Abstract
An intracellular bacterium from Ixodes ricinus ticks collected in Italy was characterized by electron microscopy (EM), PCR sequencing of the 16S rRNA gene, molecular phylogenetic analysis, and in situ hybridization (ISH). This bacterium was shown by EM to be present in the cytoplasm, as well as in the mitochondria of ovarian cells. When universal 16S rRNA bacterial primers were used, PCR amplification of ovarian DNA followed by cloning and sequencing resulted in the same sequence being found in each sample. Phylogenetic analysis of this sequence showed that the bacterium from which it was derived, tentatively designated IricES1, is part of a novel clade in the alpha subdivision of the Proteobacteria. ISH and PCR assays of various tissues performed with oligonucleotides specific for the IricES1 16S rRNA showed that IricES1 is restricted to ovarian cells. Based on the results obtained, we inferred that the bacteria seen by EM in ovarian cells are a single type of bacteria, corresponding to IricES1. PCR screening of 166 ticks from various parts of Italy and one site in England showed that IricES1 was present in 96% of adult females and 44% of nymphs (unsexed). No adult males were found to be infected. Despite the apparent parasitism of host mitochondria by IricES1, the available information suggests that the bacterium has an obligate relationship with its host, although this must be confirmed.
Bacteria that reside within the cells of eukaryotic organisms, especially arthropods, are common in nature (6, 14, 21). Many of these bacteria have been shown to be parasites, while others have been shown to have an obligate, mutualistic relationship with their hosts. Intracellular bacteria are usually found within a host-derived vacuole or phagosome or in the cytoplasm (21, 23); their presence in other parts of the cell is apparently rare. Some Rickettsia spp. are known to invade the nucleus (23), while other bacteria whose taxonomic status is unknown have been located in mitochondria. The presence of bacteria in mitochondria was first demonstrated in the 1970s with two ciliate (unicellular eukaryote) species (10, 29). Following these initial discoveries, there has apparently been only one additional demonstration of this phenomenon, in the European hard tick Ixodes ricinus. Electron microscopy (EM) studies of I. ricinus collected in England and Switzerland by Lewis (17) and Zhu et al. (30) revealed that oval bacteria were present in mitochondria of ovarian cells of female adults, as well as in primordial ovarian tissue of female larvae and nymphs. To obtain further information about these bacteria, we examined Italian populations of I. ricinus. Our goals were (i) to determine how many 16S rRNA sequence types were present in the ovary, (ii) to determine the phylogenetic position of the bacteria from the ovary, (iii) to confirm the distribution of the bacteria in different tick tissues by using PCR and in situ hybridization (ISH), and (iv) to examine how widespread the bacteria are in tick populations.
MATERIALS AND METHODS
Tick collection.
A total of 158 I. ricinus ticks (76 females, 46 males, and 36 nymphs [unsexed]) were collected in Italy between 1997 and 2002, were identified by using standard taxonomic keys, and were preserved frozen or in acetone or used immediately. This collection effort was part of a separate study of intracellular pathogens in ticks (4). Specifically, 127 ticks were collected in the Trento Province between 1997 and 2001 either from infested goats or by dragging vegetation; 9 and 16 ticks were collected from the Veneto and Marche regions, respectively, by dragging vegetation in 2000; and 6 ticks were collected in the Tuscany region from infested ruminants during 2002. An additional eight I. ricinus adults (four females and four males) were collected in 2001 by dragging vegetation in Cheddar (Somerset, United Kingdom).
EM.
The ovaries of three engorged females from Tuscany and two engorged females from Trento Province were dissected in a saline solution under sterile conditions. Each of the five ovaries was then divided into two pieces. One piece was fixed in 0.1 M cacodylate buffer (pH 7.2) containing 2.5% glutaraldehyde for 3 h at 4°C, while the other (smaller) piece was set aside for molecular work. The fixed samples were then postfixed in the same buffer containing 1% OsO4 for 1.5 h at 4°C. Samples were then dehydrated in ethanol (EtOH) and embedded in Epon 812. Thin sections (80 nm) were stained with uranyl acetate and lead citrate and examined with a Zeiss EM900 transmission electron microscope.
PCR, cloning, and sequencing.
