Abstract
Penicillium paneum is an important contaminant of cereal grains which is able to grow at low temperature, low pH, high levels of carbon dioxide, and under acid conditions. P. paneum produces mycotoxins, which may be harmful to animals and humans. We found that conidia in dense suspensions showed poor germination, suggesting the presence of a self-inhibitor. A volatile compound(s) produced by these high-density conditions also inhibited mycelial growth of different species of fungi belonging to a variety of genera, suggesting a broad action range. The heat-stable compound was isolated by successive centrifugation of the supernatant obtained from spore suspensions with a density of 109 conidia ml−1. By using static headspace analyses, two major peaks were distinguished, with the highest production of these metabolites after 22 h of incubation at 25°C and shaking at 140 rpm. Gas chromatography coupled with mass spectra analysis revealed the compounds to be 3-octanone and 1-octen-3-ol. Notably, only the latter compound appeared to block the germination process at different developmental stages of the conidia (swelling and germ tube formation). In this study, 1-octen-3-ol influenced different developmental processes during the P. paneum life cycle, including induction of microcycle conidiation and inhibition of spore germination. Therefore, the compound can be considered a fungal hormone during fungal development.
The spore is an important vehicle for distribution or long-term survival of fungi. Conidia are nonmotile asexual spores, which can be produced in very large numbers by fungi belonging to the order Eurotiales. This order, which includes the genera Aspergillus and Penicillium (14), contains many species of food-related fungi. Conidia are dispersed into the air but do not germinate before they reach a suitable substrate. Invariably, germination is inhibited when spores are present in high densities, an effect observed for example in Aspergillus niger and the zygomycete Syncephalastrum racemosum (3, 19). This is designated as the crowding effect. Also, in the formation of an adhesive bud on spores of the nematophagous fungus Drechmeria coniospora, there is a clear crowding effect (35). These observations suggest that intercellular signaling prevents premature germination.
Acervuli of Colletotrichum spp. form large numbers of conidia embedded in mucilage but not germinating there. From the mucilage that surrounds the conidia of this fungus, a self-inhibitor of germination named mycosporine-alanine has been identified and characterized (26). Germination is also retarded in pustules of rust fungi (Basidiomycetes) where cis-ferulic acid methyl ester and cis-3,4-dimethoxycinnamic acid methyl ester have been identified (28, 36).
Self-inhibitors have been characterized in Puccinia, Uromyces, Colletotrichum, Dictyostelium, Fusarium oxysporum, and Aspergillus and can be volatile or nonvolatile (2, 3, 16, 24, 28). They also can influence other fungal processes, for example, mycosporine-alanine produced by Colletotrichum graminicola prevents appressorium formation (25, 26). The self-inhibitors produced by Glomerella cingulata and Dictyostelium discoideum inhibit protein synthesis (2, 27).
Self-inhibitors inhibit spore germination reversibly. After removal of the compound from the spore or its environment, germination is initiated. The major function of self-inhibitors is stated as prevention of premature germination of spores directly after spore formation and before dispersal. This mechanism guarantees that spores only germinate after dispersal into environments that favor outgrowth to establish mycelium. Self-inhibitors can be localized outside the conidial cells, for instance, inside the mucilage of acervuli. The compound also can be localized and associated with the cell wall of the spore (29) and can be removed by washing with water (17, 34).
Penicillium paneum has been assigned to the Penicillium roqueforti group on the basis of morphological studies, genetic characterization, and secondary metabolite profiles (7). This fungus is an important contaminant of cereal grains and is able to grow at low oxygen levels, low pH, and high levels of carbon dioxide, as well as under acid conditions (6, 32). P. paneum can produce mycotoxins such as roquefortine C and patulin, which are harmful to animals and humans (7). Studies of self-inhibitors are of interest in understanding the regulatory mechanisms involved in germination. Investigation of self-inhibitors is relevant to the development of techniques that prevent food spoilage and crop diseases. Here we report the identification of 1-octen-3-ol as a volatile self-inhibitor in P. paneum.
MATERIALS AND METHODS
Preparation of conidial suspensions.
The strain used in this work was recently classified after morphological studies and genetic characterization (β-tubulin) as P. paneum and previously described as P. roqueforti LU 510 (11). P. paneum was grown on malt extract agar (MEA) medium (CM59; Oxoid Ltd., Hampshire, United Kingdom) inside glass tubes at 25°C for 7 days. Spore suspensions were prepared by addition of 9 ml of peptone physiological salt solution (8.5 g of NaCl liter−1 with 1 g of bacteriological peptone [Oxoid] liter−1, supplemented with 0.1% Tween 80) to the culture. Suspensions were prepared from 25 tubes and filtered through a 17-μm-pore-size nylon filter, reaching a final volume of 180 ml. Subsequently, the suspension was centrifuged (4,000 × g) for 3 min. Conidia were resuspended in 2 ml of malt extract broth (MEB) medium (CM57; Oxoid), and the suspension was adjusted to pH 4.0 with lactic acid. The spore suspensions were adjusted to densities of 109, 108, 107, and 106 conidia ml−1, and cells were counted in a hemocytometer (Bürker Türk).
