Abstract
When erythrocyte hemoglobin (Hb) is fully saturated with O2, nitric oxide (NO) covalently binds to the cysteine 93 residue of the Hb β-chain (B93-CYS), forming S-nitrosohemoglobin. Binding of NO is allosterically coupled to the O2 saturation of Hb. As saturation falls, the NO group on B93-CYS is transferred to thiols in the erythrocyte, and in the plasma, forming circulating S-nitrosothiols. Here, we studied whether the changes in ventilation during and following exposure to a hypoxic challenge were dependent on erythrocytic B93-CYS. Studies were performed in conscious mice in which native murine Hb was replaced with human Hb (hB93-CYS mice) and in mice in which murine Hb was replaced with human Hb containing an alanine rather than cysteine at position 93 on the Bchain (hB93-ALA). Both strains expressed human γ-chain Hb, likely allowing a residual element of S-nitrosothiol-dependent signaling. While resting parameters and initial hypoxic (10% O2, 90% N2) ventilatory responses were similar in hB93-CYS mice and hB93-ALA mice, the excitatory ventilatory responses (short-term potentiation) that occurred once the mice were returned to room air were markedly diminished in hB93-ALA mice. Further, short-term potentiation responses were virtually absent in mice with bilateral transection of the carotid sinus nerves. These data demonstrate that hB93-CYS plays an essential role in mediating carotid sinus nerve-dependent short-term potentiation, an important mechanism for recovery from acute hypoxia.
Keywords: hemoglobin, S-nitrosothiol, S-nitrosylation, ventilation, hypoxia, posthypoxia
the hypoxic ventilatory response (HVR) in conscious mice (19, 44) is likely to be dependent on the carotid bodies (CBs): in anesthetized mice, hypoxia stimulates carotid sinus nerve (CSN) activity (2), and HVR is markedly attenuated after bilateral CSN transection (25). Hypoxia stimulates chemoafferent activity in isolated mouse CB-CSN preparations (7), suggesting that peripheral mechanisms are sufficient to generate the HVR. The prevailing concept is that a hypoxemia in the CBs stimulates the release of “neurotransmitters” from primary glomus cells (PGCs), which in turn activate adjacent chemoafferents (29, 55). However, there is evidence that these chemoafferent neurons themselves might function as (low) O2 detectors, or at least that their activation is independent of PGC neurotransmitter release (53). Thus deoxygenated blood may act directly on chemoafferent neurons to increase CSN activity during hypoxic challenge in vivo.
In mice, ventilatory drive diminishes substantially during hypoxic challenge (19, 44). This ventilatory “roll-off” has been ascribed to mechanisms including 1) neurochemical processes in the nuclei tractus solitarii (nTS), which receive CB chemoafferent input (19), 2) a decrease in metabolic rate (58), 3) increases in cerebral blood flow (15), and 4) the direct depressive effects of hypoxia on brain stem neurons regulating respiratory burst rhythm and amplitude (37). It is now evident that CSN activity in mice (27, 47, 48) and other animals (16) displays substantial roll-off during brief exposure to hypoxia in vivo. This temporal decrease in CSN activity may result from decreased release of excitatory neurotransmitters from PGCs and/or diminished responsiveness of chemoafferents to these neurotransmitters or circulating factors (16). Our recent data show that murine roll-off is entirely dependent on the presence of S-nitrosoglutathione (GSNO) reductase (44), which degrades GSNO and decreases tissue S-nitrosylation status (34), suggesting that central or peripheral generation of GSNO sustains the ventilatory response to hypoxia. GSNO, known to increase minute ventilation (Vm), is formed by nitric oxide synthase (NOS) and by erythrocytic deoxygenation (6, 33). The cessation of a hypoxic challenge can result in an increase in ventilatory drive. This short-term potentiation of ventilation (STP), which occurs in humans, mice, rats (19, 26, 44, 55), and cats (39, 40), may be activated by chemoreceptor afferent input and mediated by a pontomedullary neural mechanism that lasts beyond the period of afferent input (39, 40). Although little is known about the exact neurochemical processes, STP is markedly diminished in 1) cats by blockade of central serotonin receptors (40), and 2) in neuronal NOS (nNOS) knock-out mice (26), the latter suggesting a role for NO or S-nitrosothiols (22, 36).
