Abstract
The dorsal cochlear nucleus (DCN) is a cerebellum-like auditory brain stem region whose functions include sound localization and multisensory integration. Although previous in vivo studies have shown that glycinergic and GABAergic inhibition regulate the activity of several DCN cell types in response to sensory stimuli, data regarding the synaptic inputs onto DCN inhibitory interneurons remain limited. Using acute DCN slices from mice, we examined the properties of excitatory and inhibitory synapses onto the superficial stellate cell, a poorly understood cell type that provides inhibition to DCN output neurons (fusiform cells) as well as to local inhibitory interneurons (cartwheel cells). Excitatory synapses onto stellate cells activated both NMDA receptors and fast-gating, Ca2+-permeable AMPA receptors. Inhibition onto superficial stellate cells was mediated by glycine and GABAA receptors with different temporal kinetics. Paired recordings revealed that superficial stellate cells make reciprocal synapses and autapses, with a connection probability of ∼18–20%. Unexpectedly, superficial stellate cells co-released both glycine and GABA, suggesting that co-transmission may play a role in fine-tuning the duration of inhibitory transmission.
Keywords: interneuron, auditory, cochlear nucleus, inhibition
the dorsal cochlear nucleus (DCN) is a mammalian auditory brain stem region composed of diverse neuronal cell types (Oertel and Young 2004). The contribution of the DCN to auditory processing is not clear, but previous studies suggest that it may be involved in sound localization in the vertical plane and orientation of the head toward sounds of interest (Kanold and Young 2001; May 2000; Sutherland et al. 1998). Interestingly, the local circuitry of the DCN closely resembles that of the cerebellum and cerebellum-like nuclei found in mammals and fish (Oertel and Young 2004), and this homology suggests that the DCN may also function to generate “negative images” of predictable auditory input, thereby filtering out self-generated sounds (Bell et al. 2008; Requarth and Sawtell 2011).
Regardless of the precise role of the DCN in auditory processing, previous studies have established that the glutamatergic afferent neurons (fusiform and giant cells) integrate auditory information from the ear with sensory input from nonauditory brain regions (Oertel and Young 2004). Sound information is relayed via the auditory nerve and perhaps from T-stellate cells of the ventral cochlear nucleus (Oertel et al. 2011) that synapse onto the basal dendrites of fusiform cells. Nonauditory information enters via an array of cerebellum-like granule cell parallel fibers that synapse onto fusiform cell apical dendrites. DCN parallel fibers also contact two types of molecular layer interneuron: glycinergic Purkinje-like cartwheel cells and GABAergic superficial stellate cells (henceforth “stellate cells”) that closely resemble the stellate/basket cells of the cerebellum (Bell et al. 2008; Mugnaini 1985; Wouterlood et al. 1984).
Previous studies have shown that parallel fibers recruit feedforward glycinergic inhibition onto fusiform cells, presumably mediated by cartwheel cells (Davis et al. 1996; Davis and Young 1997; Doiron et al. 2011; Kanold and Young 2001; Kuo and Trussell 2011; Roberts and Trussell 2010). Given that even a single cartwheel cell can control the firing rate of fusiform cells (Roberts and Trussell 2010), these findings imply that feedforward inhibition from interneurons effectively determines whether the DCN relays signals to downstream targets. Interestingly, in vivo work has suggested that GABAergic inhibition also controls the response properties of cartwheel and fusiform cells (Davis and Young 2000), and a recent in vitro study suggested that GABAergic inhibition controls the spread of parallel fiber excitation in the DCN (Middleton et al. 2011). Since cartwheel cell transmission is predominantly glycinergic (Apostolides and Trussell 2013a; Golding and Oertel 1997; Mancilla and Manis 2009; Roberts et al. 2008), these results suggest that stellate cells are a likely source of GABAergic inhibition.
Little is known about chemical synaptic transmission onto DCN stellate cells. This lack of information is surprising, because stellate cells are common components of the outer layers of the DCN (Wouterlood et al. 1984) and may share homology with the GABAergic stellate and basket cells known to be crucial for controlling the output of the cerebellar cortex (Barmack and Yakhnitsa 2008; Callaway et al. 1995; Coddington et al. 2013; Oldfield et al. 2010). Therefore, a comparison of cerebellar and DCN stellate cell properties might provide clues to DCN function. Nevertheless, there are no confirmed in vivo recordings from DCN stellate cells, and in vitro recordings are few (Apostolides and Trussell 2013b; Golding and Oertel 1997; Zhang and Oertel 1993). We performed whole cell recordings from visually identified DCN stellate cells in acute mouse brain slices and examined the properties of excitatory and inhibitory synapses. We found that, similarly to cerebellar stellate/basket cells, parallel fibers activate fast, Ca2+-permeable AMPA receptors on DCN stellate cells. Unlike cerebellar stellate cells (Clark and Cull-Candy 2002), however, single spikes in parallel fibers led to activation of both AMPA and NMDA receptors in DCN stellate cells. We also found that DCN stellate cells inhibit one another via reciprocal synapses as well as autapses. Although it has been previously suggested that DCN stellate cells are GABAergic, we found that they express the glycine transporter GlyT2 required for functional glycinergic transmission (Gomeza et al. 2003; Rousseau et al. 2008), and most stellate cells tested co-released both glycine and GABA. Our findings shed light on the synaptic properties to DCN stellate cells, highlighting significant functional similarities and differences between the DCN and the cerebellum.
METHODS
Slice preparation.
All protocols involving animals were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee. Mice 15–24 postnatal days of age of wild-type (C57/Bl6 or CBA), GlyT2-GFP, or GAD65-GFP background were used for the majority of these experiments. For optogenetic experiments in Fig. 7, we used two mutant mouse lines expressing channelrhodopsin2 in DCN fusiform cells [Thy1-ChR2-YFP line 18 (JAX stock no. 007612) and VGluT2-ChR2-YFP (JAX stock no. 017978); Apostolides and Trussell 2013b]. Mice were anesthetized with isoflurane and decapitated, and slices (200–250 μm thick) containing the DCN were cut in an ice-cold sucrose solution containing (in mM) 87 NaCl, 25 NaHCO3, 25 glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, and 7 MgCl2 and bubbled with 5% CO2-95% O2. After being cut, slices were allowed to recover for 30–45 min at 34°C in an artificial cerebrospinal fluid (ACSF) solution containing (in mM) 130 NaCl, 2.1 KCl, 1.7 CaCl2, 1 MgSO4, 1.2 KH2PO4, 20 NaHCO3, 3 Na-HEPES, and 10–12 glucose, bubbled with 5% CO2-95% O2 (300–310 mosM). This solution was used as the standard perfusate for all experiments. For experiments in 6-wk-old animals, mice were anesthetized with an intraperitoneal injection of 2% Avertin and the brain was sliced in an ice-cold solution composed of (in mM) 93 N-methyl-d-glucamine (NMDG), 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 10 MgSO4, 0.5 ascorbic acid, 2 thiourea, 3 Na-pyruvate, 25 glucose, 0.5 CaCl2, and 12 n-acetyl-l-cysteine, pH adjusted to 7.3–7.4 with HCl (300–310 mosM). Slices subsequently recovered at 34°C in NMDG solution for 10–15 min, followed by a 40–60 min recovery period at room temperature in standard ACSF. In some experiments 5 μM 3-[(R)-2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid (R-CPP) or 50 μM d-aminophosphonovaleric acid (d-APV) were added to the cutting solution and/or recovery chamber. After recovery, slices were maintained at ∼22°C until recording. Experiments were typically performed within 5 h of slice preparation.
Fig. 7.
