Abstract
Electrophysiology in primates has implicated long-range neural coherence as a potential mechanism for enhancing sensory detection. To test whether local synchronization and long-range neural coherence support detection performance in rats, we recorded local field potentials (LFPs) in frontal and parietal cortex while rats performed an auditory detection task. We observed significantly elevated power at multiple low frequencies (<15 Hz) preceding the target beep when the animal failed to respond to the signal (misses), in both frontal and parietal cortex. In terms of long-range coherence, we observed significantly more frontal-parietal coherence in the beta band (15–30 Hz) before the signal on misses compared with hits. This effect persisted after regressing away linear trends in the coherence values during a session, showing that the excess frontal-parietal beta coherence prior to misses cannot be explained by slow motivational changes during a session. In addition, a trend toward higher low-frequency (<15 Hz) coherence prior to miss trials compared with hits became highly significant when we rereferenced the LFPs to the mean voltage on each recording array, suggesting that the results are specific to our frontal and parietal areas. These results do not support a role for long-range frontal-parietal coherence or local synchronization in facilitating the detection of external stimuli. Rather, they extend to long-range frontal-parietal coherence previous findings that correlate local synchronization of low-frequency (<15 Hz) oscillations with inattention to external stimuli and synchronization of beta rhythms (15–30 Hz) with voluntary or involuntary prolongation of the current cognitive or motor state.
Keywords: attention, oscillation, synchrony
detection of a sensory signal is arguably the most fundamental perceptual ability. Although neurophysiologists have studied sensory detection for decades (Naatanen and Picton 1987; Palva et al. 2005; Picton and Hillyard 1974), its cellular brain mechanisms have yet to be fully characterized in primates (de Lafuente and Romo 2005, 2006), rodents (Broussard et al. 2006; Carandini and Churchland 2013), or any animal. In humans and other primates, detection in multiple sensory modalities is believed to rely on a frontal-parietal network that subserves attention and detection including conscious perception (Naghavi and Nyberg 2005). The frontal and parietal cortex are specifically active during a number of different attention and detection tasks, across sensory modalities, as shown by both positron emission tomography (Corbetta et al. 1993; Coull et al. 1996; Pardo et al. 1991) and functional magnetic resonance imaging (Coull and Nobre 1998; Fritz et al. 2007; Mayer et al. 2006; Shomstein and Yantis 2006; Wu et al. 2007). Lesions of parietal or frontal cortex in both humans and monkeys cause impairments in attention (Posner 1980), and there are numerous anatomical connections between frontal and parietal cortex in the primate brain (Marconi et al. 2001). Specifically, prefrontal (Pardo et al. 1991), dorsal supplementary motor (Coull et al. 1996), and premotor (Pesaran et al. 2008) frontal areas were among those implicated in target detection and functional coupling with parietal areas.
An analogous frontal-parietal network for detection of behaviorally relevant stimuli may exist in rats. Lesions of medio-dorsal frontal cortex (King and Corwin 1990; Vargo et al. 1988) or posterior parietal cortex (King and Corwin 1993) produce visual and auditory neglect. Single-unit recordings in posterior parietal cortex suggest that neurons there signal detection of a target visual stimulus (Broussard et al. 2006), and this area is known to have anatomical connections to the medial dorsal frontal cortex (Reep et al. 1994; called Fr2 in that paper). However, it remains unclear whether the frontal (Uylings et al. 2003) or posterior parietal (Rosner and Mittleman 1996; Ward and Brown 1997) cortex of rats is homologous to that of primates. Establishing the rat as a model of the primate detection network would be valuable because the cellular mechanisms are potentially more accessible to experimental manipulation in rodents (Carandini and Churchland 2013; Hromádka and Zador 2007) and the rodent is rapidly becoming an important model for many behavioral and neural processes that historically have been addressed in primates (Andermann et al. 2011; Carandini and Churchland 2013; Kerlin et al. 2010; Meier et al. 2011; Meier and Reinagel 2011). We were therefore interested in better characterizing interactions between these frontal and parietal areas during rats' sensory detection behavior.
Local and interarea oscillatory neural synchronization at various frequencies have been proposed as potential mechanisms for modulating interarea functional connectivity for routing, modulating, or binding distributed representations (Bollimunta et al. 2011; Buffalo et al. 2011; Cooper et al. 2003; Gregoriou et al. 2009b; Jones et al. 2010; Kolev et al. 2001; Macdonald et al. 2011; Mazaheri et al. 2011; Melloni et al. 2007; Niedermeyer 1997; Palva and Palva 2007; Raghavachari et al. 2001; Ray and Cole 1985; Sauseng et al. 2005; Scheeringa et al. 2011; Singer 1999, 2011; Tiesinga et al. 2004; Womelsdorf and Fries 2007; Worden et al. 2000). Long-range neural coherence between frontal cortical areas and parietal sensory areas in particular has been reported to be modulated during attentional tasks in monkeys (Buschman and Miller 2007; Gregoriou et al. 2009b) and during sensory detection in humans (Guntekin and Basar 2010; Melloni et al. 2007). Task-relevant coherence across areas has also been observed in rats and cats during tasks that require processing external stimuli (Fries et al. 2008; Gutierrez et al. 2010; Jones and Wilson 2005a, 2005b; Koralek et al. 2013; Pesaran et al. 2008; Siapas et al. 2005; von Stein and Sarnthein 2000), but to our knowledge frontal-parietal coherence has not been investigated in rats during sensory detection behavior.
To better characterize the frontal-parietal network in rats, and further assess their potential applicability to the primate system, we used multielectrode arrays to simultaneously record local field potentials (LFPs) from medio-dorsal frontal and posterior parietal cortex in rats performing a simple auditory detection task that prompted them to respond to an unpredictably timed target tone by licking for water reward. To test the hypothesis that frontal-parietal coherence at particular frequencies is elevated prior to successful detection of signal beeps, we compared frontal-parietal LFP coherence prior to the signal on “hit” trials, which elicited a behavioral response from the rat, with “miss” trials. If high coherence in the frontal-parietal network improves detection of the target tones, prestimulus coherence would on average be higher before hit trials (successful behavioral detection) than before misses (failure to respond to target stimulus). Against this prediction, we observed significantly elevated frontal-parietal coherence before miss trials, in the beta band (15–30 Hz). In addition, local power and interarea coherence at frequencies below 15 Hz were also associated with miss trials. These effects survived a regression to subtract out potential slow changes in coherence associated with waning motivation toward the end of a session, so they reflect trial-to-trial variations in brain state that bias the probability of successful detection.
MATERIALS AND METHODS
Animals
Neurophysiological data were obtained from nine male Long-Evans rats (Rattus norvegicus, 500–700 g; Charles River Laboratories, Wilmington, MA). The animals were housed in pairs (before surgery) or individually (after surgery) on a 12:12-h light-dark schedule (lights on at 6 AM/off at 6 PM). Training took place once per weekday during either a morning (10 AM) or an afternoon (1:30 PM) session. Approximately 1 h after training, rats received free access to water for 15–20 min. Rats were allowed free access to water over the weekend. All rats were weighed daily during water scheduling to ensure that body weight did not drop below 85% of ad libitum weight, as measured after 48 h of free access to water. If this happened, rats temporarily ceased training and were given free access to water until their weight reached their ad libitum weight the previous Sunday. All rats were allowed food ad libitum.
All procedures were approved by the Wellesley College Institutional Animal Care and Use Committee, in accordance with the guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Auditory Detection Task
Behavioral training and LFP recording took place in a standard operant chamber (80003NS, Lafayette Instrument). Rats were trained to lick at a lickometer in order to receive a water reward. Auditory stimuli (2,500 Hz, 75 dBa, 100-ms duration) were generated at randomly varying intervals by ABET-II software (Lafayette). After each beep, the rat was able to obtain water for 3 s, followed by another 3 s in which the rat could keep licking without penalty. Licks that took place in the intertrial interval after this 6-s window were penalized by a 10-s penalty period before a new trial could be generated. Trials during which the rat received water were classified as hit trials, while trials during which the rat did not receive water were classified as miss trials. Training or recording sessions typically lasted 30–60 min.
Multielectrode Array Implantation Surgery
Rats were removed from water scheduling at least 3 days before surgery. For implantation surgery, rats were anesthetized with isoflurane (1–2% in O2) and placed in a stereotaxic apparatus. Chronic 32-microelectrode arrays (Innovative Neurophysiology) were implanted in right frontal (2.0 mm anterior to bregma, 0.75 mm dextrolateral to the midline, and 1.5 mm beneath the brain surface) and right parietal (4.15 mm posterior to bregma, 3.5 mm dextrolateral to the midline, and 1.2 mm beneath the brain surface) cortex. The frontal array was a 2 × 16 grid and the parietal array a 4 × 8 grid, both with an interelectrode spacing of 150 μm and a row spacing of 300 μm. Arrays were fixed in place with dental cement. After surgery, rats were allowed to recover for 1 wk with ad libitum access to food and water. All rats were weighed and assessed for signs of pain daily for 1 wk after surgery.
Electrophysiological Recordings
LFP activity was recorded with a Cerebus Data Acquisition System (Blackrock Microsystems) at a 1,000-Hz sampling rate and band-pass filtered off-line between 0.5 and 250 Hz. Headstage amplifiers were Triangle Biosystems International M62 (input impedance 50 MΩ at 1 kHz; 19 recording sessions) or Blackrock Cereplex digital M64 (input impedance >10 GΩ, 3 pF; 6 recording sessions). The LFPs were referenced to anterior and posterior skull screws that made contact with cerebrospinal fluid. In one coherence analysis (noted in results), frontal and parietal LFPs were rereferenced to the average of the frontal and parietal arrays, respectively. Analysis of LFPs was done in MATLAB (MathWorks) as described below.
Data Preprocessing
Computer memory constraints prevented us from analyzing pairwise coherences among all 64 simultaneously recorded electrodes at once. We sampled every other electrode for our analysis, that is, 31 even-numbered channels from each session. Because channels sometimes malfunctioned in the recording headstage or in the implanted arrays themselves, or rat movements could lead to mechanical recording artifacts, we devised an automated procedure for eliminating bad channels and bad trials. Trials in which the signal exceeded ±1.5 mV or contained a flat line in the time period of interest were discarded. Sixty-hertz line noise was removed with rmlinesc.m from the Chronux Spectral Analysis Toolbox (Bokil et al. 2010). If >35% of trials at a particular electrode were discarded, then the whole channel was discarded from the analysis as potentially corrupt. If <75 trials in a session remained after artifact trials were discarded as described above, then the session was omitted from the analysis. This procedure resulted in the rejection of 11% of recorded LFP channels and 16% of trials on the remaining electrodes for movement or other artifacts, on average, such that the average number of LFPs was 28 and the average number of trials was 231 per session. After data preprocessing, trials were sorted into successful signal detections (hits) and failures to respond (misses).
