Abstract
Objective
To assess the efficacy of a peer-delivered intervention to promote short-term (6-month) and long-term (12-month) adherence to HAART in a Mozambican clinic population.
Design
A 2-arm randomized controlled trial was conducted between October 2004 and June 2006.
Participants
Of 350 men and women (≥18 years) initiating HAART, 53.7% were female, and 97% were on 1 fixed-dose combination pill twice a day.
Intervention
Participants were randomly assigned to receive 6 weeks (Monday through Friday; 30 daily visits) of peer-delivered, modified directly observed therapy (mDOT) or standard care. Peers provided education about treatment and adherence and sought to identify and mitigate adherence barriers.
Outcome
Participants' self-reported medication adherence was assessed 6 months and 12 months after starting HAART. Adherence was defined as the proportion of prescribed doses taken over the previous 7 days. Statistical analyses were performed using intention-to-treat (missing = failure).
Results
Intervention participants, compared to those in standard care, showed significantly higher mean medication adherence at 6 months (92.7% vs. 84.9%, difference 7.8, 95% confidence interval [CI]: 0.0.02, 13.0) and 12 months (94.4% vs. 87.7%, difference 6.8, 95% CI: 0.9, 12.9). There were no between-arm differences in chart-abstracted CD4 counts.
Conclusions
A peer-delivered mDOT program may be an effective strategy to promote long-term adherence among persons initiating HAART in resource-poor settings.
Keywords: randomized control trial (RCT), HIV/AIDS, peer support, modified directly observed therapy (mDOT), adherence, HAART, Mozambique
Highly active antiretroviral therapy (HAART) has been associated with improved clinical response to treat-ment;1,2 however, achieving and maintaining high levels of adherence is challenging for most HIV-positive patients.3–7 Levels of adherence <90% of prescribed doses are associated with decline in increases in HIV-1 RNA viral load (VL) and CD4 count.8–13 Though newer regimens are more potent and may permit lower levels of adherence, nonadherence remains strongly associated with mortality.14,15 Long-term adherence is particularly challenging—adherence often fluctuates16 and may decrease over time.3,17,18 The HIV Epidemiology Research Study (HERS) found that adherence rates declined from 64% at 1 month to 45% at 6 months after initiation of HAART.19 Although Parruti et al found adherence remained fairly stable after up to 24 months of follow-up, adherence then declined about 5% every 6 months thereafter.3
A rigorous meta-analysis of randomized control trials (RCT) of HAART adherence interventions,20 all conducted in the US.21–26 or Europe,27–30 indicated a variety of successful strategies, such as didactic information on HAART; interactive discussions addressing cognitions, motivations, and expectations about taking HAART (eg, motivational interviewing, group therapy for HIV-related stigma); and behavioral strategies (eg, cue-dosing, cognitive-behavior therapy). However, the most effective intervention components and the best methods for implementing them in real-world settings remain ambiguous, especially in the context of resource-constrained health care systems.
One potentially useful strategy for a resource-constrained setting is modified directly observed therapy (mDOT) that is delivered by peers. mDOT involves observed-dosing and self-dosing of daily medications. Based on the success of directly observed therapy (DOT) for controlling the tuberculosis epidemic and for administering HAART in Haiti,31 mDOT is increasingly used for HAART programs for injection drug users32 and in resource-poor settings.33–35 Our own preliminary data suggest that peer-delivered mDOT is an acceptable and feasible method of promoting HAART adherence, especially with the confines of limited resources.36
In the present study, we evaluated the effect of a 6-week intervention of peer-delivered mDOT on adherence at 6 and 12 months after initiating HAART among men and women in Beira, Mozambique. The intervention was designed to identify and overcome adherence barriers, provide psychosocial support, and strengthen the links between patients and clinical staff. We hypothesized that patients receiving this short-term intervention at the start of HAART would have superior adherence to HAART over the long term. In addition, we examined several psychosocial variables that may mediate the relation between peer support and improved adherence.
Methods
Setting and Recruitment
The RCT was conducted between October 2004 and May 2006 at the HIV care clinic in the Beira Central Hospital, a large-volume public institution providing free specialized HIV care and antiretroviral medications to all affected Mozambicans in Beira and the surrounding area. As part of routine clinic procedures, clinic staff assessed patients' HAART eligibility based on standard Mozambican norms for starting therapy, which are CD4 <200 cells/mm3 regardless of WHO stage, CD4 200 to 349 cells/mm3 if in WHO stage 3 or pregnant, and WHO stage 4 regardless of CD4 count.
