Abstract
Vanadium can be an important contaminant in groundwaters impacted by mining activities. In order to determine if microorganisms of the Geobacteraceae, the predominant dissimilatory metal reducers in many subsurface environments, were capable of reducing vanadium(V), Geobacter metallireducens was inoculated into a medium in which acetate was the electron donor and vanadium(V) was the sole electron acceptor. Reduction of vanadium(V) resulted in the production of vanadium(IV), which subsequently precipitated. Reduction of vanadium(V) was associated with cell growth with a generation time of 15 h. No vanadium(V) was reduced and no precipitate was formed in heat-killed or abiotic controls. Acetate was the most effective of all the electron donors evaluated. When acetate was injected into the subsurface to enhance the growth and activity of Geobacteraceae in an aquifer contaminated with uranium and vanadium, vanadium was removed from the groundwater even more effectively than uranium. These studies demonstrate that G. metallireducens can grow via vanadium(V) respiration and that stimulating the activity of Geobacteraceae, and hence vanadium(V) reduction, can be an effective strategy for in situ immobilization of vanadium in contaminated subsurface environments.
Vanadium contamination of groundwater resulting from industrial, mining, or natural sources can be an environmental concern. Vanadium is the most abundant transition metal in the aquasphere and is widely distributed in the Earth′s crust, with an average concentration similar to that of zinc (21). The primary source of vanadium is titanomagnetite deposits in which vanadium is present as a minor replacement for iron (8). Vanadium is also a trace element in fossilized organic matter, such as crude oils, coal, and carbonaceous fossils (2, 22, 34), and can be found in uranium-bearing minerals (7). Vanadium is used widely in metallurgy, the atomic energy industry, space technology, pharmaceutical industrial processes, and other high-tech industries, making vanadium one of the most important metals for modern technology (20). In addition, vanadium assumes an exceptional position among the biometals in that both its anionic and cationic forms can participate in biological processes (21, 22), serving as a competitive substrate with phosphate, thereby inhibiting and/or stimulating many phosphate-metabolizing enzymes.
Vanadium can exist in different oxidation states from −2 to +5, but the forms found in the natural environment are +3, +4, and +5 (7). Vanadium toxicity varies considerably with the nature of the compound but generally increases as the redox state increases, pentavalent vanadium being the most toxic and the most mobile form (7, 11, 19). In oxic waters the dominant vanadium species are the vanadate(V) compounds, while in reducing environments the vanadyl ion VO2+(IV), considered the most stable diatomic ion known (26), has been detected. This cation has a tendency to hydrolyze, and in the pH range of natural waters it is rather insoluble and strongly adsorbed on particles and forms stable complexes with humic acids. Adsorption and possible subsequent incorporation of vanadium(IV) as a solid solution is a probable inorganic sink for vanadyl in reducing sediments (31). Therefore, promoting reduction of vanadium(V) to lower redox states may be a remediation strategy for immobilizing vanadium, thereby removing it from contaminated groundwaters.
It has previously been reported that cell extracts (33) or whole cells (3, 4, 6, 35) of various microorganisms can reduce vanadium(V). Of most interest in this regard are two Pseudomonas species which can conserve energy to support growth with hydrogen, carbon monoxide, various sugars, and organic acids as the electron donor (18, 35). However, these Pseudomonas species could not grow with Fe(III) as an electron acceptor (18) and only incompletely oxidized organic substrates to acetate (35). These physiological characteristics could limit their ability to compete with other dissimilatory metal-reducing microorganisms in many subsurface environments. This is because acetate is likely to be the major electron donor supporting anaerobic respiration in subsurface environments (12) and because, even in vanadium-contaminated environments, the concentrations of vanadium(V) are likely to be orders of magnitude lower than those of Fe(III).
The reduction of U(VI) to U(IV) can support cell growth in Geobacter metallireducens and some other dissimilatory Fe(III)-reducing microorganisms (16, 17), and stimulating the in situ activity of Geobacteraceae in the subsurface can promote the reductive precipitation of uranium from contaminated groundwater (1, 9). Here we report that G. metallireducens can also conserve energy to support growth from vanadium(V) reduction and that vanadium is effectively removed from groundwater when the in situ activity of Geobacteraceae is enhanced.
MATERIALS AND METHODS
Pure culture studies.
