Abstract
Many approaches that use viral vectors to deliver transgenes have limited transduction efficiency yet require high levels of transgene expression. In particular, infection via axon terminals is relatively inefficient but is a powerful means of achieving infection of specific neuron types. Combining this with optogenetic approaches requires high gene expression levels that are not typically achieved with nontoxic retrogradely infecting vectors. We generated rabies glycoprotein-pseudotyped lentiviral vectors that use a positive feedback loop composed of a Tet promoter driving both its own tetracycline-dependent transcription activator (tTA) (“TLoop”) and channelrhodopsin-2-YFP (ChR2YFP). We show that TLoop vectors strongly express proteins in a drug-controllable manner in neurons that project to injection sites within the mouse brain. After initial infection, the virus travels retrogradely, stably integrates into the host genome, and expresses gene products. The expression is robust and allows optogenetic studies of neurons projecting to the location of virus injection, as demonstrated by fluorescence-targeted intracellular recordings. ChR2YFP expression did not cause observable signs of toxicity and continued for up to 6 mo after infection. Expression can be reversibly blocked by administration of doxycycline, if necessary, for expression of gene products that might be more toxic. Overall, we present a system that will allow researchers to achieve high levels of gene expression even in the face of inefficient viral transduction. The particular vectors that we demonstrate may enhance efforts to gain a precise understanding of the contributions of specific types of projection neurons to brain function.
Keywords: equine infectious anemia virus, channelrhodopsin(H134R), tTA, rabies glycoprotein
recent advances in molecular, genetic, and viral methods provide powerful tools for studying the organization and function of brain circuits (Luo et al. 2008). These include methods that allow neurons projecting to a particular location to be selectively infected and genetically modified so that their inputs can be identified or their activity monitored or manipulated (Lima et al. 2009; Osakada et al. 2011; Stepien et al. 2010; Yonehara et al. 2011). Nevertheless, transduction of neurons by viral uptake from their axon terminals (retrograde infection) is much less efficient than direct infection, presumably because of the small presynaptic surface area and restricted access to the very small synaptic space. As a result, approaches that require high levels of transgene expression, such as optogenetic control of neuronal activity, are difficult to achieve with retrograde infection. Current methods for robust virus-mediated gene expression via retrograde infection are not suitable for long-term studies of neural circuitries because of toxicity or latency-induced suppression of gene expression (Lima et al. 2009; Wickersham et al. 2007). Rabies glycoprotein pseudotyping of lentiviral vectors has proven successful in a number of studies to retrogradely infect neurons from injected sites (Azzouz et al. 2004; Kato et al. 2007, 2011; Kinoshita et al. 2012; Mazarakis et al. 2001). Although this is a useful method for chronic manipulation through retrograde infection, gene expression with this technique is not robust enough for many applications. Robust expression is required for applications such as fluorescence-guided targeted whole cell recordings or use of optogenetic tools such as channelrhodopsin, halorhodopsin, or archearhodopsin that require high levels of protein expression in order to generate enough photocurrent to sufficiently depolarize or hyperpolarize neurons and control their firing (Boyden et al. 2005; Chow et al. 2010; Nagel et al. 2003; Zhang et al. 2007).
A combination of approaches that would require an additional transgene and Cre-mediated amplification has been utilized in order to amplify the gene expression after retrograde infection (Aronoff et al. 2010; Chaudhury et al. 2013; Chen et al. 2013; Kuhn et al. 2012; Znamenskiy and Zador 2013). However, these methods require additional vectors or germline modification in order to express the genes of interests. Moreover, the use of pseudorabies virus (PRV) and herpes simplex virus (HSV) vectors, which have been utilized in several of these studies, suffers from toxicity, immune responses, and latency-induced inhibition.
Here we present a combination of methods that allowed us to strongly and stably express gene products in a drug-controllable manner for both local and retrograde infections. We achieved this by using a lentiviral vector system based on equine infectious anemia virus (EIAV) (Mazarakis et al. 2001; Olsen 1998). The viral vector encodes a transcription factor tetracycline-dependent transcription activator (tTA) driven by a tTA-responsive promoter (Baron et al. 1997) (“TLoop”). We show here that, after an initial leak from the expression cassette, tTA induces transcription from its own promoter, generating a positive feedback loop. This loop can be interrupted by application of a small molecule, doxycycline, that readily crosses the blood-brain barrier.
Using this strategy, we were able to activate neurons that project to barrel cortex via channelrhodopsin expression as early as 6 days after infection. When tested for longer periods of time, expression remained robust and stable up to 6 mo, the longest time point studied.
MATERIALS AND METHODS
All experiments using live animals were performed in accordance with protocols approved by the Salk Institute's Institutional Animal Care and Use Committee.
Plasmid construction.
The initial EIAV UNC-SIN6.1 CMV EGFP W and the Helper packaging plasmid PEV53B were kind gifts of John Olsen (Olsen 1998). The cytomegalovirus (CMV) promoter from the UNC-SIN6.1 CMV EGFP W vector was replaced with an improved synapsin promoter (iSynP), which consists of a 469-bp human synapsin promoter and a splice donor/splice acceptor site that belongs to the human beta globin gene (Nathanson et al. 2009), in order to generate the EIAV-iSynP-GFP vector. GFP was further replaced with channelrhodopsin-2-YFP (Chr2YFP) to generate the EIAV-iSynP-Chr2YFP vector. The TLoop EIAV Chr2YFP vector was generated by multiple cloning steps that produced an expression plasmid containing tetracycline binding operon promoter containing 7 repeats of tet elements (Gossen and Bujard 1992), a Chr2YFP with the H134R mutation (Boyden et al. 2005) linked to a tTA (Baron et al. 1997) with a 2A peptide that contains a double proline glycine sequence in order to enhance the separation of the two genes while being coexpressed (Tang et al. 2009), and a woodchuck virus posttranscriptional regulatory element (Zufferey et al. 1999) after the open reading frame. SalI and NheI restriction sites were added to the 5′ and 3′ ends of the Chr2YFP open reading frame for versatility of cloning other genes of interest. Replacing the Chr2YFP within the TLoop EIAV-Chr2YFP with DsRedX using these restriction sites generated the EIAV-TLoop DsRedX vector. A previously described double inverted open reading frame (DIO) strategy (Atasoy et al. 2008; Schnutgen et al. 2003; Sohal et al. 2009) was used to generate the Cre-dependent version EIAV-DIO-TLoop-CHR2YFP. The maps of all the clones described in this study will be available at Addgene.
