Summary
Adherence to highly active antiretroviral therapy (HAART) is generally suboptimal, limiting the effectiveness of HAART. This meta-analytic review examined whether behavioral interventions addressing HAART adherence are successful in increasing the likelihood of a patient attaining 95% adherence or an undetectable HIV-1 RNA viral load (VL). We searched electronic databases from January 1996 to September 2005, consulted with experts in the field, and hand searched reference sections from relevant articles. Nineteen studies (with a total of 1839 participants) met the selection criteria of describing a randomized controlled trial among adults evaluating a behavioral intervention with HAART adherence or VL as an outcome. Random-effects models indicated that across studies, participants in the intervention arm were more likely than those in the control arm to achieve 95% adherence (odds ratio [OR] = 1.50, 95% confidence interval [CI]: 1.16 to 1.94); the effect was nearly significant for undetectable VL (OR = 1.25; 95% CI: 0.99 to 1.59). The intervention effect for 95% adherence was significantly stronger in studies that used recall periods of 2 weeks or 1 month (vs. ≤7 days). No other stratification variables (ie, study, sample, measurement, methodologic quality, intervention characteristics) moderated the intervention effect, but some potentially important factors were observed. In sum, various HAART adherence intervention strategies were shown to be successful, but more research is needed to identify the most efficacious intervention components and the best methods for implementing them in real-world settings with limited resources.
Keywords: adherence, antiretroviral therapy, HIV/AIDS, interventions, meta-analysis, review
Highly active antiretroviral therapy (HAART) has demonstrated remarkable success in inhibiting HIV viral replication and reducing morbidity, mortality, and overall health care costs for HIV-positive persons.1,2 Optimal results of HAART, however, are most common at high levels of adherence. As adherence decreases, HIV-1 RNA viral load (VL) and the risk of progression to AIDS generally increase,3–5 as does the likelihood of generating drug-resistant strains of HIV6 and of infecting others.7 Despite these risks, non-adherence to HAART is widespread in the United States and in Europe, with estimates of the percentage of prescribed doses taken ranging from 60% to 70%.8–14 Clearly, strategies for increasing adherence are urgently needed, especially as HAART becomes more widely available in relatively resource-poor health care settings.
The literature on HAART adherence interventions has been reviewed several times. Earlier qualitative reviews noted that reports were based primarily on small pilot and feasibility studies and, although innovative, offered few prescriptive guidelines with any empiric validity.15–17 Later qualitative reviews highlighted the improved methodologic quality of the studies and noted considerable variation in sampling and assessment strategies, intervention components, and findings.18–20 Recently, the first quantitative (ie, meta-analytic) review of published HAART adherence interventions appeared.21 That analysis, which combined data from randomized controlled trials (RCTs) and noncontrolled studies that assessed pre-to-post intervention change in behavior, yielded a significant (P < 0.05) aggregated effect size (d = 0.35, odds ratio [OR] = 1.88) based on 26 findings that varied considerably across studies. Interventions targeting individuals with poor adherence had stronger effects than interventions not restricting eligibility.
The present meta-analytic review updates this prior work through September 2005 and extends it in several respects. We focused exclusively on findings from RCTs to determine effect sizes based on the interventions evaluated with the most rigorous methodology.22 In addition to adherence, we examined VL as a virologic indicator of intervention effects. Furthermore, by eliciting supplemental information from the original authors of the studies, we are able to analyze standardized versions of these 2 outcomes (ie, percentage of participants who attained 95% adherence and undetectable VL).
METHODS
Data Sources
We implemented multiple search strategies to minimize the bias of missed published interventions. First, we searched the electronic databases MEDLINE, PubMed, PsycInfo, ERIC, and EMBASE from January 1996 through September 2005. We crossed multiple search terms (ie, key words and medical subject heading terms) reflecting 3 categories: (1) HAART (ie, HAART, highly active antiretroviral therapy, antiretroviral therapy, combination therapy, HIV treatment), (2) adherence (ie, adherence, nonadherence, compliance, noncompliance), and (3) intervention (ie, intervention, randomized controlled trial). Second, we searched on-line trial registry databases (ie, the Cochrane Library and the Database of Systematic Reviews22 and the Computer Retrieval of Information on Scientific Projects [CRISP] database, hosted by the US National Institutes of Health). Third, we contacted experts in the field and put out a call for relevant studies on a popular HAART research “listserv” (http://mailman1.u.washington.edu/mailman/listinfo/haart_adherence_research). Finally, we reviewed the references of all pertinent articles.
Study Selection
Studies (published in any language) were included in the meta-analysis if they met all 4 of the following criteria: (1) described a behavioral intervention, (2) targeted individuals 18 years of age or older, (3) randomly assigned individual participants to intervention and control groups, and (4) reported outcome data on adherence or VL.
Data Abstraction
Using standardized coding forms, pairs of reviewers abstracted information from the published articles. Each study was coded for study, sample, and intervention characteristics. The interrater agreement was 93% for 17 key variables; discrepancies were reconciled via discussion. Key variables were dichotomized for use in stratification analyses. Specific intervention components were coded as (1) didactic provision of generic information about HIV, HAART in general, and the patient’s prescribed regimen; (2) interactive discussion involving patient-specific information addressing cognitions, motivations, and expectations about taking HAART; (3) behavioral strategies, including the provision of external rewards or the implementation of cue dosing; and (4) external reminders in the form of pagers, diaries, or calendars. We then rated the extent of intervention in the comparison group (received any of these intervention components [coded as 1] or received only standard of care [coded as 0]).
Assessment of Measurement Variables and Methodologic Quality
Several measurement variables were assessed: recall period for 95% adherence, threshold for establishing undetectable VL, and timing of outcome assessment. Additionally, we examined the following methodologic quality variables: sample size, length of follow-up, overall retention, differential retention by trial arms, treatment of missing data, and method for measuring adherence.
Outcome Variables and Analytic Approach
Studies varied in how they defined adherence. For example, Rigsby et al23 operationalized adherence as the percentage of prescribed doses taken within 2 hours of scheduled dosing times over a 1-week period according to electronic data monitoring, whereas Tuldra et al’s24 main outcome was percentage of prescribed doses taken in the last month according to self-report. To reduce the measurement variance and optimize the comparison of outcomes across studies, we contacted authors and requested data on 2 standardized outcome measures. One was the percentage of participants who achieved 95% or better adherence to their treatment. This cutoff point was chosen because it has been associated with the best virologic outcomes.5 The second outcome measure was the percentage of participants with an undetectable VL according to the assay used in the original research.
The following rules guided the calculation of the overall intervention effect size. First, separate meta-analyses were conducted for each outcome (95% adherence and undetectable VL). Some studies provided outcome data only immediately after the intervention, some provided outcome data only at follow-up, and some provided outcome data at both time points. Multiple or longer term follow-ups were rare. Therefore, we used outcome data from the first follow-up when available because it was the assessment period most comparable across studies. If follow-up outcomes were not available, we used the immediate postintervention outcomes. Second, in the 2 studies with multiple intervention arms, we report only 1 contrast to ensure that all data points are independent. For the study by Rigsby et al,23 we used the arm involving the more comprehensive intervention, and for the study by Rotheram-Borus et al,25 we used the arm in which treatment was delivered in person (as opposed to by telephone) to make it consistent with the other studies. Third, a hierarchic approach was used in decisions about data inclusion; that is, we used data provided directly from the authors when available. In 1 of the 2 instances in which authors did not send data to us, we were able to use information published in the original report. If data from a study were not available from either source for a particular outcome of interest, that study was omitted from the analysis of that outcome.