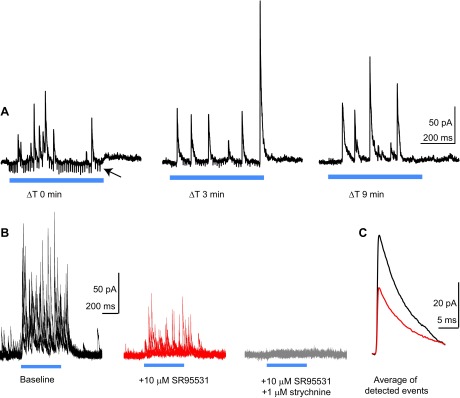
Reciprocal connections between neighboring stellate cells are also mixed GABA/glycinergic. A: single traces from an experiment in a Thy1-ChR2 mouse, recorded at different time points (ΔT) after the cell was clamped at +33 mV. The ΔT 0 min trace was recorded ∼3 min after the whole cell recording was initiated. Blue traces denote optogenetic activation of fusiform cells. The arrow points to gap junction-mediated spikelet events that represent action potentials in prejunctional fusiform cells (Apostolides and Trussell 2013b). Note the marked decrease in spikelet amplitude in the ΔT 3 min trace and the absence of spikelet events in the ΔT 9 min trace. B: example experiment from a Thy1-ChR2 mouse. The stellate cell is held at +33 mV, and fusiform cells are activated by blue light stimuli (500 ms) denoted by the blue bar. Four example sweeps are shown superimposed, recorded during baseline and in the presence of SR95531 (red) and SR95531 + strychnine (gray). C: average of IPSCs at baseline (black) and in SR95531 (red), detected with a template algorithm. Data are from the same cell as in B.
Electrophysiology.
Slices were mounted in a recording chamber and continuously perfused at 3–5 ml/min with ACSF heated to 31–33°C (total chamber volume: 1–2 ml). Neurons were visualized using Dodt contrast optics with a ×40 or ×63 objective on a Zeiss Axioscop2 microscope. For paired recordings, the pipette internal solution contained 60 K-gluconate, 10 K-glutamate, 70 KCl, 4.8 MgCl2, 4 ATP, 0.5 GTP, 10 Tris-phosphocreatine, 0.1–0.2 EGTA, and 10 HEPES, pH adjusted between 7.2 and 7.3 with KOH (∼290 mosM). This high intracellular Cl− solution increased the driving force for Cl− and thus generated larger absolute GABAA and glycine currents. For recording of miniature events and broadening action potentials shown in Fig. 7, K-gluconate, K-glutamate, and KCl were replaced by 120 mM CsCl. For evoked and spontaneous excitatory postsynaptic current (EPSC) recordings, the internal contained (in mM) 118 CsMeSO3, 5 tetraethylammonium-Cl, 5 QX314-Cl, 5 Cs4-BAPTA, 10 HEPES, 4 Mg-ATP, 0.5 GTP, and 10 Tris-phosphocreatine. In some experiments, 30 mM CsF was substituted for equimolar CsMeSO3 to facilitate gigaseal formation.
Whole cell recording pipettes (final resistance: 2.5–5 MΩ) were made from borosilicate glass (no. 8250, World Precision Instruments) using a Narishige P97 puller. Parallel fibers were stimulated by delivering voltage pulses (50–200 μs) through a theta glass pipette filled with ACSF, positioning within 20–40 μm of the cell. Series resistance in voltage-clamp (<30 MΩ) was monitored frequently and compensated 60–80% “correction,” 90% “prediction” (bandwidth: 3 kHz), and data were not analyzed if series resistance varied >20–25% over the course of the recording. Optogenetic experiments were performed using light from a custom-made, transistor-transistor logic (TTL)-gated blue light-emitting diode (LED) passed through the epifluorescence port of the microscope.
Identification of stellate cells.
Most experiments were performed using wild-type mice. Stellate cells were identified by their location in the slice (at the outer edge of the molecular layer and below the ependymal surface) and basic membrane properties. The main types of neurons found in the DCN superficial molecular layer are stellate and cartwheel cells (Osen and Mugnaini 1981; Wouterlood et al. 1984). Stellate cells were easily distinguished from cartwheel cells by the size of their soma and input resistance, which typically differed by an order of magnitude (Apostolides and Trussell 2013b). In experiments with transgenic mice, green fluorescent protein (GFP) fluorescence was observed using illumination from a 100-W Hg bulb placed in the epifluorescence port of the microscope and passed through a GFP filter set.
Data acquisition and analysis.
Traces were recorded with a Multiclamp 700B amplifier and a Digidata 1322A analog-to-digital converter board using pClamp 9 software. Signals were low-pass filtered at 10–20 kHz and digitized at 20–50 kHz. Data were analyzed offline after the traces were filtered at 2–10 kHz. For voltage-clamp data, all measurements were made on the peak amplitude of averaged traces unless otherwise stated. Spontaneous and miniature events were detected using a template algorithm or amplitude threshold in Axograph X or Clampfit, and each event was visually inspected for false positives or malformed events. Events were not included for averaging if they overlapped significantly with the decay or rising phase of other events. Decay kinetics were measured from averages of aligned events fitted with a single, double, or triple exponential decay function. Weighted decay time constants were calculated from double or triple exponential fits as (Aslow·τslow + Amedium·τmedium + Afast·τfast)/(Aslow + Amedium + Afast). All values are means ± SE. Statistical significance was determined using paired or unpaired Student's t-tests where appropriate. For AMPA/NMDA ratios, the AMPA component was calculated by measuring the amplitude of EPSCs recorded at +33 mV at a time point corresponding to the peak amplitude of events at −67 mV. The NMDA component was calculated by measuring the amplitude of EPSCs at +33 mV at 5–7 ms after the time point used to calculate the AMPA component.
Histology.
Mice were transcardially perfused with warm (38°C) 100 mM phosphate-buffered saline (PBS) solution, pH 7.4, followed by ice-cold 4% paraformaldehyde in PBS. The brains were dissected from the skull and incubated overnight in 4% paraformaldehyde for complete tissue fixation. Next, the brains were rinsed in PBS, and brain stem coronal sections were acquired at 30 μm on a vibratome (VT1000S; Leica). After being sectioned, the tissue was washed in PBS solution for 30 min. Sections were then incubated for 1 h in block solution [2% goat serum, 0.2% Triton X-100 (in PBS), and 0.3% BSA] and subsequently incubated overnight at 4°C with anti-GFP rabbit polyclonal antibody Alexa Fluor 488 conjugate (1:200 in block solution; Invitrogen Molecular Probes). The next day, sections were washed in PBS for 1 h and mounted on 0.3% gelatin-coated slides. After drying, sections were dehydrated in ascending alcohols and delipidized in xylenes. The tissue was then rehydrated and coverslipped using Fluoromount G medium (Southern Biotechnology Associates). Fluorescence images were acquired using a confocal microscope (Olympus FV1000) by sequential scanning of GFP signals using an oil-immersion objective (×60, numerical aperture 1.42) with Olympus Fluoview-1000 software. Image analysis was conducted using NIH ImageJ software.
Reagents.
2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX), d-APV, R-CPP, and SR95531 were purchased from Ascent Scientific/Abcam. Strychnine was purchased from Sigma-Aldrich.
RESULTS
Single parallel fibers activate AMPA and NMDA receptors.