Virus production.
Briefly, low-passage HEK293-T cells were grown to 95–100% confluence in medium containing 10% fetal bovine serum (FBS; SH3007002, Fisher) and antibiotics (MT30004Ci, Fisher) in 15-cm plates. The night before transfections, cells were split to three 15-cm plates. Twelve plates were used for the productions. On the day of transfection, 3 h prior to the transfections cells were washed twice with warm DMEM (GIBCO 11995) containing 10% FBS lacking antibiotics in order to remove the antibiotics from the medium for the duration of transfection. At the time of transfection for each plate 8 μg of pEV53B helper plasmid, 8 μg of SADB19 rabies glycoprotein pseudotyping plasmid (Sena-Esteves et al. 2004), or 8 μg of vesicular stomatitis virus (VSV) glycoprotein (VSVgp) pseudotyping plasmid (Naldini et al. 1996) and 8 μg of the TLoop expression plasmid were mixed with Opti-MEM (31985-070, GIBCO) at room temperature. One hundred ten microliters of pH 4.5 polyethylenimine (PEI; 9002-98-6, Polyscience) dissolved in Dulbecco's phosphate-buffered saline (DPBS; 14190-144, GIBCO) was added to the mixture, which immediately was vortexed for 5 s and left at room temperature for 5 min. The mixture was then added to the plates. All plates were left for incubation for 6 h in a 37°C incubator with 5% CO2. Cells were washed twice with warm DMEM containing 10% FBS. A final volume of 20 ml of DMEM containing antibiotics, 10% FBS, 1 μg/ml doxycycline (D9891, Sigma), and 5 mM sodium butyrate (B5887, Sigma) was added on cells and housed in a 35°C incubator with 3% CO2 for ∼40 h. On the day of harvest, the viral supernatants were spun at 1,000 g and 4°C for 5 min to remove the debris, followed by filtration through 0.45-μm Millipore filters (SCHVUO2RE). Six round-bottom Beckman centrifugation tubes (326823) were washed briefly with 100% ethanol and twice with PBS. Forty milliliters of virus solution was added per tube, followed by sealing with Parafilm. Virions were harvested at 25,000 rpm with the Beckman SW-28 rotor for 2 h at 4°C. The supernatants were carefully removed, and the inner walls of the tubes were dried with sterile tissue papers in order to avoid excess liquid. Thirty microliters of cold HBSS (14170112, GIBCO) was added to each tube, tubes were sealed with Parafilm, and the viruses were left for reconstitution overnight at 4°C. The next morning virus solutions were collected via pipetting multiple times, avoiding any bubbles, pooled, divided into 10-μl aliquots, and stored at −80°C until use or titer analysis. Titration was carried out by QPCR. The titers of the viruses used in this study, as transducing units per milliliter, were 3.2 × 109 for rabies glycoprotein-pseudotyped iSynP GFP (RG-EIAV-iSynP-GFP), 2.8 × 109 for rabies glycoprotein-pseudotyped iSynP Chr2YFP (RG-EIAV-iSynP-ChR2YFP), 1.7 × 109 for rabies glycoprotein-pseudotyped TLoop Chr2YFP (RG-EIAV-TLoop-CHR2YFP), 4.8 × 109 for VSVgp-pseudotyped TLoop DsRedX (VSVgp-EIAV-TLoop-DsRedX), and 7.4 × 108 for VSVgp-pseudotyped TLoop DIO-Chr2YFP (VSVgp-EIAV-DIO-TLoop-ChR2YFP).
Virus injections and drug treatment.
All injections were carried out as described previously (Cetin et al. 2006). For the electrophysiology and doxycycline control experiments 500 nl of virus was injected to barrel cortices of P6 C57BL/6 pups. For all other experiments, the same amounts of viruses were used with mice aged P20–P30. For the doxycycline control experiments in EIAV TLoop ChR2YFP-injected animals to suppress gene expression, doxycycline was delivered in the drinking water at a final concentration of 2 mg/ml, also mixed with 1% sucrose.
Electrophysiology and optogenetics.
Recordings were made from P20 mice coronal brain slices that were cut to a thickness of 300 μm with a VF-300 Microtome (Precisionary Instruments). Slices were preincubated at 35°C for 30 min in ACSF containing kynurenic acid, and all recordings were performed at room temperature in the absence of kynurenic acid with biocytin-containing internal solutions as described previously (Yoshimura et al. 2005). Data were collected with a dual patch-clamp amplifier (Axon Instruments MultiClamp 700B) and a data acquisition interface (CED Power 1401 MKII). Data were stored and analyzed with Spike6 software. Blue light was delivered with an in-house-built LED fiber-optic system controlled by TTL input that synchronized to data acquisition. To photoactivate recorded neurons, the tip of the light fiber was brought to ∼1 mm from the tip of the recording electrode and the recorded cells were stimulated with an initial light intensity output of 85 mW/mm2. To avoid voltage deflection due to the photoelectric effect the silver electrode wire was shortened and long glass capillaries (BF150-86-10, Sutter) were used so that the interaction of light with the electrode wire was minimized.
Tissue processing.
Brain slices used for electrophysiology were processed as whole mounts. For all other experiments, brains were sectioned with a freezing microtome to a thickness of 50 μm and kept in PBS containing 0.05% sodium azide. For the recombinant (r)EIAV iSynP GFP-injected animals, staining was performed with a rabbit primary anti-GFP antibody (ab290) at a dilution of 1:10,000 overnight and an Alexa 488 conjugate as a secondary donkey anti-rabbit antibody at a dilution of 1:200 (Invitrogen A21206) for 2 h. For electrophysiology, EYFP was visualized from native fluorescence and slices were not stained with an anti-GFP antibody. Biocytin-filled cells were stained with a streptavidin conjugate (DyLight 649 Streptavidin, Jackson Immunoresearch, 016-490-084). All slices were mounted with Vectashield with DAPI (Vectashield H1200) to label cell nuclei.
RESULTS
Retrograde infection with standard RG-pseudotyped EIAV results in low levels of gene expression.
