.
Oncolytic virotherapy for GBM
■ The success of any antiglioma oncolytic virotherapy will rely on a delicate interplay of tumor, virus and immune response.
Host immune system
■ In general, replicative viruses and the immune system are very intricately related, since the host immune response is primed and equipped to fight viral infections.
■ One of the main reasons why oncolytic virotherapy failed to deliver the promised therapeutic efficacy is the in situ premature viral inactivation by the host immune system.
■ Systemically immunosuppressed patients generally respond better to oncolytic viruses (OVs) than those with an intact immune response.
Clinically relevant animal models
■ The majority of preclinical work in the field of oncolytic virotherapy has been performed in immunodeficient nude mice implanted with human-derived GBM xenografts.
■ Such a model completely ignores one of the major players in the development of an effective anticancer virotherapy: the patient's immune system.
■ The oncolytic virotherapy research field needs novel orthotopic cancer models in immunocompetent animals, in which species-specific OVs could be employed in order to mirror the clinical scenario.
Intratumoral viral spread
■ In order to achieve a robust clinical response, it is absolutely essential for a viral vector to homogeneously spread within the tumor.
■ The intratumoral microenvironment, such as hypoxia or the connective tissue network, can act as a barrier to therapeutic viral distribution and reduce the therapeutic efficacy of the antiglioma OV therapy.
Noninvasive clinical monitoring of viral replication
■ When designing a successful OV regimen, it is very important to monitor and follow intratumoral viral replication and spread.
■ The failure of clinical trials has been partially attributed to nonspecificity of the viral vector and the failure of the OV to efficiently replicate and infect surrounding tissues.
■ Such outcomes must be conclusively monitored by the presence of a noninvasive virus-tracking system, which will allow us to adjust dosing of the OV in real time in order to maximize the therapeutic efficacy.
Combination therapy
■ Recent results demonstrate the synergistic effect of OVs with radiation therapy.
■ The shortest route to the clinic for this approach seems to be combining an OV with a pre-existing standard-of-care chemotherapy or radiation treatment.
Acknowledgments
This work was supported by the NCI (grants K99CA160775, R01CA122930 and R01CA138587).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Koonin EV, Senkevich TG, Dolja VV. The ancient virus world and evolution of cells. Biol. Direct. 2006;1:29. doi: 10.1186/1745-6150-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurtado A, Tseng JC, Boivin C, et al. Identification of amino acids of Sindbis virus E2 protein involved in targeting tumor metastases in vivo. Mol. Ther. 2005;12(5):813–823. doi: 10.1016/j.ymthe.2005.06.476. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol. Sci. 2007;28(7):326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanai R, Zaupa C, Sgubin D, et al. Effect of gamma34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J. Virol. 2012;86(8):4420–4431. doi: 10.1128/JVI.00017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin. Cancer Biol. 2000;10(6):453–459. doi: 10.1006/scbi.2000.0336. [DOI] [PubMed] [Google Scholar]
- 7.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Sesma A, Marukian S, Ebersole BJ, et al. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 2006;80(13):6295–6304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly EJ, Russell SJ. MicroRNAs and the regulation of vector tropism. Mol. Ther. 2009;17(3):409–416. doi: 10.1038/mt.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10■.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [Comprehensive report discussing the history of the development of oncolytic virotherapy.] [DOI] [PubMed] [Google Scholar]
- 11■■.Hoster HA, Zanes RP, Jr, Von Haam E. Studies in Hodgkin's syndrome; the association of viral hepatitis and Hodgkin's disease; a preliminary report. Cancer Res. 1949;9(8):473–480. [One of the earliest reports demonstrating that certain viruses can function as anticancer agents.] [PubMed] [Google Scholar]
- 12.Asada T. Treatment of human cancer with mumps virus. Cancer. 1974;34(6):1907–1928. doi: 10.1002/1097-0142(197412)34:6<1907::aid-cncr2820340609>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13■.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–856. doi: 10.1126/science.1851332. [One of the earliest publications demonstrating the antitumor activity of oncolytic viruses against human glioblastoma in an animal model.] [DOI] [PubMed] [Google Scholar]
- 14.Markovitz NS, Baunoch D, Roizman B. The range and distribution of murine central nervous system cells infected with the gamma(1)34.5- mutant of herpes simplex virus 1. J. Virol. 1997;71(7):5560–5569. doi: 10.1128/jvi.71.7.5560-5569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 16.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65(7):2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 17.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc. Natl Acad. Sci. USA. 2000;97(5):2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreansky S, He B, van Cott J, et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5(1):121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 19.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl Acad. Sci. USA. 2001;98(11):6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 21■.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [Original report describing ONYX-015, an oncolytic adenovirus genetically modified by deletion to selectively replicate in and kill cells that harbor a p53 mutation.] [DOI] [PubMed] [Google Scholar]
