Abstract
Stroke survivors often exhibit abnormally low motor unit firing rates during voluntary muscle activation. Our purpose was to assess the prevalence of saturation in motor unit firing rates in the spastic-paretic biceps brachii muscle of stroke survivors. To achieve this objective, we recorded the incidence and duration of impaired lower- and higher-threshold motor unit firing rate modulation in spastic-paretic, contralateral, and healthy control muscle during increases in isometric force generated by the elbow flexor muscles. Impaired firing was considered to have occurred when firing rate became constant (i.e., saturated), despite increasing force. The duration of impaired firing rate modulation in the lower-threshold unit was longer for spastic-paretic (3.9 ± 2.2 s) than for contralateral (1.4 ± 0.9 s; P < 0.001) and control (1.1 ± 1.0 s; P = 0.005) muscles. The duration of impaired firing rate modulation in the higher-threshold unit was also longer for the spastic-paretic (1.7 ± 1.6 s) than contralateral (0.3 ± 0.3 s; P = 0.007) and control (0.1 ± 0.2 s; P = 0.009) muscles. This impaired firing rate of the lower-threshold unit arose, despite an increase in the overall descending command, as shown by the recruitment of the higher-threshold unit during the time that the lower-threshold unit was saturating, and by the continuous increase in averages of the rectified EMG of the biceps brachii muscle throughout the rising phase of the contraction. These results suggest that impairments in firing rate modulation are prevalent in motor units of spastic-paretic muscle, even when the overall descending command to the muscle is increasing.
Keywords: spasticity, stroke, PIC, saturation, motor unit
individuals who have sustained a stroke often exhibit spasticity that limits daily function. Clinically, spasticity in stroke is characterized by increased stretch reflex responses that can be recorded with the muscles at rest (Lance 1980), suggesting enhanced excitability of the motoneuron pool (Chung et al. 2008). In addition, stroke survivors often exhibit an impaired ability to relax the muscle, especially following simple imposed movements such as extending the joint (Lewek et al. 2007). Their muscles often exhibit prolonged (spontaneous) firing of motor units following either voluntary or reflex muscle activation, suggesting that there is impaired control of motor unit firing at rest when the stroke survivor is not consciously recruiting the muscle (Lewek et al. 2007; Lukacs 2005; Mottram et al. 2010).
Paradoxically, in contrast to the apparent enhanced excitability of the motoneuron pool observed in “resting” or passive muscle of stroke survivors (Mottram et al. 2010), the firing rates of individual motor units may show minimal rate increases when the muscle is voluntarily activated (Gemperline et al. 1995). For example, in stroke survivors, motor unit firing rates are often abnormally low (Rosenfalck and Andreassen 1980; Young and Mayer 1982), even for a designated force level (Gemperline et al. 1995), and motor unit recruitment thresholds are compressed in the paretic vs. contralateral muscle (Gemperline et al. 1995). Motor unit firing rates in the biceps brachii may be as much as 6 pulses per second (pps) lower in the paretic vs. contralateral muscle of stroke survivors for matched absolute torque levels that correspond up to ∼50% of maximum (Gemperline et al. 1995). Lower firing rates of motor units will require the recruitment of more motor units to produce a given force (inefficiency), which has been reflected as an abnormally high level of EMG (electromyogram) activity for a given muscle force in the paretic vs. contralateral muscle of stroke survivors (Tang and Rymer 1981).
The origins of this disturbed firing rate modulation are unclear. The rate saturations may result from limitations in descending voluntary excitatory drive, changes in intrinsic motoneuron properties [such as persistent inward currents (PICs)], or both. In fact, recent evidence from simulations in motoneuron models suggest that a mixture of excitation and inhibition can result in saturation in firing rates (Powers et al. 2012) that closely resemble the firing profiles observed in stroke survivors during voluntary increases in force (Mottram et al. 2009). Thus, in contrast to the spontaneous firing of motor units observed in the muscles of stroke survivors while at rest (Mottram et al. 2010), firing rates are abnormally low during purposeful activation of the muscle (Gemperline et al. 1995).
Accordingly, in this study, we question whether the modulation of firing rates is impaired during voluntary contractions of elbow flexor muscles of stroke survivors, even when the drive to the motoneuron pool is increasing. We hypothesize that modulation of motor unit firing rates during voluntary increases in force with the elbow flexor muscles will be less in the spastic-paretic compared with the contralateral muscle of stroke survivors, and with analogous muscles of matched healthy control subjects at similar force levels. Portions of these data have been presented previously in abstract form (Mottram et al. 2008).
METHODS
Ten stroke survivors (5 men, 5 women; 62.3 ± 4 yr; range, 55–77 yr; Mottram et al. 2009) with a unilateral brain lesion resulting in spastic hemiparesis of greater than 6 mo duration, and 10 age- and sex-matched healthy subjects (5 men, 5 women; 61.9 ± 4 yr; range, 53–73 yr; Mottram et al. 2009) participated in the study. Demographic and clinical measures for the stroke subjects are detailed in Table 1. Clinical assessments included spasticity measures at the elbow using the Modified Ashworth Scale (0–4) (Gregson et al. 2000) and magnitude of the biceps tendon jerk (0–4+) (Litvan et al. 1996).
Table 1.
Subject characteristics for the 10 stroke survivors who participated in the experiments
| Subject ID | Stroke Type | Sex | Age, yr | Age of Sex-Matched Control, yr | Ashworth | Fuglmeyer | Chedoke | Involved Limb |
|---|---|---|---|---|---|---|---|---|
| 1 | Hemorrhagic | M | 58 | 58 | 3 | 28/66 | Stage 3 | R |
| 2 | Hemorrhagic | F | 61 | 56 | 2 | 31/66 | Stage 3 | L |
| 3 | Hemorrhagic | M | 55 | 57 | 1+ | 13/66 | Stage 3 | R |
| 4 | Hemorrhagic | M | 67 | 61 | 3 | 21/66 | Stage 2 | L |
| 5 | Hemorrhagic | F | 57 | 56 | 1+ | 30/66 | Stage 3 | L |
| 6 | Hemorrhagic | F | 58 | 61 | 2 | 7/66 | Stage 2 | R |
| 7 | Ischemic | F | 55 | 53 | 1+ | 33/66 | Stage 3 | L |
| 8 | Ischemic | M | 68 | 72 | 2 | 27/66 | Stage 3 | R |
| 9 | Ischemic | F | 67 | 72 | 3 | 28/66 | Stage 3 | R |
| 10 | Ischemic | M | 77 | 73 | 3 | 24/66 | Stage 3 | R |
M, male; F, female; R, right; L, left.
Upper arm impairment was assessed using the Fugl-Meyer test (Fugl-Meyer et al. 1975) and the Chedoke-McMaster assessment (Gowland et al. 1993). The lower boundary for spasticity was an Ashworth score of 1+ and a tendon jerk score of 3+ or higher. Subjects were excluded if they were unable to maintain the testing position, perform ramp isometric contractions with the elbow flexor muscles, or remain alert during testing. All subjects were withdrawn from anti-spasticity medications for at least 2 wk prior to testing. All procedures were performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at Northwestern University. Prior to participation in the study, all subjects gave written, informed consent.
Experimental Arrangement
Subjects were seated comfortably in a chair with their forearm, wrist and fingers secured in a cast. The arm was abducted 30–40° from the sagittal plane, and the elbow flexed to 90°. The casted forearm was fixed to a ring-mount interface attached to a six degrees-of-freedom load cell (ATI FT-4227, Woodland, CA). The load cell apparatus was connected to a plastic elbow rest mounted on a steel table. The subject's forearm was in mild pronation within the ring-mount interface. The subjects' shoulders and waist were secured tightly to the chair to minimize accessory trunk and shoulder movements: specifically, a 6-in.-wide Velcro belt was secured tightly around the subject's waist and chair, and a heavy Velcro strap was placed vertically over the anterior portion of each humeral head and then secured around the back of the chair.
Forces about the elbow joint were recorded online using a Power 1401 analog-to-digital converter and Spike2 (version 5.12) software (Cambridge Electronics Design, Cambridge, UK), and the elbow force (resultant of Fx and Fz) was displayed on a computer monitor.
Motor unit action potentials in the biceps brachii were recorded with Teflon-coated double-stranded wires (i.e., two insulated wires bonded to each other; bifilar 50-μm diameter; California Fine Wire, Grover Beach, CA). The double-stranded wire was inserted into a 27-gauge hypodermic needle, and the tip bent back to form a barb. The recording ends of the electrode were cut, so the pair served as a differential recording electrode. The electrodes were connected to preamplifiers located at the muscle that connected to an eight-channel amplifier system (Delsys, Boston, MA). Single motor unit recordings were amplified (1,000–2,000×), band-pass filtered (20–2,000 Hz), displayed on a computer monitor, and digitized for later analyses.