Previous studies in cerebellar stellate/basket cells showed that EPSCs evoked by parallel fiber stimulation lack a prominent NMDA receptor component (Glitsch and Marty 1999). Consequently, NMDA receptors on cerebellar stellate cells are thought to be extrasynaptic and primarily activated by glutamate spillover during sustained activity of multiple parallel fibers (Carter and Regehr 2000; Clark and Cull-Candy 2002; Nahir and Jahr 2013). We tested whether NMDA receptors were similarly excluded from postsynaptic sites in DCN stellate cells by evoking EPSCs while voltage-clamping the cell at negative or positive potentials. All recordings were performed in 10 μM SR95531 and 0.5–2 μM strychnine to block GABAA and glycine receptors, respectively. Single parallel fiber shocks from a bipolar stimulating electrode (0.5–6 V), delivered while the cell was held at −67 to −77 mV, evoked fast EPSCs typical of AMPA receptor-mediated transmission (Fig. 1A, bottom trace). By contrast, EPSCs at +33 mV displayed a prominent slow component (Fig. 1A, top trace) that was blocked by the selective NMDA receptor antagonist R-CPP (5 μM, n = 6 cells; Fig. 1B). NBQX (10 μM) blocked the fast EPSC component (n = 5 cells; Fig. 1C), confirming that it was mediated by AMPA receptors.
Fig. 1.
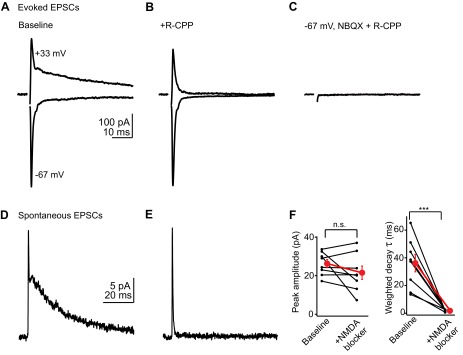
Parallel fibers activate AMPA and NMDA receptors. A: example average excitatory postsynaptic currents (EPSCs) recorded in a stellate cell at negative (bottom trace) and positive holding potentials (top trace). Of note is the slow decay component at +33 mV, typical of NMDA receptor-mediated transmission. B: in the same cell, bath application of the NMDA receptor antagonist 3-[(R)-2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid (R-CPP; 5 μM) selectively blocks the slow component. C: subsequent application of the AMPA receptor blocker 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX; 10 μM) abolishes the fast EPSC. D: average of spontaneous EPSCs (sEPSCs) recorded at +33 mV, highlighting a slow component similar to that of evoked EPSCs. These traces are from a different cell from that shown in A–C. E: average of sEPSCs recorded at +33 mV in the presence of R-CPP, showing that the slow decay is due to NMDA receptor activation. F: quantification of 6 experiments similar to those of D and E. Left graph shows the peak amplitudes of average sEPSCs before and after NMDA receptor blockade, indicating that NMDA receptors contribute minimally to the peak. Right graph shows the effect of NMDA receptor blockers on the weighted decay time constant of sEPSCs. Black lines connect data from individual experiments; red dot is mean ± SE. ***P < 0.001; n.s., not significant.
However, even relatively weak shocks employed in these experiments likely activated more than one presynaptic parallel fiber (Roberts and Trussell 2010). It could be argued that pooling of glutamate from even a few closely spaced synapses, or the repetitive activation of single parallel fibers (Isope et al. 2004; Nahir and Jahr 2013), might suffice to activate high-affinity, extrasynaptic NMDA receptors. We therefore tested whether NMDA receptors were activated by single parallel fibers by analyzing spontaneous EPSCs (sEPSCs) occurring due to random firing of presynaptic granule cells. At +33 mV, sEPSCs displayed a prominent slow component, similar to evoked EPSCs (Fig. 1D). Given that the decay phases of the average sEPSCs were best fit with the sum of two or three exponentials (4/8 cells fit with 2 exponents), we quantified the decay kinetics of average sEPSCs by calculating a weighted decay time constant before and after bath application of a NMDA receptor antagonist. Under baseline conditions, the average weighted decay time constant of sEPSCs was 36.2 ± 6.3 ms (n = 8 cells). This value was not significantly different from the weighted decay time constant of evoked EPSCs recorded in absence of NMDA receptor blockers (39.5 ± 9.2 ms; P = 0.76, unpaired t-test). R-CPP (5 μM) or d-APV (50 μM) selectively blocked the slow decay component, thereby reducing the weighted decay time constant to 1.8 ± 0.2 ms (Fig. 1, E and F; n = 8, P < 0.001, paired t-test). However, NMDA receptor antagonists had minimal effect on the peak amplitude of spontaneous events (Fig. 1F; baseline: 26.3 ± 2.1 pA, NMDA blockers: 21.5 ± 3.4 pA, n = 8, P = 0.20). Furthermore, the ability to resolve the submillisecond rise kinetics of AMPA sEPSCs (10–90% rise time: 0.48 ± 0.05 ms, n = 8 cells) suggested that the slow decay of the NMDA component is unlikely to be affected by voltage-clamp error in these experiments. We also tested whether the synaptic localization of NMDA receptors in DCN stellate cells was developmentally stable by comparing the AMPA/NMDA ratio of EPSCs in 2- to 3-wk-old mice vs. 6-wk-old mice. The AMPA/NMDA ratio was similar between the two age groups (AMPA/NMDA ratio in 2- to 3-wk old mice: 1.6 ± 0.3; 6-wk-old mice: 2.3 ± 0.4, P = 0.2, unpaired t-test). Altogether these results show that in contrast to cerebellar stellate/basket cells, glutamate released from single parallel fibers can readily access both AMPA and NMDA receptors on DCN stellate cells.
DCN stellate cells contain fast, Ca2+-permeable AMPA receptors.
Cerebellar stellate cells express Ca2+-permeable, GluA2-lacking AMPA receptors with submillisecond decay kinetics (Carter and Regehr 2002; Jackson and Nicoll 2011; Liu and Cull-Candy 2000). Interestingly, previous studies in the cochlear nuclei found that the biophysical properties of AMPA receptors correlate with the identity of the presynaptic terminal: AMPA receptors at auditory nerve synapses have rapid kinetics and are typically Ca2+ permeable, whereas AMPA receptors at parallel fiber synapses gate slowly and are impermeable to Ca2+ (Gardner et al. 1999, 2001). Thus we asked whether DCN stellate cells contained Ca2+-permeable AMPA receptors with rapid kinetics, similar to cerebellar stellate cells, or if their AMPA receptor properties followed the pattern proposed by Gardner et al. (2001).
We first quantified the kinetics of synaptic AMPA receptors by recording miniature EPSCs (mEPSCs) in DCN stellate cells. d-APV, SR95531, and strychnine were added to the bath solution to isolate AMPA mEPSCs, and 1 μM tetrodotoxin (TTX) was added to block spikes. Quantal events typically ranged in amplitude between 20 and 200 pA, with an average amplitude of 45.2 ± 6.5 pA, a 10–90% rise time of 0.17 ± 0.01 ms, and a weighted decay time constant of 0.71 ± 0.07 ms (n = 12 cells). This decay time is somewhat faster than that for evoked AMPA EPSCs described above, presumably because of dispersion of the EPSC by the release time course. Data from an example cell are shown in Fig. 2A, with the corresponding amplitude histogram of its individual events shown in Fig. 2B. mEPSCs in this cell could exceed 200 pA, which, given the high input resistance of stellate cells (Apostolides and Trussell 2013b), indicate that even small synapses are relatively potent. These amplitude and kinetic values are similar to those reported in cerebellar stellate/basket cells (Carter and Regehr 2002; Crowley et al. 2007) and suggest that AMPA receptors on DCN stellate cells might also lack the GluA2 subunit and be Ca2+ permeable (Jackson and Nicoll 2011; Liu and Cull-Candy 2000).
Fig. 2.