- 22.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 23.DeWeese TL, van der Poel H, Li S, et al. A Phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61(20):7464–7472. [PubMed] [Google Scholar]
- 24.Ramesh N, Ge Y, Ennist DL, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor – armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 2006;12(1):305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 25.Koski A, Kangasniemi L, Escutenaire S, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010;18(10):1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghi M, Martuza RL. Oncolytic viral therapies – the clinical experience. Oncogene. 2005;24(52):7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 27.Lee SS, Eisenlohr LC, McCue PA, Mastrangelo MJ, Lattime EC. Intravesical gene therapy: in vivo gene transfer using recombinant vaccinia virus vectors. Cancer Res. 1994;54(13):3325–3328. [PubMed] [Google Scholar]
- 28.Hermann LL, Coombs KM. Inhibition of reovirus by mycophenolic acid is associated with the M1 genome segment. J. Virol. 2004;78(12):6171–6179. doi: 10.1128/JVI.78.12.6171-6179.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comins C, Heinemann L, Harrington K, Melcher A, De Bono J, Pandha H. Reovirus: viral therapy for cancer ‘as nature intended’. Clin. Oncol. (R. Coll. Radiol.) 2008;20(7):548–554. doi: 10.1016/j.clon.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Lorence RM, Rood PA, Kelley KW. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J. Natl Cancer Inst. 1988;80(16):1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- 31.Reid T, Galanis E, Abbruzzese J, et al. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a Phase I trial. Gene Ther. 2001;8(21):1618–1626. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasey PA, Shulman LN, Campos S, et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J. Clin. Oncol. 2002;20(6):1562–1569. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 33.Rudin CM, Cohen EE, Papadimitrakopoulou VA, et al. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J. Clin. Oncol. 2003;21(24):4546–4552. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34■.Chiocca EA, Abbed KM, Tatter S, et al. A Phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [Clinical trial evaluating ONYX-015 as a treatment for malignant glioma.] [DOI] [PubMed] [Google Scholar]
- 35■.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [Along with [36–38], this trial tested different genetically modified HSV vectors as oncolytic viruses for malignant glioma.] [DOI] [PubMed] [Google Scholar]
- 36■.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a Phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [Along with [35,37,38], this trial tested different genetically modified HSV vectors as oncolytic viruses for malignant glioma.] [DOI] [PubMed] [Google Scholar]
- 37■.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5−) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [Along with [35,36,38], this trial tested different genetically modified HSV vectors as oncolytic viruses for malignant glioma.] [DOI] [PubMed] [Google Scholar]
- 38■.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre- and post-tumor resection for recurrent GBM. Mol. Ther. 2009;17(1):199–207. doi: 10.1038/mt.2008.228. [Along with [35–37], this trial tested different genetically modified HSV vectors as oncolytic viruses for malignant glioma.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forsyth P, Roldan G, George D, et al. A Phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol. Ther. 2008;16(3):627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 40.Lorence RM, Roberts MS, O'Neil JD, et al. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr. Cancer Drug Targets. 2007;7(2):157–167. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- 41.Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172(1):27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 42.Batliwalla FM, Bateman BA, Serrano D, et al. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol. Med. 1998;4(12):783–794. [PMC free article] [PubMed] [Google Scholar]
- 43.Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 2002;20(9):2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 44.Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J. Neurooncol. 2004;67(1–2):83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 45.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006;13(1):221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 47.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a Phase I trial. Lancet Oncol. 2008;9(6):533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 48.Au GG, Lindberg AM, Barry RD, Shafren DR. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int. J. Oncol. 2005;26(6):1471–1476. doi: 10.3892/ijo.26.6.1471. [DOI] [PubMed] [Google Scholar]