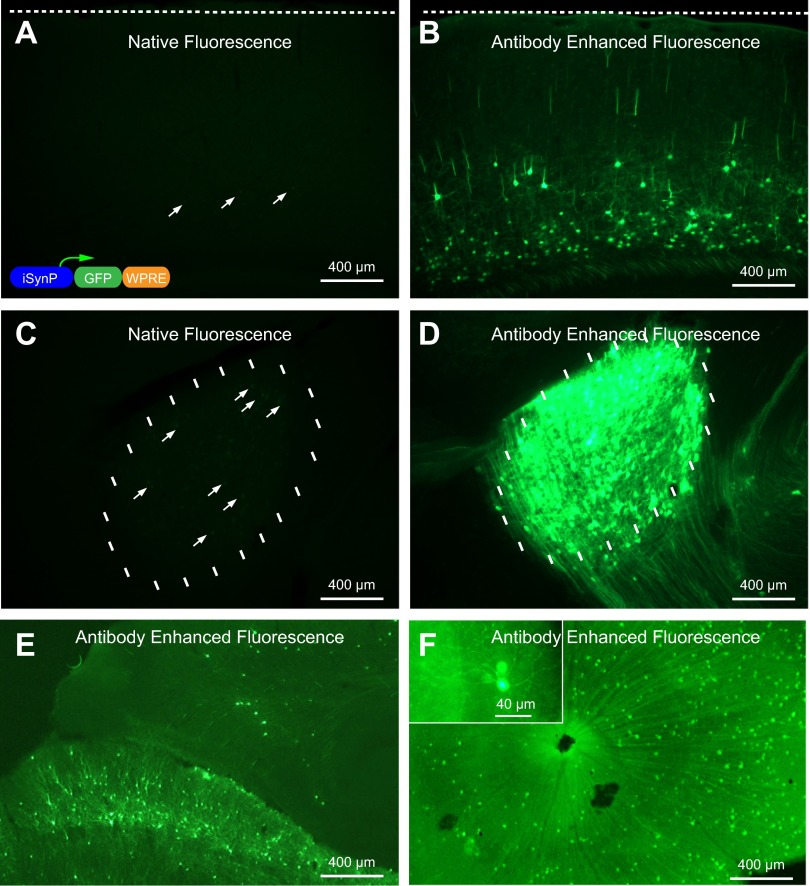
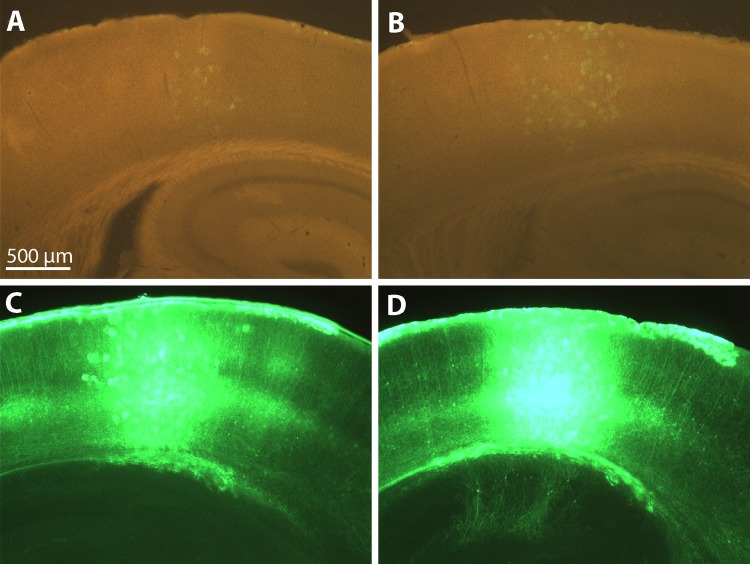
In one of our earlier reports we documented that gene expression through retrograde infection with rabies glycoprotein pseudotyping of lentiviral vectors is not as efficient as when glycoprotein-deleted rabies virus is used instead (Wickersham et al. 2007). This is most likely due to low multiplicity of infection through axon terminals and the ability of the rabies virus, but not the lentivirus, to replicate and produce many viral copies from an initially low copy number following infection. In this study we first sought to determine whether using strong and ubiquitous promoters would increase the yields as opposed to the weak CMV promoter (Wickersham and Callaway 2007). We generated an EIAV construct that expresses GFP with iSynP, since in our experience iSynP consistently yields high expression levels when used in other lentiviral vectors. To test the extent of retrograde infection and gene expression we pseudotyped this lentiviral vector with rabies glycoprotein (RG-EIAV-iSynP-GFP) and injected this virus into the dorsolateral geniculate nucleus (DLG) of mice. Three weeks after injection, brains and retinas were extracted and analyzed for GFP fluorescence. Initially with the direct observation of the GFP fluorescence, only a few neurons were barely visible within V1 and DLG (Fig. 1, A and C), which is in agreement with the previous observations with the CMV promoter (Wickersham et al. 2007). To assess whether the low yield was due to a lack of retrograde infection or if instead neurons had been infected but expression levels were low, we performed antibody staining to amplify the GFP signal. Indeed, only after this procedure was it apparent that GFP expression was present in large numbers of neurons (Fig. 1, B, D–F), consistent with previous studies demonstrating efficient retrograde transduction with sensitive staining methods (Azzouz et al. 2004; Mazarakis et al. 2001). The results were dramatic when visualized with the same epifluorescence exposure settings for stained and unstained neighboring sections side by side (Fig. 1, A–D). iSynP yielded large numbers of retrogradely infected neurons observed in numerous brain areas after anti-GFP staining. This is exemplified by images of visual cortex (Fig. 1B), superior colliculus (Fig. 1E), and retina (Fig. 1F) that are known to project to the virus injection site in the DLG.
Fig. 1.
Rabies glycoprotein pseudotyping of recombinant equine infectious anemia virus (rEIAV) improved synapsin promoter (iSynP) GFP does not allow robust expression but allows immunodetection of neurons projecting to the injection site. Three weeks after virus injection into dorsolateral geniculate nucleus (DLG), the expression of GFP is barely visible under native fluorescence at the site of injection (C) and visual cortex (A), which projects to DLG. The expression can be readily detected in vast numbers of neurons after immunoenhancement with a GFP antibody at the injection site (D) and V1 (B). Other neurons labeled that are known to project to DLG include superior colliculus neurons (E) and retinal ganglion cells (F). The schematic of the viral construct is depicted in the inset in A.