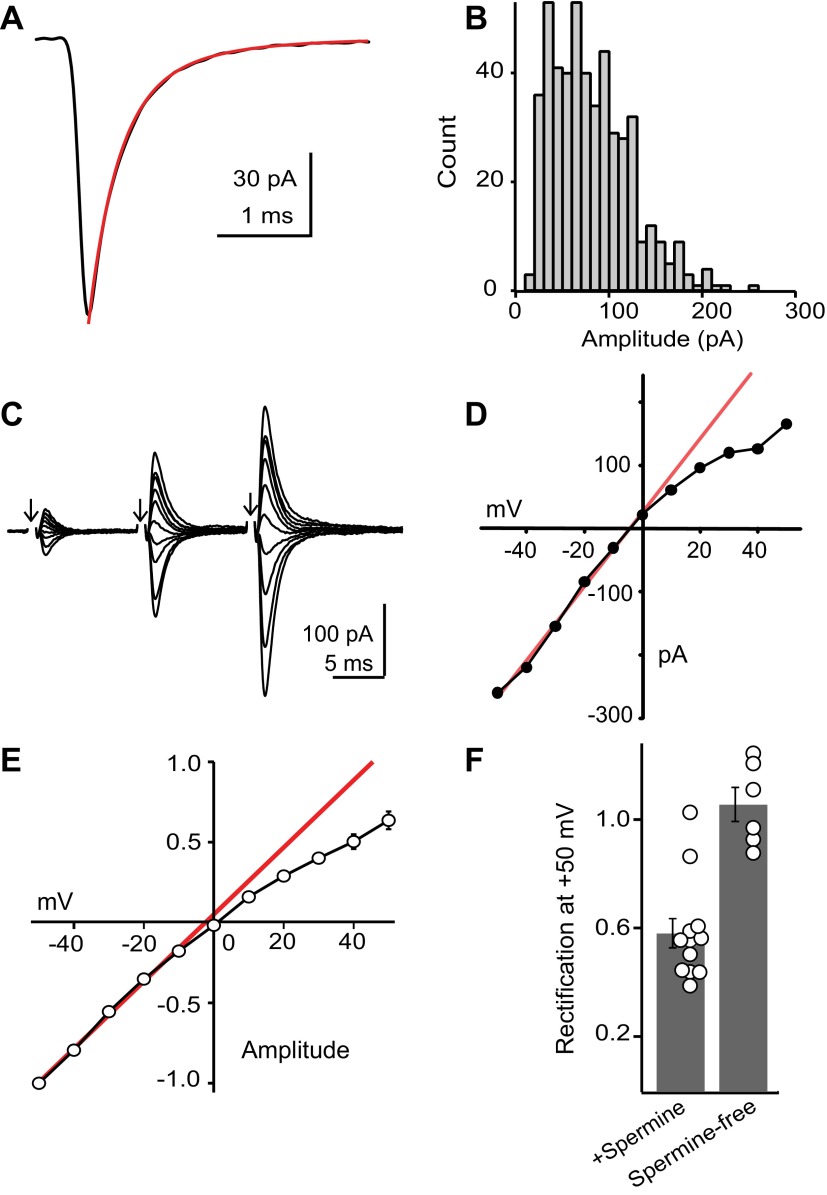
AMPA receptors are Ca2+ permeable. A: example average miniature EPSC (mEPSC) from a dorsal cochlear nucleus (DCN) stellate cell. The decay phase is fit with a double exponential decay (weighted decay τ = 0.42 ms). B: amplitude histogram of individual mEPSCs from the same cell as in A. C: example average EPSCs evoked at different holding potentials. Arrows denote time of parallel fiber stimulation. The shock artifact is blanked for clarity. D: current-voltage (I–V) curve for the third EPSC in the example traces shown in A. The red line is a linear fit to the data recorded at negative holding potentials. E: summary I–V curve for 12 cells, normalized to the amplitude at −50 mV. Error bars are generally smaller than the symbols. F: group data showing the rectification index (ratio of response recorded at +50 mV to that predicted from linear fit to points at negative potentials) of individual stellate cells. Note that all cells recorded with a spermine-free internal solution displayed a linear I–V.
We further tested for expression of Ca2+-permeable AMPA receptors by evoking EPSCs with a stimulating electrode while voltage-clamping stellate cells at different holding potentials. Ca2+-permeable AMPA receptors exhibit an inward rectification due to intracellular polyamine block at positive potentials, whereas GluA2-containing AMPA receptors are Ca2+ impermeable and have a linear current-voltage (I–V) relationship (Isaac et al. 2007). AMPA EPSCs were isolated with d-APV/SR95531/strychnine, and exogenous polyamines (spermine, 100 μM) were added to the pipette internal solution. Parallel fibers were stimulated 3 times at 100 Hz (delivered once every 10–15 s) while the postsynaptic cell was held between −50 and +50 mV. AMPA EPSCs exhibited paired-pulse facilitation, typical of parallel fiber synapses in the DCN (Kuo and Trussell 2011; Manis 1989; Tzounopoulos et al. 2004) (Fig. 2C). The amplitude of the third EPSC at −50 mV was, on average, 6.28 ± 0.56 times larger than that of the first EPSC (n = 12 cells). As expected from Ca2+-permeable AMPA receptors, the I–V curve of EPSCs showed significant inward rectification (Fig. 2, D and E). We calculated a “rectification index” (RI) for each cell, defined as the actual EPSC amplitude recorded at +50 mV divided by the value extrapolated from a linear fit of EPSCs recorded at negative potentials. Ten of 12 DCN stellate cells tested exhibited significant inward rectification (RI = 0.58 ± 0.05, P < 0.0001, 1-sample t-test; Fig. 2, E and F), and rectification was absent when EPSCs were recorded without exogenous polyamines added to the pipette solution (RI = 1.06 ± 0.06, n = 6, P = 0.5; Fig. 2F) (Kamboj et al. 1995). Thus inward rectification was due to neither poor voltage clamp at positive potentials nor the activation of glutamatergic synapses on electrically coupled neurons (Apostolides and Trussell 2013b). Altogether, these data suggest that approximately half of the AMPA EPSCs in stellate cells are generated by Ca2+-permeable receptors. The degree of inward rectification we observed was comparable to that reported in rat cerebellar stellate cells (Liu and Cull-Candy 2000; Soto et al. 2007). Given the low release probability of parallel fiber synapses, we measured the final EPSC in a train of 3 parallel fiber stimuli delivered at 100 Hz. This analysis may therefore underestimate the degree of inward rectification if the receptors experienced use-dependent unblock by spermine (Rozov and Burnashev 1999). However, paired-pulse facilitation was not significantly different between EPSCs recorded at negative and positive potentials (EPSC3/EPSC1 at −50 mV was 6.27 ± 0.47 vs. 6.79 ± 0.93 at +50 mV; P = 0.47, paired t-test), suggesting that any use-dependent unblock of GluA2-lacking receptors occurring during these short trains was negligible.
Stellate cells are GABA/glycine co-releasing interneurons.