When erythrocyte hemoglobin (Hb) is fully saturated with O2, NO is covalently bound to the cysteine 93 residue of the β-chain (B93-CYS) forming S-nitroso-Hb (6, 14, 18, 45). Binding of NO is allosterically coupled to the O2 saturation of Hb. As Hb desaturates in a hypoxemic environment (6, 45), NO is transferred from Hb to thiol-containing compounds in erythrocyte membranes and cytosol, and in plasma thiols, forming circulating S-nitrosothiols (6, 14, 42). This B93-CYS-dependent process transfers NO+ to thiols thereby providing a source of vasodilator SNOs (1, 20, 51) to improve blood flow, and thereby augmenting O2 delivery to tissues from the erythrocytes. S-nitrosothiols exert positive effects on ventilatory function and pulmonary gas-exchange mechanisms (12–14). Moreover, the SNO, S-nitroso-N-acetyl-penicillamine (SNAP), increases CSN chemoafferent activity in isolated CB-CSN preparations from cats (24, 41).
Therefore, we hypothesized that B93-CYS might be involved in ventilatory control. To our knowledge, hypoxic ventilatory signaling from a specific erythrocytic Hb residue with the ability to report blood/Hb O2 content, as opposed to dissolved O2 gas tension, has not previously been studied. We first confirmed the role of the CB-CSN complex in HVR and STP in conscious wild-type mice. Second, we tested whether HVR and STP were dependent on the presence of B93-CYS (hB93-CYS), comparing mice expressing normal human hB93-CYS in place of murine Hb (hB93-CYS mice) to wild-type mice and to those in which murine Hb was replaced with human Hb containing an alanine at position 93 (hB93-ALA mice) (23). The B93-ALA substitution should diminish S-nitrosothiol-Hb (SNO-Hb) signaling (1, 51), although signaling through SNO-γ-Hb may persist (43).
MATERIALS AND METHODS
Mice and genotyping.
All studies were carried out in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the University of Virginia Animal Care and Use Committee. Adult male C57 black 6 (C57BL6) mice were obtained from Jackson Laboratories (Bar Harbor, ME). Breeding pairs of 1) C57BL6 mice in which native murine Hb was replaced with human Hb (hB93-CYS), and 2) C57BL6 mice in which murine Hb was replaced with human Hb in which the cysteine in the 93 position of the β-chain was replaced with an alanine (hB93-ALA), were kindly provided by Dr. Tim Townes (Univ. of Alabama, Birmingham) (23). Note that these mice also expressed human γ-chain gene. The hB93-CYS and hB93-ALA mice and their off-spring were maintained in a breeding colony at the University of Virginia.
Genotypes of hB93-CYS or hG93-ALA were confirmed using the following primers: human γ-chain, 5′-GTTTAGCCAGGGACCGTTTCAG and 5′-AATTCTGGCTTATCGGAGGCAAG; wild-type human β-chain, 5′-TTGAGCAATGTGGACAGAGAAGG and 5′-GTCAGAAGCAAATGTGAGGAGCA. Additionally, the restriction endonuclease MaeIII digests human wild-type, but not human B93-cysteine to B93-alanine. Post-digest B93-cysteine to B93-alanine primers were included with the β-chain primers: 5′-ACAAGACAGGTTTAAGGAGACC-3′ and 5′-CTGTACCCTGTTACTTCTCCCC-3′. PCR conditions were 2 min at 95°C, 30 s at 95°C, 20 s at 63°C, 1 min at 68°C, with 31 cycles after step two, followed by 10 min at 68°C. Human Hb electrophoresis was performed in our clinical laboratory on a subpopulation of mice at different ages showing a loss of fetal Hb in hB93-CYS mice, but a steady level of ∼1% fetal Hb over time in the hB93-ALA mice.
Surgical transection of the carotid sinus nerves.