Effect Size Calculation
For each meta-analysis, effect sizes were estimated with ORs, because outcome variables were dichotomous. An OR >1 indicates that participants in the intervention arm were more likely to achieve the desired outcome than participants in the control arm.
Standard meta-analytic methods26–28 were applied for aggregating individual effect sizes across studies. We first used the natural logarithm to obtain log OR (lnOR) and calculated its corresponding weight (ie, inverse variance) for each study. In estimating the overall effect size, we multiplied each lnOR by its weight, summed the weighted lnOR across studies, and then divided by the sum of the weights. The aggregated lnOR was then converted back to the OR by exponential function, and a 95% confidence interval (CI) was derived. We also tested the magnitude of heterogeneity of the individual effect sizes by using the Q statistic, an approximate χ2 distribution with degrees of freedom (df) equal to the number of findings (k) – 1. Fixed-effects models and random-effects models were examined; both yielded highly similar results. The final presentation is based on a random-effects model, which provides a more conservative estimate of variance and generates more accurate inferences about a population of adherence intervention trials beyond those analyzed here.29
Sensitivity and Stratification Analyses
Sensitivity analyses were conducted to determine whether the aggregated effect size changed appreciably after deleting any specific finding. We compared the aggregated effect size based on all studies with successive iterations using k – 1 findings; that is, we removed a finding and calculated the aggregated effect size based on the remaining findings. We then replaced that finding, removed another, and repeated the process.
Additionally, we conducted stratified analyses to examine whether study, sample, measurement, methodologic quality, or intervention characteristics moderated the strength of the aggregated effect size. For example, we compared the aggregated effect size for US studies with that of non-US studies. These subgroup aggregated effect sizes were compared with the between-group heterogeneity statistic QB.27
Analysis of Publication Bias
Publication bias favoring studies with significant findings was ascertained by inspection of a funnel plot of standard error estimates versus effect-size estimates from individual samples28 and also by a linear regression test.30 For the linear regression test, the standardized effect-size estimate (effect-size estimate divided by the corresponding standard error estimate) is regressed against the weight (the inverse of the standard error). If the intercept used to measure asymmetry is significantly different from 0, this provides evidence of publication bias.
RESULTS
Study and Sample Characteristics
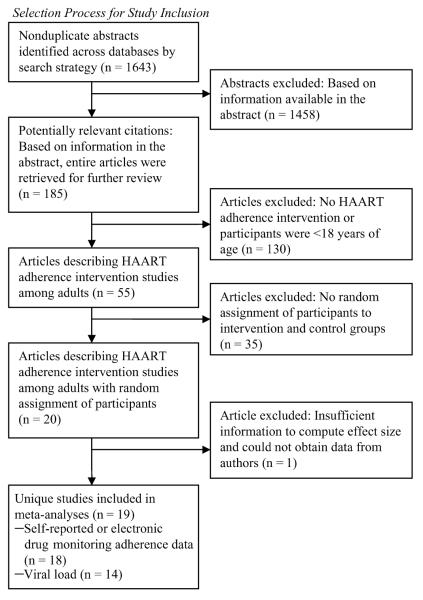
As shown in Figure 1, of the 1891 citations originally identified through the comprehensive search, 19 RCTs met eligibility criteria and were included in the meta-analyses. The studies are described in Table 1. They were published from 1999 to 2005 and were conducted mainly in the United States (74%). Most (84%) took place at outpatient HIV primary care clinics and were conducted with convenience samples, with baseline total population numbers ranging from 33 to 262 (median = 116). Eligibility criteria varied widely, although 37% of the studies restricted inclusion to patients exhibiting some marker of risk for nonadherence, such as poor baseline adherence or detectable VL. The percentage of participants who were men ranged from 0% to 91% (median = 75%); from 0% to 77% of participants in each study were men who have sex with men (MSM; median = 53%). Participants in the US studies were mostly racial/ethnic minorities (median = 54% African Americans and 19% Latino/a Americans).
FIGURE 1.
Selection process for study inclusion.
TABLE 1.
RCTs of Behavioral Interventions to Improve Adherence to HAART or Reduce HIV-1 RNA VL
| Source | Sample Location Setting Sample Size % Male % MSM at Baseline % AA % LAT % WH |
Inclusion Criteria | Description of Intervention Arm Duration (Code for Intervention Component) Baseline n Components/Intensity Intervener |
Description of Control Arm Duration, if Different (Code for Intervention Component if Given to Control Group) Baseline n Components/Intensity |
Postintervention Retention Rates Retention Rate per Arm Immediately After Intervention (Post) F/U Time Point (Length of Time Since End of Intervention) |
Outcome VL (Y/N) VL Sensitivity Adherence Method: Assessment Interval; Definition |
|---|---|---|---|---|---|---|
| Andrade et al, 200538 |
Baltimore, MD HIV Clinic N = 64 58.6% Male N/R MSM 87.9% AA N/R LAT N/R WH |
Age 18 years or older, able to self-medicate, currently receiving medical care at the study site, previously treatment naive or HAART experienced and switching regimens (only those who had received ,3 previous HAART regimens) |
24 weeks (d) Baseline n = 32 –SC adherence counseling –Use a portable, battery-powered, electronic device (Disease Management Assistance System [DMAS]) that produces a timed programmed voice message to prompt participants to take a medication dose |
Baseline n = 32 –Monthly, individualized, pharmacist-delivered 30- minute counseling session about adherence, including feedback on EDM adherence as well as providing general education about adherence issues and the pt’s prescribed regimen |
Post R I: 75% R C: 84% |
VL (Y) baseline <400 copies/mL F/U <50 copies/mL Adherence Self-report + EDM: 4 days; prescribed − missed doses divided by prescribed doses |
| DiIorio et al, 200336 |
Southeastern city, United States HIV clinic N = 20 52.9% Male N/R MSM 87.5% AA N/R LAT 11.8% WH |
English-speaking, have access to telephone and VCR, mentally stable |
6 weeks (a, b, c) Baseline n = 10 –5 days/week of directly observed therapy –3 biweekly motivational interview sessions on adherence –Additional motivational materials such as videotapes, journal, and calendar Delivered by health care provider |
(a) Baseline n = 10 –Standard care –Information component (usual adherence education provided by clinic) |
First F/U (14 days) R I: 80% R C: 90% |
VL (N) Adherence Self-report: 4 days, 14 days, 30 days; percent of doses taken |
| Goujard et al, 200341 |
Multiple sites in France University clinics N = 262 80% Male N/R MSM N/R AA, LAT, WH |
Understand French, not pregnant, did not have a partner in the study, no active psychiatric diagnoses |
12 months (a, c, d) Baseline n = 137 –3 1-hour educational sessions to develop a personalized educational plan based on anticipated problems with adherence –Planning card with stickers –Pill boxes Delivered by health care provider |