Power Spectra and Coherence Analysis
Power spectra were calculated at each frontal or parietal electrode for the 500 ms before auditory stimuli with a multitaper Fourier transform (Chronux Spectral Analysis Toolbox; mtspectrumc.m) (Bokil et al. 2010). Spectrograms were also calculated with a sliding 500-ms window in 50-ms steps, comprising 1 s before to 1 s after the auditory target. The time-bandwidth product and number of tapers were set to [5 9] for spectral analysis including the coherence calculations described below. With a 500-ms analysis window and a 1,000-Hz sampling rate, these parameters result in spectra with frequency bins ∼2 Hz wide. We focused our analysis on frequencies up to 50 Hz.
Interarea coherence was calculated for all frontal-parietal electrode pairs for the 500 ms before auditory stimuli with a multitaper Fourier transform (Chronux Spectral Analysis Toolbox; coherency.m) (Bokil et al. 2010). Coheregrams were also calculated with a sliding 500-ms window in 50-ms steps, comprising 1 s before to 1 s after the auditory target.
The difference between the coherence spectrum for hit trials and that for miss trials was calculated with the signal coherence for each trial averaged over interarea electrode pairs.
To compare coherence values on hit and miss trials from individual sessions, we first transformed coherence values with a hyperbolic tangent function so that the values would be normally distributed (Bokil et al. 2007). The transformed coherence values were compared with a two-tailed unpaired t-test to assess whether coherence in each frequency bin on hit trials was significantly different from misses. An analogous procedure was used for testing differences between hit and miss power spectra, except that the spectral power values were transformed by a logarithm (Bokil et al. 2007). To distinguish regions in the time-frequency plots (spectrograms and coheregrams) or count sessions in which higher coherence was associated with hits or misses, respectively, we used one-tailed paired t-tests based on the variability across sessions.
Choice Probability Calculation
To quantify how well coherences averaged across frontal-parietal electrode pairs at each time window of our coheregrams could predict the animal's behavior on individual trials, we applied a receiver operating characteristic (ROC) analysis. This method gives a “choice probability” index (CP) between 0 and 1 that measures how well an ideal observer could predict the animal's behavior given only that coherence value. Most commonly used to analyze the activity of single neurons, choice probability allows for a direct connection of neural behavior with the subject's choice, without assumptions about the shape of the distribution of the neural responses (Britten et al. 1996). A CP of 0.5 means that the observer would perform at chance. A CP significantly greater than 0.5 means that greater coherence predicts hits, while a CP significantly less than 0.5 indicates that lower coherence predicts hits (i.e., higher coherence predicts misses). To determine whether CP values deviated significantly from chance, we ran a permutation test (n = 2,000). On each permutation, CP values were calculated from the coherence on trials randomly assigned as hits or misses. Experimental CP values were determined to be significant if they were outside the 95% confidence intervals for that session. All CP values were calculated following a hyperbolic tangent (Bokil et al. 2007) and z-score correction of the coherence values (Britten et al. 1996).
Cross-Frequency Coupling
Cross-frequency amplitude correlations.
To assess potential inhibitory coupling between the power of alpha- and gamma-band interarea coherence, which has been reported in local power spectra (Buffalo et al. 2011; Jensen and Mazaheri 2010), we calculated matrices of linear correlation coefficients between each pair of frequency bins based on the trial-to-trial variability of prebeep coherence spectra in each session and cumulated the results across sessions. These were based on mean-subtracted coherences, so the result was a symmetrical covariance matrix whose elements ranged from 0 to 1. The significance of the correlation coefficient at each frequency pair in individual sessions was assessed at the 95% confidence level based on the variability across trials, and the numbers of sessions with significantly positive or negative correlation were counted at each frequency pair.
Cross-frequency phase-amplitude comodulation.
To assess the possibility that the phase of slow oscillations might modulate the power of higher-frequency oscillations (Canolty et al. 2006; Scheffer-Teixeira et al. 2012; Tort et al. 2008, 2009), we also calculated comodulograms that plot an established modulation index (MI) for each high-low frequency pair (Tort et al. 2008). This analysis comprised four cases: 1) local phase-amplitude comodulation in frontal cortex, 2) local phase-amplitude coupling in parietal cortex, 3) interarea comodulation of frontal high-frequency amplitude by parietal low-frequency phase, and 4) interarea comodulation of parietal high-frequency amplitude by frontal low-frequency phase. MI at each phase-frequency amplitude-frequency pair was calculated following Tort et al. 2008 (see also Tort et al. 2010). Briefly, phase is extracted from the phase-LFP by using a Hilbert transform on the band-pass filtered LFP, and amplitudes of the amplitude-LFP at each frequency bin are histogramed with respect to phase from the phase-LFP. MI is then calculated as a normalized entropy of the phase histogram, which reflects how nonuniform the phase histogram is (i.e., flat vs. peaked) by a number between 0 (constant amplitude across phase bins) and 1 (strongest possible phase-to-amplitude modulation). We assessed the statistical significance of MI values at each phase-amplitude frequency pair based on an empirical null distribution of 600 trial-shuffled composite time series of phase and amplitude values for each trial from an interarea electrode pair in each session. MIs greater than the 95th percentile of the empirical null distribution were considered significant in a given session, and significant MIs at each phase-amplitude frequency pair were counted across sessions.
RESULTS
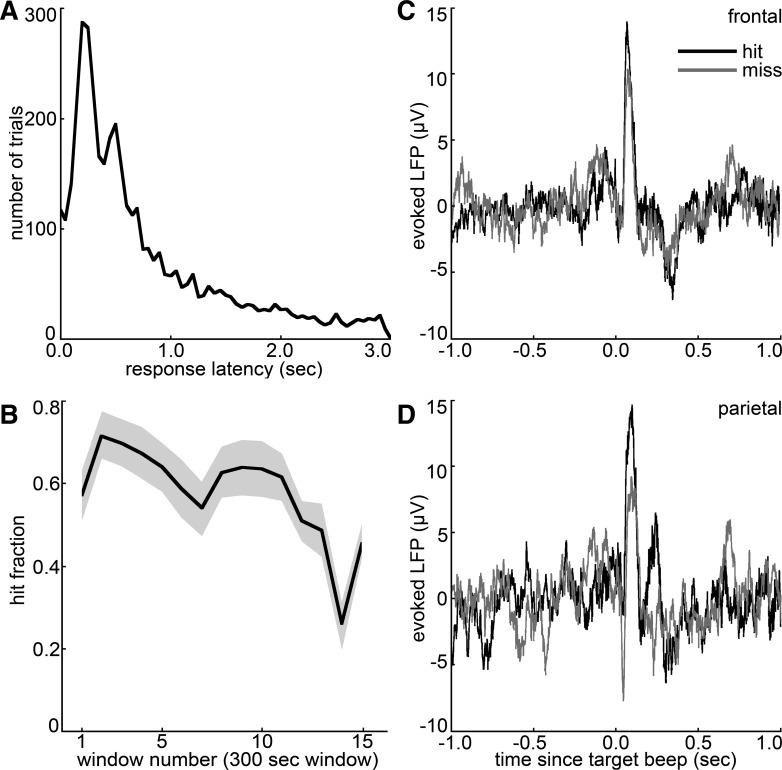
To test the hypothesis that increased frontal-parietal LFP coherence improves detection of auditory stimuli, LFPs were recorded in medio-dorsal frontal and posterior parietal cortical areas as nine rats performed an auditory detection task in 25 separate sessions. Because the target tones were presented unpredictably, the peaked response latency distribution shows that rats were actively responding to the beep event at time 0 (Fig. 1A). Because licks during the intertrial interval were punished by a 10-s time-out, there were no licks prior to 0 during any of the hit trials shown in the response latency histogram of Fig. 1A. The rats' performance during recorded sessions averaged 56% correct responses (SD 25%); the fraction of hit trials in a moving 5-min window is shown in Fig. 1B, averaged across sessions. Evoked frontal and parietal LFPs (Fig. 1, C and D) show that these areas responded to the target stimulus in the context of the signal detection task, tending to evoke larger-amplitude responses on hit trials, which is consistent with a role for these cortical areas in registering the stimulus, coordinating the learned licking response, and registering a water reward.
Fig. 1.
Auditory detection task and evoked local field potentials (LFPs). A: response latency histogram (50-ms bins) combining all 25 behavioral recording sessions. Response latencies represent the time between the signal beep and the first subsequent lick. Peaked response latency distributions demonstrate nonrandom licking; rats generally responded within 1 s of unpredictably timed auditory cues during the detection task. B: fraction of hit trials in sliding 5-min windows, averaged over recorded detection sessions. Shading denotes SE over sessions; 5 sessions lasted until the 15th window (70–75 min) or longer. C and D: session-averaged frontal (C) and parietal (D) LFPs triggered on the target tone, for hit and miss trials separately.