The staff pharmacist attempted to refer all persons initiating HAART to the study team from October 2004 to March 2005. The research manager, in consultation with clinic staff, assessed the eligibility of potential participants according to the following criteria: at least 18 years of age, living near the clinic, and free of severe mental illness or dementia (as assessed by clinic staff). The study protocol was reviewed and approved by the Institutional Review Boards of the Mozambique Ministry of Health and the University of Washington (randomized control trial number ISRCTN78797542; http://www.controlled-trials.com/ISRCTN78797542). All participants provided written consent. A data safety monitoring board of physicians and a statistician monitored unblinded participants'safety outcomes throughout the study. No serious adverse events related to the study were identified.
Procedures
Enrolled participants were scheduled for a baseline appointment on the day they started HAART. Participants were randomly assigned to either the mDOT intervention or standard care and completed a 45-minute interviewer-administered questionnaire. Random assignment to condition was based on a computer-generated allocation sequence prepared by an external statistician. Allocation concealment involved the use of sequentially numbered, opaque, sealed envelopes containing the group assignment, which the research manager opened at the moment of randomization after enrolling participants. Due to the nature of the intervention, participants and the study team could not be blinded to intervention. Participants were compensated 25,000 meticais (approximately US$1) for completing each interview (at baseline, 6-week, 6-month, and 12-month assessments) and had the option of conducting the interview either with a male or female Mozambican interviewer, in Portuguese or 1 of 2 local languages.
Measures
To ensure content and conceptual validity and reliability, most items and all scales were selected from published and validated measures, and all items were pretested for cultural appropriateness in Mozambique.
Adherence Outcomes
Owing to limited resources to conduct the study, the primary adherence outcome measure was self reported. Specifically, we assessed the percentage of prescribed HAART medication doses taken (without regard to timing) at 6 and 12 months with the commonly used question “How many of your HIV medication doses did you miss in the last 7 days?”37 A similar wording with a 30-day assessment period also was included.10 The secondary outcome was adherence at 12 months (assessed with the same 2 questions).
Clinical Outcomes
Participants provided a 4 to 5 mL blood sample for a CD4 cell count as part of standard care immediately before and 4 and 10 months after initiating HAART. The absolute CD4 cell count was determined through flow cytometry. A chart review was conducted to record the CD4 test result closest to the assessment point but within 2 months. If a CD4 result was not available during this time frame, the participant was excluded from the intent-to-treat (ITT) and change-score analyses of CD4 outcome (missing at 6 months, n = 46; at 12 months, n = 31). Mortality was verified by either medical records or by a family member who witnessed the death. If death was not verified by either of these 2 methods, then participants were assumed alive.
Soicodemographics
Variables such as gender, age, income, and education were recorded at baseline via questionnaire.
Potential Mediators
Depression was measured using the short-version (10-item) Centers for Epidemiological Studies Depression (CES-D) Scale,38 a nondiagnostic screening measure for examining the prevalence of nonspecific psychological distress in community samples. Cronbach's alpha for this sample was 0.74, lower then the published range of 0.85 to 0.92.38 A 21-item stigma scale adapted from Berger et al39 captured the social and emotional aspects of living with HIV. Three subscales were assessed: social stigma (fear and rejection), self stigma, and disclosure concerns. Published reliability scores range from 0.90 to 0.93.39 For this sample, Cronbach's alphas for the subscales were 0.78, 0.86, and 0.66, respectively. Social support was measured using the Medical Outcomes Study-Social Support (MOS-SS) survey that assesses perceptions of support in the past 30 days. The 18-item scale distinguishes among 4 domains of support (affirmational, informational, tangible, and emotional) and has demonstrated good reliability (α = 0.78 to 0.89).40–42 In the present sample, Cronbach's alpha for the overall scale was 0.92 and for each domain was 0.80, 0.78, 0.72, and 0.72, respectively. Community and peer support measures included whether the participant attended outside community support groups, number of visits with a peer after the 6-week intervention, and content of discussions with peers (ie, adherence and treatment issues, general health concerns, and social problems such as food handouts and questions pertaining to sex and pregnancy).