Surface EMGs of the biceps brachi and triceps brachii muscles were monitored simultaneously with the unitary recordings. Active differential surface EMG electrodes (Delsys) were placed on the biceps brachii short and long heads and the triceps brachii, in avoidance of the innervation zone to minimize signal cancellation (Merletti et al. 2001). All surface EMG signals were led to the same preamplifiers as the intramuscular EMG recordings, were amplified (1,000–2,000×), band-pass filtered (20–450 Hz), displayed on a computer monitor, and digitized for later analyses.
Experimental Procedures
Each stroke survivor participated in one to two sessions for the contralateral limb and one to two sessions for the spastic-paretic limb, with testing sessions separated by >1 wk. The order of testing was randomized. Control subjects participated in one to two sessions for the matched limb to ensure adequate trials for comparison to their spastic-paretic counterpart.
In total, 79, 88, and 75 pairs of motor units were collected during the voluntary ramp contractions for the spastic-paretic, contralateral, and control limb, respectively. During each session, subjects performed the following ordered tasks.
Maximum voluntary contraction force.
Subjects first performed three isometric maximum voluntary contraction (MVC) trials with both the elbow flexor and extensor muscles. Subjects were asked to gradually increase their voluntary effort to maximal levels over 3 s, with forces held for 3 s. The MVC force was quantified as the peak force obtained during the MVC task.
Motor unit isolation.
To isolate two single motor units, subjects were verbally cued to slowly increase their force until two units were visualized in the intramuscular record. The first unit isolated was referred to as the “lower-threshold motor unit,” and the second unit isolated was referred to as the “higher-threshold motor unit.” Single motor unit potentials were monitored online and on a digital oscilloscope during data collection. Up to three double-stranded fine-wire electrodes were inserted in widely separated locations of the biceps brachii muscle to help the experimenter locate suitable pairs of motor units. Once two suitable single motor units were isolated, a target force was set just slightly above the higher-threshold motor unit.
Isometric voluntary ramp contractions.
During performance of the isometric voluntary ramp contraction, subjects viewed a triangle on the computer monitor placed in front of them. The subject performed the triangular isometric voluntary ramp contractions by viewing his or her exerted resultant elbow flexion on the computer monitor and increasing the elbow flexion force at a predetermined rate to the target force slightly above the threshold force level of the higher-threshold motor unit. Subjects were instructed to make the rate of increase in force similar to the rate of relaxation.
The desired target force and desired rate of contraction were controlled by program software written in Matlab (Mathworks, Natick, MA). All trials were separated by ∼30 s. Sessions lasted 2–3 h, depending on the ease with which the motor units were isolated.
Final MVC.
A posttask MVC was measured to verify that the results were not contaminated by fatigue.
Data Analysis
Force, intramuscular, and surface EMG signals were collected online and digitized (A/D converter, 16-bit resolution) and analyzed offline using the Spike2 (version 5.12) data analysis system (Cambridge Electronic Design, Cambridge, UK). The surface and intramuscular EMGs were digitized at 2,013 Hz and 18,000 Hz, respectively. The elbow force signals (resultant of Fx and Fz) were digitized at 200 Hz. The EMG of the elbow flexor and extensor muscles during the voluntary ramp contraction was quantified as averages of the rectified EMG (aEMG) over the first and last second, and for 1/2-s intervals surrounding every 5% of contraction time. The aEMG was expressed as a percentage of the initial EMG at the start of the contraction. The coactivation ratios of the antagonist elbow extensor and elbow flexor musculature (elbow extensor muscle EMG/elbow flexor muscle EMG × 100) were determined every 5% of the contraction time.
Action potentials discharged by single motor units in biceps brachii were discriminated using a computerized, spike-sorting algorithm (Spike2, version 5.12; Cambridge Electronic Design, Cambridge, UK) (Fig. 1). Waveforms that were 20% less or greater in the vertical or horizontal direction than the “template” spike were not considered as the spike of interest. To further ensure discrimination accuracy, the interspike intervals and waveforms of identified motor units were visually examined for every trial. Trials that contained abnormally long or short interspike intervals were visually discriminated on a spike-by-spike basis.
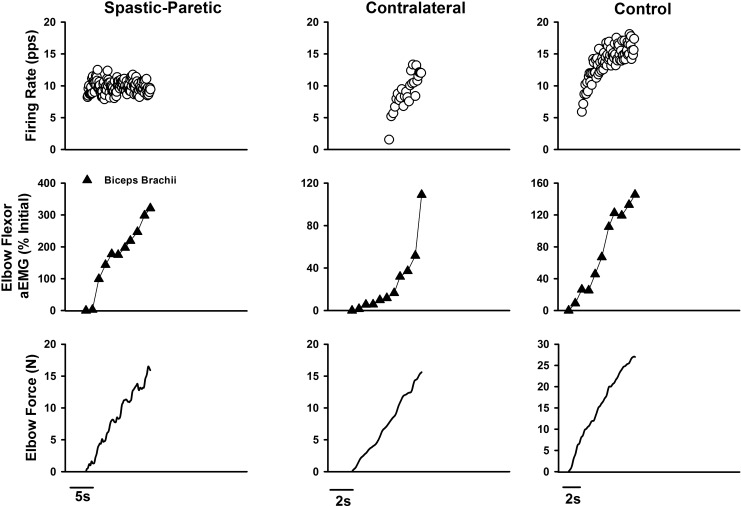
Fig. 1.
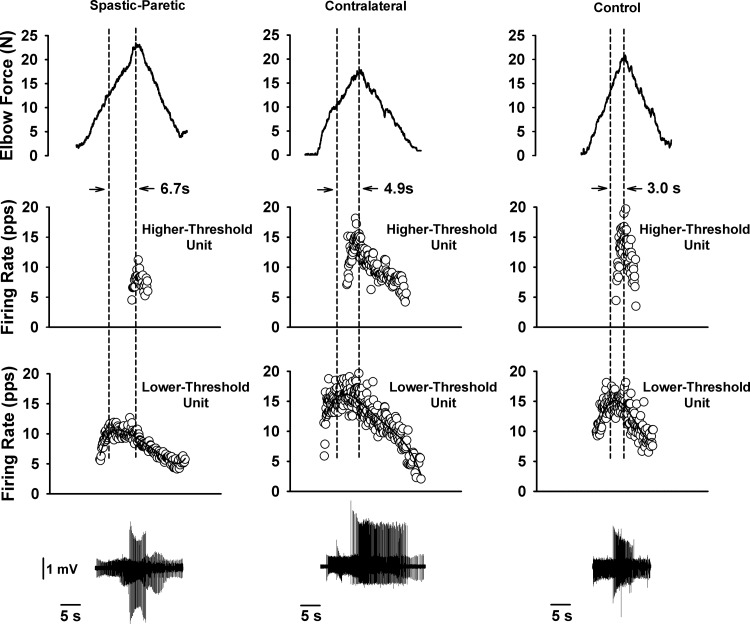
The duration of saturation in firing profiles was greater for the spastic-paretic (first column) than the contralateral (second column) or matched limb of a healthy control subject (third column). First column: two biceps brachii motor units recorded during a triangular isometric contraction with the elbow flexor muscles for the spastic-paretic limb of a stroke survivor. Bottom row denotes instantaneous firing frequency of a lower-threshold motor unit. Middle row denotes instantaneous frequency of a higher-threshold motor unit recorded during the contraction. Top row denotes force of elbow flexor muscles during the voluntary ramp contractions. Second and third columns: two biceps brachii motor units recorded from the contralateral limb of the stroke survivor and from the limb of an age-sex matched healthy control subject, respectively, during the same protocol as in the spastic-paretic limb. The duration of saturation in firing rate is shown between the vertical dashed lines for the respective limb types: spastic-paretic: 6.7 s; contralateral: 4.9 s; matched healthy control limb: 3.0 s. Note that the firing profiles of the higher-threshold motor units were increasing during the time that the lower threshold units were saturating, indicating an increase in descending drive to the respective motoneuron pools. Corresponding trains of motor unit action potentials for the lower- and higher-threshold motor units are shown for each respective muscle type. pps, Pulses per second.
For the firing rate analyses, a pair of clearly distinguishable motor unit potentials was selected from each trial. The lower-threshold unit fired during recruitment and de-recruitment of the higher-threshold unit (Fig. 1; middle row, higher-threshold unit; bottom row, lower-threshold unit). Initial discharge of lower- and higher-threshold units corresponded to the time of the first discharge in a train of spikes during the ascending ramp of the triangular contraction for which the interspike intervals were <500 ms (Fuglevand et al. 2006). The initial firing rates of the lower- and higher-threshold units were determined from the average of three spikes (2 interspike intervals). The force at recruitment of the lower- and higher-threshold units corresponded to the resultant elbow flexor force at the time of the first motor unit discharge, as determined above.