DNA was extracted (4) from individual ovary portions obtained from five adult females (the ticks used for the EM study). A PCR was performed with each sample as previously described (20) by using universal eubacterial primers fD1 and rP2, which are specific for the 5′ and 3′ ends of 16S rRNA (28). The five PCR products were separately ligated into pGEM-T Easy vectors and transformed into Escherichia coli NM522 competent cells according to the manufacturer's instructions (Promega). Ten cloned DNA fragments for each ovarian sample (a total of 50 fragments) were then sequenced by using the vector-specific T7 and SP6 forward and reverse primers. One clone from each ovarian sample (a total of five clones) was sequenced in its entirety by using internal 16S rRNA primers (16). The ovarian DNA samples were also subjected to PCR with primers specific for the genera Rickettsia (4), Wolbachia (20), Ehrlichia (7), and Borrelia (7).
Phylogenetic analysis.
All sequences obtained from the PCR and cloning experiments described above were found to represent a single 16S rRNA sequence. This sequence was subjected to BLAST analysis (http://www.ncbi.nlm.nih.gov/blast) and aligned with close relatives, as well as with other proteobacterial sequences. Alignment with the corresponding 16S rRNAs was performed by using software available at the Ribosomal Database Project website (8); secondary structure was taken into account when this was done. Phylogenetic analyses were performed by using maximum-likelihood and maximum-parsimony criteria with the programs TreePuzzle 5.0 (25) and PAUP*4b10 (26), respectively. In the TreePuzzle analyses, the default settings were used except that the TN+G model of sequence evolution was selected. In the PAUP* analyses, a 50% majority rule bootstrap consensus tree (1,000 replicates) was generated by using the heuristic search option with five random-addition replicates per bootstrap replicate. Gaps were treated as a fifth base.
ISH.
Two oligonucleotides (designated S-*-IrES1-0066-a-A-18 [5′-GCTACAGCTCTTGCCCGT-3′] and S-*-IrES1-1410-a-A-19 [5′-CAAAACCGACTCCCATGGC-3′]) were designed to target variable regions of the 16S rRNA sequence from the bacterium being investigated and were 5′ labeled with digoxigenin (DIG) (Qiagen). The four-digit number in each probe designation indicates the position in the E. coli 16S rRNA sequence (1). Target sites were selected in variable regions based on the results of previous studies (12) and were analyzed for sequence matches with Check Probe (8), which showed that the probes had unique sequences. Probe S-*-IrES1-0066-a-A-18 had 11, 12, and 8 mismatches with 16S rRNA sequences of Rickettsia conorii (accession no. AF541999), Anaplasma phagocytophilum (AY055469), and Borrelia burgdorferi (AE001147), respectively, while probe S-*-IrES1-1410-a-A-19 had 3, 5, and 10 mismatches, respectively, with the same organisms. All experimental procedures were conducted under RNase-free conditions. Three whole engorged females from Tuscany, which had been stored in acetone (13), were examined. These ticks were divided into halves with a razor, and one half of each tick was set aside for PCR screening. For ISH, tick halves were fixed in 4% phosphate-buffered paraformaldehyde overnight at 4°C, dehydrated, cleared by using an EtOH-xylene series (three changes [20 min each] of 50% EtOH, three changes [20 min each] of 70% EtOH, three changes [20 min each] of 95% EtOH, three changes [20 min each] of 100% EtOH, and then one quick change of 50% EtOH-50% xylene followed by three changes [10 min each] of xylene), and embedded in paraffin. Serial tissue sections that were 5 μm thick were cut with a rotary microtome and mounted on positively charged glass slides (ProbeOn Plus; Fisher Scientific). The slides were subjected to the following steps: dewaxation and rehydration in a xylene-EtOH series (three changes [2 min each] of xylene and then 100% EtOH, one change [5 min] of 95% EtOH, one change [5 min] of 70% EtOH, one change [5 min] of 50% EtOH, a quick rinse in diethyl pyrocarbonate-treated distilled H2O, and then two changes [5 min each] of 1× phosphate-buffered saline), pretreatment with 10 μg of proteinase K per ml (15 min at 37°C), refixation in 4% paraformaldehyde (5 min at 4°C), treatment for 20 min with 0.2 M HCl, treatment with two changes (5 min each) of 0.25% acetic anhydride in 0.1 M triethanolamine, dehydration through an EtOH series (one change [15 s] of 70% EtOH, one change [15 s] of 95% EtOH, and one change [15 s] 100% EtOH), and air drying. They were incubated in 50 μl of hybridization buffer (20 mM Tris-HCl, 0.9 M NaCl, 0.01% sodium dodecyl sulfate, 20% formamide) containing the two specific DIG-labeled probes, each at a concentration of 2 ng/μl. After incubation for 4 h at 42°C, the tissue sections were washed in buffer (20 mM Tris-HCl, 180 mM NaCl, 5 mM EDTA, 0.01% sodium dodecyl sulfate) at 44°C for 10 min. Maximally stringent hybridization and washing conditions were chosen by using the optimization procedures described by Manz et al. (18). Slides were subjected to immunological detection of bound probes with anti-DIG antibodies conjugated with alkaline phosphatase by using the manufacturer's instructions (Boehringer Mannheim) and were stained with a 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium substrate (Vector Laboratories). The tissue sections were then permanently mounted on Vectashield mounting medium (Vector Laboratories) and observed with a light microscope. In order to control for the possibility that a nonspecific oligonucleotide labeled with DIG could result in a signal, a section from each tick sample was also incubated with a probe specific for the gamma subdivision of the Proteobacteria (gamma-proteobacteria) (DIG-Gam1019) (19). Additional negative control experiments involved either treatment of slides with RNase prior to the probe hybridization step or the absence of probes. A positive control experiment with the universal bacterial probe EUB338 (2) was also performed.