Baseline n = 125 –Planning card only for the first 12 months |
Post R I: 80% R C: 88% First F/U (6 months) R I: 74% R C: 68% |
VL (Y) <200 copies/mL Adherence Self-report: 7 days; global adherence score based on PMAQ7 and qualitative criteria related to instructions and timing |
| Jones et al, 200348 |
Miami-Dade County, New York, NY, New Jersey Metropolitan Area Setting N/R N = 174 0% Male 0% MSM 54% AA 36% LAT 7% WH |
Female, met CDC criteria for AIDS, not substance dependent, no psychosis, no major depression |
3 months (a, b, c) Baseline n = 92 –10 weekly group-based, 2-hour cognitive behavioral stress management sessions (not specifically designed to influence adherence) –Expressive supportive individual psychotherapy to decrease stress and increase coping Delivered by psychologist |
Baseline n = 82 –10 weekly 120-minute time and content-equivalent individual education videotapes for time and content equivalence, including 1 45-minute education tape and 1 75- minute entertainment tape |
Post R I: 100% R C: 100% |
VL (N) Adherence Self-report: 7 days; percent of doses taken |
| Knobel et al, 199932 |
Spain Setting N/R N = 186 73% Male N/R MSM N/A AA, LAT, WH |
VL >5000 copies/mL, CD4 <500 cells/mm |
1 session (a, b) Baseline n = 65 –Psychosocial education session covered information about medications, side effects, and how to incorporate pill taking into daily activities with optional telephone support Delivered by counselor/social worker |
Baseline n = 121 –SC |
First F/U (24 weeks) R I: 92% R C: 91% |
VL (Y) <50 copies/mL Adherence Self-report: 30 days; percent of doses taken |
| Margolin et al, 200331 |
New Haven, CT Methadone clinic N = 90 70% Male N/R MSM 49% AA 16% LAT 36% WH |
IDU, opioid dependence, abuse or dependence on cocaine |
6 months (a, b, c) Baseline n = 45 –Minimal care plus 48 sessions of manualguided group therapy sessions that address medical, emotional, and spiritual needs. Topics included harm reduction skill training, increasing medication adherence, relapse prevention, coping with stigma and grief. Delivered by psychologist and peer |
(c) Baseline n = 45 –Minimal care for 6 months, which included weekly individual substance abuse counseling and case management and 6 sessions on HIV risk reduction |
Post R I: 82% R C: 71% |
VL (Y) <400 copies/mL Adherence Self-report, assessed weekly: 7 days; percent of doses taken |
| Murphy et al, 200249 |
United States HIV clinic N = 52 88% Male N/R MSM 46% AA 3% LAT 18% WH |
English-speaking, not in another study, sufficiently psychiatrically stable to participate in group experience, self-reported missing a dose once a week or more |
7–8 weeks (a, b, c) Baseline n = 27 –5 group and individual sessions offering behavioral strategies and simplified patient information and social support Delivered by psychologist and heath care provider |
(a) Baseline n = 25 –SC plus if reported problems, pts received 1 30- minute cognitive session |
Post R I : 63% R C: 64% |
VL (N) Adherence Self-report: 3 days; percent of doses taken |
| Pradier et al, 200334 |
Nice, France University hospital N = 244 73% Male N/R MSM N/A AA, LAT, WH |
On HAART at least 1 month, no hospitalization in the previous month, not included in another study |
6 months (a, b, c) Baseline n = 123 –3 bimonthly 45–60-minute education and counseling sessions based on motivational interviewing and client-centered therapy that address cognitive, emotional, behavioral, and social determinants of adherence Delivered by heath care provider |
Baseline n = 121 –SC, defined as medical consultation every 2 to 3 months as determined by the provider |
Post R I: 81% R C: 80% First F/U (2 months) R I: 52% R C: 58% |
VL (Y) <400 copies/mL Adherence EDM: 4 days; percent of doses taken |
| Rathbun et al, 200535 |
Oklahoma University-based HIV Clinic N = 43 85% Male 70% MSM 21% AA 9% LAT 70% WH |
HAART-naive pts or pts initiating a new HAART regimen who were responsible for self-administration of their medication |
12 weeks (a, b, c) Baseline n = 21 –1 pharmacist-delivered, individually tailored education session, including visual aids at regimen initiation (1–1.5-hour visit) –1 telephone F/U, 1 in-person F/U for all pts –Additional telephone and F/U visits conducted through week 12 for patients reporting adherence difficulties Delivered by pharmacist and by counselor/ social worker |
(a) Baseline n = 22 –SC, defined as the usual adherence education given by the patient’s primary care provider |
First F/U (4 weeks) R I: 73% R C: 81% |
VL (Y) <50 copies/mL Adherence EDM: percent of doses taken over entire monitoring period |
| Rawlings et al, 200342 |
United States 25 outpatient sites N = 195 65% Male 43% MSM 71% AA 21% LAT 7% WH |
40 < VL <100,000, HAART naive or limited experience, under- represented group, likely to comply with study schedule, no AIDS diagnosis |
4 weeks (a, b, c) Baseline n = 96 –4 weekly educational sessions focusing on patient empowerment, HIV pathogenesis and treatment, medication management, and adherence Delivered by heath care provider |
(a) Baseline n = 99 –At each visit, provision of names and descriptions of medications and advice about how best to take them, considering pt’ s schedule |
Post R I: 73% R C: 80% First F/U (20 weeks) R I: 53% R C: 58% |
VL (Y) <40 copies/mL Adherence EDM: 7 days; percent of doses taken |
| Remien et al, 200533 |
New York, NY 2 HIV outpatient clinics N = 215 serodiscordant couples Among HIV+ pts: 54% Male 18% MSM 62% AA 24% LAT N/R WH |
HIV-serodiscordant couples in which the HIV+ partner was in care, on HAART for >1 month and had <80% baseline adherence according to a 2-week EDM pre- enrollment observation period; relationship duration of 6 months or more; both partners were English-speaking adults (>18 years of age) |
5 weeks (a, b, c) Baseline n = 106 –4-session, nurse practitioner-delivered, couple-focused adherence intervention consisting of education about treatment and adherence, identifying adherence barriers, developing communication and problemsolving strategies, optimizing partner support, and building confidence for optimal adherence Delivered by heath care provider |
(a) Baseline n = 109 –SC consists of basic adherence education and support from a multidisciplinary treatment team –F/U usually occurs within 2–4 weeks after initiating a new regimen –Patients can contact the clinic if difficulties emerge and have monthly appointments if necessary |
Post (8 weeks) R I: 91% R C: 92% First F/U (20 weeks) R I: 85% R C: 86% Second F/U (32 weeks) R I: 83% R C: 86% |
VL (Y) <50 copies/mL Adherence EDM: 14 days; percent of doses taken |
| Rigsby et al, 200023 |
West Haven and Hartford, CT HIV clinic and study center N = 40 92.5% Male N/R MSM 72.5% AA N/R LAT 18% WH |