Standard Care
Standard care at the Beira Central Hospital is carried out by a team of clinicians, including 2 of the authors (Drs. Matediane and Micek), social workers, and peers. It includes no-cost medications, clinical and laboratory follow-up, psychosocial adherence support by a trained social worker, and referral to community-based peer support groups. Mandatory pre-HAART counseling involves education about dosing, side effects, nutritional requirements, and the importance of adherence. Before a patient is prescribed HAART, the health care team must endorse the patient's eligibility and readiness. Patients were encouraged to identify a treatment partner to help with adherence, provided with information on community-based support groups and nutritional resources, and instructed to contact their medical provider, nurse, pharmacist, or peer if they have any difficulties or concerns about their medication regimen. Peers were HIV-positive, chosen from among patients at the clinic and participants in community-based groups through self-nomination or nominations by clinic staff, and were paid a small stipend for their work. Patients met with the pharmacist and peer for pharmacy refills at week 2, 4, and 6 for the first 2 months and monthly thereafter.
Intervention
Consistent with the Fisher and Fisher42a Informational, Motivation, and Behavioral Skills (IMB) model, peers were taught to provide medication-related information and a set of core support strategies to the participants with the goal of developing skills to incorporate taking medication into their daily lives. Peers individually administered the 6-week mDOT intervention at the Beira Day Clinic to mDOT participants during their morning weekday dose. Evening and weekend doses were not observed. (For more details of the intervention, see Pearson et al.36) Nighttime and weekend doses were self-administered. As part of the daily interaction with participants, peers provided social support, information about the benefits and side effects of HAART, how to address stigma's effect on adherence, and encouragement to participate in community support groups. The peers also provided an important link between the individual and other members of the HIV clinic team and the community. All peers involved in the intervention successfully completed 1-week training and worked alongside social workers before meeting with participants. Peers also attended a 1-day refresher training every 3 months and weekly debriefing meetings with the pharmacist, social workers, and other clinic staff.
Intervention and Assessment Fidelity
Interviewers attended a 5-day training session that emphasized proper interviewing techniques such as reading items verbatim, probing, and respect for confidentiality. Interviewers were periodically observed by the principal investigator (PI) and the research manager to ensure proper interviewing technique. All data were doubled-entered by trained staff in the field. No cross-over of participants from the standard care arm to the mDOT arm was observed. Recall participants in the standard care arm had the option of accessing the peers more than the expected 4 visits (initiating HAART and pharmacy refills at week 2, 4, and 6); however, only 2 standard care participants saw the peers 1 additional time during the first 6 weeks after initiating HAART.
Data Analysis
Power calculations designed to detect a 10% difference in mean adherence between the standard care and intervention arms at the 6-month assessment indicated the need for a sample size of N = 367 based on 0.8 power to detect a significant difference (α = 0.05).
Analyses of outcomes at 6 and 12 months were intent-to-treat with missing = failure imputations (ITTm=f), where failure = 0% adherence. ITTm=f was determined reasonable, inasmuch as review of pharmacy records indicated that 92% of the participants with missing assessment data failed to pick up their ARV refills at least 30 days before the assessment period, indicating they did not have their medication in the 30 days before assessment. Because significantly more participants attrited in the control arm than in the intervention arm, it is possible that the use of a 0% adherence imputation may lead to an overestimation of the impact of the intervention. Therefore, ITT analysis without imputation of missing data was also conducted. Independent samples t tests with unequal variance were used to evaluate the efficacy of the intervention by comparing the standard-care arm to the intervention arm with respect to self-reported adherence at each assessment point. Repeated measures analyses of variance were used to assess change in adherence over time between the 2 arms.
Additional ITT analyses without imputation for missing data were conducted. Specifically, we used independent samples t tests with unequal variance to assess the outcomes of self-reported adherence, and for mean CD4 count, we calculated a change score from baseline to the 6-month assessment point and from baseline to the 12-month assessment point. In a post hoc unadjusted logistic analysis, we examined group differences in the outcomes of mortality, 90% adherence, and 100% adherence.
Analyses to assess correlates of adherence (including CD4) and possible moderators and mediators of the intervention effect were conducted. Specifically, we assessed group differences at each assessment point in sociodemographic variables, stigma, social support, mental health indicators, and use of other social support services. Standard statistics (ie, t tests, χ2, and unadjusted odds ratios [OR]) were used where appropriate. Rank correlation was used to test association when the adherence outcome was highly skewed.