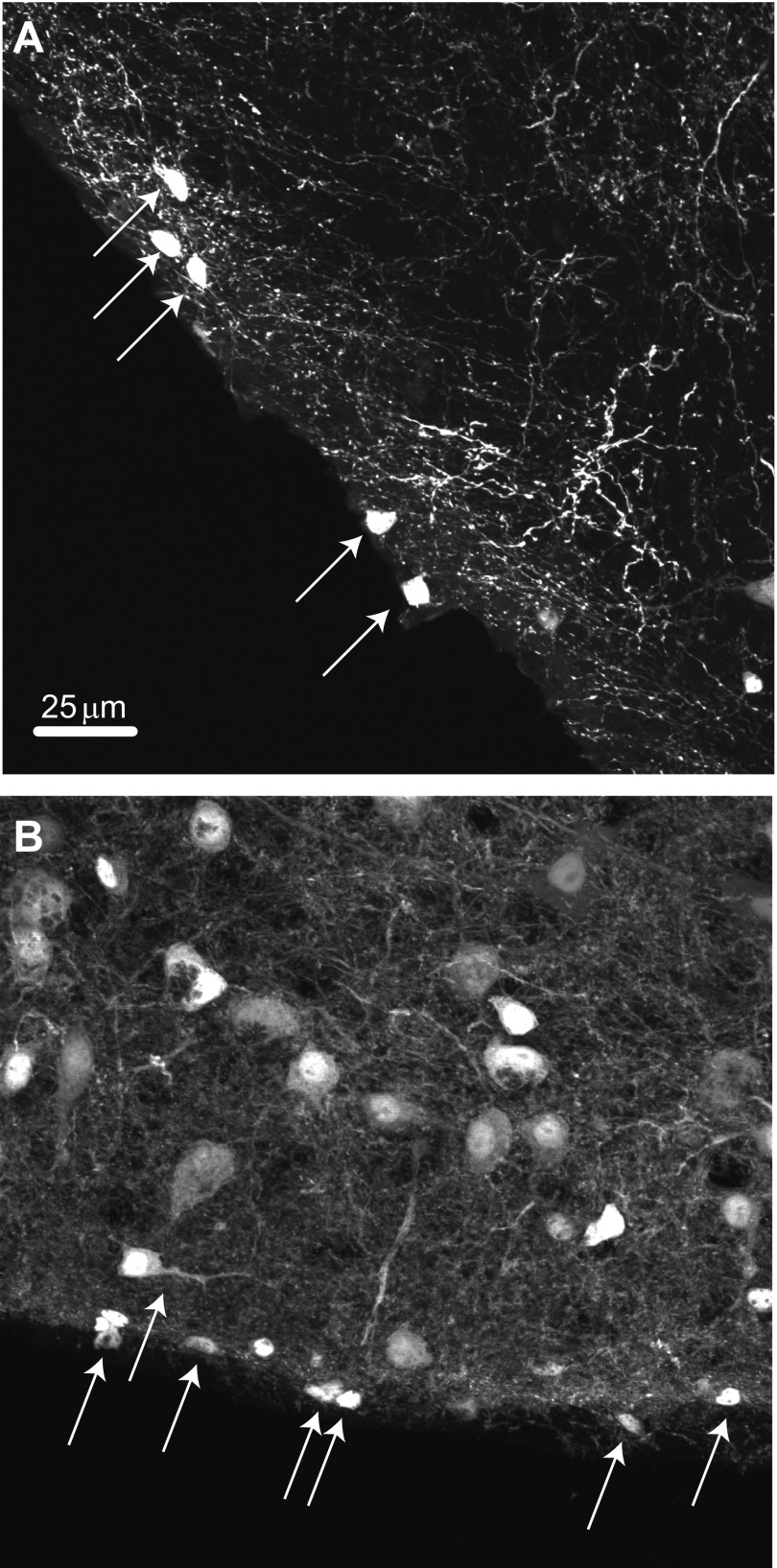
DCN stellate cells receive inhibitory inputs both from cartwheel cells and other stellate cells (Manis et al. 1994; Wouterlood et al. 1984). Whereas cartwheel cells are dual GABA-glycinergic neurons (Apostolides and Trussell 2013a; Roberts et al. 2008), previous immunohistochemical and anatomic studies have suggested that stellate cells are GABAergic interneurons (Mugnaini 1985; Wouterlood et al. 1984). Indeed, to train ourselves to recognize these tiny neurons for patch clamping in thick brain slices, we initially utilized a mouse line in which GFP expression was driven by the promoter for GAD65 (Hughes et al. 2005), a synthetic enzyme for GABA. In the DCN molecular layer in this mouse line, small cells could be observed lying close to the ependymal layer, with numerous fine processes extending toward the cell body layer (Fig. 3A), consistent with superficial stellate cells. Labeled small cells are also very common in the molecular layer of the cerebellum of this mouse (not shown). However, we were surprised that small cells can also be readily observed in a mouse line (Zeilhofer et al. 2005) in which GFP expression is driven by the promoter for GlyT2, a marker of glycinergic neurons (Fig. 3B). These observations suggested that inhibition by stellate cells may be mediated by both GABA and glycine.
Fig. 3.
Stellate cells express molecular markers of GABA/glycine co-releasing interneurons. A: photomicrograph of the DCN in a GAD65-GFP mouse, showing that stellate cells express the GABA-synthesizing enzyme GAD65. Arrows point to stellate cells located at the ependymal edge of the DCN. B: photomicrograph of the DCN in a section from a GlyT2-GFP mouse. Arrows point to small labeled neurons at the ependymal edge of the brain stem, suggesting that stellate cells also express the plasma membrane transporter required for functional glycine release. The larger neurons in the DCN molecular layer are presumably cartwheel cells, whereas the intense background reflects the high density of glycinergic fibers throughout the DCN. Scale bar in A applies also to B.
GABAergic and glycinergic transmission onto stellate cells.
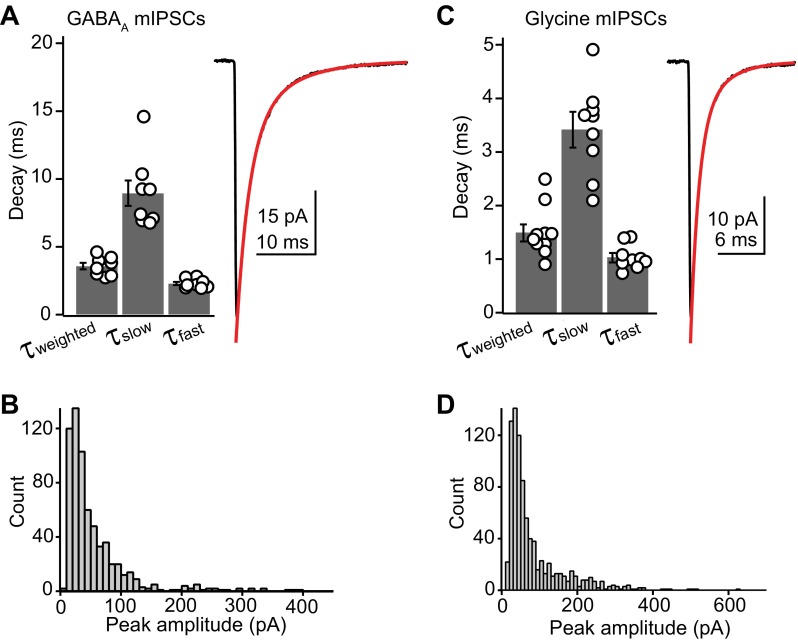
We therefore quantified the amplitude and kinetics of GABAergic and glycinergic miniature inhibitory postsynaptic currents (mIPSCs). GABAA mIPSCs were isolated with 10 μM NBQX, 50 μM d-APV, 500 nM strychnine, and 1 μM TTX. The decay phase of averaged GABAA mIPSCs were fit by the sum of two exponentials (Fig. 4A). The fast component decayed with a time constant of 2.2 ± 0.1 ms and represented 82 ± 2% of the total amplitude, whereas the slow component decayed with a time constant of 9.8 ± 1.3 ms. The weighted decay time constant for GABAA mIPSCs was 3.4 ± 0.2 ms (n = 12 cells). GABAA mIPSCs had an average amplitude of 44.4 ± 7.1 pA and a 10–90% rise time of 0.24 ± 0.01 ms (n = 9).
Fig. 4.
Inhibition is mediated by glycine and GABA. A, left: summary data showing the distribution of fast, slow, and weighted decay time constants for average GABAA miniature inhibitory postsynaptic currents (mIPSCs). Right, example average GABAA mIPSC. Red curve is the double exponential fit to the decay phase. Rate constants for the fit were as follows: τfast, 2.1 ms (73%); τslow, 7.1 ms (27%); τweighted, 3.41 ms. B: histogram showing the amplitude distribution of GABAA mIPSCs from a single cell. These data are from a different cell from that shown in A. C, left: summary data showing the distribution of fast, slow, and weighted decay time constants for average glycine mIPSCs. Right, example average glycine mIPSC. Red curve is the double exponential fit to the decay phase. Rate constants for the fit were as follows: τfast, 0.9 ms (75%); τslow, 3.0 ms (25%); τweighted, 1.5 ms. D: histogram showing the amplitude distribution of glycine mIPSCs from a single cell. The data are from a different cell from that shown in C.