Male C57BL6 mice were anesthetized with isoflurane (2% in room air) and a midline neck incision was made to reveal both carotid sinus nerves (CSNs). In some mice, the CSNs were isolated but not transected (SHAM mice) whereas in other mice, the CSNs were isolated and transected (CSNX mice). The mice were given 10–12 days to recover from surgery before use.
Protocols for hypoxic challenge.
Ventilatory parameters were recorded in conscious unrestrained mice via whole body plethysmography (PLY3223; BUXCO, Wilmington, NC) as described previously (44). The parameters were frequency of breathing (fr); tidal volume (Vt); minute ventilation (Vm); inspiratory time (Ti); expiratory time (Te); Vt/Ti, an index of respiratory drive (see 44); peak inspiratory flow (PIF); and peak expiratory flow (PEF). The provided software (Fine Pointe, BUXCO) constantly corrected digitized values for changes in chamber temperature and humidity. A rejection algorithm was included in the breath-by-breath analysis to exclude episodes of nasal breathing. Pressure changes associated with the respiratory waveform were converted to volumes (Vt, PIF, and PEF) using the algorithm of Epstein and colleagues (8, 9). More specifically, factoring in chamber temperature and humidity, the cycle analyzers filtered the acquired signals, and proprietary algorithms (Fine Pointe, BUXCO) generated an array of box flow data that identified a breath. From that data vector, the minimum and maximum values were determined. The flows at this point were “box flow” signals. From this array, the minimum and maximum box flow values were determined. Minimum and maximum box flows were then multiplied by the compensation factor provided by the selected algorithm (8, 9), thus producing the Vt, PIF, and PEF parameters. The mice were placed in the plethysmography chambers and allowed 45–60 min to acclimatize and then exposed to a hypoxic (10% O2, 90% N2) or hypercapnic-normoxic challenges (5% CO2, 21% O2, 76% N2) for 15 min after which time they were reexposed to room air for 15 min (44).
Body temperature recordings.
Male C57 mice (n = 9, 86.8 ± 1.2 days of age, 23.1 ± 0.2 g body wt), hB93-CYS mice (n = 6, 84.8 ± 0.9 days, 23.3 ± 0.2 g) and hB93-ALA mice (n = 6, 86.3 ± 1.0 days, 23.0 ± 0.2 g) and colonic body temperature (Tc) recording equipment, namely, a thermistor probe (YS 451, Yellow Springs Instruments, Yellow Springs, OH) connected to a battery-operated thermometer unit (YSI 400), were placed in a Hypoxic Work Station (Coy, Grass Lake, MI), which initially had room air flowing through its housing chamber. This chamber allowed access to the mice and recording equipment through air-tight arm holes. After 5 min, the thermistor probe was inserted 1.5–2.0 cm into the rectum to record Tc. The mice were then removed and 10% O2 was established in the chamber using a Pro:Ox Model 350 unit (Biospherix, Lacona, NY), which controlled the fractional concentration of O2 in inspired gas by solenoid controlled infusion of N2 balanced against an inward leak of air. The mice were again placed in the chamber maintained at 10% O2, and Tc was recorded 5 and 15 min during hypoxic challenge and 5 and 15 min after return to room air (induced rapidly by turning off the flow of N2 and fully opening the chamber).
Statistics.
All recorded data and derived-parameters, namely, Vt/Ti and total responses (cumulative %changes from prehypoxia values), are presented as means ± SE. The following steps were taken to determine the total responses during hypoxic challenge and return to room air. For each animal, we summed the 15 values (1 per min) recorded before the hypoxic challenge, and the 15 values recorded both during the hypoxic change and subsequent return to room air. We then determined the cumulative response by the formula, total response (%change) = {[(sum of values during hypoxic challenge or return to room air) − (sum of values before hypoxic challenge)]/sum of values before hypoxic challenge} × 100. We then determined the mean and SE of the group data. All data were analyzed by one-way or two-way ANOVA followed by Student's modified t-test with Bonferroni corrections for multiple comparisons between means (Palmer et al., 2013).
RESULTS
Baseline parameters and body temperature responses in C57BL6, hB93-CYS, and hB93-ALA mice.