Results
Study Flow
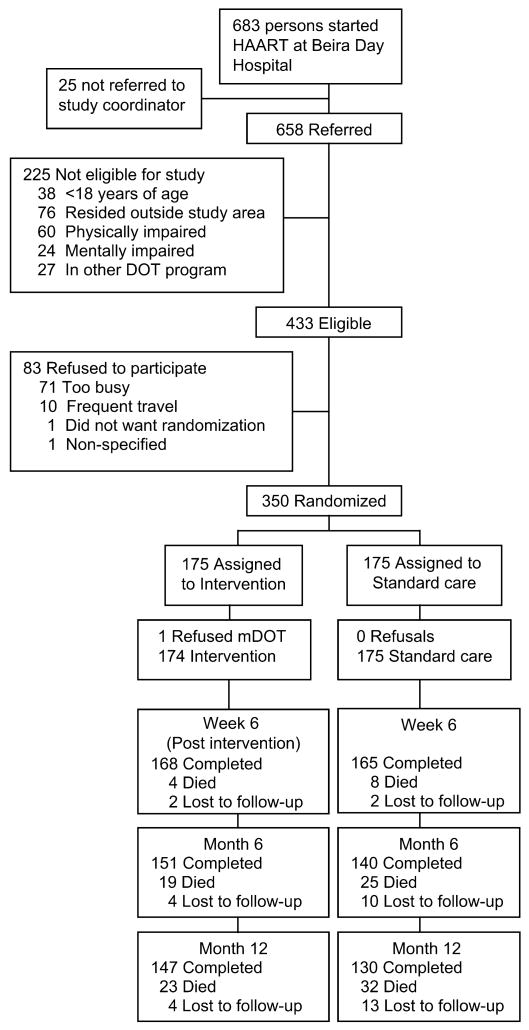
Depicted in Figure 1 are the recruitment, screening, and retention results. Ineligibility was primarily because of individuals residing outside of the study area and having severe physical and mental impairments. Most (81%) agreed to participate, and only 1 person refused study group assignment after randomization and was dropped from the study. Retention for the study appointment by intervention and standard-care arm, respectively, was 96.6% versus 94.3% at 6 weeks (OR = 1.7, 95% CI: 0.54, 5.8); 86.8% versus 80.0% at 6 months (OR = 1.6, 95% CI: −0.89, 3.1); and 84.5% versus 74.3% at 12 months (OR = 1.8, 95% CI: 1.1, 3.3). mDOT participants reported spending an average of 15 minutes (standard deviation [SD] = 15 min, range = 1 min to 2 h) at each visit with their peers and on average kept 93% of their 30 peer visits.
Figure 1.
Flow of study participants.
Participant Sociodemographic and Clinical Characteristics
Baseline sociodemographic and clinical information on the 350 participants is presented by intervention condition in Table 1. The sample consisted almost equally of men and women, most of whom (69%) were employed. There were no differences between the 2 study arms in the sociodemographic and clinical variables examined. Almost all participants (97%) were taking 1 fixed-dose non-nucleoside reverse transcriptase inhibitor (NNRTI) combination pill (ie, d4T+3TC+NVP) twice daily.
Table 1. Comparison of Sociodemographic and Clinical Characteristics at Baseline by Study Arm Among 350 HIV-positive Mozambicans.
| Standard Care (n = 175) | mDOT (n = 175) | Difference (95% CI) | |
|---|---|---|---|
| Mean age, y | 36.1 | 35.6 | 0.5 (−1.4, 2.3) |
| Mean monthly income, $USD | 54 | 58 | −4 (−23, 11) |
| Female | 53.1 | 54.3 | −1.2 (−11.7, 9.4) |
| Currently employed | 69.7 | 69.0 | 0.7 (−0.10, 0.10) |
| More than 8 years of education | 58.8 | 60.0 | −1.2 (−11.5, 9.2) |
| Currently married | 40.0 | 38.8 | 1.2 (−9.2, 11.4) |
| Treatment partner | 60.0 | 62.1 | −2.1 (−17.4, 3.2) |
| CD4 cell count <200 × 106 cells/L | 87.4 | 84.0 | 3.4 (−3.9, 10.8) |
| WHO-defined disease Phase 4 | 10.3 | 12.7 | −2.4 (−9.1, 4.3) |
CI indicates confidence interval.
