Abstract
The formation of hydroxyectoine in the industrial ectoine producer Halomonas elongata was improved by the heterologous expression of the ectoine hydroxylase gene, thpD, from Streptomyces chrysomallus. The efficient conversion of ectoine to hydroxyectoine was achieved by the concerted regulation of thpD by the H. elongata ectA promoter.
Under hyperosmotic stress, a variety of microorganisms accumulate organic low-molecular-weight compounds such as polyols, amino acids, sugars, betaines, and ectoine. These so-called compatible solutes enable the organisms to survive under conditions of high osmotic pressure (2). Since it has been demonstrated that compatible solutes, especially ectoine, are able to protect enzymes, membranes, and whole cells against stresses caused by exposure to salt, heating, freezing, and desiccation, there is an increasing interest in the use of compatible solutes for various applications in biotechnology (8, 12, 21, 20). Today, ectoine is already used as a moisturizer in cosmetics and skin care products (RonaCare Ectoin; Merck KGaA, Darmstadt, Germany) (12). In addition, ectoine's hydroxyl derivative, hydroxyectoine, has gained attention as a protein-protecting agent which, in a number of applications, has properties superior to those of ectoine (8, 4). Moreover, hydroxyectoine has also been investigated as a protectant of healthy cells during chemotherapy (1). Thus, the development of hydroxyectoine as a by-product of ectoine is foreseeable.
The biotechnological production of ectoine, a process called bacterial milking, is carried out with the halophilic gram-negative bacterium Halomonas elongata, which responds to a hypoosmotic shock by the rapid release of the accumulated solutes (15). After growth at 25°C, the typical product solution consists of more than 93% ectoine, 4% of the ectoine precursor N-acetyl-diaminobutyric acid, and only traces of glutamate, alanine, and hydroxyectoine. However, the relative proportion of hydroxyectoine can be increased to 50% by using considerably higher salinities or elevated temperatures, factors increasing costs for both the equipment and the process (15). Moreover, the costly separation of ectoine and hydroxyectoine is still necessary. Therefore, the biotechnological production of hydroxyectoine is carried out with the gram-positive Marinococcus sp. strain M52, which produces predominantly hydroxyectoine but is less amenable to the milking process than H. elongata.
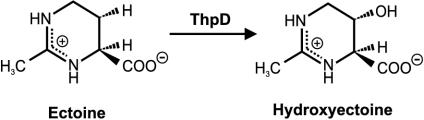
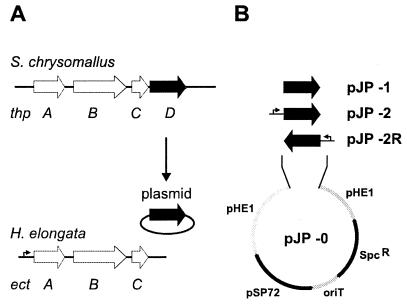
The in vivo formation of hydroxyectoine has been described for a variety of bacteria (7, 10, 18). The in vitro conversion of ectoine to hydroxyectoine by a specific hydroxylase from Streptomyces chrysomallus ATCC 11523 (Fig. 1) has been shown previously (5, 6). The corresponding gene (thpD) has been shown to be part of the ectoine-hydroxyectoine gene cluster as shown in Fig. 2. Unlike the streptomycetes, the moderately halophilic bacteria H. elongata and Marinococcus halophilus lack a thpD-like gene in the ect gene cluster (3, 9).
FIG. 1.
Reaction catalyzed by ThpD, the ectoine hydroxylase from S. chrysomallus.
FIG. 2.
(A) Map of the ectoine and hydroxyectoine biosynthesis genes of H. elongata (ect) and S. chrysomallus (thp). The thpD gene coding for the S. chrysomallus ectoine hydroxylase, which was transferred to H. elongata, is shown in black. (B) Mobilizable pHE1-derived shuttle vectors for the expression of the S. chrysomallus thpD gene in H. elongata. The ectA promoter region is indicated by small arrows.
For the industrial production of hydroxyectoine, a microorganism with a broad salt tolerance is favored to perform the bacterial milking process (15). Since H. elongata is already used for the industrial production of ectoine, there would be commercial interest in broadening its industrial application. We therefore expressed the ectoine hydroxylase gene of S. chrysomallus, thpD, in H. elongata to improve this industrially used ectoine producer so that it may serve as a hydroxyectoine producer.
Construction of H. elongata expression plasmids.