Coherence Spectra
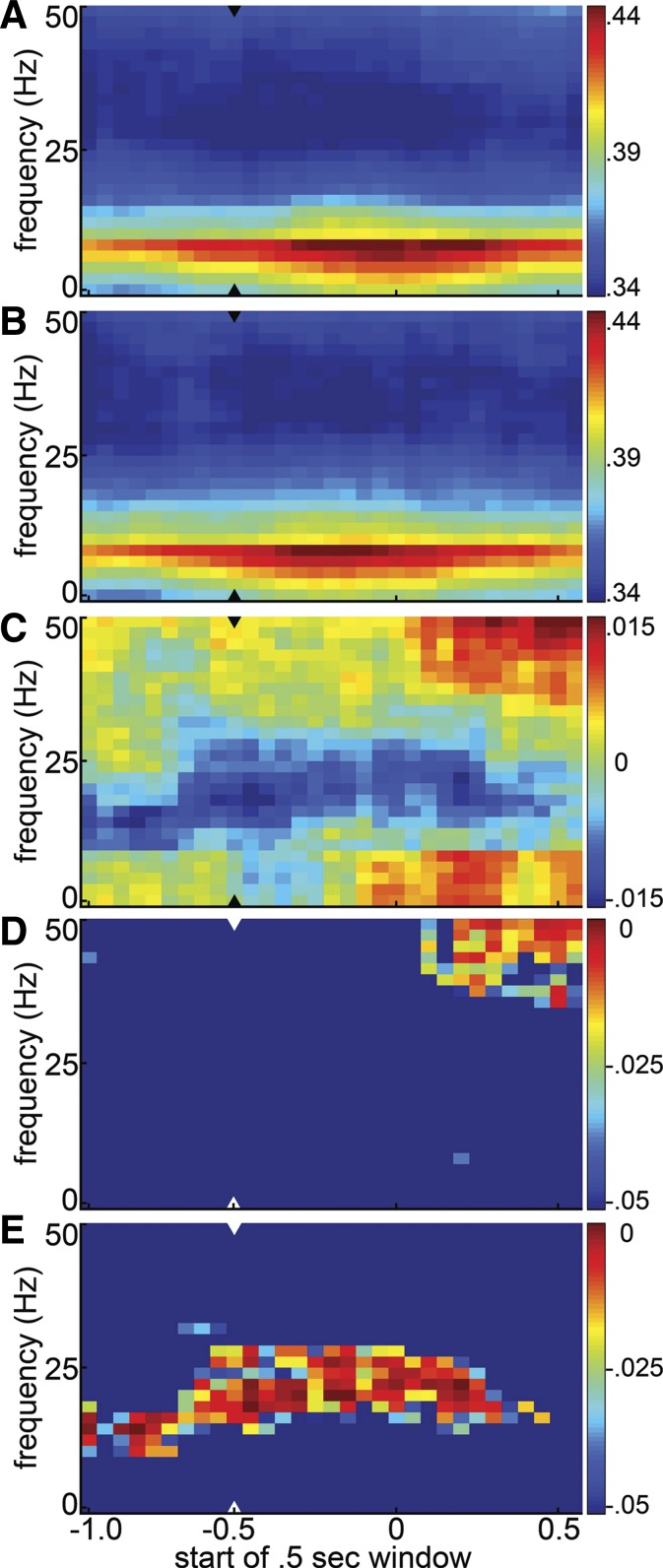
To determine whether trial-to-trial variations in frontal-parietal LFP coherence are associated with rats' detection of an auditory tone, interarea coheregrams were calculated based on single-trial coherences averaged across frontal-parietal electrode pairs in each session. The single-trial coheregrams were averaged over hit and miss trials in each session, and these hit and miss coheregrams were averaged across sessions to generate the average hit and miss coheregrams shown in Fig. 2, A and B. These plots show that both before and after the target stimulus coherence between the frontal and parietal LFPs is dominated by lower frequencies, peaking in the theta and alpha range. The difference coheregram (Fig. 2C) shows a trend toward elevated coherence at gamma frequencies and at alpha frequencies and below on hit trials compared with misses. These trends are noticeably weaker prior to the signal beep (the last entirely prebeep window starts at −0.5 s), so their increase after the beep could reflect coherence induced by the licking response on hits, or motor preparation for that response. A trend toward higher coherence in the beta range on miss trials, on the other hand, is of similar magnitude before and after the time of the beep. Figure 2, D and E, show which cells of the difference coheregram are significantly positive, indicating greater coherence on hit trials (Fig. 2D), or negative, indicating greater coherence on miss trials (Fig. 2E).
Fig. 2.
Session-averaged evolution of frontal-parietal coherence on hit vs. miss trials. A and B: average coheregrams for hit (A) and miss (B) trials. Coherence spectra were calculated in 500-ms windows shifted in 50-ms steps, averaged across interarea electrode pairs and then across hit and miss trials separately. The hit and miss coheregrams from each session were then averaged (n = 25) to form the grand coheregrams shown. As the x-axis denotes the start of each half-second window, the last prestimulus window is at −0.5, marked by the arrowheads. C: hit-miss difference coheregram: the difference between the hit and miss coheregrams shown in A and B. D and E: statistical significance and sign of differences between the average hit and miss coheregrams is shown as the P value of a 1-tailed t-test asking whether hit coherence was significantly greater than miss coherence (D) or whether miss coherence was greater than hit coherence (E). Hotter colors indicate greater statistical significance (i.e., smaller P values). In each panel the arrowhead denotes the last prestimulus window.
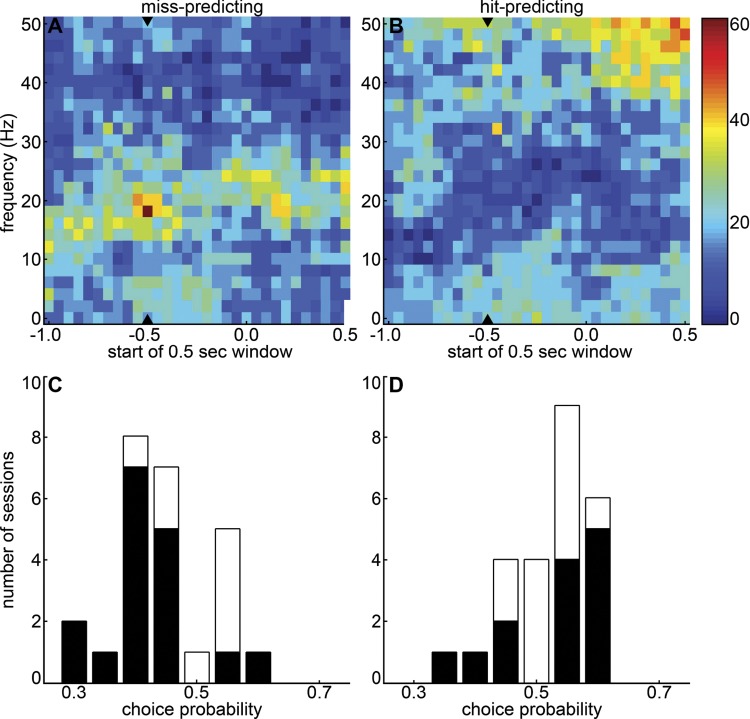
To quantify the extent to which prestimulus frontal-parietal coherences on individual trials could enable an ideal observer to predict hits and misses, we applied a choice probability analysis to the coheregram data (Fig. 3). As in the grand average coheregrams (Fig. 2), the most consistent differences between hit and miss coherences in the beta range were observed roughly between 15 and 25 Hz (Fig. 3, A and B). In almost two-thirds of sessions, elevated frontal-parietal coherence near 18 Hz in the half-second before the target tone predicted miss trials, as evidenced by CPs significantly different from 0.5 (Fig. 3A; P < 0.05 by permutation test; see materials and methods). At the same time-frequency bin, high coherence predicted hits in <10% of sessions (Fig. 3B). Consistent with the nonsignificant trend in the grand coheregrams, poststimulus coherence at theta, alpha, and gamma frequencies predicts hit trials in a substantial fraction of sessions, possibly reflecting neural correlates of licking and reward consumption (Fig. 3B).
Fig. 3.
Choice probability analysis of frontal-parietal coherence. To quantify the extent to which single-trial frontal-parietal coherences predict hit or miss trials, choice probabilities (CPs) were calculated based on coherence in each time-frequency bin (500-ms time windows, shifted by 50 ms), averaged over frontal-parietal electrode pairs. A and B: significant difference of CPs from 0.5, indicating that high coherence predicts hit or miss trials, was calculated with a permutation test (P < 0.05, see materials and methods). Color represents % of sessions in which high coherence at each time-frequency bin significantly predicted misses (A) or hits (B). Arrowheads denote the last completely prestimulus half-second window. C and D: distribution of CPs across sessions at 18 Hz for the last prestimulus window (C) and at 46 Hz for the penultimate prestimulus window (−0.45 to −0.05 s) (D), with CPs significantly different from 0.5 in black. A CP significantly greater than 0.5 means that greater coherence predicts hits, while a CP significantly less than 0.5 indicates that higher coherence predicts misses.
Figure 3C shows the distribution of CPs across sessions at the 18-Hz bin in the half-second before the target tone. In this time-frequency bin most of the significant CPs are <0.5 (meaning high coherence predicts misses, 17 sessions), while the sessions with CP significantly greater than 0.5 are few (high coherence predicts hits, 2 sessions) and closer to 0.5. In contrast, Fig. 3D shows that significant CPs at a prestimulus gamma bin are more likely to be >0.5, with nine sessions in which high prestimulus gamma coherence predicted hits (4 sessions showed the reverse), supporting the trend in the grand coheregrams (Fig. 2).
To sidestep difficulties associated with interpreting poststimulus activity, when movement-related activity could dominate or obscure sensory-related activity, we focused a more detailed analysis on the last time window before the beep, marked by the arrowheads in Figs. 2 and 3.