G. metallireducens (ATCC 53774) was obtained from our laboratory culture collection. Cells were cultured under strict anaerobic conditions at 30°C under an atmosphere of N2-CO2 (80:20) in a freshwater, bicarbonate-buffered medium (13). Acetate (10 mM) was added from a 1 M stock solution of sodium acetate as the sole electron donor, and vanadium(V) (1 to 5 mM) was provided from a 100 mM sodium metavanadate stock (Sigma Chemical Company, St. Louis, Mo.) as the sole terminal electron acceptor. All cell manipulations were carried out under N2-CO2 after passing the gases through a column of hot copper filings to remove traces of oxygen. To evaluate the potential use of other electron donors, cells were also grown with 10 mM formate and 1 mM benzoate provided from a 1 M stock solution of sodium formate and a 0.5 M stock solution of sodium benzoate, respectively.
Cells were visualized and counted using acridine orange staining and epifluorescence microscopy, as previously described (14).
Field trial.
As previously described (1), an in situ test plot was installed at a former vanadium and uranium ore processing facility in Rifle, Colo. Groundwater beneath this site contains residual metal contamination stemming from continued leaching of large mill tailing piles previously located on-site. No groundwater standards have yet been established for vanadium; however, concentrations up to 0.77 mg/liter (15 μM) are present near the former tailings pile footprint, which exceeds the 0.33-mg/liter (6 μM) human health risk-based concentration (29).
The complete description of the test plot can be found in the work of Anderson et al. (1). Briefly, an acetate and bromide solution stored in an oxygen-free tank was injected into the subsurface via an injection gallery composed of 20 wells positioned perpendicular to groundwater flow. Acetate was added to the subsurface to provide an in situ concentration of approximately 1 to 3 mM, and bromide (100 to 300 μM in situ) served as a conservative tracer for the injected solution. Changes within the subsurface were evaluated in groundwater samples collected from 15 monitoring wells installed downgradient in three rows of five wells each, and the results were compared to those of three control wells positioned upgradient from the injection gallery.
Analytical techniques.
Soluble vanadium(V) was analyzed selectively using a spectrophotometric method adapted from the method of Budevsky and Johnova (5), with a resorcinol indicator [4-(2-pyridylazo)resorcinol] after pH adjustment with ammonium acetate. Soluble vanadium(IV) was determined spectrophotometrically upon the oxidation of aniline blue with potassium bromate (25). Groundwater samples were filtered with 0.2-μm-pore-size polytetrafluoroethylene (Teflon) syringe filters (Alltech Associates Inc., Deerfield, Ill.). In order to collect solid-phase vanadium for microprobe analysis, liquid samples were centrifuged, the pellet was placed on a carbon substrate, and the water was evaporated prior to analysis of the solid with a CAMECA SX50 electron microprobe using a PGT energy-dispersive analyzer for qualitative elemental determination. The conditions were 15-kV accelerating potential, 15-nA beam current, and a focused beam.
RESULTS
Growth with vanadium(V) as sole electron acceptor.
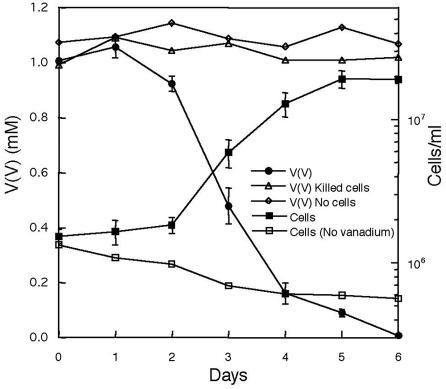
When G. metallireducens was inoculated into freshwater medium containing 10 mM acetate as the electron donor and 1 mM vanadium(V) as the sole electron acceptor, vanadium(V) was reduced over time (Fig. 1). Vanadium(V) was fully transformed into vanadium(IV), and the color of the medium changed to blue, attributable to the presence of vanadium(IV) in the form of the vanadyl ion. Cell growth coincided with vanadium(V) reduction and stopped as vanadium(V) became depleted, with a generation time of 15 h. No vanadium(V) reduction was observed when the freshwater medium was not inoculated with live cells or if the cells were heat killed before incubation. Cells did not grow in the absence of vanadium.
FIG. 1.
Cell growth and vanadium(V) concentrations when G. metallireducens that had been previously grown in acetate-vanadium(V) medium was inoculated into a freshwater medium with 10 mM acetate as the electron donor and 1 mM vanadium(V) as the electron acceptor. Results are the means and standard deviations for triplicate cultures.