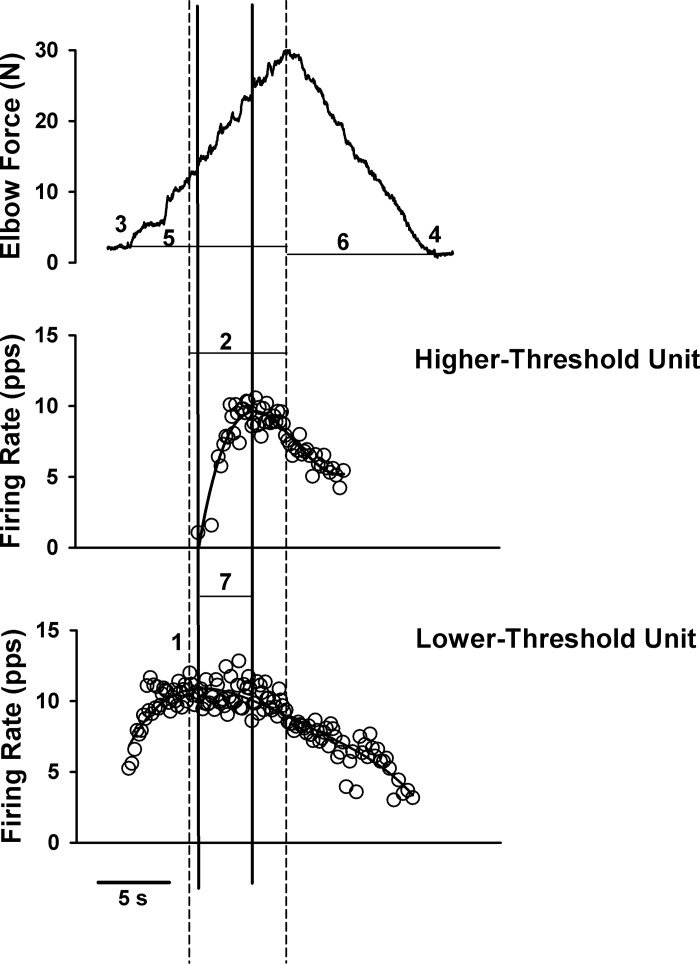
Modulation in firing rate profiles was examined by the following measures: first, the peak firing rates of the lower- and higher-threshold units were determined from the average of three spikes surrounding the first maximum firing rate attained (2 interspike intervals; Fig. 2, number 1 and vertical dashed line). Second, the incidence and duration of impaired lower- and higher-threshold unit firing rate modulation (no increase or a decrease in firing rate, despite increasing force) were determined: automated analyses followed by visual inspection of each firing profile were conducted by the same examiner. For the automated analyses, a fifth-order polynomial was set to the firing profile. Next, the peak firing rate and peak force were chosen (Fig. 2; first and second dashed vertical lines, respectively). For the visual inspection, each profile was examined following the above automated analyses to ensure that the saturation in firing was adequately captured (Fig. 2; duration between first and second dashed vertical lines). Next, the incidence of impaired modulation in firing rate was determined from slope analyses of firing rates from the time at peak firing rate (first dashed vertical line) until the time at peak force (second dashed vertical line). Firing rate slopes that were ≤0.5 pps/s, despite increasing force, were considered as impaired rate modulation. Because the force profile during the voluntary ramp contractions may not adequately represent the descending drive equally in the different muscle types (Fellows et al. 1994; Klein et al. 2010; Madhaven et al. 2011), we performed a secondary analysis in which we used the firing rate profile of the higher-threshold unit instead of the peak force as an index of the descending drive. Furthermore, because maximal firing rates may be variable poststroke (Hu et al. 2012), we used the peak of the smoothed lower-threshold unit's firing rate profile instead of the average of three spikes surrounding the first maximum firing rate as the start of the saturation in firing rates. Specifically, the incidence of impaired modulation in firing rate was also determined from the slope of the smoothed firing rate profile of the lower-threshold unit between the peak in the smoothed firing rate of the lower-threshold unit's firing profile (first solid vertical line, Fig. 2) and the peak in the smoothed rate of the higher-threshold unit's firing profile (second solid vertical line, Fig. 2). Firing rate slopes of the lower-threshold unit that were ≤0.5 pps/s during this duration were considered as impaired rate modulation.
Fig. 2.
Elbow force (top panel) and instantaneous firing frequency of a higher-threshold motor unit (middle panel) and a lower-threshold motor unit (bottom panel) during the triangular isometric contraction with the elbow flexor muscles. Adaptation in firing rate profiles was examined by the following measures. 1, Peak firing rates of the lower- and higher-threshold units were determined from the average of 3 spikes surrounding the first maximum firing rate attained (2 interspike intervals). Vertical dashed lines indicate the duration of impaired firing rate modulation (2; no increase or a decrease in firing rate despite increasing force). For the automated analyses to determine the duration of impaired firing rate modulation, a fifth-order polynomial was set to the firing profile of the motor units. Next, the lower-threshold peak firing rate and peak force were chosen (first and second dashed vertical lines, respectively). For the visual inspection, each profile was examined manually following the above automated analyses to ensure that the saturation in firing was adequately captured (duration between first and second dashed vertical lines). The incidence of impaired modulation in firing rate was determined from slope analyses of firing rates from the time at peak firing rate (first dashed vertical line) until the time at peak force (second dashed vertical line). Firing rate slopes that were ≤0.5 pps/s, despite increasing force, were considered as impaired rate modulation. Initial (3) and final (4) forces were calculated when the force left and returned to baseline after the rising and falling ramp contractions. The time to peak force (5) and time to final force (6) for each trial were determined for each subject. Because the force profile during the voluntary ramp contractions may not adequately represent the descending drive equally in the different muscle types, and because firing rates poststroke may be variable, we performed a secondary and more conservative analysis to determine the duration of impaired modulation in firing rates. Specifically, the duration of impaired modulation in firing rate of the lower-threshold unit was determined as the duration between the peak of the smoothed firing rate profile of the lower-threshold unit (first solid vertical line), and the peak of the smoothed firing rate of the higher-threshold unit (second solid vertical line). Slopes of the smoothed firing rate profiles of the lower-threshold unit between the two solid vertical lines (7) that were ≤0.5 pps/s were considered as impaired rate modulation. Note that the duration of impaired modulation in firing rate was ∼6 s when using the primary analysis method (duration between dashed vertical lines; 2), and ∼4 s when using the more conservative analysis method (duration between solid vertical lines; 7).
All force measurements were presented relative to baseline force. Initial and final forces were calculated when the force left and returned to baseline after the rising and falling ramp contractions. If the final force did not return to baseline, the final force observed was used (Fig. 2, number 4). The time to peak force and time to final force for each trial were determined for each subject to ensure similar time for the ascending and descending ramps within and across subjects (Fig. 2, numbers 5 and 6). The rates of increase and decrease in force during the ramp contractions were also determined for each muscle type, and trials in which the rate of contraction during the ascending portion of the contraction was not similar to the rate of relaxation during the descending portion of the triangular isometric contraction were removed from further analyses.
Ensuring Similarities In Task Performance Across Muscle Types
Three measures were taken to allow for comparison across muscle types (spastic-paretic, contralateral, and control muscles). First, to ensure adequate firing time of the higher-threshold units and adequate firing time of the lower-threshold unit prior to recruitment of the higher-threshold unit, trials in which the higher-threshold unit did not fire for at least 2 s or the lower-threshold unit fired for less than 1 s before the higher-threshold unit was recruited were removed from further analyses (10 trials removed for spastic-paretic limbs, 17 trials removed for contralateral limbs, 11 trials removed for control limbs). This was to ensure that, if PICs were present in the lower- or higher-threshold units, they were fully activated (Bennett et al. 2001a, 2001b; Li et al. 2004). Second, individual trials in which the rate of increase and decrease in force were significantly different from the mean rates of force increase and decrease were also removed (10 trials were removed for spastic-paretic limbs, 2 trials for contralateral limbs, 5 trials for control limbs). This was to ensure similar rates of force increase and decrease across limb types. Third, to allow for comparison across muscle types for the impaired modulation analyses, the duration of firing rate from onset to peak firing rate was determined for the lower- and higher-threshold units to determine that they were similar across muscle types. No trials had to be removed from analyses, as motor unit firing times for the rising phase of the contraction for the lower- and higher-threshold units were similar across muscle types (Table 2).
Table 2.