Although extensive labeling with RG-EIAV-iSynP-GFP could only be seen after antibody staining, we remained interested in the possibility that the levels of gene expression with this vector might be sufficient for optogenetic experiments. For this purpose we swapped GFP with channelrhodopsin-2-YFP(H134R) (ChR2YFP), which is a light-gated cation channel that can depolarize cells via sodium influx when blue light changes its conformation (Nagel et al. 2005). We pseudotyped this expression vector with rabies glycoprotein (RG-EIAV-iSynP-ChR2YFP) and injected the virus into various brain regions, hoping to get sufficient levels of expression in order to perform fluorescence-mediated targeted recordings and directly photoactivate the recorded neurons. Although the titers of the produced viruses were similar to that of the GFP-expressing viruses (see materials and methods), 3 wk after injection there was no detectable fluorescence in 300-μm brain slices at the injection sites and elsewhere. Despite the inability to directly identify and record from potentially infected neurons we attempted to evoke photocurrents in neurons recorded at the injection sites, as well as synaptic currents that might be generated by direct activation of neurons connected to the recorded neurons, and none were detected. Since we could not record directly from neurons that were potentially infected with invisible levels of the gene product we decided to build an approach that will allow us to do that.
Strong ChR2YFP expression levels are needed in order to optogenetically drive action potentials in neurons (Boyden et al. 2005; Nagel et al. 2003; Zhang et al. 2008). Our goal was to develop a retrogradely infecting viral tool that would allow us to both do fluorescence-mediated targeted electrophysiology and optogenetic photostimulation experiments. Retrograde infection in large numbers of neurons was observed, and the method revealed inputs to injection sites; however, it was clear that further development of the technique was required in order to express genes at higher levels.
TLoop EIAV vectors produce high levels of gene expression with an early onset.
Negative-strand RNA viruses (such as rabies virus and vesicular stomatitis virus) have evolved polymerases that continuously transcribe vast copies of their mRNAs, including those that encode their own polymerase; this creates a positive feedback loop. We thought that by mimicking such a positive feedback transcription loop (“TLoop”) strategy we should be able to dramatically increase the promoter readouts. To generate this loop we picked a small drug-controllable strategy. This way, using a blood-brain barrier-penetrating small drug, we would then be able to turn the gene expression on or off to interrupt the loop in the event that long-term expression of a particular gene product might be toxic or if reversibility might be desirable for particular experimental approaches.
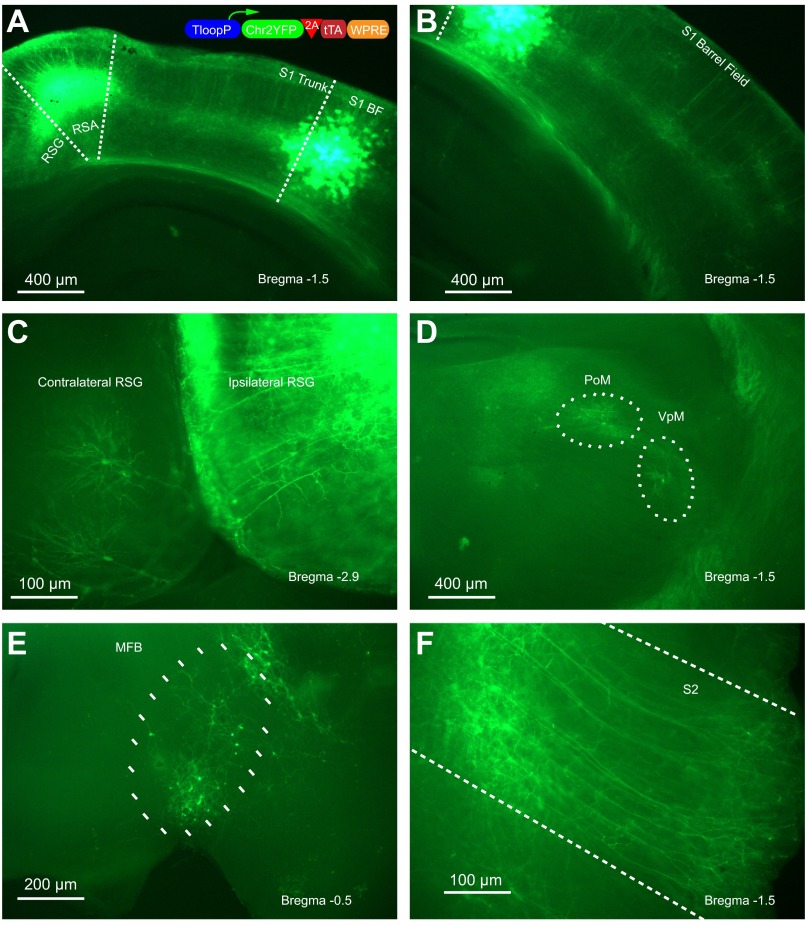
Tetracycline-dependent transcriptional activator (tTA) seemed to be a good choice fitting this criterion. tTA binds to a unique 42-bp-long DNA sequence called Tet (Baron et al. 1997). It also contains acidic domains that help recruit the transcription machinery in the presence of a minimal promoter that is only basally active, which is called a Tet promoter (Baron et al. 1997). We thought if we could coexpress tTA together with ChR2YFP downstream of a Tet promoter, this would start a positive feedback loop that would generate sufficient amounts of ChR2YFP in order to allow photoactivation of retrogradely infected neurons. We put this idea to test by using a Tet promoter cassette followed by a single open reading frame containing ChR2YFP and tTA linked with a ribosome skipping 2A peptide (Szymczak et al. 2004; Tang et al. 2009). We then pseudotyped this virus with rabies glycoprotein (RG-EIAV-TLoop-ChR2YFP) and tested the resulting vector by injecting barrel cortices of mice. Three weeks later we analyzed the brains of these mice postmortem. As seen in 300-μm-thick unstained live brain slices, ChR2YFP expression is readily visible in the neurons projecting to the area of injection (Fig. 2). This made it possible to do electrophysiological recordings from retrogradely infected neurons.
Fig. 2.
TLoop rEIAV vector is able to express robust channelrhodopsin-2-YFP (ChR2YFP) after retrograde uptake as visualized with native fluorescence. Injection site at the S1 barrel field (BF) is conveniently marked with the glial expression since the virus is taken up also by glia only locally (A). Cell bodies and dendrites of neurons projecting to the site of injection are labeled within the S1 barrel field (B), granular and agranular retrosplenial cortices (RSG, RSA) (C), posterior medial complex (PoM) and ventral posteromedial nucleus (VPM) (D), Meynert nucleus of the basal forebrain (MFB) (E), and secondary somatosensory cortex (S2) (F). The schematic of the viral construct is depicted in the inset in A.