G. metallireducens was transferred more than 50 times (10% inoculum) into the same freshwater medium, demonstrating the potential for sustained, long-term growth on vanadium. Cell growth and vanadium(V) reduction were also observed in the presence of 2, 3, 4, and 5 mM vanadium(V).
Formate and benzoate could also serve as electron donors for vanadium(V) reduction, but growth was slower than with acetate. Generation times were 24 h with formate and 36 h in medium that contained benzoate.
Vanadium precipitation.
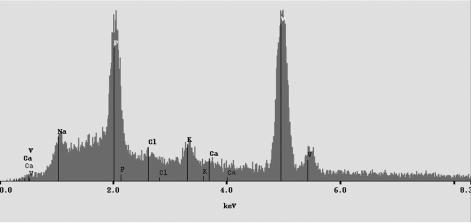
When G. metallireducens reduced vanadium(V), a green precipitate accumulated. No precipitate formed in heat-killed or abiotic controls (Fig. 2). This precipitate was observed when acetate, formate, or benzoate was the electron donor and in the presence of 1 to 5 mM vanadium(V). Once the precipitate was formed, a loss of vanadium(IV) was measured that accounted for ca. 90% of the concentration determined prior to the formation of the precipitate. Electron microprobe analysis indicated that the precipitate was mainly comprised of vanadium and phosphorous (Fig. 3), suggesting that it could be a vanadyl phosphate, such as the green mineral sincosite [CaV2(PO4)2(OH)4 · 3H2O].
FIG. 2.
Precipitation of vanadium as the result of vanadium(V) reduction.
FIG. 3.
X-Ray energy spectrum of the vanadium precipitate obtained via electron microprobe analysis.
In situ removal of vanadium(V) from contaminated groundwater.
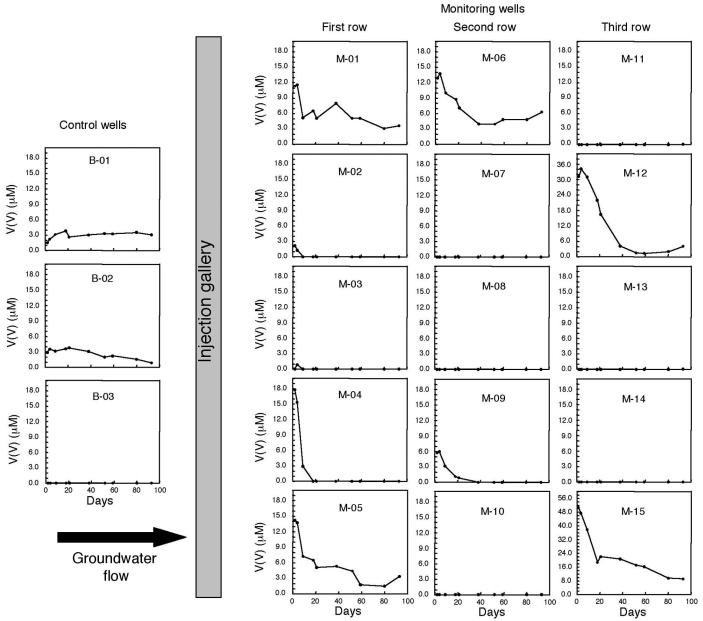
Prior to acetate injection at the Rifle, Colo., field site, soluble vanadium(V), ranging in concentration from 2.2 to 50 μM, was detected in most of the monitoring wells (Fig. 4). Once the acetate injection began, soluble vanadium(V) concentrations in wells downgradient of the injection gallery began to decrease within 9 days, whereas there was little change in vanadium(V) concentrations in the upgradient, background wells (Fig. 4). Vanadium(V) concentrations decreased to nondetectable levels in four of the downgradient monitoring wells (M-02, M-03, M-04, and M-09) within 39 days. In other downgradient wells, vanadium(V) concentrations diminished to levels below the 0.33-mg/liter (6 μM) human health risk-based concentration towards the end of the acetate injection.
FIG. 4.
Vanadium(V) in groundwater samples within the monitoring-well field. B-01 to B-03, upgradient control wells; M-01 to M-15, monitoring wells downgradient from the point of acetate injection. Groundwater flow is from left to right, and the injection gallery is positioned between the control wells and the first row of monitoring wells.
