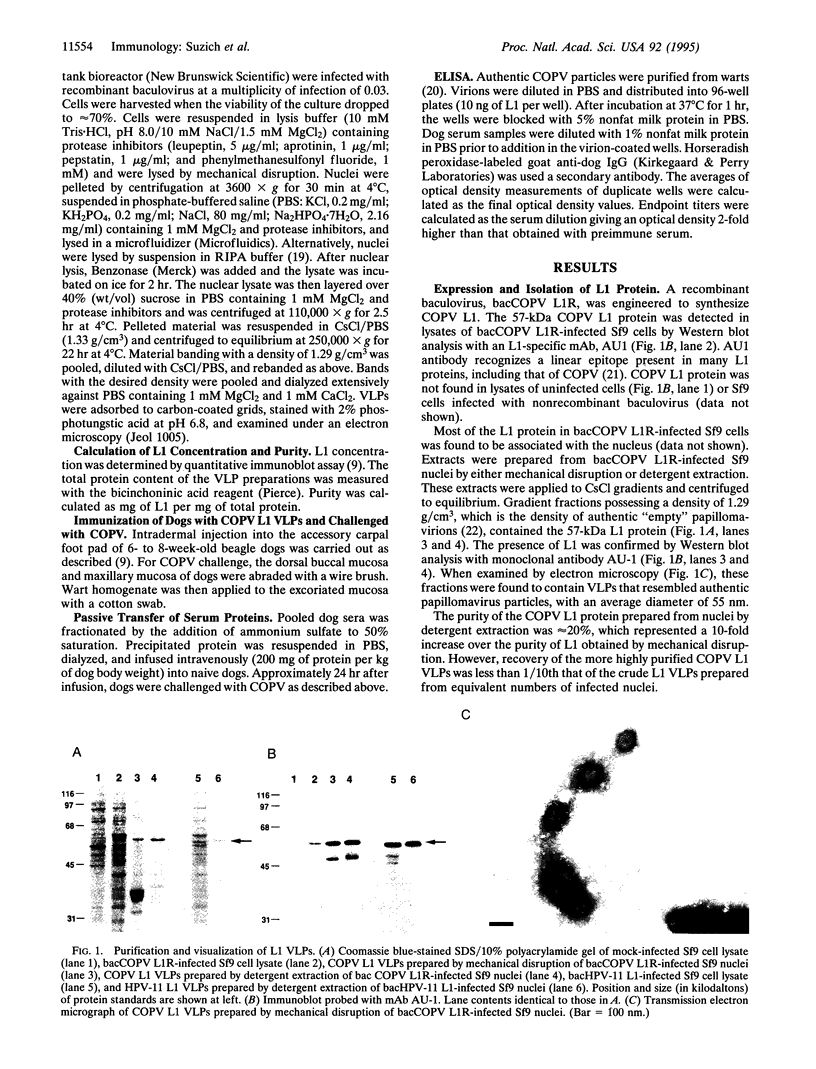
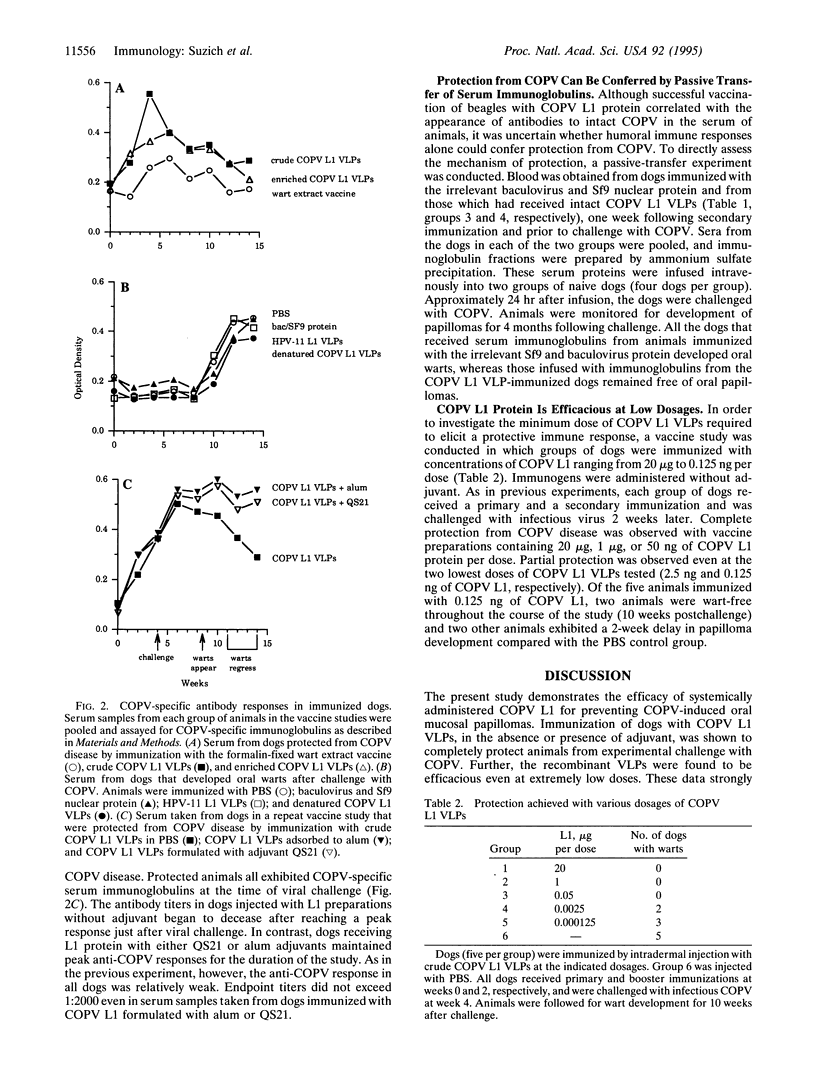
Abstract
Infection of mucosal epithelium by papillomaviruses is responsible for the induction of genital and oral warts and plays a critical role in the development of human cervical and oropharyngeal cancer. We have employed a canine model to develop a systemic vaccine that completely protects against experimentally induced oral mucosal papillomas. The major capsid protein, L1, of canine oral papillomavirus (COPV) was expressed in Sf9 insect cells in native conformation. L1 protein, which self-assembled into virus-like particles, was purified on CsCl gradients and injected intradermally into the foot pad of beagles. Vaccinated animals developed circulating antibodies against COPV and became completely resistant to experimental challenge with COPV. Successful immunization was strictly dependent upon native L1 protein conformation and L1 type. Partial protection was achieved with as little as 0.125 ng of L1 protein, and adjuvants appeared useful for prolonging the host immune response. Serum immunoglobulins passively transferred from COPV L1-immunized beagles to naive beagles conferred protection from experimental infection with COPV. Our results indicate the feasibility of developing a human vaccine to prevent mucosal papillomas, which can progress to malignancy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREEDIS C., BERWICK L., ANDERSON T. F. Fractionation of Shope papilloma virus in cesium chloride density gradients. Virology. 1962 May;17:84–94. doi: 10.1016/0042-6822(62)90084-3. [DOI] [PubMed] [Google Scholar]
- Bell J. A., Sundberg J. P., Ghim S. J., Newsome J., Jenson A. B., Schlegel R. A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology. 1994;62(4):194–198. doi: 10.1159/000163910. [DOI] [PubMed] [Google Scholar]
- Breitburd F., Kirnbauer R., Hubbert N. L., Nonnenmacher B., Trin-Dinh-Desmarquet C., Orth G., Schiller J. T., Lowy D. R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995 Jun;69(6):3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Campo M. S., Grindlay G. J., O'Neil B. W., Chandrachud L. M., McGarvie G. M., Jarrett W. F. Prophylactic and therapeutic vaccination against a mucosal papillomavirus. J Gen Virol. 1993 Jun;74(Pt 6):945–953. doi: 10.1099/0022-1317-74-6-945. [DOI] [PubMed] [Google Scholar]
- Christensen N. D., Höpfl R., DiAngelo S. L., Cladel N. M., Patrick S. D., Welsh P. A., Budgeon L. R., Reed C. A., Kreider J. W. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J Gen Virol. 1994 Sep;75(Pt 9):2271–2276. doi: 10.1099/0022-1317-75-9-2271. [DOI] [PubMed] [Google Scholar]
- Ghim S. J., Jenson A. B., Schlegel R. HPV-1 L1 protein expressed in cos cells displays conformational epitopes found on intact virions. Virology. 1992 Sep;190(1):548–552. doi: 10.1016/0042-6822(92)91251-o. [DOI] [PubMed] [Google Scholar]