For expression of the thpD gene, we assembled an autonomous replicating plasmid for H. elongata by combining the Escherichia coli pUC derivative pSP72 (Promega) with the H. elongata plasmid pHE1 (17) by the insertion of a spectinomycin resistance cassette for selection in Halomonas and an oriT gene for plasmid transfer. First, oriT of Pseudomonas aeruginosa was PCR amplified as an 0.8-kb PstI cassette from plasmid pHM10a (11) with oligonucleotides prim1a (5′-ATT ACT GCA GTC GGT CTT GCC TTG CTC GTC GG-3′) and prim1b (5′-TTT CTG CAG TGC ATA ACC CTG CTT CGG GG-3′), subcloned into plasmid pLITMUS28 (New England Biolabs) cleaved with NsiI, reisolated as a BglII-XhoI fragment, and cloned into pSP72 cleaved with BamHI and XhoI. The next step was insertion of the spectinomycin cassette, which was obtained as an EcoRI fragment from plasmid pHP45omega (14), and insertion of the BglII-linearized plasmid pHE1 into the respective sites of the pSP72 backbone. The resultant plasmid, pJP-0, harbors a single PstI site located in the pHE1 region, which was used for inserting two different thpD gene cassettes (Fig. 2B). A promoterless thpD gene cassette was generated by PCR from a genomic thpD clone (plasmid pAF1sub10) (unpublished data) with oligonucleotide prim2a (5′-GCC GAA TTC CAT ATG ACC ACC GAA GTA CGC GCC GAT-3′), introducing an NdeI site encompassing the ATG translation start codon of the thpD stop codon, and with oligonucleotide prim2b (5′-AGC GAA TTC CCC TGC AGG GGC CGG GAC GGC GTA CCC GTC CCG G-3′), introducing a PstI and EcoRI site about 50 bp downstream of the thpD gene. The NdeI-EcoRI fragment was subcloned into pSL1180 (Amersham Biosciences) cleaved with NdeI and EcoRI, thus maintaining untouched the PCR-introduced PstI site downstream of thpD and generating a second PstI site upstream of thpD located in the pSL1180 polylinker. We also generated a promoter-driven variant of the thpD gene cassette by inserting the ectA promoter of H. elongata DSM 3043 as a 250-bp NotI-NdeI fragment immediately upstream of thpD by using a single NotI site of the pSL1180 polylinker. The ectA promoter was PCR amplified from genomic DNA with oligonucleotides prim3a (5′-CGG GGA TCC GCG CCG ACG AGC GCT CGA TCG-3′) and prim3b (5′-AGC GAA TTC CCC TGC AGG GGC CGG GAC GGC GTA CCC GT-3′) to generate restriction sites. All PCR-generated fragments were sequenced to confirm the entire DNA sequence. Insertion of these thpD gene cassettes into the PstI site of plasmid pJP-0 generated plasmids pJP-2 (thpD with ectA promoter) and pJP-1 (promoterless thpD gene) (Fig. 2B). In both plasmids, the orientation of the inserted thpD gene was chosen in such a way that a known pHE1 promoter region (16) providing basal gene expression was always located upstream of the thpD gene cassette. Thus, we also expected the promoterless thpD gene in pJP-1 to be expressed to some extent.
Plasmids were transferred from E. coli S17-1 to H. elongata by conjugation. To eliminate E. coli cells after plasmid transfer, a spontaneous rifampin-resistant mutant of H. elongata DSM 304 was isolated by serial plating on solid medium saline (SWYE) containing rifampin (25 μl/ml) (17, 13). Comparison of the resulting rifampin-resistant strain H. elongata AD-98 with the wild-type strain H. elongata DSM 3043 did not reveal any dissimilarities regarding the production of ectoine or hydroxyectoine, even in the presence of plasmid pJP-D-0 (data not shown). We therefore routinely used H. elongata AD-98 for studying the effect of the introduction of thpD on the formation of hydroxyectoine in that strain.
ThpD-dependent hydroxyectoine production in H. elongata.
To determine the effect of the thpD gene on the formation of hydroxyectoine, H. elongata/pJP-2 (thpD with ectA promoter) and H. elongata/pJP-1 (promoterless thpD) were cultivated at 30°C in 10 ml of SWYE medium in 100-ml flasks, with shaking at 200 rpm, to an optical density at 600 nm of 2.4 to 2.6. Bacterial milking was carried out by lowering the salt concentration from 10 to 2% (wt/vol) with distilled water. An aliquot of the cell-free supernatant was incubated at 95°C for 15 min, cleared by centrifugation, and subjected to high-pressure liquid chromatography (HPLC) analysis (LiChroCART 250-4, LiChrospher 100 NH2; 5-μm particle size with 70% acetonitrile as the eluent). Ectoine and hydroxyectoine were quantified by using commercial ectoine and hydroxyectoine standards (Biomol). At 30°C and with 10% (wt/vol) salts, H. elongata/pJP-2 converted 76% of the ectoine into hydroxyectoine with a yield of 340 nmol of hydroxyectoine per ml (Fig. 3B, left chromatogram). In contrast, the control strain H. elongata/pJP-0 (plasmid without insert) did not convert significant amounts of ectoine into hydroxyectoine under these conditions (Fig. 3A, left chromatogram). Also, in the case of H. elongata/pJP-1, none or only traces (<3%) of the ectoine were converted into hydroxyectoine. These results show that the thpD gene of S. chrysomallus was actively expressed in H. elongata in the presence of a suitable promoter.