Not cognitively impaired, does not rely on others to administer medications, can accommodate EDM |
4 weeks (c) Baseline n = 15 (only arm 2 [CD + CR] used in present analysis) Arm 1, cue dosing: 5 sessions, pts identity cues to help them remember to take their meds and are shown EDM-generated calendar of previous weeks’ dosing; counselor discusses alternative cues for consistently missed doses Arm 2, cue dosing + cash reinforcement: 5 sessions, paid for doses taken within 2 hours of correct time up to $280/month Delivered by research assistant |
Baseline n = 18 C: EDM used but no calendar generated Pts were asked about adherence and encouraged to take medications |
Post R CD-CR: 100% R C: 89% First F/U (8 weeks) R CD-CR:100% R C: 67% |
VL (Y) <50 copies/mL Adherence EDM: 7 days; percent of doses taken |
| Rotheram-Borus et al, 2004 25 |
Los Angeles, CA, New York, NY, San Francisco, CA Community agencies N = 175 78% Male 69% MSM 26% AA 42% LAT 23% WH |
Using illicit drugs at least 5 times in last 3 months, 45-minute “screener” on sexual and substance use risk acts |
3 months (a, b, c) Baseline n = 31 –In-person delivery –6 sessions of 2 hours each delivered in person. Adherence sessions focused on improving physical health regimen, particularly utilization and adherence to antiretroviral medications –Another arm was the identical intervention but delivered by telephone, which was not included in our analyses Delivered by counselor |
Baseline n = 25 –Wait list control –Delayed group until after assessment period at 15 months; was identical to intervention arms |
Post R I: 87% R C: 76% |
VL (N) Adherence Self-report: 3 days; percent of doses taken |
| Safren et al, 200137 |
Boston, MA GLBT clinic N = 56 89% Male 70% MSM 30% AA 19% LAT 44% WH |
Starting or changing medications or <100% adherent in last 2 weeks |
1 session (a, b, c, d) Baseline n = 30 –Cognitive-behavioral, problem-solving, and motivational interviewing techniques, including an educational videotape with 1-week telephone F/U Delivered by heath care provider |
(d) Baseline n = 26 –Self-monitoring use of a daily diary to record number of pills taken |
First F/U (2 weeks) R I: 100% R C: 100% Second F/U (12 weeks) R I: 93% R C: 96% |
VL (N) Adherence Self-report: 14 days; percent of doses taken |
| Safren et al, 200344 |
Boston, MA GLBT clinic N = 70 80% Male 67% MSM 30% AA 17% LAT N/R WH |
Self-reported adherence problems and had adherence ≤90% during 2-week EDM monitoring phase |
12 weeks (d) Baseline n = 34 –EDM monitoring –Pager text-messaging system for reminders of doses, meals, and appointments |
Baseline n = 36 –EDM monitoring alone |
Post R I: 56% R C: 69% |
VL (N) Adherence EDM: 14 days; percent of doses taken |
| Samet et al, 200543 |
Boston, MA Medical center N = 151 80% Male 35% MSM 75% AA N/R LAT 45% WH |
History of alcohol problems, English- or Spanish-speaking, Mini Mental Status score ≥21, plan to stay in area next 2 years |
3 months (a, b, c, d) Baseline n = 74 –4 sessions of motivational interviewing addressing alcohol problems, individually tailored assistance to facilitate medication use –Watch with a programmable timer Delivered by heath care provider |
Baseline n = 77 –SC |
Post R I: 80% R C: 78% |
VL (Y) >500 copies/mL Adherence Self-report: 30 days; percent of doses taken |
| Smith et al, 200339 |
Chapel Hill, NC Hospital clinic N = 43 91% Male 53% MSM N/R AA N/R LAT 26% WH |
Initiating new HAART regiment that included a PI or switching to a new PI-containing regimen |
12 weeks (a, b, c, d) Baseline n = 22 –6 session self-management program consisting of information exchange, skills development, self-monitoring, goal setting, social support, and self-incentives enlistment Delivered by heath care provider and pharmacist |
(d) Baseline n = 21 EDM reminder |
Post R I: 36% R C: 43% |
VL (Y) >50 copies/mL Adherence EDM: continuous monitoring during study period; percent of doses taken |
| Tuldra et al, 200024 |
Barcelona, Spain HIV clinic N = 116 75% Male 28% MSM N/A AA, LAT, WH |
First patient each day initiating first- or secondline HAART |
1 session (a, b, c) Baseline n = 55 –Psychoeducational intervention based on self-efficacy theory and clinical practice aimed to improve pts’ knowledge and habits in handling medications; includes addressing doubts, developing dosage scheduling, strategies to handle problems, and reinforcing adherence Delivered by psychologist |
Baseline n = 61 –Usual clinic F/U, which included 1 session with a psychologist, who recorded variables related to medication adherence |
First F/U (4 weeks) R I: 56% R C: 52% Second F/U (24 weeks) R I: 56% R C: 52% Third F/U (48 weeks) R I: 58% R C: 59% |
VL (Y) >400 copies/mL Adherence Self-report: month before assessment; percent of pills taken |
| Weber et al, 200440 |
Zurich, Switzerland HIV clinic and psychotherapists in private practice; N = 58 83% Male 55% MSM N/A AA, LAT, WH |
In Swiss HIV cohort, stable HAART regimen, VL <50 copies/mL in past 3 months, no active IDU |
12 months with 3-25 sessions (b, c) Baseline n = 31 –Individual cognitive-behavioral therapy to address medication adherence and 1 other individually determined goal Delivered by psychologist |
Baseline n = 27 –SC –Included 1 30-minute cognitive session |
Post R I: 94% R C: 89% |
VL (Y) >50 copies/mL Adherence EDM: 30 days; percent of doses taken |
AA indicates African American; AT, as-treated analysis; C, control; CD, cue dosing; CDC, US Centers for Disease Control and Prevention; CD4, CD4+ lymphocyte count; CR, cash reinforcement; EDM, electronic drug monitoring; F/U, follow-up; GLBT, gay, lesbian, bisexual, and transgender; I, intervention; IDU, injection drug use; ITT, intent-to-treat analysis; LAT, Latino; PI, protease inhibitor; PMAQ7, patient medication adherence questionnaire-7; Pt, participant; SC, standard care; WH, white.
Intervention component codes: (a) didactic information on HAART; (b) interactive discussion of cognitions, motivations, and expectations about taking HAART; (c) behavioral strategies; (d) external reminders such as pagers.
Intervention Characteristics and Components
An examination of intervention characteristics revealed that the most common delivery method was 1-on-1 counseling (55%); an additional 16% of the studies used a group format. The most common interveners were health care providers such as physicians or nurses (47%) or mental health counselors such as trained psychologists (26%), with 53% of studies using research staff (rather than clinic staff) to provide the intervention. The median number of intervention sessions was 2 (range: 1–54 sessions), the median amount of time for each session was 60 minutes (range: 45 minutes to 2.5 hours), and the median intervention duration was 70 days (range: 1 day to 1 year).