Screening of I. ricinus populations.
The alignment of the novel 16S rRNA sequence with related bacterial sequences was used to design primers that specifically amplified a fragment of the new sequence. During the process of designing primers, regions in which there was high variability were chosen, and the BLAST program was used to examine the number of mismatches that candidate primers had with all other known 16S rRNA sequences, in order to avoid potential amplification from other bacteria. The forward and reverse primers were IricES1-F (5′-TGTAGCGATACAGAGTTCTGC-3′) and IricES1-R (5′-CACCCCAGTCGTCAACCTTAC-3′), respectively. IricES1-F had no matches with any bacterial or invertebrate sequences in GenBank at the time of checking, while IricES-R had at least four mismatches with all known sequences. IricES1-F and IricES-R correspond to positions 78 to 98 and 1470 to 1490, respectively, in the 16S rRNA sequence of E. coli strain K-12 (accession number AE000452) and amplify a 1,374-bp fragment of the newly discovered sequence.
A total of 166 ticks, including those examined by EM and ISH, were then screened by PCR. DNA was extracted from individual, whole bodies of 155 adults and nymphs collected in Italy and England by using a QIAamp tissue kit (Qiagen). The same method was used to extract DNA from the three half-bodies prepared during ISH. Amplification for the screening process was performed in 30 μl of buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin) containing each deoxynucleoside triphosphate at a concentration of 0.2 mM, 20 pmol of each primer, 1 U of Taq polymerase (Perkin-Elmer), and 1 μl of DNA sample. The cycling conditions were as follows: 94°C for 1.5 min; five cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 2 min; 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 2 min; and 5 min at 72°C. DNA samples from R. conorii ATCC VR-613, A. phagocytophilum Webster strain, and B. burgdorferi ATCC 35211, which are bacteria that are known to infect I. ricinus, were included as negative controls for the PCR screening. To test the quality of each tick DNA sample, PCR was performed with primers specific for the mitochondrial cytochrome oxidase III gene (CO3-F2 [5′-CCTAAYATTGAAATTGG-3′] and CO3-R [5′-TCTACRAAATGTCARTATCA-3′], modified from the study of Simon et al. [24]). The quality of the DNA of negative controls was checked by using the relevant specific primers (4, 7, 20). Finally, to complement ISH experiments, the salivary glands, gut, and ovary were dissected from five females from Trento Province (including the two ticks used in the EM study) under sterile conditions, and the DNA was extracted (4) from each organ, as well as from the remaining body. PCR was then performed as described above.
Nucleotide sequence accession number.
The 16S rRNA sequence determined in this study has been deposited in the GenBank database under accession no. AJ566640.
RESULTS
EM.