DISCUSSION
Growth via vanadium(V) reduction and precipitation of vanadium.
These results demonstrate that G. metallireducens can obtain energy for growth by coupling the oxidation of organic compounds to the reduction of vanadium(V). This is the first example of an organism which can grow via Fe(III) reduction, also conserving energy for growth from the reduction of vanadium(V), and the first example of an acetate-oxidizing vanadium(V) reducer. The ability of Geobacter species to reduce vanadium(V) is also significant because Geobacter species are the predominant microorganisms in a variety of subsurface sediments when dissimilatory metal reduction becomes an important process (1, 10, 23, 24, 27, 28). Thus, there is the potential for vanadium(V) reduction in numerous subsurface environments.
Vanadium(V) was reduced to at least vanadium(IV), as indicated by both the chemical analysis and the characteristic blue color developed in the cultures. The vanadium(IV) concentration decreased, and the blue color disappeared as a vanadium precipitate, probably a vanadium(IV) phosphate, formed. This demonstrates that G. metallireducens has the potential to precipitate vanadium from solution. The cell yield during growth on vanadium(V) averaged 1.8 × 107 cells per μmol of e− transferred to vanadium(V), which is 2.4-fold higher than the yield per μmol of e− transferred to Fe(III) in the same medium with Fe(III)-citrate serving as the electron acceptor. This higher yield may be due, at least in part, to the high redox potential (1.3 V) of the V(V)/V(IV) couple.
In situ removal of vanadium(V) from groundwater.
Precipitation of vanadium as a result of vanadium(V) reduction is the likely explanation for the removal of vanadium(V) when acetate was injected into the groundwater of the Rifle, Colo., site. The loss in vanadium(V) from the treatment zone during the first 9 days of acetate injection corresponded with the previously reported (1) stimulation of microbial Fe(III) and U(VI) reduction in the treatment zone, which was evident from the accumulation of Fe(II) and a decrease in U(VI) in the groundwater. The stimulation of dissimilatory metal reduction was associated with a dramatic increase in the proportion of microorganisms from the family Geobacteraceae within the acetate injection zone, which suggested that Geobacteraceae were responsible for the Fe(III) and U(VI) reduction (1). The finding that G. metallireducens can grow via vanadium(V) reduction suggests that the Geobacteraceae were also responsible for the removal of vanadium(V) in the treatment zone via vanadium(V) reduction.
However, in addition to this enzymatic mechanism for vanadium(V) removal, the possibility of an in situ abiotic reduction of vanadium(V) cannot be definitively ruled out. Previous studies have shown that structural Fe(II) in magnetite and ilmenite reduces vanadium(V), particularly when the pH is below 5 (32), and addition of soluble Fe(II) reduced vanadium(V) in culture media (unpublished data). Thus, a potential alternative explanation for the removal of vanadium(V) from the groundwater is abiotic reduction of vanadium(V) by the Fe(II) that was produced as microbial Fe(III) reduction was stimulated. However, it should be noted that prior to the injection of acetate, the aquifer contained dissolved Fe(II) and ca. half of the iron in the sediments was in the form of Fe(II) (1). Thus, if abiotic Fe(II) reduction of vanadium(V) was an important process in these sediments, it would be expected that vanadium(V) would have been depleted from the groundwater prior to acetate injection into the test plot and would also not have persisted in the background wells. It may be that although there is some abiotic Fe(II) reduction of vanadium(V), microbial reduction is required in order to remove the residual vanadium(V) from the groundwater.
As previously reported (1), with continued addition of acetate to the aquifer beyond 50 days, the predominant terminal electron-accepting process switched from Fe(III) reduction to sulfate reduction, presumably due to depletion of Fe(III) near the site of injection. The switch from Fe(III) reduction to sulfate reduction was accompanied by an increase in dissolved U(VI) in the groundwater, which was attributed to poor reduction of U(VI) by acetate-oxidizing sulfate reducers (1). In contrast to U(VI), vanadium(V) levels in the groundwater did not increase after 50 days of acetate injection when sulfate reduction became important. One possible explanation for this is that acetate-oxidizing sulfate reducers can reduce vanadium(V), but this has not been evaluated and seems unlikely due to the poor ability of these same organisms to reduce U(VI) (1, 17).