There were no between-group differences in age, body weight, or baseline ventilatory parameters in C57BL6, hB93-CYS, or hB93-ALA mice used in the ventilatory studies (Table 1). Tc values prior to the hypoxic challenge were similar in C57BL6, hB93-CYS, and hB93-ALA mice (Table 2). In C57BL6 mice, Tc fell slightly during exposure to hypoxia (maximal response at 15 min of −0.41 ± 0.05°C) and gradually recovered to prehypoxia levels upon return to room air. The changes in Tc recorded in hB93-CYS and hB93-ALA mice were similar to one another and those recorded in the C57BL6 mice (P > 0.05, for all between-group comparisons).
Table 1.
Animal numbers, ages, weights, and resting ventilatory parameters
| Parameter | C57BL6 | hB93-CYS | hB93-ALA |
|---|---|---|---|
| Number | 13 | 15 | 16 |
| Age, days | 87 ± 2 | 85 ± 2 | 86 ± 2 |
| Body weights, g | 23.1 ± 0.3 | 23.2 ± 0.4 | 22.9 ± 0.3 |
| Frequency, breaths/min | 227 ± 11 | 221 ± 10 | 217 ± 6 |
| Tidal volume, ml | 0.27 ± 0.02 | 0.25 ± 0.02 | 0.28 ± 0.01 |
| Minute ventilation, ml/min | 60 ± 6 | 56 ± 5 | 61 ± 4 |
| Inspiratory time, s | 0.096 ± 0.006 | 0.104 ± 0.006 | 0.111 ± 0.008 |
| Expiratory time, s | 0.196 ± 0.011 | 0.201 ± 0.013 | 0.192 ± 0.007 |
| Tidal volume/Inspiratory time, ml/s | 2.77 ± 0.24 | 2.43 ± 0.22 | 2.63 ± 0.17 |
| Peak inspiratory flow, ml/s | 4.6 ± 0.4 | 4.8 ± 0.5 | 4.8 ± 0.4 |
| Peak expiratory flow, ml/s | 3.6 ± 0.3 | 3.7 ± 0.4 | 4.1 ± 0.3 |
Data are presented as means ± SE. There were no between-group differences in any parameter (P > 0.05, for all comparisons).
Table 2.
Changes in body temperature during hypoxic challenge and upon return to room air
| Actual Body Temperature, oC |
Arithmetic Change, oC |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypoxia |
Room air |
Hypoxia |
Room air |
||||||
| Group | Pre | 5 min | 15 min | 5 min | 15 min | 5 min | 15 min | 5 min | 15 min |
| C57BL6 | 36.6 ± 0.1 | 36.5 ± 0.1 | 36.1 ± 0.1 | 36.3 ± 0.1 | 36.5 ± 0.1 | −0.10 ± 0.08* | −0.41 ± 0.05* | −0.21 ± 0.06* | −0.02 ± 0.06 |
| B93-CYS | 36.5 ± 0.1 | 36.3 ± 0.1 | 36.1 ± 0.1 | 36.2 ± 0.1 | 36.6 ± 0.1 | −0.14 ± 0.09* | −0.36 ± 0.06* | −0.22 ± 0.07* | +0.13 ± 0.08 |
| B93-ALA | 36.5 ± 0.1 | 36.4 ± 0.1 | 36.2 ± 0.1 | 36.3 ± 0.1 | 36.6 ± 0.1 | −0.11 ± 0.06* | −0.34 ± 0.07* | −0.20 ± 0.08* | +0.08 ± 0.07 |
Data are presented as means ± SE. There were 6 mice in each group.
P < 0.05, significant change from prevalues. There were no between-group differences at any time point (P > 0.05, for all comparisons).
Ventilatory responses during hypoxic challenge for hB93-CYS and hB93-ALA mice.