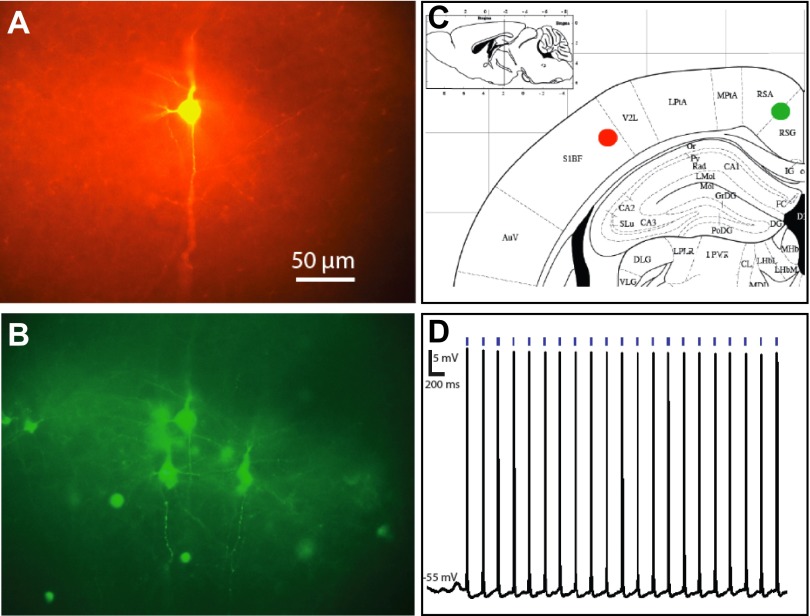
To show that the ChR2YFP expression levels driven by the TLoop rEIAV are sufficient for photoactivation, we performed electrophysiological recordings 12 days after injection. We prepared 300-μm-thick brain slices and were able to readily visualize ChR2YFP expression in neurons within the areas that project to the site of initial infection (Figs. 2 and 3). We then patched these neurons and performed whole cell recordings to determine whether action potentials could be reliably evoked in these retrogradely infected neurons after blue light stimulation. In retrogradely infected neurons we were able to evoke action potentials with 2-ms-duration LED pulses delivered by a millimeter-diameter fiber-optic cable positioned ∼1 mm above the recorded neurons with an initial output power of 80 mW/mm2 (Fig. 3). The reliability of action potential generation in recorded neurons suggests that the amount of expression is suitable for future optogenetic studies of projection neurons. Figure 3 illustrates results from a retrogradely infected neuron in retrosplenial granular cortex after viral injection into S1 barrel cortex. We performed similar experiments in brain slices from a total of four mice in which barrel cortices were injected. We recorded from neurons in a broad range of projection neurons and successfully obtained light-induced responses in all of them, including neurons in the following structures: substantia innominata, primary motor area layer V, secondary motor area layer V, posterior parietal association area layer V, lateral preoptic area, ventral posteromedial nucleus, external globus pallidus, secondary motor area layer VI, and secondary somatosensory cortex layer V.
Fig. 3.
Retrograde infection using TLoop rEIAV ChR2YFP viruses allows efficient optogenetic control. A recorded biocytin-filled neuron (A) is among many other neighboring ChR2YFP-expressing neurons (B) located in RSG (green filled circle in C) that are retrogradely infected from the injection site (red filled circle in C). Action potentials are reliably evoked by stimulating this recorded neuron with 2-ms pulses of blue light (D).
Long-term effects of ChR2YFP expression with TLoop EIAVs.
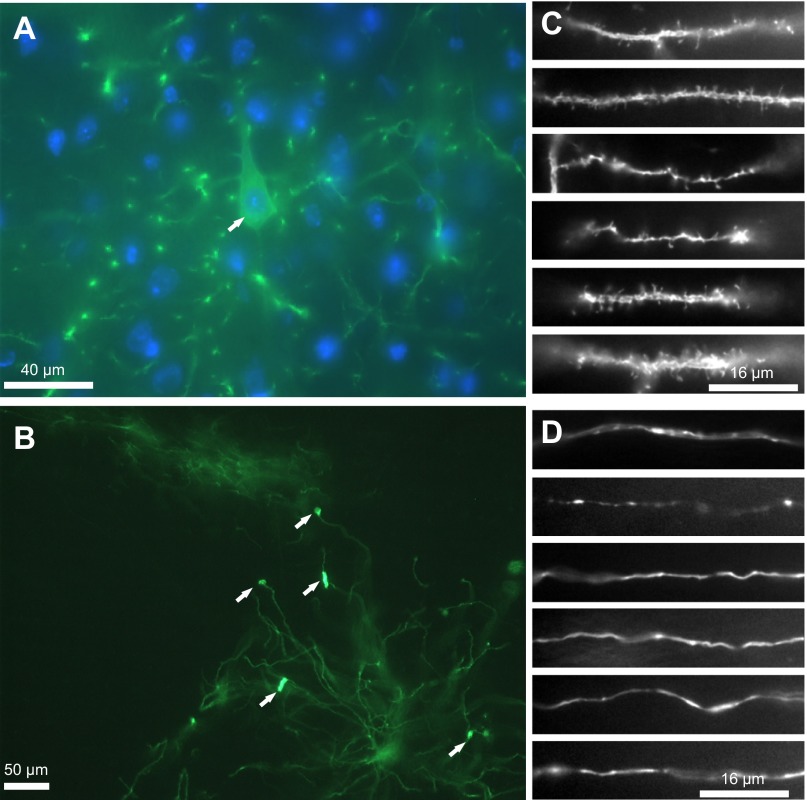
Although we developed the TLoop vectors in order to make them controllable by doxycycline application, we were interested in whether the uncontrolled positive transcriptional feedback loop might lead to overexpression of ChR2YFP at levels that are toxic to neurons. Therefore, in one case we allowed expression for a long period of time to determine whether the TLoop generated complications. Six months after injection the nuclei of the retrogradely infected neurons were indistinguishable from their neighboring neurons that had not been infected (Fig. 4A). Furthermore, in general, the morphology of dendrites and axons of the infected neurons all throughout the brain appeared normal (Fig. 4, C and D). However, we did see some notable morphological abnormalities at least in a few neurons, which looked like dendritic aggregations and swellings (Fig. 4B). Despite the overall normal appearance of neurons infected under these conditions, uncontrolled expression from TLoop vectors might lead to abnormalities, particularly under other conditions where viral transduction could be more efficient. Furthermore, other gene products that require high expression levels to be effective might be more detrimental than ChR2 and might be used with the TLoop system in the future. Therefore, even though overall the ChR2-expressing neurons appeared healthy, it may be desirable for researchers to suppress expression of other gene products or to delay expression until needed.