- 49.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol. Ther. 2010;18(11):1927–1936. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50■■.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol. 2010;12(9):928–940. doi: 10.1093/neuonc/noq046. [Along with [51], this report demonstrated that canine spontaneous glioma could be a very effective animal model for testing various antiglioma therapeutics.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51■■.Xiong W, Candolfi M, Liu C, et al. Human Flt3L generates dendritic cells from canine peripheral blood precursors: implications for a dog glioma clinical trial. PLoS ONE. 2010;5(6):e11074. doi: 10.1371/journal.pone.0011074. [Along with [50], this report demonstrated that canine spontaneous glioma could be a very effective animal model for testing various antiglioma therapeutics.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng KW, Facteau S, Wegman T, O'Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 2002;8(5):527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 53.Dingli D, Peng KW, Harvey ME, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 54■■.Barton KN, Stricker H, Brown SL, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol. Ther. 2008;16(10):1761–1769. doi: 10.1038/mt.2008.172. [Phase I study monitoring virus-mediated gene expression by noninvasive imaging techniques.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galanis E, Hartmann LC, Cliby WA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dingli D, Russell SJ, Morris JC., 3rd In vivo imaging and tumor therapy with the sodium iodide symporter. J. Cell. Biochem. 2003;90(6):1079–1086. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs A, Voges J, Reszka R, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358(9283):727–729. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- 58.Lu MY, Liao F. Interferon-stimulated gene ISG12b2 is localized to the inner mitochondrial membrane and mediates virus-induced cell death. Cell Death Differ. 2011;18(6):925–936. doi: 10.1038/cdd.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandevenne P, Sadzot-Delvaux C, Piette J. Innate immune response and viral interference strategies developed by human herpesviruses. Biochem. Pharmacol. 2010;80(12):1955–1972. doi: 10.1016/j.bcp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Schoggins JW, Wilson SJ, Panis M, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorne SH, Hwang TH, O'Gorman WE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Invest. 2007;117(11):3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherry B. Rotavirus and reovirus modulation of the interferon response. J. Interferon Cytokine Res. 2009;29(9):559–567. doi: 10.1089/jir.2009.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6(7):821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 64.Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol. Ther. 2007;15(3):588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmed AU, Ulasov IV, Mercer RW, Lesniak MS. Maintaining and loading neural stem cells for delivery of oncolytic adenovirus to brain tumors. Methods Mol. Biol. 2012;797:97–109. doi: 10.1007/978-1-61779-340-0_8. [DOI] [PubMed] [Google Scholar]
- 66.Yang X, Chen E, Jiang H, et al. Evaluation of IRES-mediated, cell-type-specific cytotoxicity of poliovirus using a colorimetric cell proliferation assay. J. Virol. Methods. 2009;155(1):44–54. doi: 10.1016/j.jviromet.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Passer BJ, Cheema T, Zhou B, et al. Identification of the ENT1 antagonists dipyridamole and dilazep as amplifiers of oncolytic herpes simplex virus-1 replication. Cancer Res. 2010;70(10):3890–3895. doi: 10.1158/0008-5472.CAN-10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyatake S, Iyer A, Martuza RL, Rabkin SD. Transcriptional targeting of herpes simplex virus for cell-specific replication. J. Virol. 1997;71(7):5124–5132. doi: 10.1128/jvi.71.7.5124-5132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57(13):2559–2563. [PubMed] [Google Scholar]
- 70.Dey M, Ulasov IV, Lesniak MS. Virotherapy against malignant glioma stem cells. Cancer Lett. 2010;289(1):1–10. doi: 10.1016/j.canlet.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ulasov IV, Zhu ZB, Tyler MA, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum. Gene Ther. 2007;18(7):589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 72.Stoff-Khalili MA, Rivera AA, Nedeljkovic-Kurepa A, et al. Cancer-specific targeting of a conditionally replicative adenovirus using mRNA translational control. Breast Cancer Res. Treat. 2008;108(1):43–55. doi: 10.1007/s10549-007-9587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oliere S, Arguello M, Mesplede T, et al. Vesicular stomatitis virus oncolysis of T lymphocytes requires cell cycle entry and translation initiation. J. Virol. 2008;82(12):5735–5749. doi: 10.1128/JVI.02601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muik A, Kneiske I, Werbizki M, et al. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J. Virol. 2010;85(11):5679–5684. doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leber MF, Bossow S, Leonard VH, et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol. Ther. 2010;19(6):1097–1106. doi: 10.1038/mt.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cawood R, Wong SL, Di Y, Baban DF, Seymour LW. MicroRNA controlled adenovirus mediates anti-cancer efficacy without affecting endogenous microRNA activity. PLoS ONE. 2010;6(1):e16152. doi: 10.1371/journal.pone.0016152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelly EJ, Nace R, Barber GN, Russell SJ. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J. Virol. 2010;84(3):1550–1562. doi: 10.1128/JVI.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, Seymour LW. Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 2009;5(5):e1000440. doi: 10.1371/journal.ppat.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79■■.Kelly EJ, Hadac EM, Greiner S, Russell SJ. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat. Med. 2008;14(11):1278–1283. doi: 10.1038/nm.1776. [Demonstrates that miRNA regulatory elements can be used to develop tumor-selective oncolytic viruses.] [DOI] [PubMed] [Google Scholar]
