Abstract
Escherichia coli and Pseudomonas putida dried in hydroxyectoine or trehalose are shown to be highly resistant to the organic solvents chloroform and acetone, and consequently, they can be encapsulated in a viable form in solid plastic materials. Bacteria are recovered by rehydration after physical disruption of the plastic. P. putida incorporated into a plastic coating of maize seeds was shown to colonize roots efficiently after germination.
Previously, we have shown how osmotic preconditioning of bacteria, followed by drying in the presence of glass-forming protectant molecules, such as trehalose or hydroxyectoine, results in a high level of desiccation tolerance, where viability is maintained throughout extended storage periods at above-ambient temperatures (8, 12). This has been termed anhydrobiotic engineering (9), in reference to anhydrobiotic organisms which naturally exhibit extreme desiccation tolerance (4, 6, 14). In this report, we describe a new approach to preserving bacteria by drying and then encapsulating the bacteria in plastic, and we demonstrate a potential application as a seed coating.
Glasses, including those derived from organic materials, are high-viscosity liquids that slow molecular diffusion and the rates of chemical reactions, including degradative processes, dramatically (7). Consequently, biological molecules embedded in some organic glasses exhibit a high degree of stability (2, 6). For example, proteins dried in a trehalose glass are protected from denaturation by organic solvents (5, 10). Whether anhydrobiotically engineered bacteria are similarly tolerant of chemical stress, as is observed for some bacterial spores (1, 13), is not known, however. We therefore tested the resistance of dried samples of Escherichia coli and Pseudomonas putida to different pure organic solvents. Growth of E. coli MC4100 and P. putida KT2440 (strains cited in reference 12) in hypersaline minimal medium (HMM), harvesting, and vacuum drying in 1 M trehalose plus 1.5% (wt/vol) polyvinylpyrrolidone (PVP; viscosity enhancer), or 1 M hydroxyectoine plus 1.5% (wt/vol) PVP, were performed as previously described (8, 12). Dried samples of E. coli and P. putida containing approximately 108 cells were mixed with 200 μl of pure acetone, chloroform, or ethanol and incubated for 5 min. Solvents were then removed under vacuum for 25 to 135 min, depending on the solvent. Control experiments were done using fresh E. coli and P. putida, and survival of solvent-treated bacteria was compared to that of untreated cells, measured by plating a dilution series and colony counting. As expected, survival of nondried E. coli and P. putida was below detection levels. Remarkably, dried bacteria tolerated acetone and chloroform treatment to a high degree, with survival rates above 90% in some instances (Table 1). The experiment was repeated three times, giving similar rates of survival. However, ethanol treatment of dried cells of both species resulted in very low or undetectable survival. This is consistent with the partial solubility of trehalose and hydroxyectoine in ethanol, which would be expected to degrade the glass matrix and attack the bacteria within.
TABLE 1.
Survival of dried E. coli and P. putida after treatment with organic solvents
| Bacterium and treatment | Survival (%)a
|
||
|---|---|---|---|
| Acetone | Chloroform | Ethanol | |
| P. putida | |||
| Not dried | ND | ND | ND |
| Dried in trehalose + PVP | 71.5 | 95.6 | 5.0 |
| Dried in HEb + PVP | 94.4 | >99 | ND |
| Dried in PVP alone | ND | ND | ND |
| E. coli | |||
| Not dried | ND | ND | ND |
| Dried in trehalose + PVP | 60.4 | 77.6 | 3.0 |
| Dried in HE + PVP | 18.7 | 53.5 | ND |
| Dried in PVP alone | ND | ND | ND |
Survival of dried E. coli and P. putida after treatment with organic solvent acetone, chloroform, or ethanol compared to survival of untreated controls. ND, not detectable (recovery rates lower than 0.001%).
HE, hydroxyectoine.