Regarding the components designed to promote adherence, almost every study provided in the intervention or control arm didactic information on HAART (79%) or interactive discussions addressing cognitions, motivations, and expectations about taking HAART (79%; eg, motivational interviewing, group therapy addressing coping with HIV-related stigma). Behavioral strategies were reported by 84% (eg, cue dosing, cognitive-behavior therapy), and 26% used external reminders such as pagers. Studies involved 1 (16%), 2 (10%), 3 (58%), or 4 (16%) of these different components.
Methodologic Quality of the Studies
All studies used an intent-to-treat analysis in which participants were analyzed based on original randomization assignment. Overall, retention rates (pooling across arms) ranged from 40% to 100% (median = 80%) immediately after the intervention and from 55% to 100% (median = 70%) at first follow-up. Retention rates did not differ significantly between arms at either assessment period for any study. Most (58%) of the studies used self-report to measure adherence; the other studies relied on electronic data monitoring. The number of follow-up assessments varied from 0 to 3 (median = 1) and ranged from 14 days to 510 days (median = 140 days) after the end of the intervention. For first follow-up, the period ranged from 14 to 365 days (median = 56 days). One third of the studies treated missing values as equivalent to failure or imputed values; the remainder omitted participants from analyses for which they had missing data.
Effect Sizes for 95% Adherence
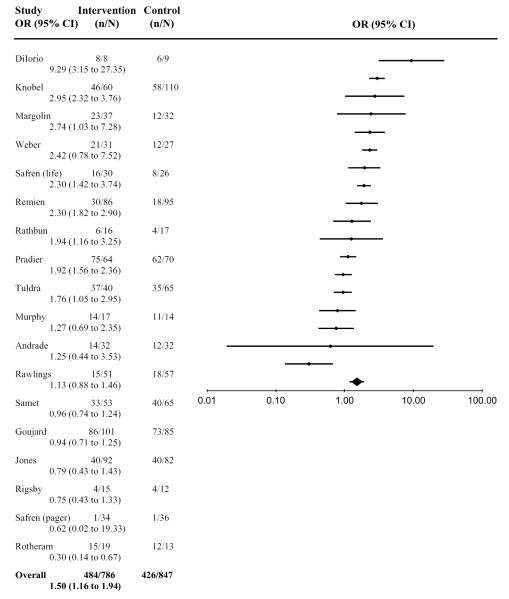
Data were available from 18 studies for 95% adherence: 5 from the immediate postintervention assessment and 13 from the first follow-up. Adherence recall periods varied from 3 to 30 days (median = 7 days). For these 18 studies, 62% (484 of 786) of intervention arm participants and 50% (426 of 847) of control arm participants achieved 95% adherence. The aggregated effect size was significant (OR = 1.50, 95% CI: 1.16 to 1.94; N = 1633) indicating that, overall, the likelihood of achieving at least 95% adherence was higher in the intervention arm than in the control arm. The effect was homogeneous (Q = 20.3, df = 18; P = 0.26), and sensitivity tests revealed that the overall significance did not change when any single finding was omitted. Figure 2 presents the individual effect-size estimates and shows that the intervention effect was significant (P < 0.05) for 8 studies.24,31–37
FIGURE 2.
Overall effect-size estimates among HAART adherence interventions for 95% adherence.
Effect Sizes for Undetectable Viral Load
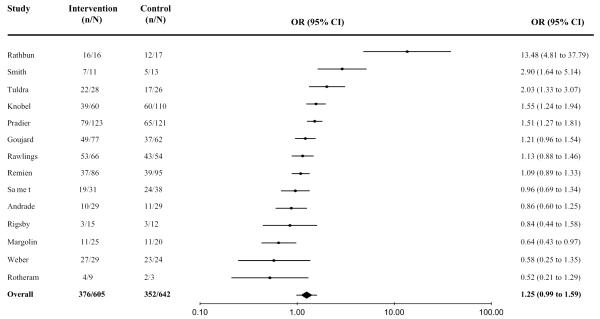
Data on undetectable VL were available from 14 studies: 4 from the immediate postintervention assessment and 10 from the first follow-up. Thresholds of detection for VL were 50 copies/mL,23,25,38–40 200 copies/mL,41 400 copies/mL,24,31,34,35,42 and 500 copies/mL.43
Overall, 62% (379 of 605) of intervention arm participants and 55% (352 of 642) of control arm participants achieved an undetectable VL. The aggregated effect size was marginally significant (OR = 1.25, 95% CI: 0.99 to 1.59, N = 1247), indicating that, overall, the likelihood of achieving an undetectable VL tended to be higher in the intervention arm than in the control arm. The effect was homogeneous (Q = 8.2, df = 14; P = 0.83), and sensitivity tests did not reveal any appreciable changes when individual findings were removed. Figure 3 presents the individual effect sizes; 5 were significant (P < 0.05).24,32,34,35,39
FIGURE 3.
Overall effect-size estimates among HAART adherence interventions for undetectable VL.
Stratified Analyses for 95% Adherence and Undetectable Viral Load
As seen in Table 2, there was only 1 significant stratification variable according to the QB statistic: the effect size was significantly larger in studies that had a 2-week or 1-month recall period for 95% adherence than in studies that had a recall period ≤7 days (QB = 3.97; P < 0.05). Additional analyses indicated that for a recall period ≤7 days, 95% adherence was similar in the intervention arm (67%) and control arm (62%), whereas for the longer recall periods, it was appreciably higher in the intervention arm (55%) than in the control arm (40%). None of the other stratification analyses conducted for 95% adherence or for undetectable VL yielded significant differences between subgroups. The relatively small number of studies in these subgroups likely decreased power to detect differences. There were several instances, however, in which the effect size was significant (ie, the 95% CI did not include 1) for one subgroup but not the other. These differences may be suggestive of potentially important moderating factors. We identified 4 variables for which these differences were consistently observed in the 95% adherence and undetectable VL outcomes. Specifically, effect sizes tended to be higher in studies conducted outside the United States (vs. domestically); in studies with interventions that included didactic information on HAART (vs. studies without this feature); in studies in which the intervention included interactive discussion of cognitions, motivations, and expectations (vs. studies without that feature); and in studies in which the outcome data came from the first follow-up (vs. immediate postintervention assessment).
TABLE 2.