Characteristics from averaged values for each of the 10 age- and sex-matched subjects during the triangular ramp contractions (paired t-tests)
| Spastic-Paretic | Contralateral | Control | |
|---|---|---|---|
| Rate of increase in force§ | |||
| N/s | 2.1 ± 0.8 | 2.2 ± 1.4 | 2.3 ± 1.2 |
| %MVC/s | 3.1 ± 2.7 | 1.1 ± 0.42‡ | 1.4 ± 0.72† |
| Rate of decrease in force§ | |||
| N/s | 1.7 ± 0.7 | 2.1 ± 1.4 | 2.0 ± 1.3 |
| %MVC/s | 3.1 ± 2.5 | 1.1 ± 0.5‡ | 1.3 ± 0.7† |
| Peak force achieved during trial, N | 15.6 ± 8.7 | 15.4 ± 4.8 | 23.6 ± 12.2‡ |
| Time from initial to peak force, s | 8.6 ± 3.2 | 8.1 ± 2.3 | 8.7 ± 2.0 |
| Time from peak to final force, s | 8.0 ± 3.0 | 8.2 ± 2.8 | 8.9 ± 1.9 |
| Lower-threshold unit | |||
| Duration of firing: onset to peak firing rate, s | 3.4 ± 1.1 | 3.4 ± 1.0 | 3.8 ± 1.6 |
| Initial firing rate, pps | 8.9 ± 3.2 | 8.5 ± 2.1 | 7.8 ± 2.0 |
| Recruitment threshold, N | 2.3 ± 2.2 | 5.8 ± 3.0* | 11.3 ± 7.8* |
| Recruitment threshold, %MVC | 2.9 ± 3.5 | 3.6 ± 2.4 | 5.4 ± 3.3 |
| Peak firing rate, pps | 13.4 ± 2.5 | 16.3 ± 2.5* | 15.2 ± 2.5 |
| Duration of impaired rate modulation using peak force, s | 3.9 ± 2.2 | 1.4 ± 0.9* | 1.1 ± 1.0* |
| Duration of impaired rate modulation using peak of higher-threshold unit, s | 2.5 ± 1.9 | 1.2 ± 0.7† | 0.8 ± 0.6‡ |
| Impaired modulation (both methods), %trials | 98.0 ± 6.3 | 83.0 ± 22.2* | 70.4 ± 38.8* |
| Higher-threshold unit | |||
| Duration of firing: onset to peak firing rate, s | 2.0 ± 0.3 | 2.0 ± 0.7 | 2.1 ± 1.1 |
| Initial firing rate, pps | 8.1 ± 2.1 | 8.0 ± 1.5 | 7.9 ± 3.5 |
| Recruitment threshold, N | 9.2 ± 5.9 | 11.1 ± 3.7 | 17.5 ± 8.9‡ |
| Recruitment threshold, %MVC | 8.7 ± 8.4 | 6.4 ± 2.5 | 8.5 ± 3.6 |
| Peak firing rate, pps | 13.2 ± 2.7 | 13.6 ± 2.2 | 13.2 ± 2.9 |
| Duration of impaired rate modulation using peak force, s | 1.7 ± 1.6 | 0.3 ± 0.3* | 0.1 ± 0.2* |
| Impaired modulation, %trials | 73.3 ± 32.4 | 32.8 ± 26.7* | 15.1 ± 22.9* |
Values are means ± SD. MVC, maximum voluntary contraction; pps, pulses per second. Variables that are significantly different across muscle types are in bold.
P ≤ 0.009 compared with spastic-paretic limb.
P ≤ 0.02 compared with spastic-paretic limb.
P ≤ 0.04 compared with spastic-paretic limb.
For comparisons across muscle types, rates of increase and decrease in force were matched using absolute force levels. Values expressed as %MVC/s are also shown for comparison across muscle types. In the higher-threshold unit, the duration of impaired modulation could only be determined using the peak force.
Following removal of the above trials, there were 59 motor unit pairs (i.e., a lower- and a higher-threshold unit) for the spastic-paretic limb, 69 motor unit pairs for the contralateral limb, and 59 motor unit pairs for the control limb. There were instances in which we identified the same motor unit pair (i.e., the same lower- and higher-threshold unit) in multiple trials of the voluntary ramp contractions. When this occurred, these duplicate motor unit pairs from the above numbers were averaged, such that 2 ± 0.3 trials were averaged for each identical motor unit pair for the spastic-paretic limb, 3 ± 0.6 trials for the contralateral limb, and 2 ± 0.9 trials for the control limb. Thus, with duplicate pairs averaged, we report on the firing properties of 51, 47, and 44 motor unit pairs for the spastic-paretic (5 ± 4.3 pairs per subject), contralateral (4.7 ± 3.0 pairs per subject), and control limb (4.3 ± 3.2 pairs per subject), respectively.
Ensuring That the Overall Descending Command to the Motoneuron Pool Was Increasing During the Saturation in Firing Profiles of the Lower-Threshold Unit
To ensure that the overall descending command to the motoneuron pool was increasing, the slope of the higher-threshold unit's firing profile was determined during the time that the lower-threshold unit's firing profile was saturating. Slope analyses of firing rates (rate of change in firing vs. time) of the higher-threshold unit were conducted from the time at peak firing rate of the lower-threshold unit (Fig. 1; first dashed vertical line) until the time at peak force (Fig. 1; second dashed vertical line). A positive slope of the higher-threshold unit's firing profile during this duration would suggest an increase in the descending command.
Statistical Analysis
Because the subjects were age- and sex-matched, separate paired Student's t-tests (SPSS version 15.0) were used to compare the dependent variables across spastic-paretic and contralateral muscle, and across spastic-paretic and control muscle (Hopkins and Glass, 1996). Dependent variables included the force at recruitment of the lower- and higher-threshold units, the initial and peak firing rates during the voluntary ramp contractions, the rate of increase and decrease in force, and the incidence and duration of impaired modulation in firing profiles during the voluntary ramp contractions. To statistically compare the duration of impaired motor unit firing across muscle types, one average value from repeated trials was determined for each subject by first determining the average of any duplicate trials, and then averaging all trials per subject for each respective limb. Thus there was one averaged value to compare for each of the 10 spastic-paretic limbs, one averaged value for each of the contralateral limbs, and one averaged value for each of the age-sex-matched control limbs for all lower-threshold units and for all higher-threshold units. Within-subject comparisons of the pre- and post-task MVC were examined with separate paired t-tests for each muscle type. Following Bonferroni corrections for two-way comparisons (spastic-paretic vs. contralateral and spastic-paretic vs. control), the α-level required for statistical significance across limb types was P ≤ 0.025. Data are reported as means ± SD within the text, and displayed as means ± SE in Figs. 3 and 5.
Fig. 3.
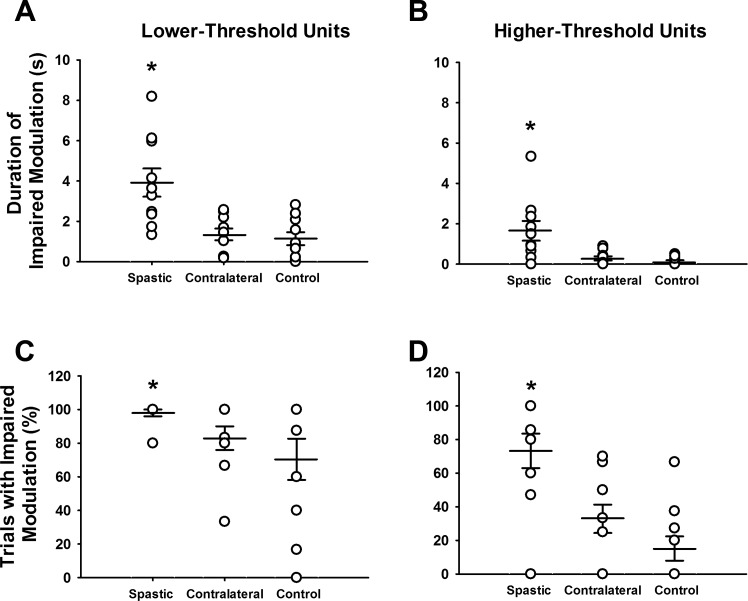
Duration of impaired modulation (A and B) and percentage of trials with impaired modulation (C and D) for the spastic-paretic, contralateral and control limb during the triangular isometric ramp contractions. A: the duration of impaired modulation in the lower-threshold unit within a trial was greater for the spastic-paretic (3.9 ± 2.2 s) than both the contralateral (1.4 ± 0.9 s; P < 0.001) and control (1.1 ± 1.0 s; P = 0.005) muscle. B: the duration of impaired modulation in the higher-threshold unit within a trial was also greater for the spastic-paretic (1.7 ± 1.6 s) than both the contralateral (0.3 ± 0.3 s; P = 0.007) and control (0.1 ± 0.2 s; P = 0.009) muscle. Each circle represents the average value from repeated trials of voluntary ramp contractions for each spastic-paretic and contralateral muscle of the 10 subjects, or the muscle of a healthy control subject; wider horizontal bars denote the average value for the group data; smaller horizontal bars denote 1 standard error of the mean (SEM) above and below mean values. C: the percentage of trials exhibiting impaired rate modulation (no increase or a decrease in firing rate, despite increasing force) in the lower-threshold unit within a trial during the ascending phase of the isometric ramp contractions was greater in the spastic-paretic (98.0 ± 6.3%) than both the contralateral (83.0 ± 22.2%; P = 0.004) and control (70.4 ± 38.8%; P = 0.002) muscle. D: the percentage of trials exhibiting impaired rate modulation in the higher-threshold unit within a trial during the ascending phase of the isometric ramp contractions was also greater in the spastic-paretic (73.3 ± 32.4%) than both the contralateral (32.8 ± 26.7%; P = 0.004) and control (15.1 ± 22.9%; P = 0.003) muscle. *P ≤ 0.009 compared with the contralateral and control limb.