FIG. 3.
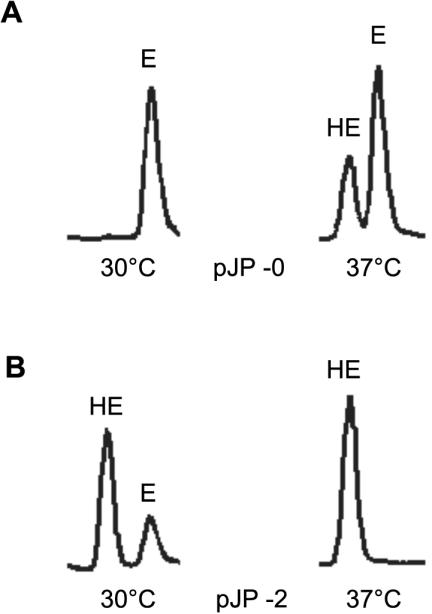
HPLC detection of ectoine (E) and hydroxyectoine (HE) produced by H. elongata strains containing plasmid pJP-0 (A) and pJP-2 (B). After growth of H. elongata at the indicated temperatures, ectoine and hydroxyectoine were released by hypoosmotic shock and subjected to HPLC analysis.
Increased yields of hydroxyectoine have been described for H. elongata at elevated temperatures and higher salinities (19). We therefore measured the hydroxyectoine formation in the transformed strains at 37°C (instead of the 30°C described above) and 10% (wt/vol) salts. As expected, the control strain H. elongata/pJP-0 produced significantly higher amounts of hydroxyectoine (37%) but still less than the ectoine produced (63%) (Fig. 3A, right chromatogram). In contrast, strain H. elongata/pJP-2 (thpD with ectA promoter) produced exclusively hydroxyectoine (100%) (Fig. 3B, right chromatogram). No ectoine could be detected under these growth conditions. Remarkably, the introduction of the promoterless thpD gene (by plasmid pJP-1), driven only by an intrinsic pHE1 promoter (16), also resulted in an enhanced formation of hydroxyectoine (80%). Ectoine (20%), however, was still present under these conditions (Table 1). Complete conversion of ectoine to hydroxyectoine was also observed when the PectA-thpD cassette was in the opposite orientation (pJP-2R) (Table 1). This clearly indicates that the PectA-thpD cassette alone is well suited for the industrial production of hydroxyectoine.
TABLE 1.
Hydroxyectoine formation by H. elongata strains at 37°C
| Plasmid | Characteristics | Relative proportiona
|
|
|---|---|---|---|
| Hydroxyectoine | Ectoine | ||
| pJP-0 | PpHE1, no insert | 37.2 | 62.8 |
| pJP-1 | PpHE1, thpD | 80.4 | 19.6 |
| pJP-2 | PpHE1, Pect, thpD | 100 | ND |
| pJP-2R | PectA, thpD | 100 | ND |
Values are given as percentages of the total amount of ectoine and hydroxyectoine and are the means of results for at least three independent cultures. ND, not detectable.
The economic benefit of the bacterial milking process, compared to fed-batch cultures, implies that at least two cycles of compatible solute extraction have to be applied (15). We therefore studied the formation of hydroxyectoine in the absence of the selection marker streptomycin at 37°C. Cultures were subjected to hypoosmotic shocks and used as seed culture every 24 h over a period of 9 days. After three cycles of bacterial milking, the amount of hydroxyectoine dropped from 100 to 96%. Prolonged cultivation led to wild-type levels of hydroxyectoine (36%) after nine cycles. In parallel, aliquots of cultures were used to assay the amount of plasmid still present. The results of the plasmid quantification indicate that pJP-2 is not stable under nonselective conditions. However, since the milking process can be repeated at least two times without the detectable appearance of ectoine, the genetically engineered H. elongata strain may be valuable for future biotechnological applications.
In this study, we showed the functional expression of the ectoine hydroxylase gene of the gram-positive bacterium S. chrysomallus, thpD, in the gram-negative host H. elongata. Introduction of the PectA-thpD gene cassette greatly stimulates production of hydroxyectoine from ectoine at 30°C and completely converts all ectoine to hydroxyectoine at 37°C. The efficient conversion of ectoine to hydroxyectoine is achieved by the concerted regulation of thpD via the H. elongata ectA promoter. Although the thpD expression plasmid is not stable under nonselective conditions, the results suggest that the bacterial milking process may be applicable for the industrial production of hydroxyectoine. To this end, we plan to transfer the thpD expression cassette stably into the genome of H. elongata.