Stratified Analyses of Aggregated Effect Sizes for 95% Adherence and Undetectable Viral Load Outcomes
| 95% Adherence |
Undetectable Viral Load |
|||
|---|---|---|---|---|
| k | OR (95% CI) | k | OR (95% CI) | |
| Study and sample characteristics | ||||
| Conducted in United States | 13 | 1.30 (0.96 to 1.71)23,25,31,33,35,36–38,42–44,48,49 | 9 | 1.06 (0.75 to 1.51)23,25,31,33,35,38,39,42,43 |
| Conducted elsewhere | 5 | 1.89 (1.28 to 2.82)24,32,34,40,41 | 5 | 1.45 (1.04 to 2.02)24,32,34,40,41 |
| 80% or more participants male | 8 | 1.21 (0.79 to 1.84)25,35,37,40,41,43,44,49 | 6 | 1.24 (0.75 to 2.05)25,35,39–41,43 |
| Less than 80% of participants male | 10 | 1.65 (1.16 to 2.34)23,24,31–34,36,38,42,48 | 8 | 1.26 (0.96 to 1.65)23,24,31,33,34,38,42 |
| 50% or more participants MSM | 5 | 1.76 (0.87 to 3.57)25,35,37,40,44 | 4 | 1.83 (0.50 to 6.67)25,35,39,40 |
| Less than 50% of participants MSM | 5 | 1.21 (0.79 to 1.84)24,33,42,43,48 | 4 | 1.15 (0.78 to 1.71)24,33,42,43 |
| HAART naive | 3 | 1.33 (0.73 to 2.43)24,35,42 | 3 | 1.22 (0.94 to 1.58)24,35,42 |
| Not HAART naive | 15 | 1.52 (1.22 to 2.07)23,25,31–34,38,40,41,48,49 | 11 | 1.66 (0.70 to 3.94)23,25,31–34,38–41,43 |
| No marker for poor baseline adherence | 14 | 1.70 (1.02 to 2.86)23–25,31,32,34–36,38,40–43,48 | 13 | 1.29 (0.99 to 1.68)23–25,31,33–35,38–43 |
| Marker for poor baseline adherence | 4 | 2.09 (1.18 to 3.69)33,37,44,49 | 1 | __33 |
| No marker for baseline detectable VL | 15 | 1.42 (1.07 to 1.87)23,25,31,33–38,40,41,43,44,48,49 | 11 | 1.19 (0.90 to 1.58)23,25,31,33–35,38–41,43 |
| Marker for baseline detectable VL | 3 | 1.83 (0.94 to 3.59)24,32,42 | 3 | 1.43 (0.91 to 2.24)24,32,42 |
| Intervention characteristics and components | ||||
| Delivered by study staff | 9 | 1.74 (1.26 to 2.40)23,25,31,33–35,37,42,49 | 8 | 1.25 (0.91 to 1.71)23,25,31,33–35,39,42 |
| Not delivered by study staff | 9 | 1.35 (0.89 to 5.05)24,32,36,38,40,41,43,44,50 | 6 | 1.26 (0.87 to 1.82)24,32,38,40,41,43 |
| 5 or more intervention sessions | 6 | 1.49 (0.82 to 2.74)23,25,31,40,48,49 | 5 | 1.24 (0.76 to 2.04)23,25,31,35,36(23,25,31,39,40) |
| Fewer than 5 sessions | 10 | 1.49 (1.13 to 1.98)24,32–37,41–44 | 7 | 1.26 (0.96 to 1.65)24,32,34,35,41–43 |
| Didactic information on HAART | 7 | 1.86 (1.25 to 2.79)25,32,36,38,40,43,48 | 7 | 1.41 (1.03 to 1.93)24,25,31,32,34,39,41 |
| No didactic information on HAART | 11 | 1.26 (0.94 to 1.68)23,24,31,33–35,37,41,42,44,49 | 7 | 1.06 (0.73 to 1.54)23,33,35,38,40–43 |
| Interactive discussion of cognitions, motivations, and expectations about adherence |
14 | 1.62 (1.21 to 2.03)24,25,31–37,40,42,43,48,49 | 11 | 1.30 (1.00 to 1.70)24,25,31–35,39,40,42,43 |
| No interactive discussion of cognitions, motivations, and expectations about adherence |
4 | 0.99 (0.55 to 1.79)23,38,41,44 | 3 | 1.07 (0.62 to 1.86)23,38,41 |
| Behavioral strategies | 15 | 1.34 (1.03 to 1.75)23–25,31,33–37,40–43,48,49 | 12 | 1.28 (0.98 to 1.68)23–25,31–35,38,40–43 |
| No behavioral strategies | 3 | 2.31 (1.41 to 3.79)32,38,44 | 2 | 1.16 (0.70 to 1.92)32,38 |
| External reminder (eg, pager) | 4 | 1.00 (0.62 to 1.63)32,41,44,37 | 4 | 1.15 (0.72 to 1.86)38,39,41,43 |
| No external reminder | 14 | 1.69 (1.24 to 2.29)23–25,31–37,40,42,48,49 | 10 | 1.29 (0.98 to 1.70)23–25,31–35,40,42 |
| Involved only 1 intervention component | 3 | 1.05 (0.45 to 2.46)23,38,44 | 1 | __38 |
| Involved (any) 2 intervention components | 9 | 1.77 (1.18 to 2.67)31–33,35,36,40,42,48,49 | 6 | 1.19 (0.84 to 1.69)31–34,40 |
| Involved (any) 3 intervention components | 6 | 1.33 (0.92 to 1.95)24,25,34,37,41,43 | 5 | 1.35 (0.94 to 1.93)24,25,34,41 |
| Involved all 4 intervention components | 1 | — | 1 | __39 |
| Control received an intervention component | 8 | 1.30 (0.90 to 1.88)33,35–37,43,48,49 | 5 | 1.19 (0.80 to 1.78)33,35,39,42,43 |
| Control received standard of care or was wait-listed |
10 | 1.75 (1.25 to 2.43)23–25,31,32,34,38,40,41,44 | 9 | 1.29 (0.96 to 1.74)23–25,31,32,34,38,40,41 |
| Methodologic quality variables | ||||
| Baseline N ≥ 50 per arm | 8 | 1.43 (0.99 to 2.04)24,32–34,41–43,48 | 7 | 1.31 (1.01 to 1.69)24,32–34,41–43 |
| Baseline N < 50 per arm | 10 | 1.73 (1.09 to 2.73)23,25,31,36–38,40,44,49 | 7 | 1.00 (0.54 to 1.84)23,25,31,35,38–40 |
| Self-report adherence measure | 11 | 1.39 (0.92 to 1.13)24,25,31,32,36,37,41,43,44,48,49 | — | — |
| Other (more “objective”) measure of adherence | 7 | 1.70 (1.22 to 2.37)23,33–35,38,40,42 | — | — |
| First follow-up <60 days | 8 | 1.49 (1.04 to 2.14)23,24,33–37,42,43 | 6 | 1.18 (0.81 to 1.73)23,24,33,35,42,43 |
| First follow-up ≥60 days | 6 | 1.60 (0.92 to 2.79)25,31,32,34,41,49 | 5 | 1.33 (0.96 to 1.85)25,31,32,34,41 |
| Retention rate <80% at immediate post or <70% at follow-up |
11 | 1.60 (1.09 to 2.34)23,31–33,35–37,40,41,43,48 | 8 | 1.16 (0.84 to 1.61)23,31–33,40,41,43,45 |
| Retention rate <80% immediately after intervention or <70% at follow-up |
6 | 1.45 (0.95 to 2.20)24,25,34,42,44,49 | 6 | 1.46 (1.00 to 2.14)24,25,34,38,39,42 |
| Differential retention rate ≤5% | 8 | 1.67 (1.07 to 2.58)31,32,35,37,40,42,44,49 | 5 | 1.23 (0.75 to 2.04)31,32,35,40,42 |
| Differential retention rate >5% | 10 | 1.44 (1.05 to 1.97)23–25,33,34,36,38,41,43,44 | 9 | 1.26 (0.94 to 1.67)23–25,33,34,38,39,41,43 |
| Imputed missing data | 6 | 1.55 (1.02 to 2.34)24,31,33,34,42,48 | 5 | 1.25 (0.91 to 1.72)24,31,33,34,42 |
| Did not impute missing data | 12 | 1.48 (1.04 to 2.10)23,25,32,35–38,40,41,43,44,49 | 9 | 1.26 (0.87 to 1.81)23,25,32,35,38–41,43 |
According to the between-group heterogeneity statistic QB, for each comparison, there were no statistically significant (P < 0.05) differences between effect sizes. Effect sizes are not given for subgroups with only 1 study.