Fig. 5.
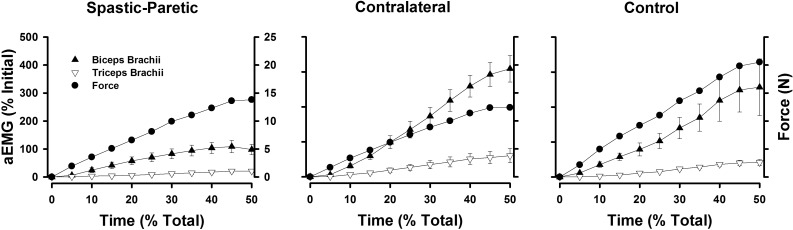
The aEMG of the biceps brachii (▲; biceps brachii short head and long head combined) and of the triceps brachii (▽) for the group data, expressed as a percentage of the initial aEMG values for the spastic-paretic, contralateral, and control muscle during the rising portion of the voluntary isometric ramp contraction. ●, The force of the elbow flexor muscles during the rising portion of the voluntary ramp contraction for the respective muscle types. For all muscle types, the force and aEMG of the elbow flexor muscles are rising during the ascending portion of the ramp contraction, whereas the aEMG of the antagonist triceps brachii muscle is fairly flat during the ascending portion of the ramp contraction. Values are means ± SE.
RESULTS
Subjects did not display fatigue during or following the contractions, as shown by the similar or greater elbow flexor MVC values before and after data collection sessions for each group (spastic-paretic muscle MVC: pre 89 ± 38 N, post 95 ± 39 N; P = 0.02; contralateral muscle MVC: pre 180 ± 43 N, post 179 ± 47 N; P = 0.42; control muscle MVC: pre 200 ± 58 N, post 196 ± 72 N; P = 0.24). Elbow extensor MVC values prior to task performance were 47.1 ± 22.1 N spastic-paretic; 78.1 ± 36.7 N contralateral; and 83.6 ± 45.0 N for control muscle. Elbow extensor MVC values were not recorded following task performance.
Figure 1 illustrates the performance of the voluntary ramp contractions with the spastic-paretic (first column) and contralateral (second column) limb of a stroke survivor and the matched limb of a healthy control subject (third column). The top row shows the force of the elbow flexor muscles during the voluntary ramp contraction, and the middle and bottom rows show the instantaneous firing frequency of a higher- and lower-threshold motor unit, respectively, recruited during performance of the voluntary isometric ramp contraction. Note the rather similar initial firing rates across muscle types and across lower-and higher-threshold units. The isometric force profiles across muscle types in Fig. 1 were also quite similar, as noted by the similar rates of increase (1.3, 1.7, and 2.0 N/s) and decrease (1.5, 1.2, and 1.7 N/s) in force for spastic-paretic, contralateral, and control muscles, respectively. The rates of increase and decrease in force were also similar for the group data (P ≥ 0.06), and corresponded to 1–3% MVC/s (Table 2). There were also similar times from initial to peak force, and from peak to final force across the spastic, contralateral, and control muscle. For the group data, the time from initial to peak force, and the time from peak to final force were also similar across muscle types (P ≥ 0.16; Table 2).
The two dashed vertical lines in each column of Fig. 1 show the duration of impaired rate modulation in the lower-threshold unit (no increase or a decrease in firing rate of the lower-threshold motor unit, despite increasing force). The durations of impaired modulation in the lower-threshold motor units for the spastic-paretic, contralateral, and control muscle in this representative example were 6.7 s, 4.9 s, and 3.0 s, respectively. Note that, as portions of the lower-threshold motor unit's firing profile were either flat or decreasing, the firing profile of the higher-threshold motor unit and the force profile were both increasing.
Despite similar initial firing rates for lower threshold motor units (8.9 ± 3.2 pps, 8.5 ± 2.1 pps, 7.8 ± 2.0 pps, spastic-paretic, contralateral, and control, respectively; Table 2), the recruitment threshold of the lower-threshold motor units was consistently lower for the spastic-paretic (2.3 ± 2.2 N) than the contralateral (5.8 ± 3.0 N; P < 0.001) or control (11.3 ± 7.8 N; P < 0.001) muscle (Table 2). Although the initial firing rates were similar for the higher-threshold units for spastic-paretic (8.1 ± 2.1 pps), contralateral (8.0 ± 1.5 pps; P > 0.05), and control (7.9 ± 3.5 pps; P > 0.05; Table 2) muscle, so too were the recruitment thresholds for the spastic-paretic (9.2 ± 5.9 N) and contralateral (11.1 ± 3.7 N; P > 0.05; Table 2) muscle. The recruitment thresholds of the higher-threshold units did, however, differ for spastic-paretic and control muscle (17.5 ± 8.9 N; P = 0.01; Table 2).
Furthermore, despite similar initial firing rates of the lower-threshold units across muscle types, the peak firing rates achieved during the isometric ramp contractions were systematically lower for spastic-paretic (13.4 ± 2.5 pps) than contralateral muscle (16.3 ± 2.5 pps; P = 0.002; Table 2). This observation was made for similar force levels achieved during the contractions for the spastic-paretic (15.6 ± 8.7 N) and contralateral (15.4 ± 4.8 N) muscle (P = 0.45, Table 2). Despite lower force levels achieved for spastic-paretic than control muscle (Table 2), the peak firing rates achieved did not differ across spastic-paretic and control muscle (15.2 ± 2.5 pps; Table 2). The peak firing rates of the higher-threshold units achieved during the isometric ramp contractions did not differ across muscle types (Table 2).
Figure 3 shows the group data for impaired modulation. In this figure, each circle represents the average value from repeated trials of voluntary ramp contractions for each spastic-paretic and contralateral muscle of the 10 subjects, or the muscle of a healthy control subject; wider horizontal bars denote the average value for the group data; smaller horizontal bars denote 1 standard error of the mean (SEM) above and below mean values. The duration of impaired modulation in the lower-threshold unit within a trial was greater for the spastic-paretic (3.9 ± 2.2 s) than for both the contralateral (1.4 ± 0.9 s; P < 0.001) and control (1.1 ± 1.0 s; P = 0.005) muscles (Fig. 3A, Table 2). The duration of impaired modulation in the higher-threshold unit within a trial was also greater for the spastic-paretic (1.7 ± 1.6 s) than both the contralateral (0.3 ± 0.3 s; P = 0.007) and control (0.1 ± 0.2 s; P = 0.009) muscle (Fig. 3B, Table 2). These findings appeared despite similar durations from initial to peak force across muscle types, and despite similar firing rate durations from onset to peak firing rate for the lower- and higher-threshold motor units across muscle types (Table 2).
We also performed a secondary analysis in which we determined the duration of rate saturation as the time between the peak in the smoothed firing rate profile of the lower-threshold unit and the peak in the smoothed firing rate profile of the higher-threshold unit (duration between solid vertical lines Fig. 2; Table 2). For the group data, the duration of impaired modulation in the lower-threshold unit within a trial was greater for the spastic-paretic (2.5 ± 1.9 s) than for both the contralateral (1.2 ± 0.7 s; P = 0.03) and control (0.8 ± 0.6 s; P = 0.01) muscles (Table 2).
The percentage of trials in which the modulation of firing rate was impaired was also determined for each muscle type, as is shown in Fig. 3, C and D, in a format similar to that shown for Fig. 3, A and B. The percentage of trials exhibiting impaired rate modulation (no increase or a decrease in firing rate, despite increasing force) in the lower-threshold unit within a trial during the ascending phase of the isometric ramp contractions was greater in the spastic-paretic (98.0 ± 6.3%) than in both the contralateral (83.0 ± 22.2%; P = 0.004) and control (70.4 ± 38.8%; P = 0.002) muscle (Fig. 3C, Table 2), and these records were dominated by instances in which firing rates decreased, despite increases in force. The impaired modulation in firing rate of the lower-threshold unit within a trial during the ascending phase of the isometric ramp contraction occurred, despite an increase in the overall descending command, as shown by the recruitment of the higher-threshold unit during the time that the lower-threshold unit was saturating. Furthermore, the slope of the higher-threshold unit's firing profile was increasing during the time that the lower-threshold unit was saturating for the spastic-paretic (3.2 ± 1.4 pps/s), contralateral (3.6 ± 1.4 pps/s), and control (3.5 ± 1.5 pps/s) muscle. For lower-threshold units, the percentage of trials in which the modulation of firing rate was impaired was the same, regardless of the analysis method.