- 80.Sugio K, Sakurai F, Katayama K, et al. Enhanced safety profiles of the telomerasespecific replication-competent adenovirus by incorporation of normal cell-specific microRNA-targeted sequences. Clin. Cancer Res. 2011;17(9):2807–2818. doi: 10.1158/1078-0432.CCR-10-2008. [DOI] [PubMed] [Google Scholar]
- 81.Le Boeuf F, Diallo JS, McCart JA, et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 2010;18(5):888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat. Rev. Cancer. 2009;9(1):64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 83.Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin® (reovirus serotype-3-dearing strain) in patients with metastatic melanoma. Mol. Ther. 2012;20(10):1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris DG, Feng X, Difrancesco LM, et al. REO-001: a Phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest. New Drugs. 2013;31(3):696–706. doi: 10.1007/s10637-012-9865-z. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen TL, Abdelbary H, Arguello M, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl Acad. Sci. USA. 2008;105(39):14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Otsuki A, Patel A, Kasai K, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol. Ther. 2008;16(9):1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 87.Kirn DH, Wang Y, Liang W, Contag CH, Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68(7):2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- 88.Reeves PM, Smith SK, Olson VA, et al. Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J. Virol. 2011;85(1):21–31. doi: 10.1128/JVI.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Israyelyan A, Chouljenko VN, Baghian A, David AT, Kearney MT, Kousoulas KG. Herpes simplex virus type-1(HSV-1) oncolytic and highly fusogenic mutants carrying the NV1020 genomic deletion effectively inhibit primary and metastatic tumors in mice. Virol. J. 2008;5:68. doi: 10.1186/1743-422X-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yun CO. Overcoming the extracellular matrix barrier to improve intratumoral spread and therapeutic potential of oncolytic virotherapy. Curr. Opin. Mol. Ther. 2008;10(4):356–361. [PubMed] [Google Scholar]
- 91.Guedan S, Rojas JJ, Gros A, Mercade E, Cascallo M, Alemany R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010;18(7):1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA. 2011;108(7):2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 94.Pipiya T, Sauthoff H, Huang YQ, et al. Hypoxia reduces adenoviral replication in cancer cells by downregulation of viral protein expression. Gene Ther. 2005;12(11):911–917. doi: 10.1038/sj.gt.3302459. [DOI] [PubMed] [Google Scholar]
- 95.Shen BH, Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Ther. 2005;12(11):902–910. doi: 10.1038/sj.gt.3302448. [DOI] [PubMed] [Google Scholar]
- 96.Minami K, Tambe Y, Watanabe R, et al. Suppression of viral replication by stress-inducible GADD34 protein via the mammalian serine/threonine protein kinase mTOR pathway. J. Virol. 2007;81(20):11106–11115. doi: 10.1128/JVI.01063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connor JH, Naczki C, Koumenis C, Lyles DS. Replication and cytopathic effect of oncolytic vesicular stomatitis virus in hypoxic tumor cells in vitro and in vivo. J. Virol. 2004;78(17):8960–8970. doi: 10.1128/JVI.78.17.8960-8970.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aghi MK, Liu TC, Rabkin S, Martuza RL. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol. Ther. 2009;17(1):51–56. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sgubin D, Wakimoto H, Kanai R, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus counteracts the hypoxia-induced modulation of glioblastoma stem-like cells. Stem Cells Transl. Med. 2012;1(4):322–332. doi: 10.5966/sctm.2011-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu C, Russell SJ, Peng KW. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol. Ther. 2010;18(6):1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ilett EJ, Barcena M, Errington-Mais F, et al. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin. Cancer Res. 2011;17(9):2767–2776. doi: 10.1158/1078-0432.CCR-10-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14(4):324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 103.Qiao J, Wang H, Kottke T, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15(8):604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dwyer RM, Khan S, Barry FP, O'Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res. Ther. 2010;1(3):25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch. Neurol. 2008;65(4):452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 106.Mader EK, Maeyama Y, Lin Y, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin. Cancer Res. 2009;15(23):7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107■■.Garcia-Castro J, Alemany R, Cascallo M, et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther. 2010;17(7):476–483. doi: 10.1038/cgt.2010.4. [Phase I clinical trial evaluating mesenchymal stem cells as carriers for targeted oncolytic virotherapy.] [DOI] [PubMed] [Google Scholar]