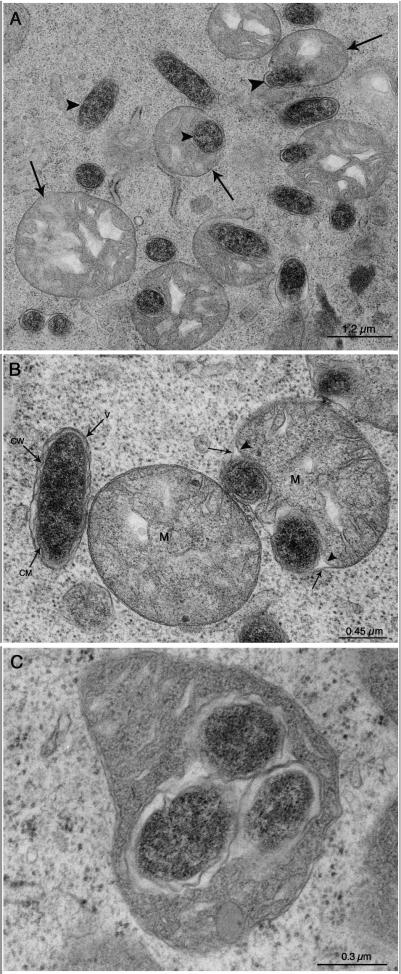
The five females examined were all found to have oval or coccoid bacteria in their ovarian cells, and the morphological characteristics were similar to those previously reported by Lewis (17) and Zhu et al. (30). Bacteria were present both in the cytoplasm and in the mitochondria (Fig. 1A). The cells were ∼0.45 μm in diameter and ∼1.2 μm long. In the cytoplasm, three membranes were associated with bacteria (Fig. 1B). The external membrane was a host membrane, while the two inner membranes were typical of gram-negative bacteria (30). Within the mitochondria, the bacteria were surrounded by what appeared to be the mitochondrial matrix (Fig. 1C).
FIG. 1.
Transmission electron micrographs showing developing oocytes from the ovary of a partially engorged adult female I. ricinus. Bacteria with the same characteristics were obtained during examination of four other tick ovaries. (A) Bacteria are present either in the cytoplasm within membrane-limited vacuoles (not clearly visible at this magnification) or inside the mitochondria. The arrowheads indicate the bacteria, and the arrows indicate mitochondria. (B) Higher magnification showing one bacterium (to the left) adjacent to a mitochondrion (M) and two other bacteria (to the right) between the outer (arrows) and inner (arrowheads) membranes of a mitochondrion. The bacterium on the left is enclosed within a cytoplasmic membrane-limited vacuole (V) and has an outer cell wall (CW) and an inner cell membrane (CM) typical of gram-negative bacteria. (C) Three bacteria enclosed within the matrix of a mitochondrion.
16S rRNA-based identification.
An alignment of the 50 sequences obtained by PCR and cloning revealed that they all were derived from a single, unique 16S rRNA sequence, 1,427 bp of which was determined. Some cloned sequences were found to have single substitutions, but these substitutions were almost certainly Taq polymerase errors due their random distribution in the alignment. PCR assays of the five ovarian samples with primers specific for Rickettsia spp. (4), Wolbachia spp. (20), Ehrlichia spp. (7), and Borrelia spp. (7) were negative in all cases.
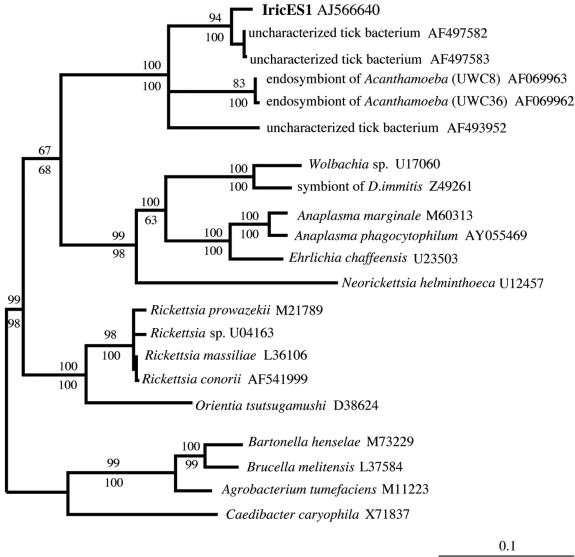
BLAST analysis showed that the novel sequence was most similar to 16S rRNAs from representatives of the alpha-proteobacteria, many of which are intracellular parasites or symbionts. The most closely related sequences (97% identity) were sequences of uncultured bacteria found in the tick Haemaphysalis wellingtoni (clones Hw191 and Hw124) (22); 91 to 92% identity with the endosymbionts of Acanthamoeba spp. strains UWC8 and UWC36 (11) and 90% identity with the uncultured bacterium Montezuma from Ixodes persulcatus (unpublished data) were also found. The next highest matches were with sequences from various Rickettsia spp. (89 to 88% identity). Phylogenetic analysis that included various representatives of the alpha-, beta-, delta-, and gamma-proteobacteria confirmed that the I. ricinus bacterium is a member of the alpha subdivision of the Proteobacteria (data not shown). A more detailed phylogenetic comparison with various alpha-protoeobacterial representatives (Fig. 2) placed the bacterium in a novel clade in this subdivision. Based on its unique 16S rRNA sequence, as well as results described below, we temporarily designate the new bacterium Ixodes ricinus endosymbiont 1 (IricES1), pending further taxonomic characterization.