Fig. 4.
Neurons that are infected retrogradely by the TLoop rEIAV ChR2YFP virus can continue expressing the gene products as long as 6 mo. A layer V pyramidal neuron cell body is shown at high magnification with a nucleus that is indistinguishable from neighboring cells [YFP-DAPI (blue) overlay, A; cell is marked with an arrow]. Neuronal processes from many different areas from retrogradely infected neurons look normal in morphology (dendrites in C and axons in D). In rare instances, neurons that are infected by the TLoop rEIAV ChR2YFP virus that continue expressing the gene products for 6 mo show signs of fluorescent aggregations (B). Dendritic processes from neurons located in the ventral medial nucleus of the thalamus that were retrogradely infected from the barrel cortex injection and have been expressing the gene products for 6 mo show rare signs of protein Chr2YFP aggregation that may be due to uncontrolled expression (indicated with arrows).
Drug-controllable expression with TLoop EIAV vectors.
The tTA transcription factor, when bound to the ligand doxycycline, should no longer induce transcription efficiently. We therefore tested whether gene expression could be suppressed in mice that were injected in the barrel cortex with RG-EIAV-TLoop-ChR2YFP by supplying doxycycline in their drinking water (n = 2) while littermates injected on the same day were kept as controls (n = 2). After 2 wk both control mice exhibited strong fluorescence in vast numbers of neurons at the injection site (somatosensory barrel cortex; Fig. 5, C and D) and sites of retrograde uptake (substantia innominata, primary motor area layer V, secondary motor area layer V, posterior parietal association area layer V, lateral preoptic area, ventral posteromedial nucleus, external globus pallidus, secondary motor area layer VI, and secondary somatosensory cortex layer V). However, the mice that were given 2 mg/ml of doxycycline supplied in the drinking water exhibited almost undetectable levels of expression, mostly in glia within the injection site at somatosensory barrel cortex (Fig. 5, A and B). These results show that expression from the TLoop rEIAV system can be effectively suppressed by doxycycline in vivo. The residual expression seen in glia may be due to high copy uptake in infected cells; therefore, increasing the dose of doxycycline or regular intraperitoneal injections might be desirable if complete shutdown is required. For many practical purposes this method might be useful whenever high levels of gene expression are not wanted.
Fig. 5.
Gene expression from the TLoop rEIAV construct in infected neurons can be tightly controlled by doxycycline administration in drinking water. Four littermates were injected with the same batch of rabies glycoprotein-pseudotyped TLoop rEIAV ChR2YFP virus in barrel cortices. For a duration of 2 wk, 2 of the animals were given doxycycline whereas the other 2 were not. Mostly faint glia expression is visible in doxycycline-treated littermates (A and B; red blank channel was used to see the anatomical landmarks), whereas strong ChR2YFP signal can be observed in the remaining untreated mice (C and D).
Early-onset and Cre-conditional expression with TLoop vectors.
Since our main purpose has been to optimize retrograde infection, we have focused on descriptions of results with rabies glycoprotein-pseudotyped lentiviral vectors. For this purpose, amplification of expression by the TLoop system is desired in order to compensate for inefficient infection. We thought that the TLoop system might also be beneficial under other conditions in which enhanced gene expression is desired. For example, developmental studies are sometimes hindered by the long time period required to obtain sufficient gene expression from nontoxic vectors such as lentiviruses. We therefore produced a TLoop EIAV lentivirus expressing DsRedX and pseudotyped with the more typical VSV glycoprotein (VSVgp-EIAV-TLoop-DsRedX). As expected from our experience with rabies glycoprotein-pseudotyped TLoop vectors, we observed that the expression with the VSVgp-pseudotyped TLoop rEIAVs was very efficient. Of particular note, we observed robust gene expression in neurons as early as 48 h in mouse barrel cortex injected with VSVgp-EIAV-TLoop-DsRedX (Fig. 6A). Therefore, in addition to the utility of the TLoop system in cases where viral infection is inefficient, this system is also likely to be advantageous for experiments requiring fast gene expression, such as developmental studies requiring short time windows.
Fig. 6.
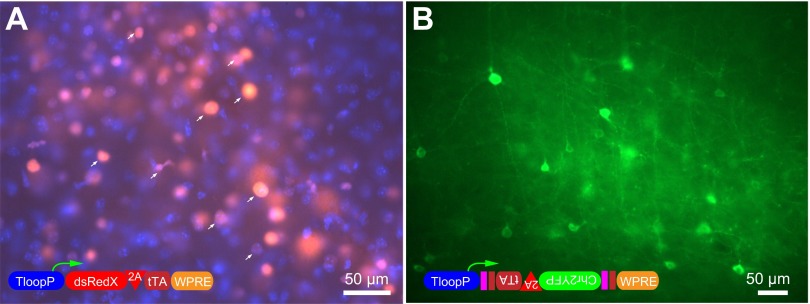
Early-onset and Cre-conditional expression with TLoop vectors. Native fluorescence can be detected as early as 48 h after injection in mouse barrel cortex with TLoop EIAVs (A). A DsRedX-expressing TLoop rEIAV virus was pseudotyped with vesicular stomatitis virus glycoprotein (VSVgp) and injected into the mouse barrel cortex. The expression can be detected after 2 days without any need of staining. Expression is mainly nuclear, indicating that the 2A skipping peptide has not been efficient enough and the majority of the DsRedX (arrows in A) was targeted to nucleus alongside with tTA, which contains a nuclear targeting signal (nuclei stained with DAPI in blue). Native ChR2YFP expression using Cre-dependent TLoop ChR2 vectors: VSVgp-pseudotyped Cre-dependent TLoop ChR2YFP vector was injected into layer V barrel cortex of a Nex-Cre mouse (B). Five days after injection, the brain was removed and sectioned. The native fluorescence from ChR2YFP is readily visible at the soma and processes of Cre-expressing neurons without any immunoenhancement. The schematics of the viral constructs are depicted in insets in A and B.
We further tested whether TLoop vectors can be used for conditional expression in Cre-expressing neurons. We produced a DIO-based (Atasoy et al. 2008; Cardin et al. 2009) Cre-conditional TLoop EIAV vector to utilize cell type-specific Cre transgenic mouse lines for the study of individual neuronal subtypes. We injected VSVgp-EIAV-DIO-TLoop-CHR2YFP into the barrel cortex of Nex-Cre mice and observed ChR2YFP expression 5 days after injection (Fig. 6B). The presence of strongly Chr2YFP-labeled pyramidal neurons without immunoenhancement and the lack of glial labeling and inhibitory interneuron morphologies at the site of injection suggested that the recombination was specific to the Nex-Cre-expressing pyramidal neurons.