Numbers of studies fluctuate across stratification variables because some studies did not report information on the variable.
Intervention components were coded as present only if they were included as part of the intervention and not the control arm.
Publication Bias
There was no evidence that our effect-size estimates were inflated because of noninclusion of studies with nonsignificant findings.
DISCUSSION
Results from this meta-analytic review indicate that HAART adherence interventions for adults can be efficacious. The magnitude of the aggregated OR indicated that participants who received an intervention were 1.5 times as likely to report 95% adherence and 1.25 times as likely to achieve an undetectable VL as participants in the control arm. These findings are encouraging because they suggest that adherence interventions can have a significant positive effect on adherence behaviors and some positive effect on biologic indicators of adherence.
In considering why the effect size was higher for 95% adherence than for the undetectable VL, one might be tempted to attribute this difference to measurement factors. The VL outcome mainly was obtained through blood draws or medical charts, whereas the 95% adherence outcome was based on self-report in most studies. Our findings do not indicate that the self-report data inflated the intervention effect, however. Indeed, the effect size for 95% adherence was somewhat larger in studies that used more objective assessments of adherence (eg, electronic drug monitoring, pill counts) than in studies using self-reports of adherence (see Table 2). Although bias cannot be completely ruled out with these more objective measures, it does not seem that the manner in which adherence was measured explains the difference. More likely, the difference may stem from clinical or biologic factors. It is possible that the HAART regimens might not have been sufficiently potent or that resistance inhibited viral suppression even in the presence of high levels of adherence (these data were not available for this review). Future research should examine these possible explanations.
Stratification analyses indicated that the intervention effect was significantly stronger in studies that used a longer recall period (ie, 2 weeks or 1 month) versus a shorter one (ie, ≤7 days) for 95% adherence. This suggests that assessment of adherence over longer periods may be more sensitive in detecting an intervention effect. There were no other statistically significant moderators for 95% adherence or undetectable VL. There were several trends that deserve attention, however. The intervention effect sizes tended to be larger in studies that provided didactic information on HAART and in studies that included interactive discussion of cognitions, motivations, and expectations regarding adherence. These findings suggest the importance of providing basic information to patients and engaging patients in discussions to help overcome cognitive factors (eg, avoidance coping), lack of motivation, and unrealistic expectations about adherence behaviors. Studies that included behavioral strategies such as external rewards and cue dosing were as efficacious as studies that did not. Also, studies that used external reminders such as pagers were no more effective than studies that did not; in fact, for 95% adherence outcomes, the latter studies did better. Although these trends are of interest, they must be viewed with caution, because many studies used multiple intervention components, thus precluding an unconfounded analysis of specific components. Also, noting the consistency of effects across outcomes of 95% adherence and undetectable VL may be informative but is an imperfect way to determine which stratification variables are most robust, especially because only 13 of the 19 studies in this review even included both outcomes.
Overall, our findings suggest that a wide variety of interventions may be efficacious. For example, in the study by Remien et al,33 a 4-session comprehensive intervention for couples delivered by a nurse practitioner demonstrated some success in increasing adherence. In contrast, in the studies by Knobel et al32 and Rathbun et al,35 a single didactic session with a pharmacist was efficacious. Because resources for adherence interventions are quite constrained in many settings and populations, it is promising that providers may choose from a diverse range of potentially effective strategies.
Our findings generally concur with those of the only other published meta-analytic review21 of HAART adherence intervention studies. Both reviews found that interventions as a whole were efficacious in improving adherence. This consistency is encouraging, especially because the prior review did not focus exclusively on RCTs and defined the outcome differently (ie, as the standardized mean difference in continuous estimates of adherence rather than the relative proportion of participants who achieved 95% adherence). Unlike our analysis, however, the prior review found that the intervention effect was significantly stronger in studies that enrolled only participants with known or anticipated adherence problems compared with studies that did not target potential participants on this dimension. Because their finding could not be fully explained by statistical regression to the mean in pre-to-postintervention comparisons of behavior change (R. Amico, PhD, personal communication, 2006), it warrants further investigation.
The limitations of our meta-analysis reflect the limitations of the primary studies. One limitation is that more than half of the studies relied solely on self-reported adherence. Although self-report has been shown to have some validity in assessing antiretroviral adherence,45,46 it may not provide the most accurate estimate of adherence and may be prone to socially desirable responding in an intervention trial. As discussed previously, however, it does not seem that the intervention effect size was biased by self-reports. Another issue is the sustainability of intervention effects over time. Current clinical guidelines recommend that patients take HAART continuously, over a period of years, to bolster immune functioning and suppress viral replication. Follow-up assessments for the studies in our review, if included at all, occurred an average of 60 days after completion of the intervention. Some studies included a follow-up assessment but no immediate postintervention assessment with which to compare the results. This omission, along with the considerable range in intervention duration and in follow-up length, makes it difficult to interpret our counterintuitive finding that effect sizes tended to be higher in studies in which the outcome data came from the first follow-up versus immediate postintervention assessment. It would be valuable if future interventions assessed behavior at multiple assessment periods and for longer periods after the intervention. Additionally, a lack of reporting on potentially important variables (eg, specific medication regimens, indicators of resistance) in the primary studies limited our ability to examine more closely clinical moderators of the intervention effects on VL. Clear and transparent reporting of key elements such as these in intervention studies would improve the quality of future meta-analyses.47
Certainly, more research in this area is needed. All the studies we reviewed targeted the individual patient, but most typologies point to at least 3 other major influences on adherence: characteristics of the provider, characteristics of the medication regimen, and macrolevel contextual factors such as clinic accessibility. Future intervention studies might successfully explore these areas. Also, all the interventions were conducted in the United States or other nations of the West. The challenges of working in severely resource-constrained settings, where there may be fewer highly educated professionals and less money for technologically sophisticated equipment, may require different intervention strategies. Fortunately, many other interventions are currently being evaluated.18 We await the results of those projects, including those investigating directly observed therapy, which was not independently evaluated in any of the studies we reviewed. Finally, there is a paucity of data to guide the implementation of adherence interventions in clinical settings. Meeting the challenge of translating interventions that are efficacious in research trials into effective clinic-based strategies that can also be used in resource-poor areas requires an ongoing operational research agenda.
Acknowledgments
Supported in part by National Institutes of Health (NIH) grants to J. M. Simoni (2 R01 MH 58986) and D. W. Pantalone (F31 MH71179) and an endowed Minority Dissertation Fellowship to C. R. Pearson.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the University of Washington, the US Centers for Disease Control and Prevention, or the NIH.