Glycine mIPSCs (isolated with NBQX, d-APV, and SR95531) were prevalent in all cells tested and had an average amplitude of 42.9 ± 5.5 pA and a 10–90% rise time of 0.17 ± 0.01 ms (n = 14). The decay of glycine mIPSCs was fit with a double exponential function in 13/14 cells, whereas 1 cell was best fit by a single exponential. Similar to GABAA mIPSCs, glycinergic events were dominated (72 ± 6% of total amplitude) by a fast decay time constant of 1.1 ± 0.1 ms and a minor slow component of 3.6 ± 0.2 ms (n = 14). The average weighted decay time constant for glycine mIPSCs was 1.7 ± 0.2 ms (Fig. 4C). Glycine mIPSCs rose significantly faster than GABAA events (P < 0.001, unpaired t-test). Furthermore, both fast and slow decay components were significantly faster for glycinergic compared with GABAA mIPSCs (P < 0.01, unpaired t-test), although the relative weighting of fast vs. slow did not differ significantly (P = 0.19). The peak amplitude of the glycine and GABAA mIPSCs did not differ significantly (P = 0.82). Figure 4, B and D, shows amplitude histograms for GABA and glycine mIPSCs recorded in two different cells, illustrating that for both classes of event, amplitudes could exceed 300–400 pA. These results show that stellate cells receive similarly potent glycinergic and GABAergic inhibition, albeit with different temporal kinetics. These results do not, however, indicate whether these different events arose from cartwheel cell or stellate cell synaptic terminals.
Unitary synaptic and autaptic connections in DCN stellate cells.
Previous anatomic studies suggested the existence of reciprocal synaptic connections between DCN stellate cells (Mugnaini 1985), in agreement with the connectivity profile of stellate/basket cells of the cerebellum (Palay and Chan-Palay 1974). We searched for reciprocal connections by making paired recordings from neighboring (intersomatic distance <80 μm) DCN stellate cells. Similar to reports on cerebellar stellate cells (Kondo and Marty 1998), we found that 11/60 (18.3%) of attempted stellate connections (each pair = 2 connections) were synaptically coupled. An example pair is shown in Fig. 5A. The presynaptic cell was held in voltage clamp, and action potentials were elicited at 10 Hz by voltage steps from −70 to +5 mV for 100 μs (top trace). Five consecutive sweeps from the postsynaptic cell are shown in black, and an average of these sweeps is shown in red. Interestingly, a similar proportion of cells also displayed a prominent autaptic connection (19/89 cells tested, 21.3%), observed as an evoked IPSC that appears after triggering an escaping action potential in the recorded neurons, thus highlighting another parallel between the DCN and cerebellar stellate cells (Pouzat and Marty 1998). An example autaptic recording is shown in Fig. 5B. The top trace is an average of 20 trials showing the Na+ action current evoked by a voltage step. The middle traces are an overlay of 20 consecutive sweeps (4 failures) evoked at 0.5 Hz shown in black, with an average of successes shown in red. The transmission probability in this recording was 83.9% (131/156 successes). Overall, transmission fidelity was highly variable between connections, with success probability ranging from 16.8% to 100% when IPSCs were evoked at 0.5–1 Hz. The mean rate of successes, i.e., when an IPSC followed a presynaptic spike, was 56 ± 9% (n = 6 autapses and 4 paired recordings). The GABAA receptor antagonist SR95531 blocked the majority of transmission in seven unitary connections (5 autapses and 2 paired recordings). The bottom traces in Fig. 5B are 20 consecutive sweeps (black) and their average (red) after the addition of 10 μM SR95531, showing that this current was predominantly due to GABA release. Figure 5C plots the amplitude of autaptic events from the recording in Fig. 5B as a function of time as the GABAA receptor blocker was added.
Fig. 5.
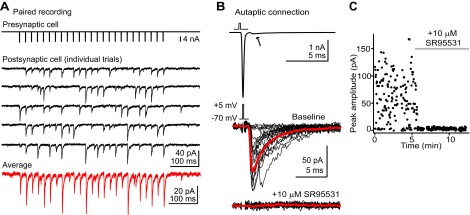
Reciprocal synaptic connections and autapses in DCN stellate cells. A: example recording of a synaptically coupled pair of stellate cells. The presynaptic cell (top trace) is held in voltage clamp and escaping spikes are triggered by short voltage steps. Middle traces (black) are 5 consecutive sweeps showing time-locked IPSCs in a postsynaptic stellate cell. Bottom trace (red) is an average of multiple trials from this cell. These experiments were performed in the presence of NBQX/R-CPP to block AMPA and NMDA receptors. IPSCs are inward due to a high-Cl− internal solution. B, top: a voltage step evokes a single escaping spike in a stellate cell. The arrow points to a small but significant synaptic current observed after the action current, typical of autaptic transmission. Middle traces are example autaptic IPSCs from this stellate cell. Note the difference in scale bar from the top trace. Black traces are individual trials; red trace is an average of successful transmission events. Bottom traces are recordings after bath application of SR95531. The escaping spike artifact was removed from these traces by digitally subtracting an average of transmission failures. C: time course of the experiment shown in B.
In some recordings, we noticed a small residual IPSC in the presence of SR95531, which together with the GlyT2 expression described above suggests that DCN stellate cells co-release glycine. Because of the high failure rate of these connections, however, it was difficult to determine whether these IPSCs originated specifically from the stellate cell of interest or if these were spontaneous glycinergic IPSCs coincidently originating from another presynaptic neuron. We therefore repeated the pharmacological experiments in stellate cells loaded with a CsCl-rich internal solution. By blocking K+ currents that would normally repolarize presynaptic action potentials, intracellular Cs+ should enhance exocytosis, thereby increasing the absolute IPSC size and reducing the variability of unitary IPSCs (Vincent and Marty 1996). Under these conditions, autaptic IPSCs showed almost no failures (Fig. 6A). Bath application of 10 μM SR95531 reduced the peak IPSC to 29 ± 7% of baseline (n = 13 cells). In 11/13 experiments, a significant SR95531-insensitive component remained, and this component was subsequently blocked by 0.5–1 μM strychnine (Fig. 6B). Thus glycine and GABA are functionally co-released from DCN stellate cells. Although transmission was mostly mediated by GABA and GABAA receptors in the majority of experiments, there was a significant variability in percentage of transmission mediated by glycine in the grouped data (Fig. 6B). This variability could reflect differential postsynaptic expression of glycine vs. GABAA receptors or different vesicular ratios of glycine and GABA. Furthermore, although we performed these pharmacology experiments soon after establishing a whole cell recording, differential washout of intracellular GABA vs. glycine (Apostolides and Trussell 2013a) may also contribute to the observed variability.
Fig. 6.
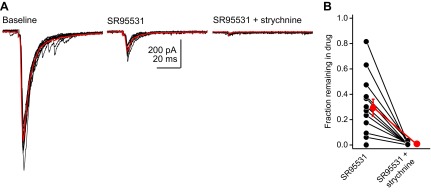
Stellate cells co-release GABA and glycine. A: example traces from a stellate cell filled with a CsCl internal solution. Black traces are individual sweeps; red trace is an average of sweeps in each individual condition. SR95531 blocks the majority of the autaptic IPSC in this cell. The remaining component is blocked by strychnine. B: summary data showing the fraction of IPSC (normalized to a baseline period) remaining after the addition of SR95531 and strychnine. Notice the spread of data points, with some cells displaying no glycine component, whereas some were predominantly glycinergic.