FIG. 2.
Phylogenetic comparison of the 16S rRNA of IricES1, an intracellular bacterium from the tick I. ricinus, with the 16S rRNAs of selected members of the alpha-proteobacterial assemblage. The tree was constructed by using TreePuzzle 5.0 with the TN+G model of substitution. A parsimony bootstrap tree estimated with PAUP* was found to have an identical topology. The GenBank accession number for each sequence is indicated. The numbers above and below each node are quartet puzzling support values and bootstrap values from parsimony analyses, respectively. The scale bar indicates the number of inferred substitutions per site. Other analyses in which various beta-, gamma-, and delta-proteobacteria were included as outgroups resulted in very similar relationships for the alpha-proteobacteria shown.
ISH.
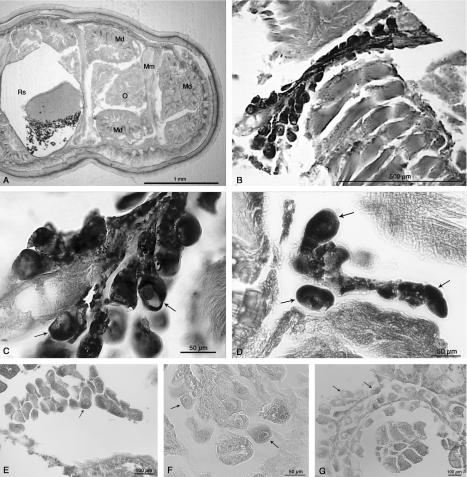
Clear signals were found in the ovarian cells (both oocytes and funicular cells) of each of the three females examined but not in other parts of the body (Fig. 3B, C, and D). A similar, although weaker, signal was found when the universal EUB338 bacterial probe was used (data not shown). The signal was not present when a gamma-proteobacterial probe was used or in probe-free experiments (Fig. 3E and F). Similarly, no signal was found following RNase treatment and addition of the specific probes (Fig. 3G). PCR screening with specific primers of DNA extracted from the bodies of the same three females provided additional confirmation that they contained IricES1 (data not shown). None of these ticks was found to be positive for Rickettsia, Ehrlichia, Wolbachia, or Borrelia, suggesting that the ISH signal obtained was entirely due to IricES1.
FIG. 3.
(A) Light microscopy image of a transverse section of a partially engorged I. ricinus adult female. O, ovary; Md, midgut diverticulum; Mm, muscles; Rs, rectal sac. (B) ISH with the 16S rRNA of IricES1 in ovarian tissue of a partially engorged I. ricinus adult female by using a probe mixture containing two DIG-labeled specific oligonucleotides, S-*-IrES1-0066-a-A-18 and S-*-IrES1-1410-a-A-19. The probes were detected with alkaline phosphatase anti-DIG antibody (Boehringer Mannheim) and were stained with the BCIP-nitroblue tetrazolium substrate (Vector Laboratories). (C) Same section shown in panel B, but at a higher magnification. In all panels, the arrows indicate oocytes. (D) ISH, as described above for panel B, for a section from a different tick sample. (E) Negative control hybridization with a general gamma-proteobacterial probe (DIG-Gam1019). (F) No-probe control. (G) RNase treatment control. The slides were treated with 20 μg of RNase per ml prior to the probe hybridization step.
Host prevalence.
A PCR assay with specific 16S rRNA primers for IricES1 revealed that 93 of the 166 field-collected ticks from Italy and England were infected by the bacterium (minimal field infection rate, 56%). Interestingly, of the 80 adult females examined, 77 (96%) were found to be positive, including all four ticks from England. All 50 adult males examined were found to be negative, and 16 of 36 (44.4%) of the nymphs were positive. Direct sequencing of PCR products obtained from 10 adult females and three nymphs (including representatives from England and various regions of Italy) showed they were identical to the IricES1 16S rRNA. For the five females whose different body parts were subjected to PCR with specific primers, signals were obtained from the ovaries but not from the gut, salivary glands, or remaining body (data not shown).