The percentage of trials exhibiting impaired rate modulation in the higher-threshold unit within a trial during the ascending phase of the isometric ramp contractions was also greater in the spastic-paretic (73.3 ± 32.4%) than both the contralateral (32.8 ± 26.7%; P = 0.004) and control (15.1 ± 22.9%; P = 0.003) muscle (Fig. 3D, Table 2).
The surface aEMG activity of the biceps brachii short head and long head were monitored during the isometric triangular contractions. Figure 4 shows representative instantaneous frequency firing profiles of the lower-threshold motor unit (top row), biceps brachii aEMG (middle row; short head and long head combined), and force of the elbow flexor muscles (bottom row) during the ascending portion of the voluntary ramp contraction for the spastic-paretic, contralateral, and control muscle. Note that the force and surface aEMG of the biceps brachii are rising, despite flat firing rate profiles for the spastic-paretic muscle. Firing rate profiles are rising as expected for the contralateral and control muscle.
Fig. 4.
Instantaneous frequency firing profiles of the lower-threshold motor unit (top row), biceps brachii (short head and long head combined; middle row), and force of the elbow flexor muscles (bottom row) during the ascending portion of the voluntary ramp contraction for the spastic-paretic, contralateral, and control muscle. Note that the force and surface averages of the rectified EMG (aEMG) of the biceps brachii are rising, despite flat firing rate profiles for the spastic-paretic muscle. Firing rate profiles are rising as expected for the contralateral and control muscle. Note that, in this spastic-paretic example, the duration of impaired modulation in firing is longer (∼13 s) than the average duration of impaired modulation (∼4 s) observed in spastic-paretic muscle. This is shown to provide an example of some of the longer durations of impaired modulation observed in this data set.
Figure 5 shows the aEMG of the biceps brachii (biceps brachii short head and long head combined), and of the triceps brachii for the group data, expressed as a percentage of the initial aEMG values for the spastic-paretic, contralateral, and control muscle during the rising portion of the voluntary isometric ramp contraction. The black circles denote the force of the elbow flexor muscles during the rising portion of the voluntary ramp contraction for the respective muscle types. Note that, for all muscle types, the force and aEMG of the biceps brachii are rising during the ascending portion of the ramp contraction, whereas the aEMG of the antagonist triceps brachii muscle is fairly flat during the ascending portion of the ramp contraction for the three muscle types. Consistent with this observation, the coactivation ratios of the antagonist elbow extensor and elbow flexor musculature during the voluntary ramp contractions did not differ across spastic-paretic (49.7 ± 26.6%), contralateral (40.2 ± 9.8%), or control muscle (43.2 ± 17.5%; P ≥ 0.19).
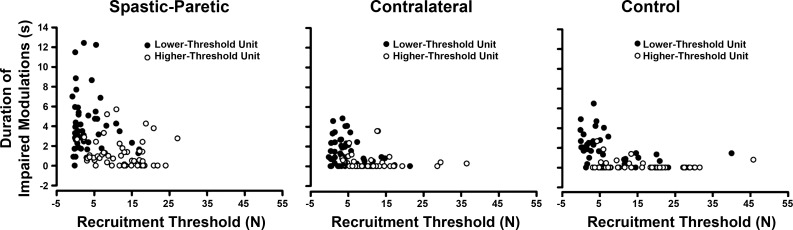
Figure 6 shows the duration of impaired modulation (defined with respect to peak force) vs. the recruitment threshold for the spastic-paretic, contralateral, and control muscle. Fifty-one motor unit pairs (i.e., a lower- and a higher-threshold motor unit) are shown for the spastic-paretic muscle, 47 motor unit pairs for the contralateral muscle, and 44 motor unit pairs for the control muscle. The black circles denote the lower-threshold motor units, whereas the open circles denote the higher-threshold motor units. Note that, in contralateral and control muscle, the duration of impaired modulation for most of the lower-threshold motor units is less than 5 s, whereas in spastic-paretic muscle, the duration of impaired modulation in many of the lower-threshold motor units is greater than 5 s. Similarly, in contralateral and control muscle, the duration of impaired modulation for most of the higher-threshold motor units is less than 0.5 s, whereas in spastic-paretic muscle, the duration of impaired modulation in most of the higher-threshold motor units is greater than 0.5 s.
Fig. 6.
The duration of impaired modulation vs. the recruitment threshold for 51 motor unit pairs in the spastic-paretic muscle, 47 motor unit pairs in the contralateral muscle, and 44 motor unit pairs in the control muscle. ●, Lower-threshold motor units; ○, higher-threshold motor units. Note the longer duration of impaired modulation for both lower- and higher-threshold motor units in spastic-paretic muscle than contralateral or control muscle.
DISCUSSION
The primary objective of this study was to search systematically for signs of saturation in firing profiles in lower- and higher-threshold motor units in the spastic-paretic muscle of stroke survivors. This task was accomplished by examining alterations in firing profiles of lower- and higher-threshold motor units during isometric voluntary ramp contractions with the elbow flexor muscles of stroke survivors and healthy matched controls.
We observed a number of features of impaired rate modulation in firing profiles in lower- and higher-threshold motor units in the spastic-paretic compared with the contralateral muscle of stroke survivors, and in the biceps brachii muscles of matched healthy control subjects. First, despite similar initial firing rates for both lower- and higher-threshold motor units, the threshold for recruitment was lower in spastic-paretic compared with healthy control muscle. Second, for lower-threshold units, peak firing rates were lower in spastic-paretic compared with contralateral muscle. Third, the duration of impaired modulation in firing rate was greater for both lower- and higher-threshold motor units in spastic-paretic than contralateral or control muscle. Fourth, the percentage of trials exhibiting impaired modulation in firing rate during the ascending phase of the contraction was greater in spastic-paretic than contralateral or control muscle. These findings were despite an increase in the overall descending command, as shown by the recruitment of the higher-threshold unit during the time that the lower-threshold unit was saturating, and by the continuous increase in aEMG of the biceps brachii muscle throughout the rising phase of the contraction. Potential mechanisms are discussed below.
PIC-based Mechanisms For Saturation In Motor Unit Firing Rates In Stroke
Increases in membrane conductance.
The PIC in spinal motoneurons is a depolarizing current, generated by voltage-sensitive Na+ and/or Ca2+ channels (termed Na and Ca PICs), that greatly augment synaptic input. The PIC current may persist for many seconds after activation and thus promote long-lasting discharge of motoneurons (Hounsgaard and Kiehn 1989; Lee and Heckman 1998; Li and Bennett 2003; Schwindt and Crill 1982). PICs, however, can have a paradoxical impact on firing patterns: enhanced excitability due to amplification, coupled to a restriction in rate modulation due to rate saturation (Heckman et al. 2008). This rate saturation likely occurs because most of the synaptic input enters via the dendrites where most of the PIC is generated. The paradoxical pattern of enhanced excitability and subsequent limited rate modulation has been observed in human motor unit firing patterns (Binder et al. 1996; Heckman et al. 2005; Hornby et al. 2002; Kernell 2006). Because estimated PIC amplitudes have been shown to be similar across spastic-paretic, contralateral, and control muscle, however (Mottram et al. 2009), it is unlikely that increased membrane conductance plays a major role in the enhanced saturation in firing profiles observed in spastic-paretic muscle.
Inactivation of the sodium PIC.
The Na PIC has been shown to be critical for normal, steady repetitive firing in motoneurons (Harvey et al. 2006a; Lee and Heckman 2001). The Na PIC is rapidly activated just subthreshold to the action potential (fast and persistent) and, therefore, plays a critical role in ensuring a rapid depolarization to securely activate action potentials (Crill 1996; Kuo et al. 2006; Lee and Heckman 2001). In fact, when the Na PIC is eliminated by administration of monoamine antagonists, motoneurons lose their ability to fire during slow current ramps (Harvey et al. 2006c), and spikes can be activated only with rapid onset stimulations. It follows that the impaired rate modulation in spastic-paretic motor units could be attributable in part to inactivation of the Na PIC (Avery and Johnston 1996; Harvey et al. 2006b, 2006c; Lee and Heckman 1998), as published evidence suggests that these channels inactivate when a motoneuron is held near threshold with a tonic, depolarizing bias current (Harvey et al. 2006b).
Subthreshold activation of the Ca PIC.