Activating fusiform cells generates GABA and glycinergic IPSCs in stellate cells.
We sought another method for determining the transmitter phenotype of stellate cells that would avoid the dialysis of presynaptic neurons, which may alter the effectiveness of inhibitory transmission (Apostolides and Trussell 2013a). The approach is based on the recent demonstration that stellate cells are electrically coupled to fusiform principal cells and that optogenetic activation of fusiform cells leads to action potentials in stellate cells (Apostolides and Trussell 2013b). This provides a means for selectively activating presynaptic stellate cells without doing presynaptic recordings. We therefore recorded from stellate cells in transgenic mice expressing channelrhodopsin2 in glutamatergic fusiform cells [Apostolides and Trussell 2013b: Thy1-ChR2 line 18 (Arenkiel et al. 2007) and VGluT2-ChR2 (Hägglund et al. 2010)]. Stellate cells were recorded with a cesium-based internal solution and voltage-clamped between 0 and +40 mV to establish an outward Cl− driving force. Pulses of blue light delivered to the slice often led to electrically conducted photocurrents and spikelets, as previously shown. However, we noticed that holding the stellate cell at positive potentials for extended periods (>5 min) of recording weakened the gap junction-mediated events on the recorded cell, leaving only chemical transmission (Fig. 7A). This enabled us to record IPSCs in stellate cells generated by the neighboring still-coupled stellate cells. Blue light pulses led to vigorous bursts of IPSCs (Fig. 7B). We determined the transmitter phenotype of stellate cells by averaging individual detected IPSCs (see methods) evoked under baseline conditions and in the presence of GABAA receptor blockers (Fig. 7C). GABA-glycine co-transmission was evident in 10/12 cells tested: SR95531 reduced the peak amplitude of average IPSCs to 47 ± 10% of baseline (range: 20–80% remaining in SR95531; n = 6 and n = 4 in Thy1-ChR2 and VGluT2-ChR2 mice, respectively). In two cells, SR95531 completely abolished light-evoked IPSCs (1 cell from each genotype). The fraction remaining in SR95531 in these experiments was not significantly different from that in the CsCl-filled autapses (P = 0.45, unpaired t-test). Together with the results of Figs. 3 and 6, these data show that DCN stellate cells co-release GABA and glycine from autaptic and reciprocal synaptic connections.
DISCUSSION
We have examined chemical synaptic transmission onto DCN stellate cells, contrasting the results with known properties of synapses onto cerebellar stellate cells. Although general parallels exist between these two types of interneuron in terms of GABAergic phenotype, subtype of AMPA receptors, and existence of gap junctions (see Apostolides and Trussell 2013b), several differences are apparent, notably in NMDA and glycine receptor activation. Given the kinetic properties of these receptors, our results suggest that the time course of synaptic excitation and inhibition may be quite different in stellate cells of the two regions.
Glutamate receptors on DCN stellate cells are Ca2+ permeable.
We observed that NMDA and AMPA receptors in DCN stellate cells could be activated by single stimuli to parallel fibers. This contrasts with cerebellar molecular layer interneurons, where the NMDA component of parallel fiber EPSCs is small (Clark and Cull-Candy 2002; Glitsch and Marty 1999) and is primarily recruited by glutamate spillover during intense presynaptic activity (Carter and Regehr 2000; Clark and Cull-Candy 2002; Nahir and Jahr 2013). However, similar to cerebellar stellate cells, intracellular polyamines cause inward rectification of current-voltage relations for AMPA receptor EPSCs, indicating the presence of GluA2-lacking, Ca2+-permeable AMPA receptors. Thus single action potentials in granule cells may activate two types of glutamate-gated, Ca2+-permeable conductance on DCN stellate cells, mediated by AMPA and NMDA receptors. DCN parallel fiber synapses onto cartwheel and fusiform cells are differentially depressed or potentiated by similar patterns of Ca2+ influx through NMDA receptors during spike-timing-dependent plasticity (Tzounopoulos et al. 2004, 2007). The presence of Ca2+-permeable AMPA receptors, which lack the outward rectification endowed by voltage-dependent Mg2+ block of NMDA receptors, suggests the possibility that parallel fiber plasticity rules in DCN stellate cells may be distinct from those previously described in cartwheel and fusiform cells (e.g., Lamsa et al. 2007). Moreover, the submillisecond decay kinetics of stellate cell AMPA receptors contrast sharply with the properties of AMPA receptors at parallel fiber synapses onto cartwheel cells and the apical dendrites of fusiform cells (Gardner et al. 1999, 2001), suggesting that similar patterns of parallel fiber activity will result in cell type-specific spike output.
Inhibitory transmission is mediated by glycine and GABA.
Glycinergic and GABAergic mIPSCs had similar amplitudes but different time courses, suggesting that the potency of both transmitter systems is approximately equal but also that the temporal profiles of inhibition may differ in a transmitter-specific manner. Based on labeling of small superficial cells similar to the ones we routinely record from, DCN stellate cells probably express the plasma membrane glycine transporter GlyT2, which transports glycine from the extracellular space into the nerve terminal for subsequent vesicular packaging (Gomeza et al. 2003; Rousseau et al. 2008). Most DCN stellate cells tested co-released glycine and GABA, although transmission was predominantly mediated by GABAA receptors. This suggests that stellate cells release more GABA than glycine or, alternatively, that postsynaptic GABAA receptors are more abundant and/or have higher conductance than glycine receptors. Although our data do not distinguish between these possibilities, cartwheel cells of the DCN appear to maintain a largely glycinergic phenotype through a preponderance of glycine receptors rather than a mismatch in GABA/glycine synthesis (Apostolides and Trussell 2013a).
Stellate cells probably also receive inhibitory inputs from cartwheel cells (Manis et al. 1994), and we have occasionally noticed large IPSC bursts typical of the cartwheel cell's “complex spike” firing pattern (data not shown; Golding and Oertel 1997; Roberts et al. 2008). Thus some proportion of glycine and GABAA mIPSCs in our data sets likely originate from cartwheel as well as stellate cell synapses.
Function of DCN stellate cells.
A recent study from Middleton et al. (2011) demonstrated that brain slices made from acoustically traumatized mice exhibited enhanced spread of electrical activity through the DCN molecular layer, apparently due to reduced GABAergic control of excitation. Given that GABA is the primary transmitter of stellate cells, it may be that reduced activity of stellate cells or their synapses may underlie this cellular correlate of tinnitus, and this possibility highlights the need to clarify how stellate cells function within the context of molecular layer circuitry. However, it should be noted that cartwheel cells of the molecular layer can also release GABA (Apostolides and Trussell 2013a; Roberts et al. 2008). Thus the relative contribution of GABAergic inhibition from stellate or cartwheel cells in controlling the spread of activity in the DCN is still unclear.
The three targets of parallel fibers-stellate cells, cartwheel cells, and fusiform cells-together form a remarkably sophisticated, interacting network. Cartwheel cells provide powerful inhibition to fusiform cells (Roberts and Trussell 2010), with synapses targeting near the fusiform soma (Rubio and Juiz 2004). Stellate cells, given their size and position, primarily reach the distal portions of fusiform dendrites (Apostolides and Trussell 2013b) and probably distal regions of cartwheel dendrites, as well. It is therefore possible that they serve to locally control excitation by parallel fibers in those distal compartments. However, the recent observation that fusiform cells communicate to stellate cells via gap junctions suggests a model in which fusiform cell activity, triggered by either parallel fibers or auditory nerve, is carried back into the molecular layer by stellate cells (Apostolides and Trussell 2013b). The inhibitory synapses stellate cells made onto one another and onto other cell types, as well as the gap junctions between stellate cells, may provide a mechanism for distributing these “retrograde” signals from fusiform cells in complex ways throughout the molecular layer. Further understanding of how such signals are processed will require clarifying the organization of these cell types with respect to the tonotopic axis and the subtypes of modalities represented by parallel fiber activity.