Exposure of hB93-CYS mice to hypoxic challenge (10% O2, 90% N2) elicited immediate responses including 1) increases in fr and Vt, and therefore Vm; 2) decreases in Ti and Te; 3) increases in Vt/Ti; and 4) increases in PIF and PEF (Figs. 1–3 and Table 3). Ventilatory responses, including fr, Vt, Vm, Ti, Vt/Ti, and PIF, displayed substantial roll-off during the hypoxic challenge whereas Te and PEF responses did not. As such, the increases in fr observed at the latter stages of the challenge were associated with a reduction in Te rather than Ti. The ventilatory responses that occurred in the hB93-ALA mice were similar to those in the hB93-CYS mice with respect to the initial maximal responses and the degree of roll-off, except for modest attenuation of PEF (Figs. 1–3, Table 3).
Fig. 1.
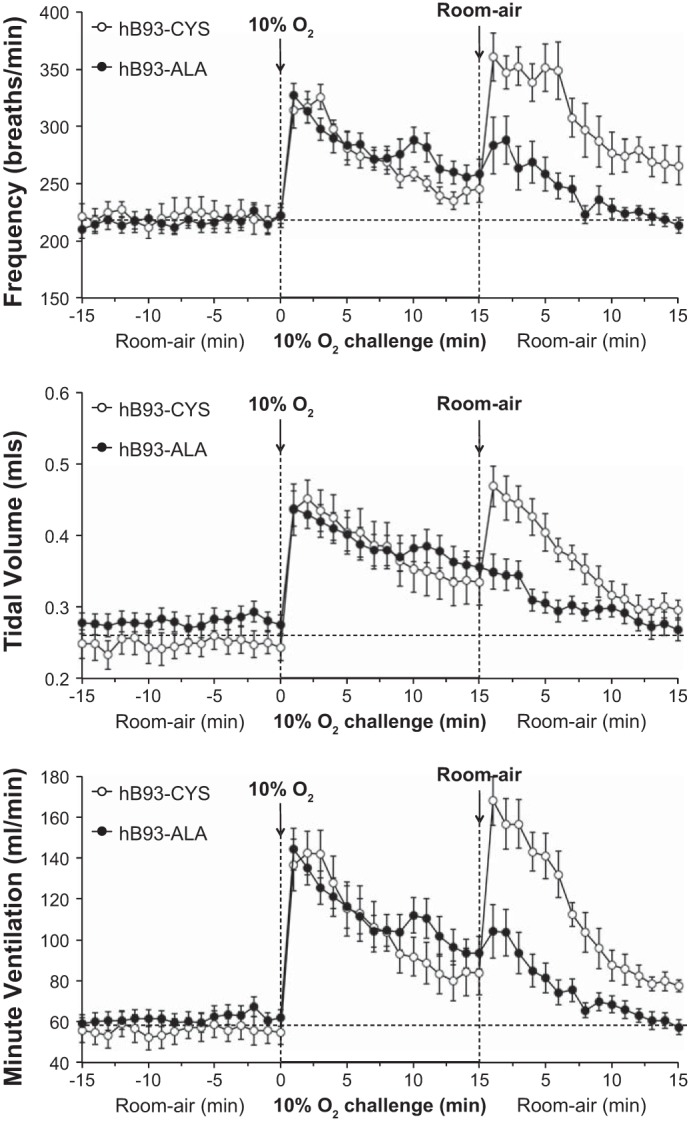
Frequency of breathing (top panel), tidal volume (middle panel), and minute ventilation (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) before and during a hypoxic challenge (10% O2, 90% N2) and upon subsequent return to room air. The data are presented as means ± SE. Note that the horizontal dashed lines in the panels of Figs. 1–3 and Figs. 5 and 6 denote the average values recorded before hypoxic challenge (data taken from both groups of mice).
Fig. 3.