Nucleotide sequence accession number.
The nucleotide sequence of the thp genes from S. chrysomallus (ATCC 11523) has been deposited in the GenBank nucleotide sequence database under accession number AY524544.
REFERENCES
- 1.Barth, S. 2000. Konstruktion, Expression und präklinische Charakterisierung rekombinanter Immuntherapeutika für einen Einsatz bei malignen Erkrankungen. Habilitationsschrift, Universität zu Köln, Cologne, Germany.
- 2.Brown, A. D. 1976. Microbial water stress. Bacteriol. Rev. 40:803-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cánovas, D., C. Vargas, M. I. Calderon, A. Ventosa, and J. J. Nieto. 1998. Characterization of the genes for the biosynthesis of the compatible solute ectoine in the moderately halophilic bacterium Halomonas elongata DSM 3043. Syst. Appl. Microbiol. 21:487-497. [DOI] [PubMed] [Google Scholar]
- 4.Cánovas, D., N. Borges, C. Vargas, A. Ventosa, J. J. Nieto, and H. Santos. 1999. Role of Nγ-acetyldiaminobutyrate as an enzyme stabilizer and an intermediate in the biosynthesis of hydroxyectoine. Appl. Environ. Microbiol. 65:3774-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grammel, N. 2000. Molekulargenetische und biochemische Analyse der Biosynthese von 2-Methyl-4-carboxy-3,4,5,6-tetrahydropyrimidin und seinem 5-Hydroxyderivat, zwei salzstreβinduzierbaren Osmolyten, in Streptomyces chrysomallus. Ph. D. thesis. Technische Universität Berlin, Berlin, Germany. [Online.] http://edocs.tu-berlin.de/diss/2000/grammel_nicolas.htm.
- 6.Grammel, N., and U. Keller. 21June2001. Tetrahydropyrimidine oxygenase gene, polypeptides encoded by said gene and method for producing the same. German patent DE19957470.
- 7.Kuhlmann, A. U., and E. Bremer. 2002. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 68:772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippert, K., and E. A. Galinski. 1992. Enzyme stabilisation by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37:61-65. [Google Scholar]
- 9.Louis, P., and E. A. Galinski. 1997. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143:1141-1149. [DOI] [PubMed] [Google Scholar]
- 10.Malin, G., and A. Lapidot. 1996. Induction of synthesis of tetrahydropyrimidine derivatives in Streptomyces strains and their effect on Escherichia coli in response to osmotic and heat stress. J. Bacteriol. 178:385-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motamedi, H., A. Shafiee, and S. J. Cai. 1995. Integrative vectors for heterologous gene expression in Streptomyces spp. Gene 160:25-31. [DOI] [PubMed] [Google Scholar]
- 12.Motitschke, L., H. Driller, and E. A. Galinski. May. 2000. Ectoin and ectoin derivatives as moisturizer in cosmetics. U.S. patent US6060071.
- 13.Nieto, J. J., R. Fernández-Castillo, M. C. Marquez, A. Ventosa, E. Quesada, and F. Ruiz-Berraquero. 1989. Survey of metal tolerance in moderately halophilic eubacteria. Appl. Environ. Microbiol. 55:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 15.Sauer, T., and E. A. Galinski. 1998. Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 57:306-313. [PubMed] [Google Scholar]
- 16.Tegos, G., C. Vargas, G. Vartholomatos, A. Perysinakis, J. J. Nieto, A. Ventosa, and C. Drainas. 1997. Identification of a promoter region on the Halomonas elongata cryptic plasmid pHE1 employing the inaZ reporter gene of Pseudomonas syringae. FEMS Microbiol. Lett. 154:45-51. [DOI] [PubMed] [Google Scholar]
- 17.Vargas, C., R. Fernández-Castillo, D. C ánovas, A. Ventosa, and J. J. Nieto. 1995. Isolation of cryptic plasmids from moderately halophilic eubacteria of the genus Halomonas. Characterization of a small plasmid from H. elongata and its use for shuttle vector construction. Mol. Gen. Genet. 246:411-418. [DOI] [PubMed] [Google Scholar]
- 18.Ventosa, A., J. J. Nieto, and A. Oren. 1998. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62:504-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wohlfarth, A., J. Severin, and E. A. Galinski. 1990. The spectrum of compatible solutes in heterotrophic halophilic eubacteria of the family Halomonadaceae. J. Gen. Microbiol. 136:705-712. [Google Scholar]
- 20.Yasuhiko Y, K. Yoshio, K. Eiichiro, T. Yasuhiro, and S. Seichi. 21October1997. Production of l-amino acid by fermentation method using ectoine. Japanese patent JP9271382.
- 21.Yasuhiro T., T. Mitsuo, Y. Hiroyuki, and Y. Kazuhiko. 18June1996. High-density culture. Japanese patent JP8154670.