Germination of conidia on different media.
For germination studies, conidia were inoculated in MEB (pH 4.0) and incubated in a water bath at 25°C with shaking at 140 rpm for 10 h. High-density spore suspensions with 109 conidia ml−1 were then diluted in MEB to 106 conidia ml−1and incubated for another 10 h. As a control, germination of P. paneum at 106 conidia ml−1 was also studied. Germination was also analyzed on solid medium. Droplets (5 μl) of conidia suspensions containing 106 to 109conidia ml−1 were placed on microscopic slides coated with a very thin layer (0.5 mm) of MEA medium, and the slides were incubated for 24 h at 25°C in a petri dish containing wet filter paper. Germination was determined by examination of at least 100 conidia harvested with adhesive tape from the surface of separate colonies, by using an Ltda BX40 microscope (×1,000 magnification; Olympus Optical Co., Tokyo, Japan). The criterion used to measure germination was the emergence of germ tubes (18). The experiments were performed in triplicate.
Activity of volatile metabolites on radial growth.
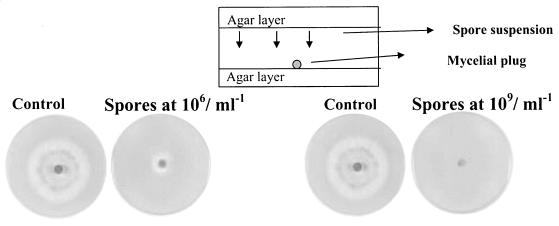
The method described here for study of the inhibition of fungal radial growth by volatile metabolites was developed by Lisa Fredlund (Swedish University of Agricultural Sciences, Uppsala, Sweden; personal communication). In the technique, one petri dish containing two layers of agar, a top and a bottom layer, is used (see illustration of the method in Fig. 3). The top side contained the inoculated spore suspension. In our study, this consisted of 106 or 109 conidia ml−1 on 3% MEA (wt/vol). For this, a volume of 50 μl of the P. paneum spore suspension was lined onto the surface of the agar by using a spiral plater (I.K.S. BV, Leerdam, The Netherlands). On the bottom side of the plate, a mycelial plug was placed that had been obtained from the periphery of a 5-day-old MEA culture. The plate was incubated for 7 days at 25°C. As a control, a plate lacking conidia on the top layer was incubated. The diameters of colonies on the bottom layer (excluding the central plug) were measured. The effect of volatile compounds produced by P. paneum conidia at 106 conidia ml−1 was tested on the other fungal species, including P. roqueforti strain LU 513, A. niger, Aspergillus parasiticus, Aspergillus flavus, Fusarium culmorum VTT D-80148, and Rhizopus oligosporus LU 575. Experiments were done with four- to sixfold replication.
FIG. 3.
Inhibition of mycelial development by P. paneum. The plates contained MEA medium with 3% agar on the top and bottom sides. On the bottom side of the plate, a mycelial plug of P. paneum was placed in the center. On the upper layer, the spore suspensions with 106 or 109 conidia ml−1 were distributed by spiral plating. The plates were incubated for 7 days at 25°C. As a control, plates were incubated without P. paneum spore suspensions.
Extraction of the volatile compounds.
For extraction of the inhibitor(s), a modified version of the procedure of Leite and Nicholson (26) was used. The compound(s) was extracted from a suspension of 109 conidia ml−1 immediately after preparation of the suspension (0 h) and after 22 and 45 h of incubation at 25°C. We tested three different solutions for incubation of conidia for further extraction: MEB at pH 4.0, MEB at pH 6.0, and distilled water. Under all conditions, the percentage of inhibition was the same (data not shown). From that point on, the experiments were done using extracted compound(s) in MEB at pH 4.0, since the germination of spores was also assessed in the same medium. The suspensions were centrifuged at 4,000 × g for 4 min at 20°C to remove the conidia. The supernatant was centrifuged at 7,000 × g for 10 min at 15°C, and the supernatant obtained was centrifuged at 13,000 × g for 20 min at 4°C and filter sterilized through a 0.2-μm-pore-size filter (FP 030/03; Schleicher and Schuell GmbH, Dassel, Germany). This extract solution was tested against fresh conidia at 106 P. paneum conidia ml−1, and the germination of conidia was assessed after 7 h. As a control, fresh conidia with a density of 106 conidia ml−1 in MEB (pH 4) were used. The results are the means of three independent experiments.
Detection and identification of the volatile compound(s).