DISCUSSION
Oval bacteria that reside in the cytoplasm and mitochondria in the ovaries of I. ricinus have previously been found in EM studies of ticks collected from England (17) and Switzerland (30). In EM investigations of I. ricinus collected in Italy, we found bacteria with the same characteristics. PCR and sequencing studies of the ovaries examined by EM strongly suggested that only one type of bacteria was present. This bacterium, temporarily designated IricES1, is a member of a novel clade of the alpha-proteobacteria. The inferred numbers of 16S rRNA substitutions that have accumulated in members of this clade (as indicated by the branch lengths in Fig. 2) are similar to the numbers that have accumulated in various alpha-proteobacterial genera (e.g., Wolbachia versus Anaplasma, Rickettsia versus Orientia), suggesting that the novel clade comprises at least one new genus. ISH and PCR screening of various tick tissues by using IricES1-specific oligonucleotides revealed that the bacterium is restricted to the ovarian tissues. Based on the results described above, we inferred that the bacteria revealed by EM in ovarian cells are a single type, corresponding to IricES1.
PCR screening of 130 adult ticks from a variety of locations in Italy and one English location revealed that only females were infected with IricES1 and that the prevalence was very high (96%). Notably, the 16S rRNA sequence from ticks collected in England was identical to the sequence from ticks collected in Italy. Together, our results and those of previous EM studies (17, 30) suggest that IricES1 is widespread in I. ricinus females in Europe, since it is present at all the locations examined so far. Forty-four percent of 36 nymphs collected in Italy were also infected. Although the sexes of these nymphs were not determined, a likely explanation for the level of prevalence is that the bacteria were present only in female nymphs. Zhu et al. (30) examined mature larvae and nymphs of both sexes and found bacteria only in ovarian tissue. Consistent with our study, Zhu et al. (30) found bacteria in 100% of all females examined (n = 40); one half of these females had been born in the laboratory, and other half had been collected in the wild.
Like many alpha-proteobacteria, IricES1 is found in the cytoplasm enclosed in a host membrane; however, it also has the remarkable characteristic of existing within mitochondria. This phenomenon has previously been demonstrated for the ciliates Halteria geleiana (29) and Urotricha ovata (10); however, the phylogenetic status of the bacteria involved has yet to be determined. It is not yet known if close relatives of IricES1 (i.e., clones Hw191 and Hw124 from the tick H. wellingtoni) are able to exist in mitochondria. The endosymbionts of Acanthamoeba spp., which are in the novel clade to which IricES1 belongs, have been shown by EM to be present only in the cytoplasm (11).
The possible types of interactions that occur between the ticks and IricES1 include (i) parasitism (the bacteria harm the hosts), (ii) commensalism or transient mutualism (the bacteria benefit the hosts or cause no harm but are not necessary for host survival), (iii) reproductive parasitism (the bacteria do not harm the hosts in the normal sense but cause reproductive alterations, such as skewed sex ratios), and (iv) obligatory mutualism (the bacteria provide essential compounds to the hosts) (21). The very high female-specific prevalence of IricES1, combined with the presence of this organism in I. ricinus obtained from all parts of Europe examined thus far, argues against parasitism and commensalism. Such a pattern could, however, be consistent with two forms of reproductive parasitism: male killing and induction of parthenogenesis. In the former case, a genetic male that inherits the bacterium from its mother would be killed, while in the latter case, only females would be produced by infected mothers. In each case, if the bacterium is very prevalent, the sex ratios are expected to be skewed towards females (21), which has been found in some butterfly populations (15). However, Zhu et al. (30) showed that an infected I. ricinus mother gave birth to both male and female larvae, and in our study, males and females were found at approximately equal levels in all regions where adult ticks were collected by dragging vegetation. The absence of IricES1 in late-stage larval and nymphal males indicates that another form of reproductive parasitism, cytoplasmic incompatibility, is an unlikely effect of the bacteria. Microbially induced cytoplasmic incompatibility is characterized by the inability of offspring to be produced in matings between infected males and uninfected females, as a result of a modification-rescue system (21). The absence of IricES1 in developing and adult males would mean that modification of sperm would not be possible.