Adherence Description
Mean adherence was high across groups, averaging more than 85% at each time point. It decreased from 6 weeks to 6 months and then increased slightly from 6 months to 12 months. This fluctuation in adherence overtime was not significant. At baseline and the 6- and 12-month assessment points, there were no significant associations between adherence and age, gender, income, education level, partner/ martial status, number of persons disclosed to, stigma, or depression. However, mean adherence was higher among participants who had attended a community support group (6 months: 97% vs. 93% [mean difference: −0.04, 95% CI: −0.08, −0.01]; 12 months: 99% vs. 96% [mean difference: −0.03, 95% CI: −0.05, −0.01]) and met with a peer at least once post intervention (6 months: 97% vs. 91% [mean difference: −0.06, 95% CI: −0.11, −0.02]; 12 months: 98% vs. 93% [mean difference: −0.05, 95% CI: −0.10, −0.01]). Additionally, at 12 months, adherence was associated with self-efficacy (ρ = 0.15, P < 0.01) and social support (ρ = 0.22, P < 0.001), specifically affirmational (ρ = 0.21, P < 0.001), tangible (ρ = 0.20, P < 0.001), emotional (ρ = 0.20, P < 0.001), and informational support (ρ = 0.18, P < 0.001).
Adherence Outcomes
Table 2 shows mean adherence data for each assessment point by study arm. As shown in the table, ITTm=f analyses indicated that, compared to participants in standard care, those in the mDOT arm reported a significantly higher mean 7-day and 30-day adherence at both the 6-month and 12-month follow-ups. ITT without imputation of missing data showed a similar pattern, with slightly higher adherence in the mDOT group compared to standard care at 6 months (7-day measure: 95.2% vs. 90.1%, difference: 4.2, 95% CI: 0.1, 9.3; 30-day measure: 96.3 versus 92.3, difference: 3.9, 95% CI: −0.1, 8.9). However, this difference was not evident at 12 months (7-day measure: 97.0% vs. 96.4%, difference: 0.6, 95% CI: 22.8, 3.9; 30-day measure: 97.8% vs. 97.4%, difference: 0.4, 95% CI: −2.6, 3.4). Change in adherence over the 12-month period differed significantly between the 2 arms (F[2276] = 3.66, P < 0.05). However, the between-arm difference at each assessment point was less than the 10% considered to be clinically meaningful (Table 2).
Table 2. Intent-to-Treat Mean Adherence (% Prescribed Doses Taken) and Mean Change in CD4 Count at Each Assessment Point by Study Arm Among 350 HIV-Positive Mozambicans.
| 6 Weeks Post-Intervention, n* (%) | 6 Months, n* (%) | 12 Months, n* (%) | |
|---|---|---|---|
| 7-day measure | |||
| mDOT | 170 (97.5) | 155 (92.7) | 151 (94.4) |
| Standard care | 167 (90.7) | 150 (84.9) | 143 (87.7) |
| % difference (95% CI) | 6.8 (2.3, 11.2) | 6.7 (1.0, 14.0) | 6.8 (0.9, 12.9) |
| 30-day measure | |||
| mDOT | 170 (97.7) | 155 (93.8) | 151 (95.2) |
| Standard care | 167 (92.8) | 150 (86.1) | 143 (88.5) |
| % difference (95% CI) | 4.9 (1.0, 8.7) | 7.6 (1.1, 14.1) | 6.6 (0.6, 12.6) |
| CD4 cell count | Mean Change (SD) Baseline–6 mo | Mean Change (SD) Baseline–12 mo | |
| mDOT | 140.6 (12.5) | 176.4 (14.3) | |
| Standard care | 144.4 (12.0) | 176.0 (13.1) | |
| % difference (95% CI) | 3.8 (−30.3, 37.9) | −0.65 (−38.9, 37.6) |
Persons who completed the interview and persons lost to follow-up (missing = failure) made up n. Excluded from the analysis were participants whose death was verified. For both 7-day and 30-day adherence outcomes, missing data was coded as failure (0% adherence). CD4 count was not available for all participants, and there was no imputation for missing data.
mDOT indicates modified directly observed treatment; CI, confidence interval; SD, standard deviation.
A more clinically meaningful outcome may be represented by the percentage of study participants who achieved a commonly accepted benchmark of adherence that is associated with good clinical outcomes. Using the 90% cutoff points with the 7-day and 30-day measures, we found the mDOT participants were more likely than the standard-care participants to achieve ≥90% adherence level at 6 months (7-day measure: 92% mDOT vs. 85%, OR = 2.0, 95% CI: 0.93, 4.5; 30-day measure: 92% mDOT vs. 87%, OR = 1.9, 95% CI: 0.83, 4.3). There was no group difference in the percentage of study participants with 100% adherence at 6 months, nor were there group differences in the percentage of study participants with ≥90% or 100% adherence using either measure at the 12-month assessment point.