- Ghim S., Christensen N. D., Kreider J. W., Jenson A. B. Comparison of neutralization of BPV-1 infection of C127 cells and bovine fetal skin xenografts. Int J Cancer. 1991 Sep 9;49(2):285–289. doi: 10.1002/ijc.2910490224. [DOI] [PubMed] [Google Scholar]
- Hagensee M. E., Yaegashi N., Galloway D. A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993 Jan;67(1):315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R., Booy F., Cheng N., Lowy D. R., Schiller J. T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R., Taub J., Greenstone H., Roden R., Dürst M., Gissmann L., Lowy D. R., Schiller J. T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993 Dec;67(12):6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsky L. A., Galloway D. A., Holmes K. K. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–163. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Demonstration of two distinct classes of bovine papilloma virus. Virology. 1978 Sep;89(2):372–379. doi: 10.1016/0042-6822(78)90179-4. [DOI] [PubMed] [Google Scholar]
- Lim P. S., Jenson A. B., Cowsert L., Nakai Y., Lim L. Y., Jin X. W., Sundberg J. P. Distribution and specific identification of papillomavirus major capsid protein epitopes by immunocytochemistry and epitope scanning of synthetic peptides. J Infect Dis. 1990 Dec;162(6):1263–1269. doi: 10.1093/infdis/162.6.1263. [DOI] [PubMed] [Google Scholar]
- Lorincz A. T., Reid R., Jenson A. B., Greenberg M. D., Lancaster W., Kurman R. J. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992 Mar;79(3):328–337. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Kirnbauer R., Schiller J. T. Genital human papillomavirus infection. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. C., Bonnez W., Reichman R. C., Garcea R. L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993 Apr;67(4):1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. C., Reichman R. C., Bonnez W. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J Gen Virol. 1994 Aug;75(Pt 8):2075–2079. doi: 10.1099/0022-1317-75-8-2075. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H., Bauer H. M., Hoover R. N., Glass A. G., Cadell D. M., Rush B. B., Scott D. R., Sherman M. E., Kurman R. J., Wacholder S. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993 Jun 16;85(12):958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- Sundberg J. P., O'Banion M. K., Schmidt-Didier E., Reichmann M. E. Cloning and characterization of a canine oral papillomavirus. Am J Vet Res. 1986 May;47(5):1142–1144. [PubMed] [Google Scholar]
- Zhou J., Stenzel D. J., Sun X. Y., Frazer I. H. Synthesis and assembly of infectious bovine papillomavirus particles in vitro. J Gen Virol. 1993 Apr;74(Pt 4):763–768. doi: 10.1099/0022-1317-74-4-763. [DOI] [PubMed] [Google Scholar]
- de Villiers E. M. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898–4903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Viruses in human cancers. Science. 1991 Nov 22;254(5035):1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]