Further control experiments were performed to test whether the protection of dried E. coli and P. putida against organic solvents was simply due to the lack of water or due to the presence of trehalose or hydroxyectoine. Bacteria suspended in 1.5% PVP alone suffered a marked drop in viability during drying, since trehalose and hydroxyectoine are required for a high degree of protection against desiccation damage, but sufficient cells survived to assess the effects of organic solvents. Dried samples containing ∼105 cells were treated as described above and compared with survival of nontreated bacteria. Table 1 shows that cells dried without trehalose or hydroxyectoine did not survive treatment with organic solvents, confirming the requirement for these excipients. Clearly, PVP alone either does not encapsulate bacteria efficiently or is soluble in organic solvents.
The fact that dried cells tolerate treatment with pure solvents suggests the possibility of incorporating microorganisms in plastic without affecting viability. To test this, ∼107 dried cells of E. coli or P. putida were crumbled to powder, which was mixed with 1 ml of chloroform and 50 mg of polystyrene, in this order. The mixture was spread on a glass plate and allowed to air dry for 15 min, when a solid plastic layer formed. When plastic layers were shredded using a sterile razor blade and incubated in Luria-Bertani medium at an appropriate temperature, growth was detected in less than 12 h. The cultures of either E. coli or P. putida were demonstrated to be pure and to consist of the same strains included in the initial plastic layer.
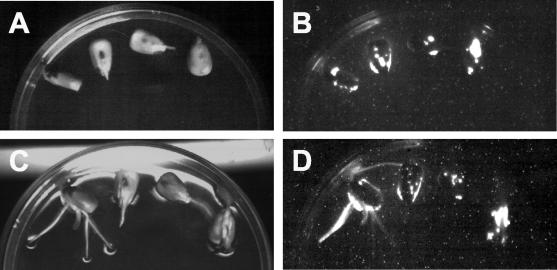
As an initial test of the applicability of this approach, a seed coating experiment was performed using P. putida, which is an efficient root colonizer and is beneficial to plants (3, 11). To monitor root colonization during the germination process, a bioluminescent strain, named P. putida MAX10, was first constructed. The promoterless luxCDABE operon from Photorhabdus luminescens was inserted into P. putida chromosomal DNA after conjugation between an E. coli strain carrying plasmid pUT mini-Tn5 ′luxCDABE Tcr and a spontaneous rifampin-resistant mutant of P. putida KT2440, as described previously (15). Maize seeds were sterilized in 70% (vol/vol) ethanol for 5 min, incubated a second time in 20% (vol/vol) bleach for 20 min, and washed five times in sterile distilled water. The seeds were air dried in a microbiological flow cabinet and then coated with bacteria by immersion in a mixture of dried, powdered P. putida MAX10, chloroform, and polystyrene and prepared as described above. After 2 min, the seeds were withdrawn from the mixture and allowed to dry in a sterile airflow for 10 min. Seeds were stored at 30°C in dark and dry conditions for 1, 30, or 90 days after coating and then sowed onto agar plates to allow germination. Plates were incubated at 30°C in the dark; germination was first detected after 4 days, and root colonization was monitored over the next week by detection of bacterial luminescence. Before germination, no light emission was observed (data not shown). However, after germination, luminescence of root processes was seen to develop, consistent with colonization of the rhizosphere by P. putida MAX10 (Fig. 1).
FIG. 1.
Two different stages of germination of maize seeds coated with luminescent P. putida MAX10 in a polystyrene layer 4 days (A and B) and 9 days (C and D) after the seeds were coated with bacteria. Pictures were taken under artificial light (A and C) or in the dark (B and D) with 30-min exposure, using a charge-coupled device camera. The seeds shown had been stored for 30 days after the plastic coating was applied, although identical results were also obtained with seeds stored for 1 or 90 days after coating (not shown).