Although the PIC is likely not the primary source of the impaired firing rate modulation, the presence of abnormal PICs in spastic-paretic motoneurons cannot be ruled out. It is conceivable that a combination of the PIC and a depolarizing descending drive contributed to the saturation in firing profiles. In fact, the greater incidence and duration of impaired rate modulation in spastic-paretic compared with contralateral and control motoneurons might be attributable to subthreshold activation of the Ca PIC. If the lower-threshold motoneurons of spastic-paretic muscles are indeed depolarized from extrinsic synaptic sources, as suggested previously (Mottram et al. 2009, 2010), this might result in preactivation of the PIC, because there is evidence that exogenous synaptic inputs assist in lowering PIC threshold when activated with subsequent current injection (Bennett et al. 1998b; Lee and Heckman 2000; Li et al. 2004).
If the PIC is activated before recruitment, motor units will start to fire with a low frequency-current gain (Bennett et al. 1998a, 1998b; Li and Bennett 2003; Li et al. 2004). The region of low frequency-current gain has been referred to as “rate limiting” (Heckman and Binder 1993), “saturation” (Bailey et al. 2007; Heckman et al. 2008; Johns and Fuglevand 2004; Monster and Chan 1977), or the “preferred firing range” (Hornby et al. 2002; Kiehn and Eken 1997). This mechanism would produce saturation at a relatively low firing rate. In animal preparations, however (Lee and Heckman 1998; Powers et al. 2012), PIC activation alone does not produce the strong saturation seen in the present results, as demonstrated by the observed prolonged period of flat or even negative firing rate modulation.
Proportional inhibition.
Thus far, only the interactions between the PIC and sources of excitatory synaptic input have been considered. The PIC, however, is also known to be highly sensitive to inhibitory synaptic input (Bui et al. 2008a, 2008b; Hounsgaard et al. 1988; Kuo et al. 2003). In fact, Powers et al. (2012) suggested that the contribution of PICs to rate modulation is likely to depend on a mixture of excitatory and inhibitory synaptic currents contributing to the net synaptic drive to the motoneuron pool. To examine the potential contributions of PIC activation and synaptic input patterns to motor unit rate modulation, Powers and colleagues examined the responses of a set of cable motoneuron models to different patterns of excitatory and inhibitory inputs. When applying “proportional inhibition” (an inhibitory input that rose and fell in parallel with the excitatory input; also referred to as “balanced inhibition”; Berg et al. 2007), with the inhibitory conductance applied uniformly across the soma and dendrites (Fig. 7 panel 2, in Powers et al. 2012), the low-rate modulation observed closely resembles that which we have observed in the spastic-paretic limb of stroke survivors (Figs. 1 and 4). Further simulation studies are necessary to assess the response of firing profiles to proportional inhibition across motoneuron models with similar PIC amplitudes.
Non-PIC-based Mechanisms for Saturation in Motor Unit Firing Rates in Stroke
Antagonist coactivation.
Although increases in antagonist coactivation have been observed poststroke (Trumbower et al. 2010), it is unlikely that antagonist coactivation played a role in the observed saturation in firing profiles in the current study, as coactivation ratios of the antagonist elbow extensor and elbow flexor muscles were similar across muscle types during the voluntary ramp contractions. This agrees with previous findings in which antagonist coactivation did not contribute to muscle weakness poststroke (Klein et al. 2010).
Reduced drive from disrupted corticospinal tracts.
Impairments in voluntary activation of the involved limb have been observed poststroke (Madhaven et al. 2011) and have been suggested to contribute to muscle weakness of the involved limb in stroke survivors (Klein et al. 2010). Although the mechanisms for voluntary activation failure are not well understood (Gandevia et al. 2001), reduced drive from disrupted corticospinal tracts (Berardelli et al. 1987) may play a role in the observed saturation in firing profiles. Furthermore, a reduced ability to recruit and fully activate the motor units still under voluntary control (Frontera et al. 1997) may contribute to the impaired modulation in firing rates observed in the spastic-paretic limb of stroke survivors.
The proposed mechanisms discussed above are based on indirect evidence, and other explanations are certainly feasible. For example, the observed impairments in rate modulation of spastic-paretic motoneurons could be attributable to an increase in the duration of the motoneuron after-hyperpolarization, resulting in enhanced input conductance and lower firing rates for these patients (Liang et al. 2010; Piotrkiewicz et al. 2007). Finally, increased postsynaptic inhibition from regional interneurons, muscle disuse (Hu et al. 2012), or alterations in afferent input (Mazzaro et al. 2007) might contribute to the altered firing profiles observed at contraction onset.
Methodological Considerations
Differences across lower- and higher-threshold motor units in spastic-paretic muscle.
Although both lower- and higher-threshold motor units in spastic-paretic muscle displayed increases in the incidence and duration of impaired rate modulation, the peak firing rate in lower-threshold units was lower in spastic-paretic than contralateral muscle, whereas in higher-threshold units, peak firing rates were similar across spastic-paretic, contralateral, and control muscle. This finding was likely attributable to the shorter firing times for higher- than lower-threshold units: to avoid recruitment of additional units during the voluntary ramp contractions, subjects were instructed to reduce their force shortly after recruiting the higher-threshold unit. This resulted in longer firing durations from onset to peak for lower-threshold (∼3.4 s) than higher-threshold (∼2.0 s) units.
Expressing force in absolute vs. relative values.
To make meaningful comparisons across spastic-paretic, contralateral, and control muscle, we ensured that the absolute rate of force development did not differ across muscle types, instead of matching rates of force development across muscle types expressed as a percentage of MVC. The MVCs in the spastic-paretic muscle were indeed approximately one-half that of the contralateral and control muscle MVC values, likely due to muscle atrophy following a stroke (Hafer-Macko et al. 2008), impaired ability to achieve high firing rates, or an inability to fully activate the available motoneuron pool voluntarily (Gemperline et al. 1995). We suggest that, due to the atrophy and impaired drive in stroke, expressing forces and rates of force development as %MVC does not necessarily provide a meaningful comparison across muscle types, as we do not know whether the entire motoneuron pool was indeed activated for the stroke survivor during the MVC conducted with the spastic-paretic muscle. This renders comparisons between muscles based on fractions of this putative maximum highly variable. Nonetheless, in Table 2, we have expressed recruitment threshold and rate of increase in force in both Newtons and %MVC.
Role of Rate Saturation in Reduced Firing Rates Observed in Spastic-Paretic Muscle
In summary, modulation in firing rates were impaired in both lower- and higher-threshold motor units in spastic-paretic compared with contralateral and control muscle during voluntary ramp contractions performed with the elbow flexor muscles, regardless of the analysis method used (Fig. 2). The observed impaired modulation in firing rates during voluntary contractions is in contrast to the enhanced excitability of spastic-paretic motoneurons observed at rest (Mottram et al. 2009, 2010). These paradoxical findings are similar to previous observations for enhanced stretch or Hoffman reflexes at rest (Burne et al. 2005; Chardon et al. 2009; Huang et al. 2006; Powers et al. 1988), yet not during a background contraction (Burne et al. 2005; Thompson et al. 2009). We suggest that the enhanced reflex responses at rest in stroke survivors are explained by the presence of a low-level tonic depolarizing synaptic drive to the spastic-paretic motoneuron pool. During voluntary activation, however, potentially higher levels of proportional inhibition (Powers et al. 2012) or reduced drive from disrupted corticospinal tracts might contribute to the impaired rate modulation observed in these patients.
Clinical Implications
Therapeutic interventions in stroke depend on the task being conducted: in contrast to the enhanced excitation of the motoneuron pool observed in the “resting” muscle of stroke survivors (Mottram et al. 2010), voluntary activation of the spastic-paretic motoneuron pool results in saturation in firing profiles of the individual motor units. To compensate, stroke survivors likely utilize motor unit recruitment over rate modulation when increasing force with the elbow flexor muscles. Whereas the therapeutic intervention during “rest” might be to reduce the tonic synaptic depolarizing drive to the resting motoneuron pool or to reduce the resting membrane potential of the motoneurons via application of 5-HT antagonists (D'Amico et al. 2013), the therapeutic intervention during voluntary force production might be to provide additional synaptic input from external sources to the motoneuron pool to assist with force production.
GRANTS
This work was supported by the Brinson Foundation Post-Doctoral Fellowship awarded to C. J. Mottram, National Institutes of Health (NIH) Grants (2T32-HD-007418-16 and 5R24-HD-050821-04) awarded to W. Z. Rymer, NIH Grant NS-071951 awarded to C. J. Heckman and R. K. Powers, and NIH Grant (NS-062200) awarded to R. K. Powers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.M. and N.L.S. conception and design of research; C.J.M. performed experiments; C.J.M. analyzed data; C.J.M., C.J.H., R.K.P., W.Z.R., and N.L.S. interpreted results of experiments; C.J.M. prepared figures; C.J.M. drafted manuscript; C.J.M., C.J.H., R.K.P., W.Z.R., and N.L.S. edited and revised manuscript; C.J.M., C.J.H., R.K.P., W.Z.R., and N.L.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Courtney Wallace and Constantin Chikando for assistance with data collection and analysis; Jennifer Moore and Tobey DeMott for assistance with data collection; Janina Madoff for technical assistance; Rochelle Bright for proofreading parts of the manuscript; and Christine R. Mottram for assistance with manuscript preparation.