DISCUSSION
In this study we demonstrated that with a positive transcriptional feedback loop (“TLoop”) based on a blood-brain barrier-penetrating drug-controllable transcription system, it is possible to achieve highly efficient retrograde delivery of gene products from rEIAV-based lentiviral vectors. This method made it possible to perform optogenetic photoactivation experiments and fluorescence-guided whole cell recordings targeted to neurons selected on the basis of their projections to the virus injection sites. The TLoop system that we have introduced here and demonstrated in the context of optogenetic control of projection neurons might also be useful for other types of experiments. For example, rapid gene expression may be utilized for developmental studies. Furthermore, the TLoop system may be used in order to amplify gene expression readouts when the efficiency of the method is low, such as in the case of bridge protein-mediated viral infection (Xu et al. 2005).
Another feature of TLoop vectors is that gene expression can be turned off upon delivery of doxycycline, which readily crosses the blood-brain barrier. There are currently two versions of the tTA system. One can be inhibited by application of doxycycline, namely, Tet OFF, which we utilized in this study, and another can be activated upon doxycycline administration, namely, reverse tTA (rtTA)-Tet ON (Toniatti et al. 2004). Using the TLoop strategy with a Tet ON promoter should also allow researchers to activate gene expression by doxycycline administration. For example, in clinical applications or long-term experiments in primates, it may be desirable to have a drug-inducible method of gene delivery.
There are several current strategies that allow retrograde infection-mediated gene expression. For example, recombinant adeno-associated virus (rAAV) vectors have been used in a variety of studies in order to retrogradely infect and express gene products in motor neurons after intramuscular vector delivery (Kaspar et al. 2003; Stepien et al. 2010). However, the efficiency of the retrograde uptake has been reported to be low with rAAVs injected into the brain (Burger et al. 2004; Hollis et al. 2010; Towne et al. 2009; Xu et al. 2005). Since studies using such reagents in the central nervous system typically require antibody staining to visualize fluorescent protein, it would seem unlikely that these approaches would be suitable for optogenetics experiments requiring high levels of gene expression unless an additional sensitive genetic amplification method such as Cre-mediated recombination (Aronoff et al. 2010; Chen et al. 2013) is utilized. While incorporation of the TLoop system into AAV vectors has the potential to overcome this limitation, in our experience the numbers of neurons retrogradely infected with AAV are much lower than with RG-pseudotyped lentiviruses, and this was the basis for our choice of lentiviral vectors in the experiments described here. Future experiments using rAAVs for retrograde infection might benefit from studies using rAAV serotype libraries to screen for more efficient rAAV serotypes for retrograde uptake, and/or incorporation of amplification strategies such as the TLoop system in order to achieve high gene expression levels.
An attenuated HSV-1 vector carrying a EF1a promoter and ChR2YFP produced by Biovex has been used for retrograde infection resulting in successful optical control of neurons (Lima et al. 2009). However, the authors report that the expression of the ChR2YFP recedes after 10 days after injection. The remaining HSV-1 genes expressed from the prepared vectors plus a possible immune response are particular concerns for long-term expression with this method. We and others have previously generated ChR2-expressing glycoprotein-deleted rabies vectors and successfully utilized them for short-term optogenetic control of retrogradely infected neurons (Apicella et al. 2012; Kiritani et al. 2012; Kress et al. 2013; Osakada et al. 2011). However, with this method long-term expression is currently not possible because of the toxicity-associated degradation of infected neurons after 12 days.
Rabies glycoprotein pseudotyping of lentiviral vectors has been previously used for retrograde gene delivery (Azzouz et al. 2004; Kato et al. 2007, 2011; Kinoshita et al. 2012; Mazarakis et al. 2001; Stepien et al. 2010). Use of VSVgp C-terminal domains in order to generate a chimeric rabies glycoprotein seems to increase transduction efficiencies of the pseudotyped HIV-based lentiviral vectors in vivo (Kato et al. 2007, 2011). A combinatorial approach that uses a tet promoter packaged in an HIV-based lentiviral vector for retrograde infection combined with a local infection of rAAV that delivers the tTA transactivator has been utilized to express a toxin in order to conditionally block neurotransmitter release and study the hand dexterity motor circuitry (Kinoshita et al. 2012). This approach amplifies the expression that might normally be obtained from the retrograde lentiviral vector by combination with a highly efficient local rAAV infection and the strong Tet system. Furthermore, efficiency of the expressed toxin in blocking synaptic release may have contributed to the successful implementation of the system. It is not known whether this same amplification approach would be suitable for reversible activation or inactivation using optogenetic approaches that likely require higher levels of gene expression. Such a combinatorial approach could, however, be advantageous when it is necessary to limit effects of gene expression to projection neurons in a particular brain area that can be defined by the location of the local AAV injection. Similar combinatorial control could be achieved with the TLoop vectors by using a locally infecting rAAV to express Cre-recombinase and combining with the Cre-dependent TLoop virus we have described (EIAV-DIO-TLoop-CHR2YFP). With this system gene expression levels would be suitable for optogenetic experiments, as we have demonstrated. Regardless of any advantages of combinatorial approaches, in cases where it is not necessary, the RG-EIAV-TLoop-ChR2YFP vector that we have described here has the advantage of requiring only a single viral injection and does not require the precise matching of a second viral injection to a site that overlaps with retrogradely infected neurons.
In this study we also demonstrated successful implementation of Cre-recombinase-mediated gene expression using EIAV-DIO-TLoop-CHR2YFP vectors. We performed this via testing recombination of a VSVgp-pseudotyped EIAV-DIO-TLoop-CHR2YFP vector in Nex-Cre mouse line neocortex. We observed that TLoop expression matched the expected Nex-Cre expression pattern. Minimal CMV promoter drives expression well in glia, and lentiviruses infect glia efficiently. This can be observed from the images of the injected sites presented in this study (e.g., Figs. 2 and 5). We reasoned that if there was nonspecific recombination with the Cre-dependent TLoop system we would see glia at the site of injection. We did not see any expression in glia at the site of injection and therefore concluded that the recombination was specific. Moreover, there was not any visible fluorescence in the virus-producing HEK293 cells, while a regular TLoop construct fluoresces brightly in these producer cells. Further evidence for selectivity is the fact that no inhibitory cortical neuronal morphologies were visible at the sites of infection as expected since Cre is only expressed in excitatory neurons in the Nex-Cre line. Given the relatively large capacity of lentiviral genome and early-onset drug-controllable Cre-dependent expression capability, this method may be useful in future studies that would benefit from these features.