A volume consisting of 2 ml of 109 conidia ml−1 of each of the suspensions was transferred to a headspace vial, which was closed with a Teflon-butyl seal and a magnetic crimp cap. Samples were analyzed by static headspace analysis using a Fisons Instruments autosampler HS 800 (Interscience, Breda, The Netherlands) gas chromatograph. The column was a 30-m by 0.54-mm-inside-diameter (film thickness = 1.0 μm) fused silica DB-WAX column (J & W Scientific), with 30 kPa for detection of the volatile compounds. The oven temperature was held at 60°C for 5 min and then programmed to 110°C at a rate of 3°C/min, 170°C at 4°C/min, and at 200°C for the 3-min isotherm. The control was MEB medium at pH 4.
For dynamic headspace analysis, 2 ml of suspensions (109 conidia ml−1) that had been incubated for 22 h was added to 8 ml of distilled water and transferred to the purge and trap. Different suspensions were incubated in polypropylene tubes (Greiner GmbH, Frickenhousen, Germany) or in glass tubes. Nitrogen gas at a flow rate of 40 ml/min was passed through spore suspensions held at 25°C in a water bath for 15 min, to trap the volatile compounds in a glass tube (160 mm by 4-mm inside diameter) filled with 90 mg of 20/35 Tenax TA mesh (Chrompack, Breda, The Netherlands). For subsequent analysis, the Tenax trap was heated for 10 min in a thermodesorption cold trapping injector (Chrompack 16200) at 250°C with a helium flow rate of 15 ml/min. Upon ballistic heating of the cold trap, the compounds were transferred to the connected DB5 capillary column (60 m by 0.25-mm inside diameter; 0.25-μm film thickness) programmed from 60 to 280°C at a rate of 6°C/min. The column was connected to a Finnigan MaT95 mass spectrometer, operated in the 70-eV electron ionization mode, and set for scanning from molecular masses ranging from 24 to 300 at 0.7 s/degree. Compounds were identified by matching the spectra against those in the NIST98 library and the Wageningen library of mass spectra and by checking their Kovats (DB5) indices. Three different experiments were performed for identification of the compound(s).
Inhibitory assay.
Spore suspensions were incubated in the presence of 4 mM 3-octanone (Fisher Scientific BV, Hertogenbosch, The Netherlands) and 1-octen-3-ol (Janssen, Geel, Belgium). After 3 and 7 h, 100 conidia were analyzed under the microscope, and the percentage of germinated spores was calculated, as described above. The control used consisted of spores suspended in MEB medium. The results are the means of three independent experiments.
RESULTS
Effect of spore density on germination efficiency.
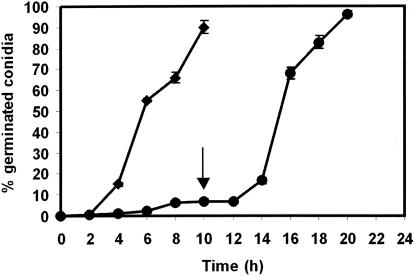
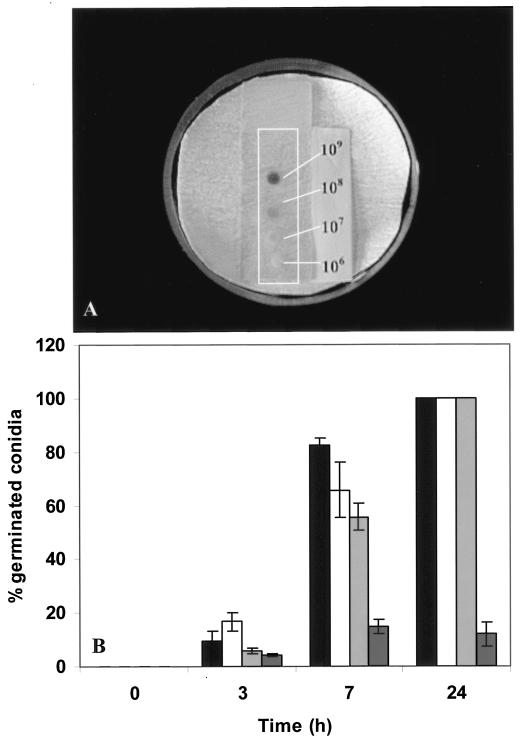
Suspensions of 106 P. paneum conidia per ml (controls) showed 90% germination within 10 h of incubation in MEB. However, at 109 conidia ml−1 only 7% germination was observed. In order to test if this reduction was a consequence of the increased spore density, a dense spore suspension was diluted to 106 conidia ml−1 with MEB (pH 4.0). Indeed, after an additional 10 h of incubation in MEB, 96% of the conidia had germinated (Fig. 1). When 5-μl volumes of suspensions ranging from 106 to 109 conidia ml−1 were placed on a very thin (0.5-mm) agar layer for 24 h (Fig. 2A), all spores germinated in inoculum densities of 106 to 108 conidia ml−1, but only 10% of conidia germinated in the case of 109 conidia ml−1 (Fig. 2B).