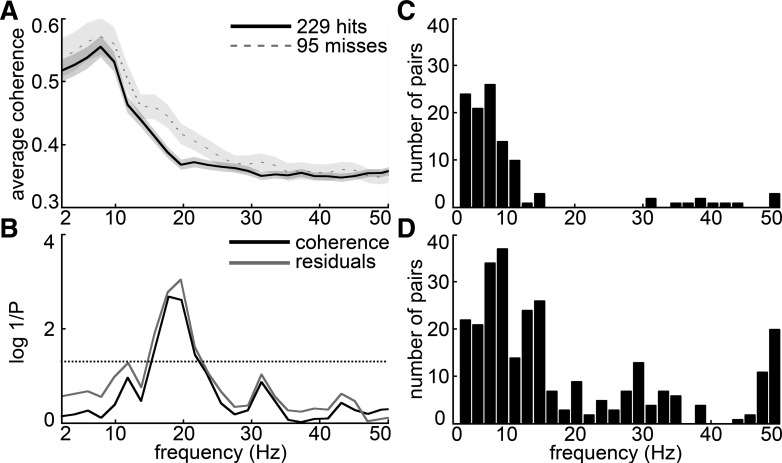
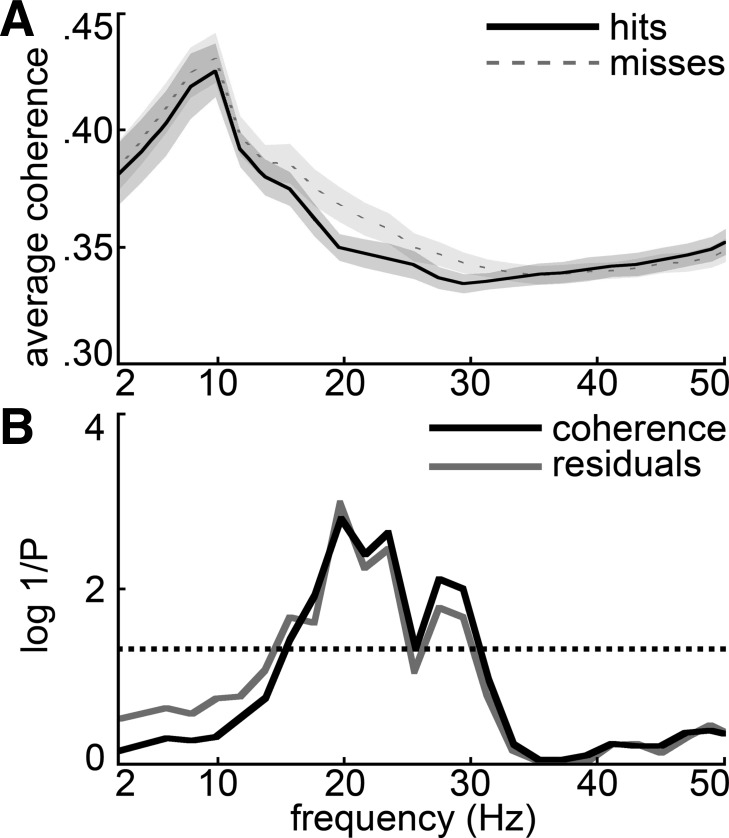
Figure 4A shows frontal-parietal coherence spectra from a typical session averaged over frontal-parietal electrode pairs and then averaged over hit and miss trials separately, for the 500-ms window just prior to the target tone. The coherences on both hit and miss trials tend to have the highest magnitudes at frequencies below 10 Hz. In this session, frontal-parietal coherence was significantly greater between 15 and 20 Hz prior to miss trials compared with hits, as shown by the black P value spectrum in Fig. 4B. Of 25 recorded detection sessions, 11 showed significantly greater (P < 0.05, by 1-tailed t-test after tanh correction; see materials and methods) coherence on misses than hits at 20 Hz in this prestimulus half-second window. Consistent with the effect in the grand coheregrams (Fig. 2), no sessions were found to have significantly greater 20 Hz coherence prior to hits. In the gamma frequency range, few sessions showed significant differences between hits and misses in the prebeep window (i.e., a maximum of 3 sessions at 38 Hz showed significantly higher hit vs. miss coherence, while 3 other sessions showed the reverse). In contrast, the picture was more mixed at frequencies below the beta range; for example, at 8 Hz seven sessions had significantly higher coherence prior to misses (consistent with the trend in the grand coheregrams) but four sessions showed the reverse. At a theta bin (6 Hz), five sessions showed the reverse: higher coherence prior to hits rather than misses.
Fig. 4.
Prestimulus hit and miss coherence spectra for an example session and individual electrode pair analysis. A: the average coherence spectrum across all frontal-parietal electrode pairs for 1 detection session is plotted for hits and misses. Shading denotes SE based on the variability across trials. B: the P value for a 2-tailed unpaired t-test comparing hit and miss coherences (after tanh transformation, see materials and methods) is plotted as log(1/P) (darker curve) so that higher plotted values indicate more significant (smaller) P values. To account for potential changes in coherence associated with slow changes in rats' motivation, the lighter curve shows the analogous P values for a hit-miss comparison based on residuals of a linear regression on coherence values during each session (i.e., after slow changes have been subtracted out). Frequency bins where either curve exceeds the dotted line were significant at the 95% confidence level; similarly, bins where the curve exceeds 2 on the y-axis were significant at the 99% confidence level [since log(1/0.01) = 2]. C and D: total number (across 25 sessions) of individual frontal-parietal electrode pairs at each frequency bin that showed prestimulus hit coherence significantly greater than miss coherence (C) or vice versa (D), according to a stringent Bonferroni-corrected criterion (t-test, uncorrected α = 0.05).
The average sliding-window hit fraction plotted in Fig. 1B reflects the fact that rats did not always respond to the target tone at a constant rate throughout a session. In some sessions the rat's response rate slowed gradually during a session, or tapered off toward the end, possibly reflecting satiation or fatigue. The significant difference in the beta range between hit and miss coherences (Fig. 4A) could be due to slow changes in motivation, if waning motivation to respond were associated with increased beta coherence in the later part of such a session, when miss trials are more common. To control for this possibility, we regressed away any linear trend in coherence over the course of each session separately in each frequency bin. This should eliminate from each session any slow trend in coherence that might be associated with slow changes in motivation. We then compared the residuals of this coherence regression on hits and misses and generated a spectrum of P values representing the significance of the hit-miss difference in the spectrum of coherence residuals. The lighter curve in Fig. 4B shows that the spectrum of P values for the hit-miss comparison on the coherence residuals is essentially equal to the unregressed result, for this session. That is, even after slow linear changes in coherence through the session have been subtracted out, the data show a significant excess of beta coherence prior to miss trials.
Averaging coherence across electrode pairs within a session, as in the above analyses, could obscure significant effects on particular pairs. To assess this possibility we also calculated the significance of hit-miss coherence differences at individual electrode pairs, using the conservative Bonferroni procedure to correct for multiple statistical comparisons (Dunn 1961). Because the average number of interarea (i.e., frontal-parietal) electrode pairs per session was ∼200, our corrected criterion corresponding to an uncorrected α of 0.05 was of order 0.05/200 = 0.00025. Figure 4, C and D, show the total number of electrode pairs for which hit coherence was significantly greater than miss coherence (Fig. 4C) or miss coherence was greater than hit coherence (Fig. 4D) in each frequency bin, summed over all 25 recording sessions. The stringent Bonferroni criterion resulted in very few pairs showing a significant difference between and miss coherence in the prebeep window, even in the beta range where we observed the most consistent hit-miss coherence differences (see above and session-averaged results below).
When we averaged the prebeep coherence spectra over all sessions, they showed a profile similar to the typical session of Fig. 4A (Fig. 5). That is, the grand average shows a significant excess of beta-frequency coherence prior to miss trials (Fig. 5A). The P value spectrum in Fig. 5B (black curve) shows that the excess coherence prior to miss trials is statistically significant between 15 and 30 Hz. Significant average hit-miss coherence differences were absent in a 500-ms control window beginning 1.95 s before the target beep. The gray curve in Fig. 5B shows that the excess frontal-parietal beta coherence prior to miss trials persists and is undiminished when slow changes in coherence that might be related to changes in motivation have been subtracted.
Fig. 5.
Session-averaged prestimulus frontal-parietal coherence spectra. A: separate frontal-parietal coherence spectra for hit and miss trials were calculated for each session based on the 500 ms prior to each signal beep and then averaged over the 25 sessions. The variability of these average coherence spectra across sessions is indicated by the shading denoting SE calculated from variability of the hit and miss coherence spectra across sessions. B: the P value for a 2-tailed t-test comparing hit and miss coherences (see materials and methods) is plotted as log(1/P) (darker curve) so that higher plotted values indicate more significant (smaller) P values. To account for potential changes in coherence associated with slow changes in rats' motivation, the lighter curve shows the analogous P values for a hit-miss comparison based on residuals of a linear regression on coherence values during each session (i.e., after slow changes have been subtracted out). Frequency bins where either curve exceeds the dotted line were significant at the 95% confidence level; similarly, bins where the curve exceeds 2 on the y-axis were significant at the 99% confidence level [since log(1/0.01) = 2].
We also ran the analysis on LFPs rereferenced to the average signal across each (frontal or parietal) array; in this case the premiss excess coherence was highly significant at all frequencies below 28 Hz (2-tailed P ∼0.01 or less; not shown). Thus the prestimulus effects were broader in frequency and more highly significant on rereferencing, which suggests that the prestimulus effects we observed were specific to the areas we recorded from rather than deriving from global states captured by our grounding skull screw. The rereferencing may also have eliminated some common noise across each array; however, we use the original ground-referenced LFPs for all other analyses because rereferencing also eliminated most of the evoked (i.e., poststimulus) response, which was also similar across electrodes on each array.
To determine whether frontal-parietal coherence predicted response latency on hit trials, we compared coherence spectra on fast vs. slow trials by breaking the response latencies into halves, thirds, or quarters and comparing prestimulus spectra from the fastest and slowest groups. The analysis did not reveal significant differences but did show a nonsignificant trend toward higher coherence prior to hits in the gamma range (30–40 Hz, 2-tailed t-test P = 0.12 near 35 Hz; data not shown).
Cross-Frequency Coupling
Cross-frequency amplitude correlations.
To assess potential inhibitory coupling between the power of alpha and gamma interarea coherence, which has been reported for local power spectra (Buffalo et al. 2011; Jensen and Mazaheri 2010), we calculated matrices of linear correlation coefficients between each pair of frequency bins based on the trial-to-trial variability of prebeep coherence spectra in each session and cumulated the results across sessions. The session-averaged cross-frequency covariance matrix showed that nearby frequency bins tended to be correlated, as expected in natural data. In addition, frequencies below ∼10 Hz tended to be highly correlated and frequencies above ∼10 Hz tended to be more correlated with each other than with frequencies below 10 Hz. This covariance structure thus suggests that frequencies above and below ∼10 Hz represent distinct functional bands.
Session-averaged cross-frequency power correlation coefficients for frontal-parietal coherence were near 0 or positive for all frequency combinations. To evaluate the statistical significance of cross-frequency coherence correlations in individual sessions, we calculated P values for each correlation coefficient at a 95% confidence level. Ten of twenty-five sessions showed significant positive correlation between high beta (20–25 Hz) and gamma (30–50 Hz), and five of twenty-five sessions showed significant positive correlation between theta or alpha (4–12 Hz) and gamma. Only two sessions showed significant negative correlation, between alpha (∼12 Hz) and gamma. Thus negative correlations between low and high frequencies were the exception rather than the rule in our experimental context.
Cross-frequency phase-amplitude comodulation.