Given the success of the TLoop strategy for obtaining high levels of ChR2 expression and optogenetic control of the activity of projection neurons, we expect that this system will also have great utility for a variety of other approaches where infection efficiency is low but high levels of gene expression are required. While we have demonstrated this system in the context of lentiviral vectors, the TLoop system should be able to be incorporated into any viral vector where transcription can be controlled by the Tet promoter, including AAV and adenoviral vectors. This system might also be used to obtain high levels of gene expression in transgenic or knockin mouse lines. In addition to expressing ChR2 with the use of the TLoop system in retrogradely infecting viruses, we expect that it will also be useful for expression of other optogenetic tools that inactivate neurons or for expression of genetically encoded calcium indicators to preferentially visualize activity of neurons that project to certain sites in the brain.
GRANTS
This work was supported by The Gatsby Foundation, National Institutes of Health Grants MH-063912, EY-010742, EY-022577, and EY-020673, the Howard Hughes Medical Institute, and the Human Frontiers Science Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C. and E.M.C. conception and design of research; A.C. performed experiments; A.C. and E.M.C. analyzed data; A.C. and E.M.C. interpreted results of experiments; A.C. prepared figures; A.C. and E.M.C. drafted manuscript; A.C. and E.M.C. edited and revised manuscript; A.C. and E.M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the director of the Gene Transfer, Targeting and Therapeutics Core Facility, Dan Gibbs, for his assistance in some of the virus preparations and titer quantification. We also thank John Olsen for kindly providing the necessary initial rEIAV components.
REFERENCES
- Apicella AJ, Wickersham IR, Seung HS, Shepherd GM. Laminarly orthogonal excitation of fast-spiking and low-threshold-spiking interneurons in mouse motor cortex. J Neurosci 32: 7021–7033, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci 31: 2221–2233, 2010 [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci 28: 7025–7030, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429: 413–417, 2004 [DOI] [PubMed] [Google Scholar]
- Baron U, Gossen M, Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res 25: 2723–2729, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005 [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10: 302–317, 2004 [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc 1: 3166–3173, 2006 [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493: 532–536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Carta S, Soldado-Magraner J, Schneider BL, Helmchen F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature 499: 336–340, 2013 [DOI] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463: 98–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89: 5547–5551, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Jamshidi P, Lorenzana AO, Lee JK, Gray SJ, Samulski RJ, Zheng B, Tuszynski MH. Transient demyelination increases the efficiency of retrograde AAV transduction. Mol Ther 18: 1496–1500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301: 839–842, 2003 [DOI] [PubMed] [Google Scholar]
- Kato S, Inoue K, Kobayashi K, Yasoshima Y, Miyachi S, Inoue S, Hanawa H, Shimada T, Takada M. Efficient gene transfer via retrograde transport in rodent and primate brains using a human immunodeficiency virus type 1-based vector pseudotyped with rabies virus glycoprotein. Hum Gene Ther 18: 1141–1151, 2007 [DOI] [PubMed] [Google Scholar]
- Kato S, Kobayashi K, Inoue K, Kuramochi M, Okada T, Yaginuma H, Morimoto K, Shimada T, Takada M. A lentiviral strategy for highly efficient retrograde gene transfer by pseudotyping with fusion envelope glycoprotein. Hum Gene Ther 22: 197–206, 2011 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature 487: 235–238, 2012 [DOI] [PubMed] [Google Scholar]
- Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci 32: 4992–5001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Yamawaki N, Wokosin DL, Wickersham IR, Shepherd GM, Surmeier DJ. Convergent cortical innervation of striatal projection neurons. Nat Neurosci 16: 665–667, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B, Ozden I, Lampi Y, Hasan MT, Wang SS. An amplified promoter system for targeted expression of calcium indicator proteins in the cerebellar cortex. Front Neural Circuits 6: 49, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PloS One 4: e6099, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron 57: 634–660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, Carter EE, Barber RD, Baban DF, Kingsman SM, Kingsman AJ, O'Malley K, Mitrophanous KA. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet 10: 2109–2121, 2001 [DOI] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284, 2005 [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 100: 13940–13945, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272: 263–267, 1996 [DOI] [PubMed] [Google Scholar]
- Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161: 441–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JC. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther 5: 1481–1487, 1998 [DOI] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71: 617–631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnutgen F, Doerflinger N, Calleja C, Wendling O, Chambon P, Ghyselinck NB. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat Biotechnol 21: 562–565, 2003 [DOI] [PubMed] [Google Scholar]
- Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods 122: 131–139, 2004 [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron 68: 456–472, 2010 [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single “self-cleaving” 2A peptide-based retroviral vector. Nat Biotechnol 22: 589–594, 2004 [DOI] [PubMed] [Google Scholar]
- Tang W, Ehrlich I, Wolff SB, Michalski AM, Wolfl S, Hasan MT, Luthi A, Sprengel R. Faithful expression of multiple proteins via 2A-peptide self-processing: a versatile and reliable method for manipulating brain circuits. J Neurosci 29: 8621–8629, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniatti C, Bujard H, Cortese R, Ciliberto G. Gene therapy progress and prospects: transcription regulatory systems. Gene Ther 11: 649–657, 2004 [DOI] [PubMed] [Google Scholar]
- Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain 5: 52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Callaway EM. Suitability of hCMV for viral gene expression in the brain—Wickersham and Callaway reply. Nat Methods 4: 379–379, 2007 [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 4: 47–49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Ma C, Bass C, Terwilliger EF. A combination of mutations enhances the neurotropism of AAV-2. Virology 341: 203–214, 2005 [DOI] [PubMed] [Google Scholar]
- Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature 469: 407–410, 2011 [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature 433: 868–873, 2005 [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature 446: 633–639, 2007 [DOI] [PubMed] [Google Scholar]
- Zhang YP, Holbro N, Oertner TG. Optical induction of plasticity at single synapses reveals input-specific accumulation of alpha CaMKII. Proc Natl Acad Sci USA 105: 12039–12044, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497: 482–485, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol 73: 2886–2892, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]