FIG. 1.
Germination of P. paneum conidia at 25°C. Densities tested were 106 (♦) and 109 (•) spores ml−1. The arrow represents the time (10 h) at which the spore suspension was diluted to 106 conidia ml−1. Three independent experiments were performed, and the error bars show the standard deviations.
FIG. 2.
Germination efficiency at different concentrations of P. paneum conidia on MEA placed on microscope slides and harvested at 3, 7, and 24 h at conidial concentrations of 106 (black bars), 107 (white bars), 108 (light grey bars), and 109 (dark grey bars) spores ml−1. (A) Conidia suspensions on the microscopic slide after 24 h. (B) Results of the assessment of germination efficiency are shown. Three independent experiments were performed, and the error bars show the standard deviations.
Inhibition of radial mycelial development by P. paneum conidia.
To investigate whether a volatile was involved in the effect noted above, mycelial growth was analyzed in a system where the conidia suspension was placed on the upper part of the plate while a mycelial plug was kept in the bottom part (Fig. 3). At density levels of 106 conidia ml−1 on the top side of the plate, the radial growth of P. paneum was already restricted, suggesting that the inhibitory compound(s) was volatile. At 109 conidia ml−1 mycelial growth was completely inhibited. Different fungal species were tested for analysis of the inhibitory spectrum. The mycelial growth of P. paneum, P. roqueforti, A. niger, A. parasiticus, A. flavus, F. culmorum, and R. oligosporus was restricted by P. paneum spore suspensions of 106 conidia ml−1 that had been distributed by spiral plating on the upper part of the plates (Table 1). These results show that a volatile compound(s) inhibits hyphal growth in different fungal species.
TABLE 1.
Inhibition of radial development of mycelia of several fungi by a spore suspension of P. paneum
| Fungi | Mycelial growth diameter (cm)a
|
|
|---|---|---|
| Control | With inhibitor | |
| P. paneum | 4.2 ± 0.1 | 2.2 ± 0.2 |
| P. roqueforti 513 | 7.1 ± 0.5 | 2.6 ± 0.2 |
| F. culmorum D-80148 | 5.8 ± 0.1 | 2.7 ± 0.2 |
| A. parasiticus | 5.6 ± 0.1 | 3.8 ± 0.1 |
| A. flavus | 7.6 ± 1.0 | 3.6 ± 1.3 |
| A. niger | 2.7 ± 0.2 | 1.5 ± 0.2 |
| R. oligosporus 575 | 8.5 ± 0.2 | 3.3 ± 0.1 |
Conidia were used at a density of 106 conidia ml−1 on the upper side of the plate. The plates were incubated for 7 days at 25°C. As a control, plates without P. paneum spore suspensions were incubated. The diameter of the growth zone of the related fungi inoculated in the middle of the plates was measured. Experiments were done with fourfold (controls) or sixfold (with inhibition) replication.
Effect of the extracted compounds on germination.
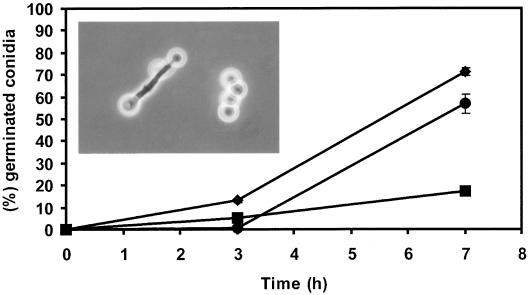
The effect of the extracted volatile compound(s) on fresh conidia of P. paneum in suspensions (106 conidia ml−1) in MEB (pH 4.0) was measured (Fig. 4). Germination efficiency in the control was 71% within 7 h. Conidia exposed to compounds that were extracted from freshly prepared spore suspension (0 h) germinated with an efficiency of 84% (data not shown). Exposure to compounds that were extracted after 22 and 45 h of incubation showed only 17 and 57% germination, respectively. Apart from inducing the highest inhibition of germination, the 22-h extract also induced microcycle conidiation (inset in Fig. 4). This phenomenon is characterized by the rapid sporulation after germination, bypassing the vegetative growth phase that usually follows germination. The extracted compounds that inhibited germination were heat stable, since exposure to 100°C for 5 min did not affect their activity (data not shown).
FIG. 4.
Germination of fresh conidia of P. paneum exposed to extracted compounds from P. paneum. Fresh conidia in the absence (♦) and in the presence of extracted compounds collected at 22 (▪) and 45 h (•) of incubation. The insert shows microcyclic conidiation of the primary conidia in the presence of extracted compounds collected at 22 h. Three independent experiments were done, and the error bars show the standard deviations.
Identification of the volatile compounds.