To assess the possibility that the phase of slow oscillations might modulate the power of higher-frequency oscillations (Canolty et al. 2006; Scheffer-Teixeira et al. 2012; Tort et al. 2008, 2009), we also calculated comodulograms that plot a phase-amplitude “modulation index” (MI) for each high-low frequency pair (see materials and methods). This analysis comprised four cases: 1) local phase-amplitude comodulation in frontal cortex, 2) local phase-amplitude coupling in parietal cortex, 3) interarea comodulation of frontal high-frequency amplitude by parietal low-frequency phase, and 4) interarea comodulation of parietal high-frequency amplitude by frontal low-frequency phase.
The analysis of local phase-amplitude coupling showed similar trends in frontal and parietal cortex. The power of frequencies above 30 Hz tended to be modulated by the phase of frequencies below 10 Hz, with the strength of modulation increasing monotonically at lower frequencies in the average across sessions. To further assess the strength and consistency of the trends we observed in individual sessions, we calculated the statistical significance of comodulation indexes at each amplitude-frequency phase-frequency pair, using a significance threshold based on an empirical null distribution (95% confidence level; see materials and methods). In 10 of 25 sessions the frontal modulation index did not increase monotonically with decreasing phase-frequency but instead peaked at phase-frequencies in the theta or beta band. The greatest number of significant single-session local phase-amplitude comodulation effects in both frontal and parietal cortex was five sessions, for modulation of frontal gamma amplitudes (near 40 Hz) by parietal theta rhythm in the 5–7 Hz range. In parietal cortex 9 of 25 sessions showed comodulograms with peaks in the theta, alpha, or beta range of phase-frequency instead of monotonically decreasing comodulation with increasing phase-frequency.
The analysis of interarea modulation of frontal gamma amplitude by parietal phase showed monotonically increasing comodulation with decreasing parietal phase-frequency in the average over sessions. In contrast to the pattern in the session average, in 10 of 25 individual sessions the comodulation was not monotonic but instead had a peak with respect to parietal phase-frequency between 5 and 10 Hz (9 sessions) or between 12 and 16 Hz (1 session). To further assess the strength and consistency of the trends we observed in individual sessions, we calculated the statistical significance of comodulation indexes at each amplitude-frequency phase-frequency pair, using a significance threshold based on an empirical null distribution with a relatively liberal criterion (α = 0.05; see materials and methods). The greatest number of significant single-session comodulation effects was five sessions, for modulation of frontal gamma amplitudes (30–55 Hz) by parietal theta rhythm in the 4–5 Hz range. Thus, although we observed a trend toward comodulation of frontal gamma amplitude by parietal theta phase in the session average and in some individual sessions, we conclude that the comodulation was not usually strong, nor was it consistent across rats.
Finally, we consider the modulation of parietal gamma amplitude by low-frequency frontal phase. The average across sessions again showed parietal gamma comodulation increasing monotonically with decreasing frontal phase. Again, 10 of 25 sessions instead showed peaked comodulograms, with peaks in the theta, alpha, or beta band of phase-frequency. We assessed statistical significance of effects in individual sessions as above and found a maximum of five significant sessions for modulation of parietal gamma (30–35 Hz) by frontal theta and alpha between 5 and 14 Hz. Thus, as above, although we observed a trend toward comodulation of parietal gamma by frontal theta or alpha phase in the session average and in some individual sessions, we conclude that the comodulation was not robust in our experimental context.
Power Spectra
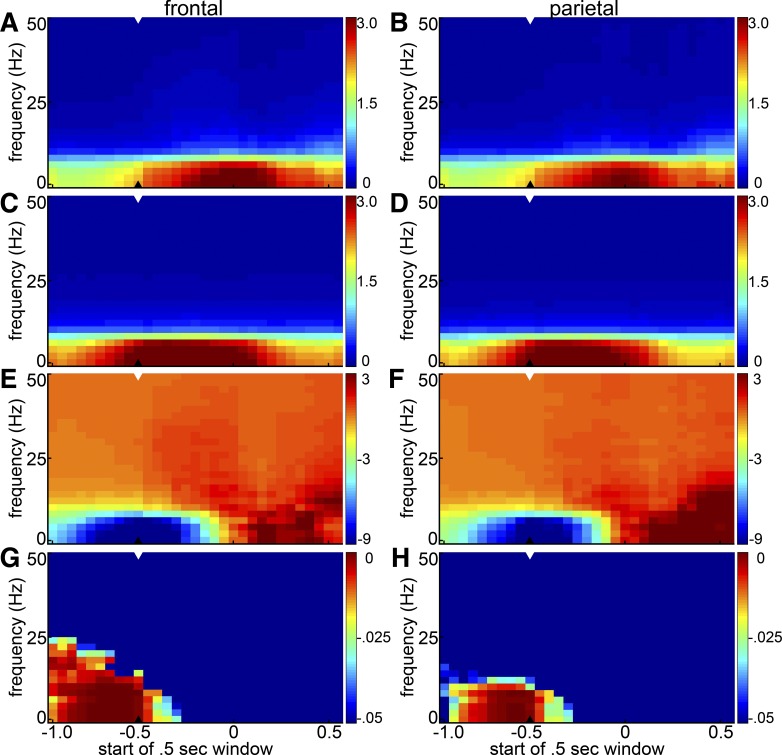
Although local spectral power can in principle vary independently of coherence with another area, it seems natural that the increased frontal-parietal beta coherence before miss trials might be related to increased beta power in frontal or parietal cortex. To also determine whether trial-to-trial variations in frontal or parietal spectral power are associated with rats' detection of an auditory tone, power spectrograms on hit and miss trials were calculated from frontal and parietal LFPs (Fig. 6, A–D). As with the coherence spectra, the power spectra were largest at low frequencies. The drop-off in power with increasing frequency was, however, steeper than the drop in coherence at higher frequencies. As in the average coheregrams of Fig. 2, low-frequency activity in the later poststimulus period tended to be associated with hit trials (Fig. 6, E–H). In the half-second window prior to stimulus presentation, the excess power on miss trials was significant at sub-beta frequencies (<10 Hz in frontal and parietal cortex; Fig. 6, E–H). The excess beta synchronization (15–30 Hz) associated with miss trials in the coherence spectra was not observed in the power spectra. We also applied a cross-frequency coupling analysis to assess potential inhibitory coupling between alpha and gamma power in frontal and parietal cortex, but as with the coherence data we found no consistent cross-frequency antagonism (not shown). A comparison of power spectra on fast and slow response trials likewise failed to reveal significant effects of prestimulus power on response latency.
Fig. 6.
Session-averaged hit and miss spectrograms for frontal and parietal cortex. Power spectra were calculated in 500-ms windows shifted in 50-ms steps, averaged across frontal and parietal electrodes separately and then across hit and miss trials separately. A: average frontal hit spectrogram. B: parietal hit spectrogram. C: frontal miss spectrogram. D: parietal miss spectrogram. Arrowheads indicate the last prestimulus time-window. Color represents power in (nV)2 × 10−4. E and F: difference between hit and miss spectrograms (hit − miss) for frontal (E) and parietal (F) electrodes. Color represents power in (nV)2 × 10−5. G and H: statistical significance of differences between the average hit and miss spectrograms is shown as the P value of a 1-tailed t-test asking whether miss power was greater than hit power for frontal (G) and parietal (H) electrodes. Hotter colors indicate greater statistical significance (i.e., smaller P values). (There were no significant time-frequency bins for the converse question asking whether hit power was greater than miss power, so the results are not shown.) In each panel the arrowhead denotes the last prestimulus window.
DISCUSSION
To test the functional role of frontal-parietal neural coherence during performance of a simple auditory detection task with unpredictably timed target stimuli, we analyzed simultaneously recorded medial dorsal frontal and posterior parietal LFPs from 25 detection sessions in nine rats. Low-frequency coherence (<15 Hz, Fig. 2) and gamma coherence (>30 Hz, Fig. 2) tended to be elevated after stimulus onset during successful signal detection, consistent with a role for these frequencies in coordinating frontal-parietal activity during perception of the target tone, selecting and executing the licking response, and processing the water reward. We also observed significant coherence differences between successful hit trials and misses prior to the onset of the target tone.
Elevated Prestimulus Coherence at Low Frequencies (<15 Hz) Is Associated with Misses
A trend toward higher low-frequency coherence prior to miss trials in the raw data became highly significant (P ∼0.01 or lower) when we reran the analysis with LFPs referenced to the mean voltage on the frontal and parietal electrode arrays respectively (Fig. 5; the rereferencing may have eliminated some noise common to electrodes on each array). In other words, elevated prestimulus theta or alpha coherence (4–15 Hz) between frontal and parietal cortex predicted miss trials (Figs. 2, 3, and 5), and this effect was undiminished after subtracting away linear trends in coherence across the duration of each session (Fig. 5B). Although the average coherence effect size was not large in absolute terms (∼0.02), small differences in pairwise neural correlations can reflect large functional differences in network state (Schneidman et al. 2006). The fact that rereferencing LFPs to the array average enhanced the prestimulus coherence excess before miss trials suggests that the effect is specific to the recorded areas rather than being inherited from the ground screw, which might reflect relatively global activity. Similarly, comparing the LFP power spectra from the half-second before signal tones, frontal and parietal activity associated with miss trials exhibited higher power in frequencies below the beta range (<15 Hz) than activity associated with hit trials (Fig. 6).
Because these prestimulus effects cannot be due to licking or reward processing, they might be interpreted in terms of states of arousal or vigilance that bias the probability of a correct behavioral response or in terms of a motor tendency or preparation to lick.
In humans, low-frequency theta (4–8 Hz) and alpha (8–13 Hz) power can be associated with drowsiness or relaxation (Cahn and Polich 2006), and the slow waves of deep sleep fall in the delta range (<4 Hz). During awake states of arousal delta, theta, and alpha synchrony are also associated with attention to internal representations such as working memory (Cooper et al. 2003; Palva and Palva 2007; Raghavachari et al. 2001; Ray and Cole 1985; Sauseng et al. 2005), and alpha/mu rhythms in particular have frequently been related to a local idling state of inattention or active inhibition of attention to external stimuli in specific brain areas (Bollimunta et al. 2011; Buffalo et al. 2011; Hanslmayr et al. 2007; Jones et al. 2010; Kelly et al. 2009; Macdonald et al. 2011; Mazaheri et al. 2011; Thut et al. 2006; van Dijk et al. 2008; Worden et al. 2000). Similar observations have related high 7- to 12-Hz cortical LFP power in rats to disengagement from external stimuli (Fontanini and Katz 2005), although as in humans the prominent alpha-range oscillations do not preclude behavioral detection of stimuli (Wiest and Nicolelis 2003). Thus in humans and rats low-frequency cortical LFP power appears to be negatively correlated with attention to external stimuli. Indeed, the arousal state of rabbits transitioning from a nonalert to an alert state has been indexed by the relative amplitude of low-frequency EEG activity (Bereshpolova et al. 2011). The amount of low-frequency power has also been used as an index for arousal state in rats (Hattori et al. 2010).