GRANTS
Funding was provided by National Institute of Deafness and Other Communication Disorders (NIDCD) Grant R01 DC004450 (to L. O. Trussell), and the Cornelia H. Stevens Achievement Rewards for College Scientist Scholar Award, the N.L. Tartar Trust Fellowship, and NIDCD Fellowship F31 DC012222 (to P. F. Apostolides).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.F.A. and L.O.T. conception and design of research; P.F.A. performed experiments; P.F.A. analyzed data; P.F.A. and L.O.T. interpreted results of experiments; P.F.A. and L.O.T. prepared figures; P.F.A. and L.O.T. drafted manuscript; P.F.A. and L.O.T. edited and revised manuscript; P.F.A. and L.O.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Carolina Borges-Merjane for preparing sections from GFP mice used for the confocal imaging in Fig. 3, Michael Bateschell and Ruby Larisch for help with mouse colony management, and Zhengquan Tang and Daniel Yaeger for comments on the manuscript.
REFERENCES
- Apostolides PF, Trussell LO. Rapid activity-independent turnover of vesicular transmitter content at a mixed glycine/GABA synapse. J Neurosci 33: 4768–4781, 2013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Regulation of interneuron excitability by gap junction coupling with principal cells. Nat Neurosci 16: 1764–1772, 2013b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54: 205–218, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci 28: 1140–1152, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci 31: 1–24, 2008 [DOI] [PubMed] [Google Scholar]
- Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci 15: 2777–2787, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Regehr WG. Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse. J Neurosci 20: 4423–4434, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Regehr WG. Quantal events shape cerebellar interneuron firing. Nat Neurosci 5: 1309–1318, 2002 [DOI] [PubMed] [Google Scholar]
- Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci 22: 4428–4436, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington LT, Rudolph S, Vande Lune P, Overstreet-Wadiche L, Wadiche JI. Spillover-mediated feedforward inhibition functionally segregates interneuron activity. Neuron 78: 1050–1062, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Carter AG, Regehr WG. Fast vesicle replenishment and rapid recovery from desensitization at a single synaptic release site. J Neurosci 27: 5448–5460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Miller RL, Young ED. Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus. J Neurophysiol 76: 3012–3024, 1996 [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Granule cell activation of complex-spiking neurons in dorsal cochlear nucleus. J Neurosci 17: 6798–6806, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol 83: 926–940, 2000 [DOI] [PubMed] [Google Scholar]
- Doiron B, Zhao Y, Tzounopoulos T. Combined LTP and LTD of modulatory inputs controls neuronal processing of primary sensory inputs. J Neurosci 31: 10579–10592, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Time course and permeation of synaptic AMPA receptors in cochlear nuclear neurons correlate with input. J Neurosci 19: 8721–8729, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci 21: 7428–7437, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J Neurosci 19: 511–519, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol 78: 248–260, 1997 [DOI] [PubMed] [Google Scholar]
- Gomeza J, Ohno K, Hülsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 40: 797–806, 2003 [DOI] [PubMed] [Google Scholar]
- Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci 13: 246–252, 2010 [DOI] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabó G, Erdélyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci USA 102: 9038–9043, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JTR, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54: 859–871, 2007 [DOI] [PubMed] [Google Scholar]
- Isope P, Franconville R, Barbour B, Ascher P. Repetitive firing of rat cerebellar parallel fibres after a single stimulation. J Physiol 554: 829–839, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. Stargazin (TARP gamma-2) is required for compartment-specific AMPA receptor trafficking and synaptic plasticity in cerebellar stellate cells. J Neurosci 31: 3939–3952, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol 486: 297–303, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci 21: 7848–7858, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Marty A. Synaptic currents at individual connections among stellate cells in rat cerebellar slices. J Physiol 509: 221–232, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SP, Trussell LO. Spontaneous spiking and synaptic depression underlie noradrenergic control of feed-forward inhibition. Neuron 171: 306–318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science 315: 1262–1266, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405: 454–458, 2000 [DOI] [PubMed] [Google Scholar]
- Mancilla JG, Manis PB. Two distinct types of inhibition mediated by cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol 102: 1287–1295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB. Responses to parallel fiber stimulation in the guinea pig dorsal cochlear nucleus in vitro. J Neurophysiol 61: 149–161, 1989 [DOI] [PubMed] [Google Scholar]
- Manis PB, Spirou GA, Wright DD, Paydar S, Ryugo DK. Physiology and morphology of complex spiking neurons in the guinea pig dorsal cochlear nucleus. J Comp Neurol 348: 261–276, 1994 [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res 148: 74–87, 2000 [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GMG, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci USA 108: 7601–7606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E. GABA neurons in the superficial layers of the rat dorsal cochlear nucleus: light and electron microscopic immunocytochemistry. J Comp Neurol 235: 61–81, 1985 [DOI] [PubMed] [Google Scholar]
- Nahir B, Jahr CE. Activation of extrasynaptic NMDARs at individual parallel fiber-molecular layer interneuron synapses in cerebellum. J Neurosci 33: 16323–16333, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Wright S, Cao XJ, Ferragamo M, Bal R. The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus. Hear Res 276: 61–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci 27: 104–110, 2004 [DOI] [PubMed] [Google Scholar]
- Oldfield CS, Marty A, Stell BM. Interneurons of the cerebellar cortex toggle Purkinje cells between up and down states. Proc Natl Acad Sci USA 107: 13153–13158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen KK, Mugnaini E. Neuronal circuits in the dorsal cochlear nucleus. In: Neuronal Mechanisms of Hearing, edited by Syka J, Aitkin L. New York: Plenum, 1981, p. 119–133 [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. New York: Springer, 1974 [Google Scholar]
- Pouzat C, Marty A. Autaptic inhibitory currents recorded from interneurones in rat cerebellar slices. J Physiol 509: 777–783, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requarth T, Sawtell NB. Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits. Curr Opin Neurobiol 21: 602–608, 2011 [DOI] [PubMed] [Google Scholar]
- Roberts MT, Bender KJ, Trussell LO. Fidelity of complex spike-mediated synaptic transmission between inhibitory interneurons. J Neurosci 28: 9440–9450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MT, Trussell LO. Molecular layer inhibitory interneurons provide feedforward and lateral inhibition in the dorsal cochlear nucleus. J Neurophysiol 104: 2462–2473, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Aubrey KR, Supplisson S. The glycine transporter GlyT2 controls the dynamics of synaptic vesicle refilling in inhibitory spinal cord neurons. J Neurosci 28: 9755–9768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401: 594–598, 1999 [DOI] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Differential distribution of synaptic endings containing glutamate, glycine, and GABA in the rat dorsal cochlear nucleus. J Comp Neurol 477: 253–272, 2004 [DOI] [PubMed] [Google Scholar]
- Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat Neurosci 10: 1260–1267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DP, Masterton RB, Glendenning KK. Role of acoustic striae in hearing: Reflexive responses to elevated sound sources. Behav Brain Res 97: 1–12, 1998 [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7: 719–725, 2004 [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron 54: 291–301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P, Marty A. Fluctuations of inhibitory postsynaptic currents in Purkinje cells from rat cerebellar slices. J Physiol 494: 183–199, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Mugnaini E, Osen KK, Dahl AL. Stellate neurons in rat dorsal cochlear nucleus studies with combined Golgi impregnation and electron microscopy: synaptic connections and mutual coupling by gap junctions. J Neurocytol 13: 639–664, 1984 [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bösl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol 482: 123–141, 2005 [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Cartwheel and superficial stellate cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol 69: 1384–1397, 1993 [DOI] [PubMed] [Google Scholar]