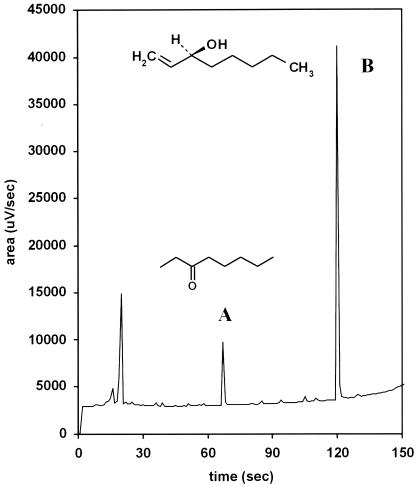
The static headspace samples of high-density conidial suspensions showed two distinct peaks of volatile compounds produced at retention times of 10.875 and 19.875 min (data not shown). The area measurements for peaks of the volatile compounds in samples obtained at 0, 22, and 45 h correlated strongly with the degree of inhibition of spore germination seen with the same extracts. The mass spectrometric identification of the volatile compounds found in the sample collected at 22 h of incubation is given in Table 2. The two dominant peaks were identified as 3-octanone and 1-octen-3-ol (Fig. 5). In Fig. 5 is an example of the given structure of S-(+)-1-octen-3-ol, but the chirality of the compound was not determined in this study.
TABLE 2.
Identification of volatile compounds produced by spore suspensions of P. paneum incubated in MEB (pH 4.0) for 22 h at 25°C
| Compound | GC peak areaa |
|---|---|
| 1,3-Octadiene (cis and trans) | 270 |
| 1,5-Octadien-3-ol | 56 |
| 1-Octen-3-ol | 3,400 |
| 3-Octanone | 900 |
| 5-Octen-3-one | 80 |
| 3-Octanol | 105 |
| Nonanal | 48 |
| Decanal | 104 |
| Sesquiterpene hydrocarbons | 43 |
GC, gas chromatography. Values are in arbitrary units.
FIG. 5.
Headspace analysis chromatogram and chemical structures of the volatile compounds produced by P. paneum with 109 conidia ml−1 incubated in MEB (pH 4) at 22 h. Peaks of 3-octanone (A) and 1-octen-3-ol (B) are indicated.
Action of 3-octanone and 1-octen-3-ol.
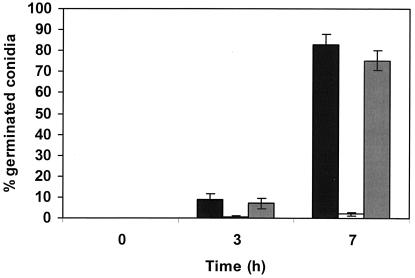
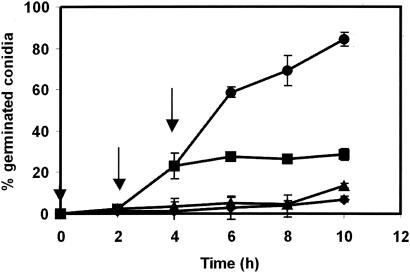
The effect of externally added pure 3-octanone and 1-octen-3-ol on P. paneum conidia was analyzed (Fig. 6). In the presence of 4 mM 1-octen-3-ol, the germination frequency after 7 h was 2%, while after the same exposure to 3-octanone it was 75%. In the control, 83% of the conidia germinated. Different concentrations of 1-octen-3-ol were tested to evaluate if a dose-dependent effect on germination could be observed. A decrease in germination efficiency in comparison with the control level was observed in all concentrations tested and in the presence of 1, 2.5, 4.0, and 5.0 mM 1-octen-3-ol, for which the germination efficiency after 10 h was 83% ± 8.1%, 35% ± 6.3%, 13% ± 2.1%, and 1% ± 0.6%, respectively. Controls containing no inhibitor showed a germination efficiency of 88% ± 5.3%. To study the effect of the inhibitor on different stages of germination, 4 mM 1-octen-3-ol was added to a conidial suspension directly after addition of the conidia to MEB and also after 2 and 4 h of incubation. These times roughly correspond with the germination stages of isotropic growth (2 h, swelling) and germ tube formation (4 h). Figure 7 shows that germination was blocked after addition of 1-octen-3-ol during swelling of the conidia, and no germ tubes were formed. Also after 4 h, the conidia that had not already formed germ tubes were blocked. The microcycle conidiation was also induced in the presence of 4 mM 1-octen-3-ol.
FIG. 6.
Action of 1-octen-3-ol and 3-octanone on P. paneum conidial germination, as shown by the percentage of germinated conidia in MEB (black bars), in the presence of 4 mM 1-octen-3-ol (white bars), and in the presence of 4 mM 3-octanone (grey bars). The results are the means of three independent experiments. Error bars show the standard deviations.
FIG. 7.