Nevertheless, it is not a general rule that low-frequency synchronization implies inattention to external stimuli, as it is well established that theta rhythms are involved in navigation including cognitive representations of the external environment in human cortex (Kahana et al. 1999; Raghavachari et al. 2001) and in monkeys (Lee et al. 2005) and rats (Buzsaki 2005). These cognitive theta rhythms must be distinguished from the sleepy rhythms that manifest in the same frequency range. Task-relevant interarea coherence in this range has been demonstrated in rats, in tasks that depend on external cues for success (Jones and Wilson 2005b; Koralek et al. 2013; Siapas et al. 2005). Thus increases in the amplitude of hippocampal theta rhythm have also been interpreted as increases in arousal in rabbits (Swadlow and Weyand 1985). Moreover, the potential significance of theta synchronization for sensory processing is not limited to spatial navigation; for example, it has been shown to improve learning of stimulus associations in a nonspatial trace conditioning paradigm (Griffin et al. 2004). These varying associations of low-frequency oscillatory synchronization with cognitive functions point to the importance of considering specifics of the experimental context, since electrode referencing, brain area and tissue layer, and task details can qualitatively change the results.
Nevertheless, the literature on alpha and theta rhythms supports interpretation of our present results in terms of states of arousal or vigilance rather than in motor terms. We have already implicitly distinguished trial-to-trial fluctuations in response probability from slower state changes that could affect our rats' varying detection performance during a session, when we regressed away linear trends in coherence across each session. Since our rats' hit rates did sometimes decline toward the end of a session (Fig. 1B), we considered motivation to be the factor that most plausibly might vary systematically during a session, if the animals became sated with water or fatigued, for example. Although attention and motivation can interact in subtle ways (Engelmann et al. 2009), our regression control analysis unambiguously assigns the prestimulus coherence effect to trial-to-trial variations in response probability, on timescales on the order of seconds to minutes, as opposed to monotonic changes throughout a session. Therefore we interpret our results in terms of lapses in arousal, vigilance, or sustained attention, which bias the probability of correctly responding, as opposed to slow changes in the motivation to respond appropriately in order to get water. Additional support for this interpretation comes from a human EEG study that reported fluctuations in alpha power on timescales of minutes that mirrored changes in attentional state (Macdonald et al. 2011)—this result is comparable to what we observed in the alpha range both in terms of the timescale of fluctuations and in terms of the attentional effect of alpha coherence (see below).
One aspect of attentional performance is the degree to which it can be sustained over time; another, independent aspect of attentional function is its degree of selectivity. Moderate levels of “arousal” refer to an awake brain state capable of responding, and “vigilance” or “alertness” tend to refer to a relatively nonselective responsivity compared with more “selective attention” in which specific responses must be chosen appropriate to particular stimuli. Our simple detection task does not establish the level of selective attention studied in typical human (Nicholls et al. 2002; Ortiz et al. 1993) or monkey (Buschman and Miller 2007; Chalk et al. 2010) attention studies, although it has been shown that rats can exhibit sustained selective attention comparable to humans in matched detection tasks (Bushnell et al. 2003). On the other hand, the rats' behavior in our simple detection task is not consistent with indiscriminate, nonselective responding, as evidenced by the peaked response time distribution in Fig. 1A. Moreover, we have observed that tapping on the door to the behavioral chamber while a rat is performing the task generally causes the rat to look toward the door rather than to lick reflexively; this further supports the idea that the rats are not licking indiscriminately in response to any sound. Thus we consider miss-hit differences in our task to represent trial-to-trial fluctuations or lapses in arousal or vigilance of a sort with relatively little selectivity. Situating our task within this space of attentional selectivity and duration is pertinent because cortical stimulus processing can be qualitatively different when rats are engaged in an active sensory task compared with when they are awake (i.e., aroused to some extent) but not engaged in a task (Otazu et al. 2009). Specifically, that study found that task engagement suppressed auditory responses, in contrast to the effect of selective attention, which enhanced the sensory responses (Otazu et al. 2009). Compared with analogous effects in the literature (Macdonald et al. 2011), the timescale of the alpha effects we observed supports the idea that they are correlates of vigilance or sustained attention, as does the (nonsignificant) trend toward faster responding that we noted in association with higher prestimulus gamma (30–50 Hz) coherence, but it is conceivable that oscillatory variations in arousal state on timescales of minutes could also account for our results. Further experiments will be needed to establish definitively whether the hit-miss differences we observed represent trial-to-trial fluctuations in alertness that would disappear under a more demanding task or correlates of voluntary sustained attention that would persist or increase with increases in task difficulty [see also Gamma frequencies (>30 Hz) below].
Thus, in the context of our simple detection task in rats, both local synchronization (LFP spectral power) and frontal-parietal coherence at sub-beta frequencies appear to represent a state of relative inattention to the target stimuli. The relative lack of attention represented by increased prestimulus low-frequency coherence could be due to involuntary dips in alertness or could reflect top-down inhibition of responding to avoid a time-out with no possibility of reward (Broussard and Givens 2010; Klimesch et al. 2007). Overall, our alpha-band frontal-parietal coherence results extend to long-range frontal-parietal coherence in rats the analogous results of a human EEG study that reported fluctuations of local alpha power that mirrored fluctuations in attention on a timescale on the order of minutes (Macdonald et al. 2011).
However, our results cannot rule out the possibility that low-frequency frontal-parietal coherence facilitates frontal-parietal communication and sensory attention in some circumstances, or at specific pairs of frontal-parietal locations. For example, at the frequency bin near 4 Hz, 5 of 25 sessions showed exhibited significantly more frontal-parietal coherence prior to hit trials. It is possible that in these sessions the rats were in a more “engaged” state of alertness, such that theta coherence increased the probability of hits, whereas in other sessions the rats were in a distinct state of arousal such that increased theta coherence antagonized correct responding. That global brain state can reverse the sign of attentional effects is attested to by a study of auditory responses in rat cortex in different behavioral contexts (Otazu et al. 2009).
Similarly, it is possible that a more demanding task requiring discrimination among competing alternatives or detection of near-threshold sensory events would force the animal into a more highly attentive state in which theta (for example) coherence would contribute more consistently to improving the chance of a successful hit trial.
Another possibility is that task-relevant coherence at these frequencies is found between specific sites, so that our averaging across frontal and parietal electrode locations obscures more localized effects. Our results do not strongly support this possibility, but we did identify multiple frontal-parietal electrode pairs showing significantly elevated coherence prior to hits under a stringent Bonferroni-corrected statistical criterion (Fig. 4). A recent demonstration of task-relevant cortico-striatal spike field coherence that was specific at the level of individual neurons recorded at the same electrode (Koralek et al. 2013) supports the plausibility of such site-specific functionally relevant coherence, at low (theta and alpha) frequencies in particular.
Elevated Prestimulus Coherence in the Beta Band (15–30 Hz) Is Associated with Misses
In other words, decreased prestimulus beta coherence (15–30 Hz) between frontal and parietal cortex predicted hit trials (Figs. 2, 3, and 5), and this effect was undiminished after subtracting away linear trends in coherence across the duration of each session (Fig. 5B).
As for the lower-frequency effects, our prestimulus beta effects might be interpreted in attentional or motor terms. Because beta frequency (15–30 Hz) rhythms can occur in similar functional contexts as gamma (Benchenane et al. 2011), we were interested to test whether interarea coherence at these frequencies supports attentional performance in our task. Our results fail to support a role of frontal-parietal beta coherence in mediating vigilance or attention in our simple detection task, and instead contribute to a body of literature in humans documenting a similar antagonism between beta synchronization and detection performance as has been found in the alpha band (Jones et al. 2009, 2010). The effect of prestimulus beta synchronization on detection performance is weaker than the effect of prestimulus alpha (Jones et al. 2009, 2010), and beta and alpha can occur independently or together (Jones et al. 2009, 2010; Palva et al. 2005; Tiihonen et al. 1989), suggesting that they represent at least partially distinct neural processes (Klostermann et al. 2007).
The literature on beta rhythm also supports interpreting our prestimulus beta effects in terms of motor or cognitive set. Beta is similar to lower-frequency mu rhythm (near 10 Hz, like alpha) (Hari 2006; Wiest and Nicolelis 2003) in that it is expressed strongly over somato-motor cortex and tends to desynchronize during voluntary movement (Pfurtscheller et al. 1997a) and even during motor imagery (Pfurtscheller and Neuper 1997). However, unlike mu, local beta synchronization and EEG-EMG coherence are characteristic of prolonged muscular contractions (Baker et al. 1997; Chakarov et al. 2009; Sanes and Donoghue 1993). In fact, beta oscillations may be composed of a component that can be considered “part of” mu (Hari 2006) and a distinct component that can be interpreted as a correlate of active movement inhibition or “idling” of motor areas (Pfurtscheller et al. 1997b). A recent review of beta-band oscillations proposes that they reflect a prolonging of the current sensorimotor or cognitive state, such that involuntary increases in beta activity can antagonize adaptive motor or cognitive flexibility (Engel and Fries 2010). Our results appear consistent with this idea and may extend it to frontal-parietal coherence, because excessive frontal-parietal beta coherence appears to maintain the rats' immobile state, leading to more frequent misses. Similarly, Zhang and colleagues studied LFP coherence between frontal motor areas and posterior parietal cortex recorded while monkeys performed a visual go/no-go task. They interpreted poststimulus frontal-parietal beta decoherence as a correlate of response preparation on both go and no-go trials and late beta coherence as a correlate of active response inhibition on no-go trials (Zhang et al. 2008). Another study concluded that synchronized beta oscillations “bind” a frontal-parietal sensorimotor network into a functional unit and govern behavioral changes while monkeys perform a task that involves motor maintenance together with visual discrimination (Brovelli et al. 2004). In our experiments, the decreased frontal-parietal beta coherence that we observed before hit trials could reflect release from the beta-dependent maintenance of the current immobile state, or a priming of the neurons needed to execute the behavioral response once the beep has been detected that results in a higher hit probability. Conversely, spontaneous involuntary upward fluctuations in frontal-parietal beta coherence could reduce the probability of correctly responding with a lick should the target tone sound at that moment. Alternatively, beta coherence might reflect active voluntary inhibition of licking (because it would lead to long time-out periods without water reward) that is inappropriately prolonged after the target tone on miss trials. Thus frontal-parietal beta coherence in our recordings may be interpreted as a signature of motor inhibition or maintenance, but since its prestimulus fluctuations from trial to trial predict detection performance (and we have ruled out an interpretation in terms of changes in motivation), its desynchronization (reduced coherence) may also represent a neural correlate of successful arousal, vigilance, or attention. In support of the notion that neural correlates of movement and attention might overlap in this way, it has been reported that dividing attention can partially disrupt EEG-EMG beta coherence during a sustained contraction task (Kristeva-Feige et al. 2002)—this is an example where clearly cognitive attention is interacting with the “pure motor” beta coherence between cortex and muscle.