Hydrogen sulfide reduces vanadium(IV) to insoluble vanadium(III) (30), and thus, sulfide production might have accounted for the continued removal of vanadium(V) under sulfate-reducing conditions in the field trial. In contrast, sulfide does not effectively reduce U(VI) (15, 16). Regardless of the mechanisms involved, it is clear that stimulating microbial Fe(III) reduction, and possibly sulfate reduction, is an effective strategy for removing vanadium(V) from contaminated groundwater.
In summary, the results presented here demonstrate that the reduction of vanadium(V) by G. metallireducens can convert soluble vanadium(V) into an insoluble form that readily precipitates from solution. Microbially mediated vanadium(V) reduction, whether a direct enzymatic reduction or an indirect abiotic mechanism, can be a suitable strategy for the in situ removal of toxic vanadium from groundwater. Further study of the biochemical and abiotic mechanisms involved in vanadium(V) reduction in sediments and groundwaters may help in better understanding the behavior of this pollutant in the environment.
Acknowledgments
This research was funded by the Natural and Accelerated Bioremediation Research program, Biological and Environmental Research, U.S. Department of Energy (grants DE-FG02-0ER62985 and DE-FG02-97ER62475), and by a postdoctoral Fellowship from the Secretaría de Estado de Educación y Universidades (Spain), cofunded by the European Social Fund, to I.O.-B.
We thank Charles T. Resch (Pacific Northwest National Laboratory) for sample collection and field-related activities. We also thank Philip E. Long (Pacific Northwest National Laboratory), Richard Dayvault (S. M. Stoller Corporation), and Donald R. Metzler (U.S. Department of Energy) for coordinating activities conducted at the Old Rifle UMTRA site, as well as Mike Jercinovic for support with the electron microprobe analysis.
REFERENCES
- 1.Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, A. Peacock, R. Dayvault, S. Marutzky, D. R. Metzler, K. Karp, M. Lowe, D. C. White, P. E. Long, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baroch, E. F. 1983. Vanadium and vanadium alloys, p. 673-687. In Kirk-Othmer encyclopedia of chemical technology, 3rd ed., vol. 23. John Wiley & Sons, New York, N.Y.
- 3.Bautista, E. M., and M. Alexander. 1972. Reduction of inorganic compounds by soil microorganisms. Soil Sci. Soc. Am. Proc. 36:918-920. [Google Scholar]
- 4.Bisconti, L., M. Pepi, S. Mangani, and F. Baldi. 1997. Reduction of vanadate to vanadyl by a strain of Saccharomyces cerevisiae. Biometals 10:239-246. [DOI] [PubMed] [Google Scholar]
- 5.Budevsky, O., and L. Johnova. 1965. Colorimetric determination of vanadium (V) with 4-(2-pyridylazo)resorcinol. Talanta 12:291-301. [Google Scholar]
- 6.Carpentier, W., K. Sandra, I. De Smet, A. Brigé, L. De Smet, and J. Van Beeumen. 2003. Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl. Environ. Microbiol. 69:3636-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crans, D. C., S. S. Amin, and A. D. Keramidas. 1998. Chemistry of relevance to vanadium in the environment, p. 73-95. In J. O. Nriagu (ed.), Vanadium in the environment. Part 1: chemistry and biochemistry. John Wiley & Sons, Inc., New York, N.Y.