Action of 1-octen-3-ol at different germination stages of P. paneum conidia. The arrows represent the time of addition of the compound. Percentages of germination in MEB (pH 4) (•), in the presence of 4 mM of 1-octen-3-ol added at time 0 (♦), after 2 h (▴), and after 4 h of incubation (▪) are shown. The results are the means of three independent measurements. Error bars show the standard deviations.
DISCUSSION
The volatile compound 1-octen-3-ol blocks the germination process of P. paneum conidia. For the first time, this substance has been demonstrated to be a volatile germination self-inhibitor. The compound was measured in dense conidial suspensions, and its effect was alleviated by dilution of the conidia. Also, the compound inhibited mycelial growth of various fungi from different genera, indicating an additional regulatory effect on the physiology and development of the fungal organism.
Large numbers of conidia are formed on conidiophores of Penicillium and Aspergillus spp. In these species, the spores and the spore-forming structures are directly exposed to the air. In these fungi, volatile self-inhibitors may be more efficient than nonvolatiles (25). C. graminicola is able to produce a nonvolatile self-inhibitor, mycosporine-alanine, and a volatile self-inhibitor, 3-hexen-1-ol (25, 26). Conceivably, the volatile 1-octen-3-ol of P. paneum is produced by conidia and released into the air in order to inhibit germination until appropriate environmental conditions prevail.
1-Octen-3-ol is well known as a major component of the odor of the mushrooms Agaricus bisporus and Pleurotus species (12, 30). 1-Octen-3-ol, though known from a wide range of fungi, has never been recognized as a germination self-inhibitor. A wide selection of important food-related fungi including Penicillium species P. camemberti, P. chrysogenum, P. commune, P. tricolor, P. viridicatum, P. aurantiogriseum, P. citrinum, P. funiculosum, and P. raistricki, Aspergillus species A. niger, A. ochraceus, A. oryzae, and A. parasiticus, and Alternaria and Fusarium species have been shown to produce this compound (5, 21, 23). Interestingly, 1-octen-3-ol is not produced in dense suspensions of Penicillium brevicompactum IBT 18329, P. commune CBS 468.84, P. crustosum CBS 101025, P. chrysogenum CBS 779.95, and P. roqueforti CBS 135.65 (data not shown).
In general, fungal volatiles have been investigated for different purposes. These compounds have been used as indicators of fungal growth on grains (5, 33) and as stimulators or inhibitors of plants, fungi, and bacteria (15, 16). Beltran-Garcia et al. (4) studied antibacterial activities of mixtures of volatile compounds (1-octen-3-ol, 3-octanol, octanol, 3-octanone, and 2-octanone) at concentrations found in the mushroom Pleurotus ostreatus and found that these mixtures inhibit growth of Bacillus cereus, Bacillus subtilis, Escherichia coli, and Salmonella enterica serovar Typhimurium. Volatile metabolites have also been used as a taxonomic criterion for classification of Penicillium species (23).
In fungi, 1-octen-3-ol is a product of the enzymatic breakdown of linoleic acid by lipoxygenase and a hydroperoxide lyase (1, 22). The biosynthesis of 1-octen-3-ol is generally considered to make a major contribution to mushroom flavor. The compound is detected in raw mushrooms, especially when they are damaged (20, 30). The compound is present in higher concentrations in mushroom gills, where the spores are formed. Peak levels of 1-octen-3-ol detected over the course of development of Agaricus bisporus fruit bodies were found in young caps about 35 mm in diameter with closed veils (13, 30). The fact that 1-octen-3-ol is produced in areas where high-density concentrations of propagules occur, e.g., on mushrooms and in conidial masses of Penicillium species, suggests that this compound has a common function as an inhibitor of premature spore germination.
Together with 1-octen-3-ol, a nonvolatile metabolite, 10-oxo-trans-8-decenoic acid, is formed during the oxidative breakdown of linoleic acid by fungi (22). This compound is considered to have an influence on the development of fungal structures in the mushroom. It stimulates growth of the mycelium, fruiting body initiation, and stipe elongation. It has been regarded as a growth-regulating substance produced by gills (10, 31). The two substances originating from the degradation of linoleic acid by lipoxygenase may act in concert as a complex of hormones (growth regulators). In addition, 1-octen-3-ol was shown to induce microcyclic conidiation in P. paneum, suggesting an additional role as a factor driving the fungal colony to spore production and dispersal. We suggest that products of linoleic acid breakdown may play an important role in the regulation of transition between vegetative and sexually and asexually reproductive structures. 10-Oxo-trans-8-decenoic acid may also influence the production of spore-forming structures on hyphae, but evidence for the latter remains to be given.