Clinical Outcomes
mDOT participants were more likely than those in the standard-care arm to get a CD4 cell count (at 6 months post HAART: 90% vs. 79%, OR = 2.5, 95% CI: 1.3, 5.3; at 12 months post HAART: 96% vs. 83%, OR = 5.3, 95% CI: 2.0, 16.2). However, mean CD4 count among those with CD4 data did not differ between arms at baseline (Table 1) or at 6 and 12 months (Table 2). A post hoc analysis with morality as the outcome indicated no significant differences between the mDOTarm and standard-care arm at the 6-month and 12-month assessment points (11% vs. 14%, OR = 0.74, 95% CI: 0.37, 1.5; 13% vs. 18%, OR = 0.68, 95% CI: 0.36, 1.3, respectively). There was a moderate correlation between the 7-day adherence measure and CD4 at 6 months (r = 0.29, P ≤ 0.001) and a weak correlation at 12 months (r = 0.15, P ≤ 0.05).
Potential Mediators
At the 6-month assessment, mDOT participants were more likely than those in standard care to attend community support groups (41% vs. 27%, OR = 1.9, 95% CI: 1.1, 3.2); to visit a peer post-intervention (56% vs. 43%, OR = 1.7, 95% CI: 1.0, 2.8); and, among those who visited a peer, to discuss adherence and treatment issues (47% vs. 34%, OR = 1.7, 95% CI: 1.0, 2.8), general health concerns (19% vs. 11%, OR = 1.7, 95% CI: 0.86, 3.6), and social problems such as food handouts and questions pertaining to sex and pregnancy (18% vs. 6%, OR = 3.5, 95% CI: 1.5, 9.4). None of these differences were significant at the 12-month assessment point. There were no significant differences reported in stigma, self-efficacy, depression, or social support between arms at the 6-month or 12-month assessments.
Discussion
This is one of the few studies to rigorously examine mDOT for HAART in a resource-constrained setting. In this RCTof 350 men and women with HIV in Mozambique, using an ITTm=f, we found that a 6-week peer-delivered mDOT intervention led to higher adherence among mDOT participants than those with standard HIV care at both 6 and 12 months. An ITT analysis without imputation showed a similar effect at 6 but not 12 months, possibly owing to slightly higher attrition in the standard care than the intervention arm.
The difference in adherence between arms was approximately 7%, slightly less than the 10% effect size originally considered clinically meaningful. However, the standard care involved a comprehensive and intensive program of adherence support, and initials levels of adherence were quite high in both arms, creating a potential ceiling effect and limiting room for improvement. Furthermore, a study14 published after our trial commenced found that even a difference in adherence as small as 5% within the range of 77% to 94% on an NNRTI regimen, as observed in our study, may decrease the likelihood of the emergence of drug-resistant HIV.
Other positive findings were that mDOT participants were more likely than standard-care participants to be active in the health care system. Specifically, they were more likely to obtain their CD4 count, attend support groups, and meet with peers after the intervention period. One of the goals of providing mDOT for 6 weeks was not just to observe dosing but to formalize adherence support within the health care system and provide a liaison (ie, an HIV-positive peer) to help patients unaccustomed to chronic care maneuver through the health care system and access available resources. This is especially pertinent in resource-limited settings such as Mozambique, where patient-centered care for chronic illness is uncommon.
This study was limited by several factors. First, universal access to free HAART had become available in Mozambique shortly before the start of this study; therefore, our sample may have consisted of a group of unusually highly motivated people seeking care.43 To address this, we started study recruitment 4 months after the introduction of universal free HAART, and we powered the study for high adherence and a small effect size. Second, the nature of the intervention precluded blinding the participants and study personnel. Lack of blinding may have influenced patients' behaviors. To minimize this limitation, we assured all participants they could access the peers as frequently as they desired. Third, because self-report is a nonobjective means to measure adherence, participants' responses may have been influenced by the intervention, with mDOT participants more likely to inflate their reports of adherence (indeed, there was no group difference in the less subjective secondary outcomes of CD4 count and mortality). To counter this, peers were not included in the team of research personnel that conducted the interviews. Self-report has shown a moderate correlation with viral load19,44,45 and moderate to weak correlations, similar to this study, with CD4.9–11,46 Finally, because this study included only 1 site in the second-largest urban center in Mozambique, the results may not be generalizable to rural areas or urban centers with differing infrastructure or cultural beliefs.