The ability to encapsulate viable cells in plastic layers should have a number of applications in biotechnology. One factor which can have a detrimental effect on dried microorganisms over the long term is humidity in the environment; increasing moisture content of the dried sample compromises viability. Storage under vacuum or in an inert atmosphere can prevent this (8, 12) but is costly and unwieldy. Long-term storage of culture collections and libraries should be facilitated by the plastic encapsulation procedure we describe, since the dried bacteria are isolated from atmospheric conditions but can be recovered easily. The use of plastic for encapsulation also allows shaping or molding into layers, sheets, films, blocks, pellets, or pills, for example, so that the procedure could be applied in many different industries.
Acknowledgments
We thank Arcadio García de Castro for useful discussions and Simon Swift for providing lux reagents.
A.T. is the Anglian Water Fellow in Biotechnology of Pembroke College, University of Cambridge. This work was partially supported by EC grant BIO4-CT98-0283 and by Merck Chemicals, Ltd.
REFERENCES
- 1.Bloomfield, S. F., and M. Arthur. 1994. Mechanisms of inactivation and resistance of spores to chemical biocides. Soc. Appl. Bacteriol. Symp. Ser. 23:91S-104S. [DOI] [PubMed] [Google Scholar]
- 2.Burke, M. J. 1986. The vitreous state and survival of anhydrous biological systems, p. 358-364. In A. C. Leopold (ed.), Membranes, metabolism and dry organisms. Cornell University Press, New York, N.Y.
- 3.Campbell, R., and M. P. Greaves. 1990. Anatomy and community structure of the rhizosphere, p. 11-34. In J. M. Lynch (ed.), The rhizosphere. Wiley & Sons, Chichester, United Kingdom.
- 4.Clegg, J. S. 2001. Cryptobiosis—a peculiar state of biological organization. Comp. Biochem. Physiol. B 128:613-624. [DOI] [PubMed] [Google Scholar]
- 5.Cleland, J. L., and A. J. S. Jones. November1994. Excipient stabilization of polypeptides treated with organic solvents. European patent EP 0 686 045 B1.
- 6.Crowe, J. H., J. F. Carpenter, and L. M. Crowe. 1998. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60:73-103. [DOI] [PubMed] [Google Scholar]
- 7.Franks, F. 1985. Biophysics and biochemistry at low temperatures. Cambridge University Press, Cambridge, United Kingdom.
- 8.García de Castro, A., H. Bredholt, A. Strøom, and A. Tunnacliffe. 2000. Anhydrobiotic engineering of gram-negative bacteria. Appl. Environ. Microbiol. 66:4142-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García de Castro, A., J. Lapinski, and A. Tunnacliffe. 2000. Anhydrobiotic engineering. Nat. Biotechnol. 18:473. [DOI] [PubMed] [Google Scholar]
- 10.Gribbon, E. M., S. D. Sen, B. J. Roser, and J. Kampinga. 1996. Stabilisation of vaccines using trehalose (Q-T4) technology. Dev. Biol. Stand. 87:193-199. [PubMed] [Google Scholar]
- 11.Lugtenberg, B. J. J., and L. Dekkers. 1999. What makes Pseudomonas bacteria rhizosphere competent? Environ. Microbiol. 1:9-13. [DOI] [PubMed] [Google Scholar]
- 12.Manzanera, M., A. García de Castro, A. Tøonderik, M. Rayner-Brandes, A. R. Strøom, and A. Tunnacliffe. 2002. Hydroxyectoine is superior to trehalose for anhydrobiotic engineering of Pseudomonas putida KT2440. Appl. Environ. Microbiol. 68:4328-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tunnacliffe, A., and J. Lapinski. 2003. Resurrecting van Leeuwenhoek's rotifers: a reappraisal of the role of disaccharides in anhydrobiosis. Philos. Trans. R. Soc. Lond. Ser. B 358:1755-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winson, M. K., S. Swift, P. J. Hill, C. M. Sims, G. Griesmayr, B. W. Bycroft, P. Williams, and G. S. A. B. Stewart. 1998. Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol. Lett. 163:193-202. [DOI] [PubMed] [Google Scholar]