Recently, the link between linoleic acid derivatives and asexual and sexual spore development was studied in Aspergillus nidulans. The authors described the “Psi factor,” a mixture of three hydroxylated linoleic acids. The proportion of the compounds designated as PsiA, PsiB, and PsiC controls the ratio of asexual to sexual spore development. PsiB and PsiC promote development of cleistothecia and inhibit asexual conidiation, while PsiA antagonizes PsiB and PsiC (8, 9).
Current investigation is focused on the mechanism of action of 1-octen-3-ol on conidia. Elucidation of this mechanism may give new insights into the regulation of germination. 1-Octen-3-ol is a promising compound to investigate for possible applications in the prevention of food spoilage and the control of crop diseases.
Acknowledgments
This work was supported by grants from CAPES Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
We thank Lisa Fredlund (Swedish University of Agricultural Sciences) for providing the system for volatile inhibition studies. We are grateful to Erik Dekker (CBS) for molecular identification of isolates and to Jan Cozijnsen (Food Chemistry, Wageningen University & Research Centre) for the headspace analyses. We thank Richard Summerbell for critical reading of the manuscript.
REFERENCES
- 1.Assaf, S., Y. Hadar, and C. G. Dosoretz. 1997. 1-Octen-3-ol and 13-hydroperoxylinoleate are products of distinct pathways in the oxidative breakdown of linoleic acid by Pleorotus pulmonarius. Enzyme Microb. Technol. 21:484-490. [Google Scholar]
- 2.Bacon, C. W., and A. S. Sussman. 1973. Effects of the self-inhibitor of Dictyostelium discoideum on spore metabolism. J. Gen. Microbiol. 76:331-344. [DOI] [PubMed] [Google Scholar]
- 3.Barrios-Gonzáles, J., C. Martinez, A. Aguilera, and M. Raimbault. 1989. Germination of concentrated suspensions of spores from Aspergillus niger. Biotechnol. Lett. 11:551-554. [Google Scholar]
- 4.Beltran-Garcia, M. J., M. Estearron-Espinosa, and O. Tetsuya. 1997. Volatile compounds secreted by the oyster mushroom (Pleurotus ostreatus) and their antibacterial activities. J. Agric. Food Chem. 45:4049-4052. [Google Scholar]
- 5.Börjesson, T., U. Stöllman, and J. Schnürer. 1990. Volatile metabolites and other indicators of Penicillium aurantiogriseum growth on different substrates. Appl. Environ. Microbiol. 56:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boysen, M. E., S. Björneholm, and J. Schnürer. 2000. Effect of the biocontrol yeast Pichia anomala on interactions between Penicillium roqueforti, Penicillium carneum, and Penicillium paneum in moist grain under restricted air supply. Postharvest Biol. Technol. 19:173-179. [Google Scholar]
- 7.Boysen, M. E., P. Skouboe, J. Frisvad, and L. Rossen. 1996. Reclassification of the Penicillium roqueforti group into three species on the basis of molecular genetic and biochemical profiles. Microbiology 142:541-549. [DOI] [PubMed] [Google Scholar]
- 8.Calvo, A. M., H. W. Gardner, and N. P. Keller. 2001. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 276:25766-25774. [DOI] [PubMed] [Google Scholar]
- 9.Calvo, A. M., L. Hinze, H. W. Gardner, and N. P. Keller. 1999. Sporogenic effect of polyunsaturated fatty acid on development of Aspergillus spp. Appl. Environ. Microbiol. 65:3668-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Champavier, Y., M. Pommier, N. Arpin, A. Voiland, and G. Pellon. 2000. 10-Oxo-trans-8-decenoic acid (ODA): production, biological activities, and comparison with other hormone-like substances in Agaricus bisporus. Enzyme Microb. Technol. 26:243-251. [DOI] [PubMed] [Google Scholar]
- 11.Chitarra, G. S., P. Breeuwer, M. J. R. Nout, A. C. van Aelst, F. M. Rombouts, and T. Abee. 2003. An antifungal compound produced by Bacillus subtilis YM 10-20 inhibits germination of Penicillium roqueforti conidiospores. J. Appl. Microbiol. 94:159-166. [DOI] [PubMed] [Google Scholar]
- 12.Cronin, D. A., and M. K. Ward. 1971. The characterization of some mushroom volatiles. J. Sci. Food Agric. 22:477-479. [Google Scholar]
- 13.Cruz, C., C. Noel-Suberville, and M. Montury. 1997. Fatty acid content and some flavor compounds release in two strains of Agaricus bisporus, according to three stages of development. J. Agric. Food Chem. 45:64-67. [Google Scholar]
- 14.Dijksterhuis, J., and R. A. Samson. 2002. Food and crop spoilage on storage, p. 39-52. In F. Kempken (ed.), Mycota XI: agricultural applications. Spring-Verlag, Berlin, Germany.