Findings suggest mDOT for HAART has the potential to promote adherence and should be considered for implementation. Although implementing this intervention requires substantial resources, the cost is minimized through the use of peers.36 (Intervention expenses approximated only USD$33 per participant for the 6-week intervention.) Also, 6 weeks of mDOT may not be necessary. Perhaps a shorter, more intensive initial intervention could reduce participant burden and overall cost of mDOT, yet still provide improvements in long-term adherence. One further caution to wider implementation is that an mDOT intervention requires clinics to restructure care to render it more patient-centered by providing social, food, and transportation assistance. It also requires sustained commitment at the institutional level (eg, clinic, hospital, government, and international). Further effectiveness research incorporating community support resources may reveal how best to disseminate this promising strategy for enhancing life-extending therapy for AIDS in resource-constrained settings.
Acknowledgments
This work was supported in part by the Stroum Endowed Minority Dissertation Fellowship funding to Pearson, an STD/AIDS Research Training Grant (NIH T32 AI07140) to Dr. Micek, a National Institutes of Health (NIH) grant to Dr. Simoni (2 R01 MH 58986), PEPFAR and TAP funding Grant No. 1440/TAP:HIV-AIDS/MS-DPC/GACOPI/04 to Dr. Gloyd.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the University of Washington, the Mozambique Ministry of Health, or the National Institutes of Health (NIH).
References
- 1.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Parruti G, Manzoli L, Toro PM, et al. Long-term adherence to first-line highly active antiretroviral therapy in a hospital-based cohort: predictors and impact on virologic response and relapse. AIDS Patient Care STDS. 2006;20:48–56. doi: 10.1089/apc.2006.20.48. [DOI] [PubMed] [Google Scholar]
- 4.Friedland GH, Williams A. Attaining higher goals in HIV treatment: the central importance of adherence. AIDS. 1999;13(Suppl 1):S61–S72. [PubMed] [Google Scholar]
- 5.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 6.Nachega JB, Knowlton AR, Deluca A, et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S127–S133. doi: 10.1097/01.qai.0000248349.25630.3d. [DOI] [PubMed] [Google Scholar]
- 7.Mouala C, Kaba-Mebri J, Wata JB, et al. Factors associated with adherence to therapy among HIV-infected patients in Bangui. Sante. 2006;16:119–130. in French. [PubMed] [Google Scholar]
- 8.Knobel H, Guelar A, Carmona A, et al. Virologic outcome and predictors of virologic failure of highly active antiretroviral therapy containing protease inhibitors. AIDS Patient Care STDS. 2001;15:193–199. doi: 10.1089/10872910151133729. [DOI] [PubMed] [Google Scholar]
- 9.Cederfjall C, Langius-Eklof A, Lidman K, et al. Self-reported adherence to antiretroviral treatment and degree of sense of coherence in a group of HIV-infected patients. AIDS Patient Care STDS. 2002;16:609–616. doi: 10.1089/108729102761882143. [DOI] [PubMed] [Google Scholar]
- 10.Mannheimer S, Friedland G, Matts J, et al. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34:1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 11.Dorz S, Lazzarini L, Cattelan A, et al. Evaluation of adherence to antiretroviral therapy in Italian HIV patients. AIDS Patient Care STDS. 2003;17:33–41. doi: 10.1089/108729103321042890. [DOI] [PubMed] [Google Scholar]
- 12.Goujard C, Bernard N, Sohier N, et al. Impact of a patient education program on adherence to HIV medication: a randomized clinical trial. J Acquir Immune Defic Syndr. 2003;34:191–194. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Maggiolo F, Ravasio L, Ripamonti D, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis. 2005;40:158–163. doi: 10.1086/426595. [DOI] [PubMed] [Google Scholar]
- 14.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 15.Gulick RM. Adherence to antiretroviral therapy: how much is enough? Clin Infect Dis. 2006;43:942–944. doi: 10.1086/507549. [DOI] [PubMed] [Google Scholar]
- 16.Carrieri MP, Raffi F, Lewden C, et al. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virological response: a 3-year follow-up study. Antivir Ther. 2003;8:585–594. doi: 10.1177/135965350300800606. [DOI] [PubMed] [Google Scholar]
- 17.Pinheiro C, De-Carvalho-Leite J, Drachler M, et al. Factors associated with adherence to antiretroviral therapy in HIV/AIDS patients: a cross-sectional study in Southern Brazil. Braz J Med Biol Res. 2002;35:1173–1181. doi: 10.1590/s0100-879x2002001000010. [DOI] [PubMed] [Google Scholar]