Gamma frequencies (>30 Hz).
We did not find higher frontal-parietal coherence in the gamma range before hit trial beeps in the grand average across sessions (Fig. 5). This could be because frontal-parietal gamma coherence is not a correlate of attention to external stimuli in rats as it appears to be in humans and monkeys (Buschman and Miller 2007; Gregoriou et al. 2009a, 2009b; Womelsdorf and Fries 2006). However, our results cannot rule out the possibility that frontal-parietal gamma coherence modulates sensory detection in some circumstances, or at specific pairs of frontal-parietal locations. The nonsignificant trend we observed toward faster responding when prestimulus gamma coherence (around 35 Hz) was elevated supports this possibility. Also, at the frequency bin near 46 Hz, elevated coherence in 9 of 25 sessions significantly predicted hit trials according to our choice probability analysis (Fig. 3D). Again, it is possible that in these sessions the rats were in a more “engaged” state of alertness, during which gamma coherence contributed to the difference between hits and misses, whereas in other sessions the rats were in a distinct state of arousal in which the hit-miss difference was dominated by lower frequencies. Similarly, it is possible that a more demanding task, requiring discrimination among competing alternatives or detection of near-threshold sensory events, would force the animal into a more highly attentive situation in which gamma coherence would contribute significantly to the difference between a successful hit trial and a miss.
The hypothesis that elevated gamma coherence is associated with external attention is contentious in part because effects can depend on stimulus properties and vary with position in cortex (Ray and Maunsell 2010). Similarly, the sign of attention-related gamma effects can vary with brain area. A recent study found that reduced gamma power and spike field coherence in V1 was associated with monkeys' correct performance on a visual task, while in the same experiments increased local coherence in V4 predicted correct attentional performance (Chalk et al. 2010). Another reported elevated prestimulus gamma power correlated with longer reaction times in humans (Reinhart et al. 2011) rather than shorter times, as was reported in monkeys (Womelsdorf et al. 2006). These varying results again suggest the importance of experimental details such as recording location and specific task demands.
Cross-frequency coupling.
We did not observe significant antagonism between the appearance of coherence or power at high (gamma) and low (especially alpha) frequencies, as has been observed in other contexts (Buffalo et al. 2011; Jensen and Mazaheri 2010). Such cross-frequency inhibitory coupling might emerge at higher levels of attentional engagement or selectivity, or might have been obscured in our experiments by positive correlations between theta and gamma frequencies, which have also been reported (Canolty and Knight 2010; Tort et al. 2008). Similarly, we observed some modulation of gamma amplitude by local or interarea theta or alpha phase, but these were not robust or consistent across rats in our experimental context.
Causes and effects of neural coherence.
Cellular-level studies have shed light on the possible mechanisms that give rise to oscillatory coherence at each of the different frequency bands. In general, these neural rhythms are generated through the coordinated firing of excitatory and inhibitory neurons. Excitatory neurons tend to drive low-frequency cortical oscillations, while inhibitory neurons tend to produce high-frequency oscillatory activity. It is believed that cortical theta and alpha rhythms arise from the rhythmic firing of pyramidal cells in the cortex and hippocampus (Alonso and Garcia-Austt 1987)—in particular, layer 5 pyramidal neurons are a main source of alpha synchrony (Silva et al. 1991; Steriade et al. 1990). At higher frequencies, stimulating excitatory pyramidal cells is not sufficient to produce coherent oscillatory activity (Cardin et al. 2009). Instead, beta activity is thought to originate from inhibitory interneurons in sensorimotor cortical regions (Jensen et al. 2005), and networks of fast-spiking inhibitory interneurons are implicated in generating gamma coherence (Cardin et al. 2009).
Because synchronized neural activity can amplify downstream responses (Tiesinga et al. 2004), it is likely to have functionally measurable effects in the brain, though these are not well characterized in behaving animals. The development of rodent models could prove essential for advancing our understanding of sensory detection and attentional processing in humans. In addition to offering a comparatively rapid, inexpensive way of studying a large number of subjects, rodents are ideal subjects for techniques that are difficult or impossible to implement in other animals (Carandini and Churchland 2013; Hromádka and Zador 2007). Moreover, optogenetic techniques that have made it possible to manipulate the activities of specific cell types in vivo in rodents (Cardin et al. 2009) could lead to the discovery of the causal mechanisms underlying detection-related neural oscillations (Knoblich et al. 2010) and perceptual judgments in general (Carandini and Churchland 2013).
Conclusion
Our observation of frontal-parietal attention-related coherence effects comparable to those seen in humans and primates, in which beta frequencies reflect motor or cognitive maintenance and lower frequencies reflect sensorimotor inhibition or idling, supports the idea that the frontal-parietal attention network functions analogously in the rat and indicates that the rat may be a viable model for studying human sensory detection. Our results failed to support the idea that frontal-parietal coherence at beta or gamma frequencies mediates efficient transformation from sensory target representations in parietal cortex into adaptive behavioral responses implemented by frontal areas. This may have been due to differences between our simple detection task and more demanding attentional tasks employed in primate experiments (Buschman and Miller 2007; Gregoriou et al. 2009a) rather than species differences between primates and rats. On the other hand, future recordings in frontal and parietal cortex during sensory detection and discrimination performance may support the existence of significant species differences in the functional roles of these areas. Nevertheless, a better understanding of principles and mechanisms of dynamic neural integration gleaned from the rat model could lead to insights into functions and dysfunctions of the primate attentional and detection system. This expectation is supported by a body of observations that neurological disorders that affect attention, such as autism (Grice et al. 2001; Orekhova et al. 2008), schizophrenia (Light et al. 2006; Minzenberg et al. 2010; Reinhart et al. 2011), and ADHD (Barry et al. 2011; Lenz et al. 2010; Yordanova et al. 2001), manifest abnormalities in oscillatory synchronization.
GRANTS
This work was supported by National Science Foundation Grant IOS-1121689 and summer research grants from Support of Mentors and Their Students from Under-Represented Minorities (SOMAS-URM) and the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.H. and M.C.W. conception and design of research; L.H., K.S., K.S.B., and M.C.W. performed experiments; L.H., K.S., K.S.B., and M.C.W. analyzed data; L.H., K.S., and M.C.W. interpreted results of experiments; L.H., K.S., K.S.B., and M.C.W. prepared figures; L.H., K.S., and M.C.W. drafted manuscript; L.H., K.S., K.S.B., and M.C.W. edited and revised manuscript; L.H., K.S., K.S.B., and M.C.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Jeremy Wilmer for suggesting the regression analysis, Caroline Dodge for help with data collection and analysis, Sam Peterson for technical assistance, Galina Gagin for help with formatting figures, Bevil Conway for helpful comments on an early version of the manuscript, and Adriano Tort for guidance in implementing the cross-frequency phase-amplitude analysis.