- 8.Evans, H. T., and J. S. White. 1987. The colorful vanadium minerals: a brief review and a new classification. Miner. Rec. 18:333-340. [Google Scholar]
- 9.Finneran, K. T., R. T. Anderson, K. P. Nevin, and D. R. Lovley. 2002. Potential for bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil Sediment Contam. 11:339-357. [Google Scholar]
- 10.Holmes, D. E., K. T. Finneran, R. A. O'Neil, and D. R. Lovley. 2002. Enrichment of Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llobet, J. M., and J. L. Domingo. 1984. Acute toxicity of vanadium compounds in rats and mice. Toxicol. Lett. 23:227-231. [DOI] [PubMed] [Google Scholar]
- 12.Lovley, D. R., and F. H. Chapelle. 1995. Deep subsurface microbial processes. Rev. Geophys. 33:365-381. [Google Scholar]
- 13.Lovley, D. R., S. J. Giovannoni, D. C. White, J. E. Champine, E. J. P. Phillips, Y. A. Gorby, and S. Goodwin. 1993. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 159:336-344. [DOI] [PubMed] [Google Scholar]
- 14.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovley, D. R., and E. J. P. Phillips. 1992. Reduction of uranium by Desulfovibrio desulfuricans. Appl. Environ. Microbiol. 58:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovley, D. R., E. J. P. Philips, Y. A. Gorby, and E. R. Landa. 1991. Microbial reduction of uranium. Nature 350:413-416. [Google Scholar]
- 17.Lovley, D. R., E. E. Roden, E. J. P. Phillips, and J. C. Woodward. 1993. Enzymatic iron and uranium reduction by sulphate-reducing bacteria. Mar. Geol. 113:41-53. [Google Scholar]
- 18.Lyalikova, N. N., and N. A. Yurkova. 1992. Role of microorganisms in vanadium concentration and dispersion. Geomicrobiol. J. 10:15-26. [Google Scholar]
- 19.Nechay, B. R., L. B. Nanninga, P. S. E. Nechay, R. L. Post, J. J. Grantham, I. G. Macara, L. F. Kubena, T. D. Philips, and F. H. Nielsen. 1986. Role of vanadium in biology. Fed. Proc. 45:123-132. [PubMed] [Google Scholar]
- 20.Nriagu, J. O. 1998. History, occurrence and uses of vanadium, p. 1-24. In J. O. Nriagu, (ed.), Vanadium in the environment. Part 1. Chemistry and biochemistry. John Wiley & Sons, Inc., New York, N.Y.
- 21.Rehder, D. 1991. The bioinorganic chemistry of vanadium. Angew. Chem. Int. Ed. Engl. 30:148-167. [Google Scholar]
- 22.Rehder, D. 1995. Inorganic considerations of the function of vanadium in biological systems, p. 1-43. In H. Sigel and A. Sigel (ed.), Metal ions in biological systems, vol. 31. Marcel Dekker, Inc., New York, N.Y. [PubMed]
- 23.Roling, W. F., B. M. van Breukelen, M. Braster, B. Lin, and H. W. van Verseveld. 2001. Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Appl. Environ. Microbiol. 67:4619-4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooney-Varga, J. N., R. T. Anderson, J. L. Fraga, D. Ringelberg, and D. R. Lovley. 1999. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl. Environ. Microbiol. 65:3056-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safavi, A., M. R. Hormozi Nezhad, and E. Shams. 2000. Highly selective and sensitive kinetic spectrophotometric determination of vanadium (IV) in the presence of vanadium (V). Anal. Chim. Acta 409:283-289. [Google Scholar]
- 26.Selbin, J. 1966. Oxovanadium (IV) complexes. Coord. Chem. Rev. 1:293-314. [Google Scholar]
- 27.Snoeyenbos-West, O. L., K. P. Nevin, R. T. Anderson, and D. R. Lovley. 2000. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb. Ecol. 39:153-167. [DOI] [PubMed] [Google Scholar]
- 28.Stein, L. Y., M. T. La Duc, T. J. Grundl, and K. H. Nealson. 2001. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 3:10-18. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Energy. 1999. Final site observational work plan for the UMTRA project Old Rifle site GJO-99-88-TAR. U.S. Department of Energy, Grand Junction, Colo.
- 30.Wanty, R. B., and M. B. Goldhaber. 1992. Thermodynamics and kinetics of reactions involving vanadium in natural systems: accumulation of vanadium in sedimentary rocks. Geochim. Cosmochim. Acta 56:1471-1483. [Google Scholar]
- 31.Wehrly, B., and W. Stumm. 1989. Vanadyl in natural waters: adsorption and hydrolysis promote oxygenation. Geochim. Cosmochim. Acta 53:69-77. [Google Scholar]
- 32.White, A. F., and M. L. Peterson. 1996. Reduction of aqueous transition metal species on the surfaces of Fe(II)-containing oxides. Geochim. Cosmochim. Acta 60:3799-3814.
- 33.Woolfolk, C. A., and H. R. Whiteley. 1962. Reduction of inorganic compounds with molecular hydrogen by Mocrococcus lactilyticus. J. Bacteriol. 84:647-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 1988. Vanadium. Environmental health criteria. No. 80. World Health Organization, Geneva, Switzerland.
- 35.Yurkova, N. A., and N. N. Lyalikova. 1991. New vanadate-reducing facultative chemolithotrophic bacteria. Microbiology 59:672-677. [Google Scholar]