In the absence of an obvious negative effect of IricES1 and on the basis of the high prevalence of this organism, it could be speculated that IricES1 is involved in an obligate mutualism with I. ricinus females. Despite the fact that many of their mitochondria are occupied by IricES1, I. ricinus oocytes give rise to male and female offspring following fertilization. Since all of the oocytes from engorged females that have been examined have been found to contain the bacteria, there is presumably ∼100% transovarial transmission. Zhu et al. (30) checked larvae several weeks after birth and found that only females harbored the bacteria. Thus, the bacteria appear to be lost in males early during their development. It will be necessary to examine newly born larvae for the presence of the bacterium to confirm whether 100% vertical transmission occurs. In any case, IricES1 continues to survive in females in primordial ovarian tissue, and it is transmitted transtadially (30) and eventually is transmitted in mature oocytes. A Rickettsia sp. from the tick Ixodes scapularis appears to have a similar life cycle. Using a PCR assay, Noda et al. (20) found that this endosymbiont was present in all early-stage I. scapularis larvae but in only one-half of the nymphs. In adults, PCR showed that the I. scapularis endosymbiont was present only in females and was restricted to ovarian tissue.
A common form of obligatory mutualism involves the provision of nutrients by one partner in exchange for accommodation, as is the case for aphids and the symbiont Buchnera aphidicola (3). The fact that IricES1 is restricted to ovarian tissue suggests that it does not play a large part in host metabolism. If it is an obligatory mutualist, it may be more like the Wolbachia that is found in European populations of Asobara tabida, which is required for oogenesis (9). It is likely that some kind of control over the level of replication of IricES1 is exerted by I. ricinus during oogenesis. Mitochondria are known to divide rapidly during oocyte development and to be swiftly turned over within the cell (5). Thus, the presence of IricES1 in a portion of the mitochondria may not compromise the energy reserves of the cell to a great extent. To test the tentative hypothesis that there is an obligatory relationship between I. ricinus and IricES1, it will be necessary to rid the host of the infection, allow the animals to breed, and then attempt to raise any offspring produced. In our study, 3 of 80 adult females were negative for IricES1 during PCR screening. Although controls indicated that the DNA in these samples could be readily amplified, it is not clear if the ticks did not contain the bacterium or whether PCR failed to detect them. Breeding experiments involving wild ticks that lack IricES1 (if such ticks exist) would facilitate our understanding of the interactions between host and symbiont.
When in the cytoplasm, IricES1 appears to reside inside a host membrane. As is the case for the primary symbionts of mealybugs (27), there appears to be very little vacuolar space between the host and bacterial membranes. Residence within a host membrane is typical of many intracellular alpha-proteobacterial genera that are closely related to IricES1, such as Wolbachia, Ehrlichia, and Anaplasma. A notable exception to this characteristic is the genus Rickettsia, which is present in the cytoplasm. Although IricES1 has been clearly demonstrated to be present in the mitochondria (17, 30; this study) and has been shown to multiply as the mitochondria degenerate (30), it is currently not clear how IricES1 enters these organelles and in what part of the mitochondria it exists. We are currently performing a more in-depth ultrastructural study of IricES1 and plan to perform other experiments to determine the nature of the symbiosis between this organism and its host.
Acknowledgments
This work was supported by grants from the Italian Ministry of Health to the Istituto Zooprofilattico Sperimentale della Sicilia A. Mirri, Palermo, and from the Centro di Ecologia Alpina, Trento. N.L. is supported by MIUR and The University of Milan (Rientro dei Cervelli program).
We thank M. Kenny and S. Shaw for providing ticks from England, V. Sambri, A. Franceschi, A. Rizzoli, V. Tagliapietra, L. Agostini, S. De Felici, R. Luise, and A. Torina for help with tick collection in Italy, T. Miura, H. Watanabe, and T. Fukatsu for advice concerning ISH, M. Myohara for help with microscopy, three anonymous referees for constructive criticism on an earlier version of the manuscript, and D. Raoult for providing the A. phagocytophilum strain.