- 15.French, R. C. 1992. Volatile chemical germination stimulators of rust and other fungal spores. Mycologia 84:277-288. [Google Scholar]
- 16.Garret, M. K., and P. M. Robinson. 1969. A stable inhibitor of spore germination produced by fungi. Arch. Microbiol. 67:370-377. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb, D. 1973. Endogenous inhibitors of spore germination. Phytopathology 63:1326-1327. [Google Scholar]
- 18.Griffin, D. H. 1994. Spore dormancy and germination, p. 375-398. In Fungal physiology, 2nd ed. John Wiley & Sons, New York, N.Y.
- 19.Hobot, J. E., and K. Gull. 1980. The identification of a self inhibitor from Syncephalastrum racemosum and its effect upon sporangiospore germination. Antonie Leeuwenhoek 46:435-441. [DOI] [PubMed] [Google Scholar]
- 20.Holtz, R. B., and L. C. Schisler. 1971. Lipid metabolism of Agaricus bisporus (Lange) Sing. I. Analysis of sporophore and mycelial lipids. Lipids 6:176-180. [Google Scholar]
- 21.Kaminski, E., S. Stawicki, and E. Wasowicz. 1974. Volatile flavor compounds produced by molds of Aspergillus, Penicillium, and fungi imperfecti. Appl. Microbiol. 27:1001-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuribayashi, T., H. Kaise, C. Uno, T. Hara, T. Hayahawa, and T. Joh. 2002. Purification and characterization of lipoxygenase from Pleurotus ostreatus. J. Agric. Food Chem. 50:1247-1253. [DOI] [PubMed] [Google Scholar]
- 23.Larsen, T. O., and J. C. Frisvad. 1995. Characterization of volatile metabolites from 47 Penicillium taxa. Mycol. Res. 99:1153-1166. [Google Scholar]
- 24.Lax, A. R., G. E. Templeton, and W. L. Meyer. 1985. Isolation, purification, and biological activity of a self-inhibitor from conidia of Colletotrichum gloeosporioides. Phytopathology 75:386-390. [Google Scholar]
- 25.Leite, B., and P. Nicholson. 1993. A volatile self-inhibitor from Colletotrichum graminicola. Mycologia 85:945-951. [Google Scholar]
- 26.Leite, B., and R. L. Nicholson. 1992. Mycosporine-alanine: a self-inhibitor of germination from the conidial mucilage of Colletotrichum graminicola. Exp. Mycol. 16:76-86. [Google Scholar]
- 27.Lingappa, B. T., Y. Lingappa, and E. Bell. 1973. A self-inhibitor of protein synthesis in the conidia of Glomerella cingulata. Arch. Microbiol. 94:97-107. [DOI] [PubMed] [Google Scholar]
- 28.Macko, V., R. C. Staples, and J. A. A. Renwick. 1971. Germination self-inhibitor of sunflower and snapdragon rust uredospores. Phytopathology 61:902. [Google Scholar]
- 29.Macko, V., R. C. Staples, Z. Yaniv, and R. R. Granados. 1976. Self-inhibitors of fungal spore germination, p. 73-98. In D. J. Weber and W. M. Hess (ed.), The fungal spore: form and function. John Wiley and Sons, London, United Kingdom.
- 30.Mau, J. L., R. B. Beelman, and G. R. Ziegler. 1992. 1-Octen-3-ol in the cultivated mushroom, Agaricus bisporus. J. Food Sci. 57:704-706. [Google Scholar]
- 31.Mau, J. L., R. B. Beelman, and G. R. Ziegler. 1992. Effect of 10-oxo-trans-8-decenoic acid on growth of Agaricus bisporus. Phytochemistry 31:4059-4064. [Google Scholar]
- 32.Petersson, S., and J. Schnürer. 1999. Growth of Penicillium roqueforti, P. carneum, and P. paneum during malfunctioning airtight storage of high-moisture grain cultivars. Postharvest Biol. Technol. 17:47-54. [Google Scholar]
- 33.Schnürer, J., J. Olsson, and T. Börjesson. 1999. Fungal volatiles as indicators of food and feeds spoilage. Fung. Genet. Biol. 27:209-217. [DOI] [PubMed] [Google Scholar]
- 34.Steele, S. D. 1973. Self-inhibition of arthrospore germination in Geotrichum candidum. Can. J. Microbiol. 19:943-947. [DOI] [PubMed] [Google Scholar]
- 35.van den Boogert, P. H. J. F., J. Dijksterhuis, H. Velvis, and M. Veenhuis. 1992. Adhesive knob formation by conidia of the nematophagous fungus Drechmeria conidiospora. Antonie Leeuwenhoek 61:221-229. [DOI] [PubMed] [Google Scholar]
- 36.Van Sumere, C. F., C. Van Sumere-De Preter, L. C. Vining, and G. A. Ledingham. 1957. Coumarins and phenolic acids in the uredospores of wheat stem rust. Can. J. Microbiol. 3:847-862. [DOI] [PubMed] [Google Scholar]