- 18.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 19.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni JM, Pearson CR, Pantalone DW, et al. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiIorio C, Resnicow K, McDonnell M, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care. 2003;14:52–62. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 22.Heckman BD, Catz SL, Heckman TG, et al. Adherence to antiretroviral therapy in rural persons living with HIV disease in the United States. AIDS Care. 2004;16:219–230. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- 23.Margolin A, Avants SK, Warburton LA, et al. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychol. 2003;22:223–228. [PubMed] [Google Scholar]
- 24.Rathbun RC, Farmer KC, Stephens JR, et al. Impact of an adherence clinic on behavioral outcomes and virologic response in treatment of HIV infection: a prospective, randomized, controlled pilot study. Clin Ther. 2005;27:199–209. doi: 10.1016/j.clinthera.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19:807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 26.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39:1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 27.Knobel H, Carmona A, Lopez JL, et al. Adherence to very active antiretroviral treatment: impact of individualized assessment. Enferm Infecc Microbiol Clin. 1999;17:78–81. in Spanish. [PubMed] [Google Scholar]
- 28.Pradier C, Bentz L, Spire B, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials. 2003;4:121–131. doi: 10.1310/brbv-3941-h1pp-ndry. [DOI] [PubMed] [Google Scholar]
- 29.Tuldra A, Fumaz CR, Ferrer MJ, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:221–228. doi: 10.1097/00126334-200011010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Weber R, Christen L, Christen S, et al. Effect of individual cognitive behaviour intervention on adherence to antiretroviral therapy: prospective randomized trial. Antivir Ther. 2004;9:85–95. [PubMed] [Google Scholar]
- 31.Farmer P, Leandre F, Mukherjee J, et al. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy) Bull World Health Organ. 2001;79:1145–1151. [PMC free article] [PubMed] [Google Scholar]
- 32.McCance-Katz EF, Gourevitch MN, Arnsten J, et al. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J Addict. 2002;11:271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- 33.Behforouz HL, Farmer PE, Mukherjee JS. From directly observed therapy to accompagnateurs: enhancing AIDS treatment outcomes in Haiti and in Boston. Clin Infect Dis. 2004;38(Suppl 5):S429–S436. doi: 10.1086/421408. [DOI] [PubMed] [Google Scholar]
- 34.Sarna A, Kaai S, Hawken M, et al. Acceptability of a Modified Directly Observed Therapy Approach to Improve Adherence to Antiretroviral Therapy. Washington, DC: Population Council; 2004. Horizons Research Summary. [Google Scholar]
- 35.Daniel OJ, Alausa OK. Treatment outcome of TB/HIV positive and TB/ HIV negative patients on directly observed treatment, short course (DOTS) in Sagamu, Nigeria. Niger J Med. 2006;15:222–226. doi: 10.4314/njm.v15i3.37217. [DOI] [PubMed] [Google Scholar]
- 36.Pearson CR, Micek M, Simoni JM, et al. Modified directly observed therapy to facilitate highly active antiretroviral therapy adherence in Beira, Mozambique. Development and implementation. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S134–S141. doi: 10.1097/01.qai.0000248339.82567.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 39.Berger B, Ferrans C, Lashley F. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24:518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 40.Kulik JA, Mahler HI. Emotional support as a moderator of adjustment and compliance after coronary artery bypass surgery: a longitudinal study. J Behav Med. 1993;16:45–63. doi: 10.1007/BF00844754. [DOI] [PubMed] [Google Scholar]
- 41.Murphy DA, Greenwell L, Hoffman D. Factors associated with antiretroviral adherence among HIV-infected women with children. Women Health. 2002;36:97–111. doi: 10.1300/J013v36n01_07. [DOI] [PubMed] [Google Scholar]
- 42.Simoni JM, Frick PA, Lockhart D, et al. Mediators of social support and antiretroviral adherence among an indigent population in New York. AIDS Patient Care STDS. 2002;16:431–439. doi: 10.1089/108729102760330272. [DOI] [PubMed] [Google Scholar]
- 42a.Fisher JD, Fisher WA, Amico KR, et al. An information-motivation-behavioral skills model of adherence to antiretroviral theraphy. Health Psychol. 2006;25:462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 43.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 44.Pearson CR, Simoni JM, Hoff P, et al. Assessing antiretroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS Behav. 2007;11:161–173. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 46.Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16:199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]