REFERENCES
- Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci 16: 5567–5582, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 97: 933–936, 2007 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2038–2045, 1998a [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998b [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001a [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001b [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Manfredi M, Zamponi A, Cecconi V, Dolce G. Cortical and cervical stimulation after hemispheric infarction. J Neurol Neurosurg Psychiatry 50: 861–865, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007 [DOI] [PubMed] [Google Scholar]
- Binder M, Heckman CJ, Powers RK. The physiological control of motor neuron activity. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 1, p 3–53 [Google Scholar]
- Bui TV, Grande G, Rose PK. Multiple modes of amplification of synaptic inhibition to motoneurons by persistent inward currents. J Neurophysiol 99: 571–582, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons. J Neurophysiol 99: 583–594, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne JA, Carleton VL, O'Dwyer NJ. The spasticity paradox: movement disorder or disorder of resting limbs? J Neurol Neurosurg Psychiatry 76: 47–54, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardon MK, Suresh NL, Rymer WZ. A new method for reflex threshold estimation in spastic muscles. Conf Proc IEEE Eng Med Biol Soc 2009: 5300–3, 2009 [DOI] [PubMed] [Google Scholar]
- Chung SG, van Rey E, Bai Z, Rymer WZ, Roth EJ, Zhang LQ. Separate quantification of reflex and nonreflex components of spastic hypertonia in chronic hemiparesis. Arch Phys Med Rehabil 89: 700–710, 2008 [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996 [DOI] [PubMed] [Google Scholar]
- D'Amico JM, Murray KC, Li Y, Finlay MG, Bennett DJ, Gorassini MA. Constitutively active 5-HT2/α1 receptors facilitate muscle spasms after human spinal cord injury. J Physiol 109: 1473–1484, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows SJ, Kaus C, Ross HF, Thilmann AF. Agonist and antagonist EMG activation during isometric torque development at the elbow in spastic hemiparesis. Electroencephalogr Clin Neurophysiol 93: 106–112, 1994 [DOI] [PubMed] [Google Scholar]
- Frontera WR, Grimby L, Larsson L. Firing rate of the lower motoneuron and contractile properties of its muscle fibers after upper motoneuron lesion in man. Muscle Nerve 20: 938–947, 1997 [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Dutoit AP, Johns RK, Keen DA. Evaluation of plateau-potential-mediated “warm up” in human motor units. J Physiol 571: 683–693, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Stegling S. The post-stroke hemiplegic patient. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001 [DOI] [PubMed] [Google Scholar]
- Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve 18: 1101–1114, 1995 [DOI] [PubMed] [Google Scholar]
- Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 24: 58–63, 1993 [DOI] [PubMed] [Google Scholar]
- Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 29: 223–228, 2000 [DOI] [PubMed] [Google Scholar]
- Hafer-Macko CE, Ryan AS, Ivey FM, Macko RF. Skeletal muscle changes after hemi-paretic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev 45: 261–272, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulations of motoneuron firing rate modulation. J Neurophysiol 69: 1005–1008, 1993 [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins KD, Glass V. Statistical Methods in Education and Psychology. Boston, MA: Allyn and Bacon, 1996, p. 296–299 [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: A preferred firing range across vertebrate species? Muscle Nerve 25: 632–648, 2002 [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol 414: 265–282, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Suresh AK, Li X, Rymer WZ, Suresh NL. Impaired motor unit control in paretic muscle post stroke assessed using surface electromyography: a preliminary report. Conf Proc IEEE Eng Med Biol Soc 2012: 4116–4119, 2012 [DOI] [PubMed] [Google Scholar]
- Huang CY, Wang CH, Hwang IS. Characterization of the mechanical and neural components of spastic hypertonia with modified H reflex. J Electromyogr Kinesiol 16: 384–391, 2006 [DOI] [PubMed] [Google Scholar]
- Johns RK, Fuglevand AJ. Evidence for intrinsic mechanisms underlying firing rate saturation in human motor neurons. In: Program No. 188.2. Neuroscience 2004 Abstracts. San Diego, CA: Society for Neuroscience, 2004. Online [Google Scholar]
- Kernell D. The Motoneurone and Its Muscle Fibres. Oxford, UK: Oxford University Press, 2006 [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997 [DOI] [PubMed] [Google Scholar]
- Klein CS, Brooks D, Richardson D, McLlroy WE, Bayley MT. Voluntary activation failure contributes more to plantar flexor weakness than antagonist coactivation and muscle atrophy in chronic stroke survivors. J Appl Physiol 109: 1337–1346, 2010 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman CJ. Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol 90: 3617–3624, 2003 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 819–834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 30: 1303–1313, 1980 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol 85: 472–475, 2001 [DOI] [PubMed] [Google Scholar]
- Lewek MD, Hornby TG, Dhaher YY, Schmit BD. Prolonged quadriceps activity following imposed hip extension: a neurophysiological mechanism for stiff-knee gait? J Neurophysiol 98: 3153–3162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004 [DOI] [PubMed] [Google Scholar]
- Liang L, Chen JJ, Wang Y, Jakubiec M, Mierzejewska J, Piotrkiewicz M. Changes in spinal motoneuron “fastness” in post-stroke spastic patients. J Med Biol Eng 30: 17–22, 2010 [Google Scholar]
- Litvan I, Mangone CA, Werden W, Bueri JA, Estol CJ, Garcea DO, Rey RC, Sica RE, Hallett M, Bartko JJ. Reliability of the NINDS Myotatic Reflex Scale. Neurology 47: 969–972, 1996 [DOI] [PubMed] [Google Scholar]
- Lukacs M. Electrophysiological signs of changes in motor units after ischaemic stroke. Clin Neurophysiol 116: 1566–1570, 2005 [DOI] [PubMed] [Google Scholar]
- Madhaven S, Krishnan C, Jayaraman A, Rymer W, Stinear J. Corticospinal tract integrity correlates with knee extensor weakness in chronic stroke survivors. Clin Neurophysiol 122: 1588–1594, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaro N, Nielsen JF, Grey MJ, Sinkjaer T. Decreased contribution from afferent feedback to the soleus muscle during walking in patients with spastic stroke. J Stroke Cerebrovasc Dis 16: 135–144, 2007 [DOI] [PubMed] [Google Scholar]
- Merletti R, Rainoldi A, Farina D. Surface electromyography for noninvasive characterization of muscle. Exerc Sport Sci Rev 29: 20–25, 2001 [DOI] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40: 1432–1443, 1977 [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Origins of spontaneous firing of motor units in the spastic-paretic biceps brachii muscle of stroke survivors. J Neurophysiol 104: 3168–3179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Wallace CL, Chikando CN, Heckman CJ, Rymer WZ. Disturbances in motor unit firing rate modulation are more prevalent in muscles of spastic-paretic stroke survivors. In: Program No. 76.6/NN14 Neuroscience 2008 Abstracts San Diego, CA: Society for Neuroscience, 2008. Online [Google Scholar]
- Piotrkiewicz M, Kudina L, Mierzejewska J, Jakubiec M, Hausmanowa-Petrusewicz I. Age-related change in duration of afterhyperpolarization of human motoneurones. J Physiol 585: 483–490, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, ElBasiouny SM, Rymer WZ, Heckman CJ. Contribution of intrinsic properties and synaptic inputs to motoneuron discharge patterns: a simulation study. J Neurophysiol 107: 808–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol 23: 115–124, 1988 [DOI] [PubMed] [Google Scholar]
- Rosenfalck A, Andreassen S. Impaired regulation of force and firing patterns of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry 43: 907–916, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol 48: 875–890, 1982 [DOI] [PubMed] [Google Scholar]
- Tang A, Rymer WZ. Abnormal force-EMG relations in paretic limbs of hemiplegic human subjects. J Neurol Neurosurg Psychiatry 8: 690–698, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Estabrooks KL, Chong S, Stein RB. Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair 23: 133–142, 2009 [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Ravichandran VJ, Krutky MA, Perreault EJ. Contributions of altered stretch reflex coordination to arm impairments following stroke. J Neurophysiol 104: 3612–3624, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Mayer RF. Physiological alterations of motor units in hemiplegia. J Neurol Sci 54: 401–412, 1982 [DOI] [PubMed] [Google Scholar]