- 108.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26(3):831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 109.Ahmed AU, Rolle CE, Tyler MA, et al. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol. Ther. 2010;18(10):1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tyler MA, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16(2):262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed AU, Thaci B, Alexiades NG, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol. Ther. 2011;19(9):1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112■.Ahmed AU, Tyler MA, Thaci B, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol. Pharm. 2011;8(5):1559–1572. doi: 10.1021/mp200161f. [Comparative study evaluating different stem cells as cell carriers for targeted antiglioma oncolytic virotherapy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thaci B, Ahmed AU, Ulasov IV, et al. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012;19(6):431–442. doi: 10.1038/cgt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stepkowski SM. Molecular targets for existing and novel immunosuppressive drugs. Expert Rev. Mol. Med. 2000;2(4):1–23. doi: 10.1017/S1462399400001769. [DOI] [PubMed] [Google Scholar]
- 115.Li H, Zeng Z, Fu X, Zhang X. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer Res. 2007;67(16):7850–7855. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- 116.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl Acad. Sci. USA. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vandenberk L, Van Gool SW. Treg infiltration in glioma: a hurdle for antiglioma immunotherapy. Immunotherapy. 2012;4(7):675–678. doi: 10.2217/imt.12.64. [DOI] [PubMed] [Google Scholar]
- 118.Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 2011;19(6):1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mastrangelo MJ, Maguire HC, Lattime EC. Intralesional vaccinia/GM-CSF recombinant virus in the treatment of metastatic melanoma. Adv. Exp. Med. Biol. 2000;465:391–400. doi: 10.1007/0-306-46817-4_34. [DOI] [PubMed] [Google Scholar]
- 120.Mukherjee S, Haenel T, Himbeck R, et al. Replication-restricted vaccinia as a cytokine gene therapy vector in cancer: persistent transgene expression despite antibody generation. Cancer Gene Ther. 2000;7(5):663–670. doi: 10.1038/sj.cgt.7700133. [DOI] [PubMed] [Google Scholar]
- 121.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 122.Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6(6):941–949. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- 123.Kottke T, Diaz RM, Kaluza K, et al. Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol. Ther. 2008;16(12):1910–1918. doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Idema S, Lamfers ML, van Beusechem VW, et al. AdDelta24 and the p53-expressing variant AdDelta24-p53 achieve potent anti-tumor activity in glioma when combined with radiotherapy. J. Gene Med. 2007;9(12):1046–1056. doi: 10.1002/jgm.1113. [DOI] [PubMed] [Google Scholar]
- 125.Lamfers ML, Grill J, Dirven CM, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62(20):5736–5742. [PubMed] [Google Scholar]
- 126.Geoerger B, Grill J, Opolon P, et al. Potentiation of radiation therapy by the oncolytic adenovirus dl1520 (ONYX-015) in human malignant glioma xenografts. Br. J. Cancer. 2003;89(3):577–584. doi: 10.1038/sj.bjc.6601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lamfers ML, Idema S, Bosscher L, et al. Differential effects of combined Ad5- delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin. Cancer Res. 2007;13(24):7451–7458. doi: 10.1158/1078-0432.CCR-07-1265. [DOI] [PubMed] [Google Scholar]
- 128.Nandi S, Ulasov IV, Tyler MA, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68(14):5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72(14):3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008;14(1):259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kottke T, Thompson J, Diaz RM, et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin. Cancer Res. 2009;15(2):561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis. Gene Ther. 2004;11(2):214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ruotsalainen J, Martikainen M, Niittykoski M, et al. Interferon-beta sensitivity of tumor cells correlates with poor response to VA7 virotherapy in mouse glioma models. Mol. Ther. 2012;20(8):1529–1539. doi: 10.1038/mt.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aghi M, Chou TC, Suling K, Breakefield XO, Chiocca EA. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59(16):3861–3865. [PubMed] [Google Scholar]
- 135.Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67(24):11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- 136.Alonso MM, Gomez-Manzano C, Jiang H, et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007;14(8):756–761. doi: 10.1038/sj.cgt.7701067. [DOI] [PubMed] [Google Scholar]
- 137.Kesari S, Randazzo BP, Valyi-Nagy T, et al. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab. Invest. 1995;73(5):636–648. [PubMed] [Google Scholar]