REFERENCES
- 1.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 4.Low-Beer S, Yip B, O’Shaughnessy MV, et al. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 6.Salomon H, Wainberg MA, Brenner B, et al. Prevalence of HIV-1 resistant to antiretroviral drugs in 81 individuals newly infected by sexual contact or injecting drug use. Investigators of the Quebec Primary Infection Study. AIDS. 2000;14(Suppl):F17–F23. doi: 10.1097/00002030-200001280-00003. [DOI] [PubMed] [Google Scholar]
- 7.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 8.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JA. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002;29(Suppl):S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- 10.Gordillo V, Amo JD, Soriano V, et al. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Fernandez J, Escobar-Rodriguez I, Campo-Angora M, et al. Evaluation of adherence to highly active antiretroviral therapy. Arch Intern Med. 2001;161:2739–2740. doi: 10.1001/archinte.161.22.2739. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwkerk PT, Sprangers MA, Burger DM, et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Arch Intern Med. 2001;161:1962–1968. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- 13.Moatti JP, Carrieri MP, Spire B, et al. Adherence to HAART in French HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance treatment. The Manif 2000 study group. AIDS. 2000;14:151–155. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- 14.Heckman BD, Catz SL, Heckman TG, et al. Adherence to antiretroviral therapy in rural persons living with HIV disease in the United States. AIDS Care. 2004;16:219–230. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- 15.Simoni JM, Frick PA, Pantalone DW, et al. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med. 2003;11:185–198. [PubMed] [Google Scholar]
- 16.Haddad M, Inch C, Glazier RH, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2000;3:CD001442. doi: 10.1002/14651858.CD001442. [DOI] [PubMed] [Google Scholar]
- 17.Fogarty L, Roter D, Larson S, et al. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns. 2002;46:93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- 18.Simoni JM, Pantalone DW, Frick PA, et al. Enhancing antiretroviral adherence: a review of published reports of randomized controlled trials and on-going NIH-funded research. In: Trafton JA, Gordon W, editors. Best Practices in the Behavioral Management of Chronic Disease. 2nd ed Institute for Disease Management; Los Altos, CA: 2005. pp. 70–95. [Google Scholar]
- 19.Ickovics JR, Meade CS. Adherence to antiretroviral therapy among patients with HIV: a critical link between behavioral and biomedical sciences. J Acquir Immune Defic Syndr. 2002;31(Suppl):S98–S102. doi: 10.1097/00126334-200212153-00002. [DOI] [PubMed] [Google Scholar]
- 20.Cote J, Godin G. Efficacy of interventions in improving adherence to antiretroviral therapy. Int J STD AIDS. 2005;16:335–343. doi: 10.1258/0956462053888934. [DOI] [PubMed] [Google Scholar]
- 21.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41:285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 22.Alderson P, Green S, Higgins JPT, editors. Cochrane Reviewers’ Handbook. 4.2.2. The Cochrane Library; Chichester, UK: 2004. [updated March 2004] [Google Scholar]
- 23.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15:841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuldra A, Fumaz CR, Ferrer MJ, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:221–228. doi: 10.1097/00126334-200011010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Rotheram-Borus MJ, Swendeman D, Comulada S, et al. Prevention for substance-using HIV-positive young people: telephone and in-person delivery. J Acquir Immune Defic Syndr. 2004;37(Suppl):S68–S77. doi: 10.1097/01.qai.0000140604.57478.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper H, Hedges LV. The Handbook of Research Synthesis. Russell Sage Foundation; New York: 1994. [Google Scholar]
- 27.Lipsey MW, Wilson DB. Practical Meta-Analysis. Sage; Thousand Oaks, CA: 2001. [Google Scholar]
- 28.Sutton AJ, Abrams KR, Jones DR, et al. Methods for Meta-Analysis in Medical Research. John Wiley & Sons Ltd; West Sussex, UK: 2000. [Google Scholar]
- 29.Hedges LV, Vevea JL. Fixed and random effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 30.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a sample, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolin A, Avants SK, Warburton LA, et al. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychol. 2003;22:223–228. [PubMed] [Google Scholar]
- 32.Knobel H, Carmona A, Lopez JL, et al. Adherence to very active antiretroviral treatment: impact of individualized assessment. Enferm Infecc Microbiol Clin. 1999;17:78–81. [PubMed] [Google Scholar]
- 33.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19:807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 34.Pradier C, Bentz L, Spire B, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials. 2003;4:121–131. doi: 10.1310/brbv-3941-h1pp-ndry. [DOI] [PubMed] [Google Scholar]
- 35.Rathbun RC, Farmer KC, Stephens JR, et al. Impact of an adherence clinic on behavioral outcomes and virologic response in treatment of HIV infection: a prospective, randomized, controlled pilot study. Clin Ther. 2005;27:199–209. doi: 10.1016/j.clinthera.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 36.DiIorio C, Resnicow K, McDonnell M, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care. 2003;14:52. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 37.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-Steps and medication monitoring. Behav Res Ther. 2001;39:1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 38.Andrade ASA, McGruder HF, Wu A, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis. 2005;41:875–882. doi: 10.1086/432877. [DOI] [PubMed] [Google Scholar]
- 39.Smith SR, Rublein JC, Marcus C, et al. A medication self-management program to improve adherence to HIV therapy regimens. Patient Educ Couns. 2003;50:187–199. doi: 10.1016/s0738-3991(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 40.Weber R, Christen L, Christen S, et al. Effect of individual cognitive behaviour intervention on adherence to antiretroviral therapy: prospective randomized trial. Antivir Ther. 2004;9:85–95. [PubMed] [Google Scholar]
- 41.Goujard C, Bernard N, Sohier N, et al. Impact of a patient education program on adherence to HIV medication: a randomized clinical trial. J Acquir Immune Defic Syndr. 2003;34:191–194. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 42.Rawlings MK, Thompson MA, Farthing CF, et al. Impact of an educational program on efficacy and adherence with a twice-daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34:174–183. doi: 10.1097/00126334-200310010-00007. [DOI] [PubMed] [Google Scholar]
- 43.Samet JH, Horton NJ, Meli S, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10:83–93. doi: 10.1177/135965350501000106. [DOI] [PubMed] [Google Scholar]
- 44.Safren SA, Hendriksen ES, Desousa N, et al. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care. 2003;15:787–793. doi: 10.1080/09540120310001618630. [DOI] [PubMed] [Google Scholar]
- 45.Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J Acquir Immune Defic Syndr. 2005;38:445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- 46.Simoni JM, Kurth AE, Pearson CR, et al. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV clinical management and research. AIDS Behav. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 48.Jones DL, Ishi M, LaPerriere A, et al. Influencing medication adherence among women with AIDS. AIDS Care. 2003;15:463. doi: 10.1080/0954012031000134700. [DOI] [PubMed] [Google Scholar]
- 49.Murphy DA, Lu MC, Martin D, et al. Results of a pilot intervention trial to improve antiretroviral adherence among HIV-positive patients. J Assoc Nurses AIDS Care. 2002;13:57–69. doi: 10.1177/1055329002238026. [DOI] [PubMed] [Google Scholar]