REFERENCES
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Exp Brain Res 67: 493–501, 1987 [DOI] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC. Functional specialization of mouse higher visual cortical areas. Neuron 72: 1025–1039, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol 501: 225–241, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, Dupuy FE, McCarthy R, Selikowitz M. EEG coherence and symptom profiles of children with Attention-Deficit/Hyperactivity Disorder. Clin Neurophysiol 122: 1327–1332, 2011 [DOI] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol 21: 475–485, 2011 [DOI] [PubMed] [Google Scholar]
- Bereshpolova Y, Stoelzel CR, Zhuang J, Amitai Y, Alonso JM, Swadlow HA. Getting drowsy? Alert/nonalert transitions and visual thalamocortical network dynamics. J Neurosci 31: 17480–17487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: a platform for analyzing neural signals. J Neurosci Methods 192: 146–151, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokil H, Purpura K, Schoffelen JM, Thomson D, Mitra P. Comparing spectra and coherences for groups of unequal size. J Neurosci Methods 159: 337–345, 2007 [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci 31: 4935–4943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996 [DOI] [PubMed] [Google Scholar]
- Broussard J, Sarter M, Givens B. Neuronal correlates of signal detection in the posterior parietal cortex of rats performing a sustained attention task. Neuroscience 143: 407–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JI, Givens B. Low frequency oscillations in rat posterior parietal cortex are differentially activated by cues and distractors. Neurobiol Learn Mem 94: 191–198, 2010 [DOI] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci USA 101: 9849–9854, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA 108: 11262–11267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862, 2007 [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Benignus VA, Case MW. Signal detection behavior in humans and rats: a comparison with matched tasks. Behav Processes 64: 121–129, 2003 [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15: 827–840, 2005 [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull 132: 180–211, 2006 [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science 313: 1626–1628, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci 14: 506–515, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Churchland AK. Probing perceptual decisions in rodents. Nat Neurosci 16: 824–831, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarov V, Naranjo JR, Schulte-Monting J, Omlor W, Huethe F, Kristeva R. Beta-range EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J Neurophysiol 102: 1115–1120, 2009 [DOI] [PubMed] [Google Scholar]
- Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron 66: 114–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NR, Croft RJ, Dominey SJ, Burgess AP, Gruzelier JH. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol 47: 65–74, 2003 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci 13: 1202–1226, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia 34: 1085–1095, 1996 [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 18: 7426–7435, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci 8: 1698–1703, 2005 [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci USA 103: 14266–14271, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons among means. J Am Stat Assoc 56: 52–64, 1961 [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 20: 156–165, 2010 [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci 3: 4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. 7 to 12 Hz activity in rat gustatory cortex reflects disengagement from a fluid self-administration task. J Neurophysiol 93: 2832–2840, 2005 [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci 28: 4823–4835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention—focusing the searchlight on sound. Curr Opin Neurobiol 17: 437–455, 2007 [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324: 1207–1210, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. Long-range neural coupling through synchronization with attention. Prog Brain Res 176: 35–45, 2009b [DOI] [PubMed] [Google Scholar]
- Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, de Haan M, Johnson MH. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport 12: 2697–2700, 2001 [DOI] [PubMed] [Google Scholar]
- Griffin AL, Asaka Y, Darling RD, Berry SD. Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav Neurosci 118: 403–411, 2004 [DOI] [PubMed] [Google Scholar]
- Guntekin B, Basar E. A new interpretation of P300 responses upon analysis of coherences. Cogn Neurodyn 4: 107–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Simon SA, Nicolelis MA. Licking-induced synchrony in the taste-reward circuit improves cue discrimination during learning. J Neurosci 30: 287–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bauml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage 37: 1465–1473, 2007 [DOI] [PubMed] [Google Scholar]
- Hari R. Action-perception connection and the cortical mu rhythm. Prog Brain Res 159: 253–260, 2006 [DOI] [PubMed] [Google Scholar]
- Hattori M, Onoda K, Sakata S. Identification of rat P3-like processes in the anterior cingulate cortex and hippocampus. Neurosci Lett 472: 43–46, 2010 [DOI] [PubMed] [Google Scholar]
- Hromádka T, Zador AM. Toward the mechanisms of auditory attention. Hear Res 229: 180–185, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage 26: 347–355, 2005 [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4: 186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus 15: 867–873, 2005a [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol 3: e402, 2005b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Kerr CE, Wan Q, Pritchett DL, Hamalainen M, Moore CI. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J Neurosci 30: 13760–13765, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Pritchett DL, Sikora MA, Stufflebeam SM, Hamalainen M, Moore CI. Quantitative analysis and biophysically realistic neural modeling of the MEG mu rhythm: rhythmogenesis and modulation of sensory-evoked responses. J Neurophysiol 102: 3554–3572, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399: 781–784, 1999 [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur J Neurosci 30: 2224–2234, 2009 [DOI] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67: 858–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V, Corwin JV. Neglect following unilateral ablation of the caudal but not the rostral portion of medial agranular cortex of the rat and the therapeutic effect of apomorphine. Behav Brain Res 37: 169–184, 1990 [DOI] [PubMed] [Google Scholar]
- King VR, Corwin JV. Comparisons of hemi-inattention produced by unilateral lesions of the posterior parietal cortex or medial agranular prefrontal cortex in rats: neglect, extinction, and the role of stimulus distance. Behav Brain Res 54: 117–131, 1993 [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev 53: 63–88, 2007 [DOI] [PubMed] [Google Scholar]
- Klostermann F, Nikulin VV, Kuhn AA, Marzinzik F, Wahl M, Pogosyan A, Kupsch A, Schneider GH, Brown P, Curio G. Task-related differential dynamics of EEG alpha- and beta-band synchronization in cortico-basal motor structures. Eur J Neurosci 25: 1604–1615, 2007 [DOI] [PubMed] [Google Scholar]
- Knoblich U, Siegle JH, Pritchett DL, Moore CI. What do we gain from gamma? Local dynamic gain modulation drives enhanced efficacy and efficiency of signal transmission. Front Hum Neurosci 4: 185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev V, Yordanova J, Schurmann M, Basar E. Increased frontal phase-locking of event-related alpha oscillations during task processing. Int J Psychophysiol 39: 159–165, 2001 [DOI] [PubMed] [Google Scholar]
- Koralek AC, Costa RM, Carmena JM. Temporally precise cell-specific coherence develops in corticostriatal networks during learning. Neuron 79: 865–872, 2013 [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige R, Fritsch C, Timmer J, Lucking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol 113: 124–131, 2002 [DOI] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45: 147–156, 2005 [DOI] [PubMed] [Google Scholar]
- Lenz D, Krauel K, Flechtner HH, Schadow J, Hinrichs H, Herrmann CS. Altered evoked gamma-band responses reveal impaired early visual processing in ADHD children. Neuropsychologia 48: 1985–1993, 2010 [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry 60: 1231–1240, 2006 [DOI] [PubMed] [Google Scholar]
- Macdonald JS, Mathan S, Yeung N. Trial-by-trial variations in subjective attentional state are reflected in ongoing prestimulus EEG alpha oscillations. Front Psychol 2: 82, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex 11: 513–527, 2001 [DOI] [PubMed] [Google Scholar]
- Mayer AR, Harrington D, Adair JC, Lee R. The neural networks underlying endogenous auditory covert orienting and reorienting. Neuroimage 30: 938–949, 2006 [DOI] [PubMed] [Google Scholar]
- Mazaheri A, DiQuattro NE, Bengson J, Geng JJ. Pre-stimulus activity predicts the winner of top-down vs. bottom-up attentional selection. PLoS One 6: e16243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P, Flister E, Reinagel P. Collinear features impair visual detection by rats. J Vis 11: 22, 2011 [DOI] [PubMed] [Google Scholar]
- Meier P, Reinagel P. Rat performance on visual detection task modeled with divisive normalization and adaptive decision thresholds. J Vis 11: 1, 2011 [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci 27: 2858–2865, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 35: 2590–2599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology 24: 375–425, 1987 [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn 14: 390–425, 2005 [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Gora J, Stough CK. Hemispheric asymmetries for visual and auditory temporal processing: an evoked potential study. Int J Psychophysiol 44: 37–55, 2002 [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol 26: 31–49, 1997 [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: relation to age, IQ, and EEG gamma oscillations. Neurosci Lett 434: 218–223, 2008 [DOI] [PubMed] [Google Scholar]
- Ortiz TA, Goodin DS, Aminoff MJ. Neural processing in a three-choice reaction-time task: a study using cerebral evoked-potentials and single-trial analysis in normal humans. J Neurophysiol 69: 1499–1512, 1993 [DOI] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci 12: 646–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Linkenkaer-Hansen K, Naatanen R, Palva JM. Early neural correlates of conscious somatosensory perception. J Neurosci 25: 5248–5258, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci 30: 150–158, 2007 [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64, 1991 [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature 453: 406–409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett 239: 65–68, 1997 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. Int J Psychophysiol 26: 121–135, 1997a [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Edlinger G. On the existence of different types of central beta rhythms below 30 Hz. Electroencephalogr Clin Neurophysiol 102: 316–325, 1997b [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA. Human auditory evoked potentials. II. Effects of attention. Electroencephalogr Clin Neurophysiol 36: 191–199, 1974 [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol 32: 3–25, 1980 [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci 21: 3175–3183, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron 67: 885–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science 228: 750–752, 1985 [DOI] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res 100: 67–84, 1994 [DOI] [PubMed] [Google Scholar]
- Reinhart RM, Mathalon DH, Roach BJ, Ford JM. Relationships between pre-stimulus gamma power and subsequent P300 and reaction time breakdown in schizophrenia. Int J Psychophysiol 79: 16–24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner AL, Mittleman G. Visuospatial attention in the rat and posterior parietal cortex lesions. Behav Brain Res 79: 69–77, 1996 [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA 90: 4470–4474, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol 57: 97–103, 2005 [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Mazaheri A, Bojak I, Norris DG, Kleinschmidt A. Modulation of visually evoked cortical FMRI responses by phase of ongoing occipital alpha oscillations. J Neurosci 31: 3813–3820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer-Teixeira R, Belchior H, Caixeta FV, Souza BC, Ribeiro S, Tort AB. Theta phase modulates multiple layer-specific oscillations in the CA1 region. Cereb Cortex 22: 2404–2414, 2012 [DOI] [PubMed] [Google Scholar]
- Schneidman E, Berry MJ, 2nd, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440: 1007–1012, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci 26: 435–439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46: 141–151, 2005 [DOI] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 251: 432–435, 1991 [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24: 49–65, 111–125, 1999 [DOI] [PubMed] [Google Scholar]
- Singer W. Dynamic formation of functional networks by synchronization. Neuron 69: 191–193, 2011 [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinas RR, Lopes de Silva FH, Mesulam MM. Report of IFCN Committee on Basic Mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol 76: 481–508, 1990 [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Weyand TG. Receptive-field and axonal properties of neurons in the dorsal lateral geniculate nucleus of awake unparalyzed rabbits. J Neurophysiol 54: 168–183, 1985 [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26: 9494–9502, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga PH, Fellous JM, Salinas E, Jose JV, Sejnowski TJ. Synchronization as a mechanism for attentional gain modulation. Neurocomputing 58–60: 641–646, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kajola M, Hari R. Magnetic mu rhythm in man. Neuroscience 32: 793–800, 1989 [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104: 1195–1210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA 106: 20942–20947, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA 105: 20517–20522, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res 146: 3–17, 2003 [DOI] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28: 1816–1823, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo JM, Corwin JV, King V, Reep RL. Hemispheric asymmetry in neglect produced by unilateral lesions of dorsomedial prefrontal cortex in rats. Exp Neurol 102: 199–209, 1988 [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38: 301–313, 2000 [DOI] [PubMed] [Google Scholar]
- Ward NM, Brown VJ. Deficits in response initiation, but not attention, following excitotoxic lesions of posterior parietal cortex in the rat. Brain Res 775: 81–90, 1997 [DOI] [PubMed] [Google Scholar]
- Wiest MC, Nicolelis MA. Behavioral detection of tactile stimuli during 7–12 Hz cortical oscillations in awake rats. Nat Neurosci 6: 913–914, 2003 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. Neuronal coherence during selective attentional processing and sensory-motor integration. J Physiol 100: 182–193, 2006 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol 17: 154–160, 2007 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra P, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439: 733–736, 2006 [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci 20: RC63, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CT, Weissman DH, Roberts KC, Woldorff MG. The neural circuitry underlying the executive control of auditory spatial attention. Brain Res 1134: 187–198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Banaschewski T, Kolev V, Woerner W, Rothenberger A. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder–evidence from event-related gamma oscillations. Clin Neurophysiol 112: 1096–1108, 2001 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience 156: 238–246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]