REFERENCES
- 1.Alm, E. W., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The oligonucleotide probe database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, P., N. A. Moran, and L. Baumann. 1997. The evolution and genetics of aphid endosymbionts. Bioscience 47:12-20. [Google Scholar]
- 4.Beninati, T., N. Lo, H. Noda, F. Esposito, A. Rizzoli, G. Favia, and C. Genchi. 2002. First detection of spotted fever group rickettsiae in Ixodes ricinus from Italy. Emerg. Infect. Dis. 8:983-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinton, L. P., and J. H. Oliver, Jr. 1971. Fine structure of oogonial and oocyte development in Dermacentor andersoni Stiles (Acari: Ixodidae). J. Parasitol. 57:720-747. [PubMed] [Google Scholar]
- 6.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. John Wiley and Sons Interscience, New York, N.Y.
- 7.Cinco, M., D. Padovan, R. Murgia, M. Maroli, L. Frusteri, M. Heldtander, K. E. Johansson, and E. O. Engvall. 1997. Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J. Clin. Microbiol. 35:3365-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dedeine, F., F. Vavre, F. Fleury, B. Loppin, M. E. Hochberg, and M. Boulétreau. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA 98:6247-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Puytorac, P., and J. Grain. 1972. Intramitochondrial bacteria and peculiarities of cytostomopharyngeal ultrastructure in the ciliate, Urotricha ovata Kahl (Ciliata). C. R. Seances Soc. Biol. Fil. 166:604-607. [PubMed] [Google Scholar]
- 11.Fritsche, T. R., M. Horn, S. Seyedirashti, R. K. Gautom, K. H. Schleifer, and M. Wagner. 1999. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl. Environ. Microbiol. 65:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs, B. M., G. Wallner, W. Beisker, I. Schwippl, W. Ludwig, and R. Amann. 1998. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 64:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukatsu, T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol. Ecol. 8:1935-1945. [DOI] [PubMed] [Google Scholar]
- 14.Hayes, S. F., and W. Burgdorfer. 1989. Interactions between rickettsial endocytobionts and their tick hosts, p. 235-251. In W. Schwemmler and G. Gassner (ed.), Insect endocyobiosis: morphology, physiology, genetics, evolution. CRC Press, Boca Raton, Fla.
- 15.Jiggins, F. M., G. D. Hurst, and M. E. Majerus. 2000. Sex-ratio-distorting Wolbachia causes sex-role reversal in its butterfly host. Proc. R. Soc. London B Biol. Sci. 267:69-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, New York, N.Y.
- 17.Lewis, D. 1979. The detection of rickettsia-like microorganisms within the ovaries of female Ixodes ricinus ticks. Z. Parasitenkd. 59:295-298. [DOI] [PubMed] [Google Scholar]
- 18.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligonucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 19.Nielsen, A. T., W. T. Liu, C. Filipe, L. Grady, Jr., S. Molin, and D. A. Stahl. 1999. Identification of a novel group of bacteria in sludge from a deteriorated biological phosphorus removal reactor. Appl. Environ. Microbiol. 65:1251-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neill, S. L., A. A. Hoffmann, and J. H. Werren. 1997. Influential passengers. Oxford University Press, New York, N.Y.
- 22.Parola, P., J. P. Cornet, Y. O. Sanogo, R. S. Miller, H. V. Thien, J. P. Gonzalez, D. Raoult, I. S. Telford, and C. Wongsrichanalai. 2003. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J. Clin. Microbiol. 41:1600-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon, C., F. Frati, A. Beckenbach, B. Crespi, H. Liu, and P. Flook. 1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87:651-701. [Google Scholar]
- 25.Strimmer, K., and A. V. Haeseler. 1996. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964-969. [Google Scholar]
- 26.Swofford, D. L. 2000. PAUP*: phylogenetic analysis using parsimony (*and other methods), 4.0beta10 ed. Sinauer Associates, Sunderland, Mass.
- 27.von Dohlen, C. D., S. Kohler, S. T. Alsop, and W. R. McManus. 2001. Mealybug beta-proteobacterial endosymbionts contain gamma-proteobacterial symbionts. Nature 412:433-436. [DOI] [PubMed] [Google Scholar]
- 28.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamataka, S., and R. Hayashi. 1970. Electron microscopic studies on the mitochondria and intramitochondrial microorganisms of Halteria geleiana. J. Electron Microsc. 19:50-62. [PubMed] [Google Scholar]
- 30.Zhu, Z., A. Aeschlimann, and L. Gern. 1992. Rickettsia-like microorganisms in the ovarian primordia of molting Ixodes ricinus (Acari: Ixodidae) larvae and nymphs. Ann. Parasitol. Hum. Comp. 67:99-110. [Google Scholar]