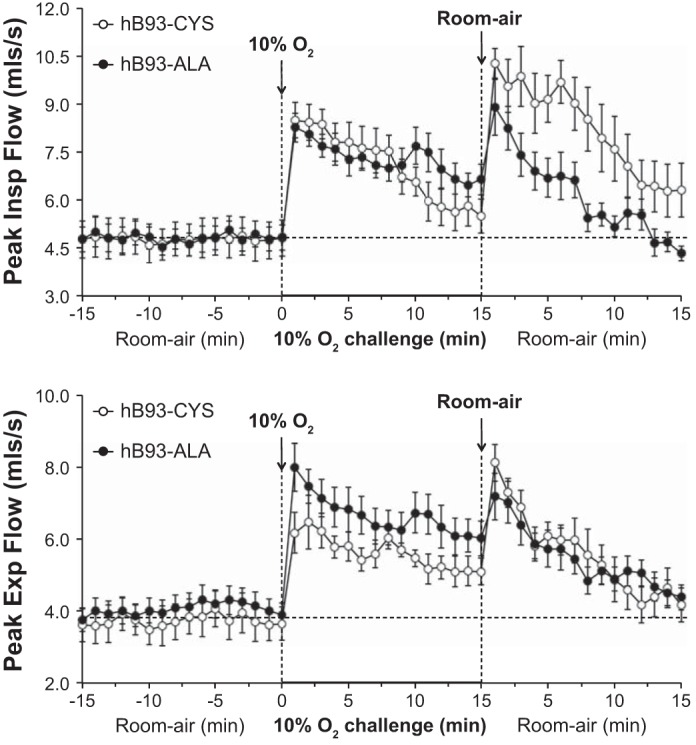
Peak inspiratory (Insp) flow (top panel) and peak expiratory (Exp) flow (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) before and during hypoxic challenge (10% O2, 90% N2) and upon return to room air. The data are presented as means ± SE.
Table 3.
Ventilatory responses (%change from pre values) in hB93-CYS and hB93-ALA mice during hypoxic and challenge and upon subsequent return to room air
| Hypoxic Challenge |
Return to Room Air |
||||
|---|---|---|---|---|---|
| Parameter | Group | Maximum | 15 min | Maximum | 15 min |
| Frequency, breaths/min | hB93-CYS | +48 ± 4* | +12 ± 4* | +81 ± 10* | +27 ± 6* |
| hB93-ALA | +51 ± 4* | +21 ± 6* | +48 ± 6*† | 0 ± 4† | |
| Tidal volume, ml | hB93-CYS | +84 ± 8* | +35 ± 4* | +98 ± 7* | +21 ± 4* |
| hB93-ALA | +67 ± 6* | +27 ± 4* | +34 ± 5*† | −3 ± 5† | |
| Minute ventilation, ml/min | hB93-CYS | +173 ± 16* | +52 ± 8* | +214 ± 18* | +47 ± 6* |
| hB93-ALA | +139 ± 14* | +55 ± 9* | +102 ± 12*† | −3 ± 7† | |
| Inspiratory time, s | hB93-CYS | −30 ± 3* | 0 ± 4 | −42 ± 2* | −12 ± 3* |
| hB93-ALA | −24 ± 3* | −8 ± 3* | −28 ± 3*† | −1 ± 3† | |
| Expiratory time, s | hB93-CYS | −30 ± 4* | −23 ± 5* | −36 ± 5* | −21 ± 3* |
| hB93-ALA | −42 ± 3* | −25 ± 3* | −1 ± 6† | −3 ± 4† | |
| Tidal volume/Inspiratory time, ml/s | hB93-CYS | +150 ± 12* | +36 ± 5* | +227 ± 19* | +38 ± 7* |
| hB93-ALA | +120 ± 15* | +42 ± 6* | +91 ± 12*† | −1 ± 6† | |
| Peak inspiratory flow, ml/s | hB93-CYS | +92 ± 8* | +18 ± 4* | +133 ± 16* | +32 ± 8* |
| hB93-ALA | +87 ± 10* | +39 ± 6*† | +108 ± 14* | −4 ± 6*† | |
| Peak expiratory flow, ml/s | hB93-CYS | +96 ± 11* | +51 ± 8* | +130 ± 14* | +6 ± 6 |
| hB93-ALA | +114 ± 14* | +53 ± 7* | +81 ± 10*† | +9 ± 5 | |
Data are presented as means ± SE. There were 16 mice in each group.
P < 0.05, significant response.
P < 0.05, B93-ALA mice vs. B93-CYS mice.
Ventilatory responses upon return to room air for hB93-CYS and hB93-ALA mice.
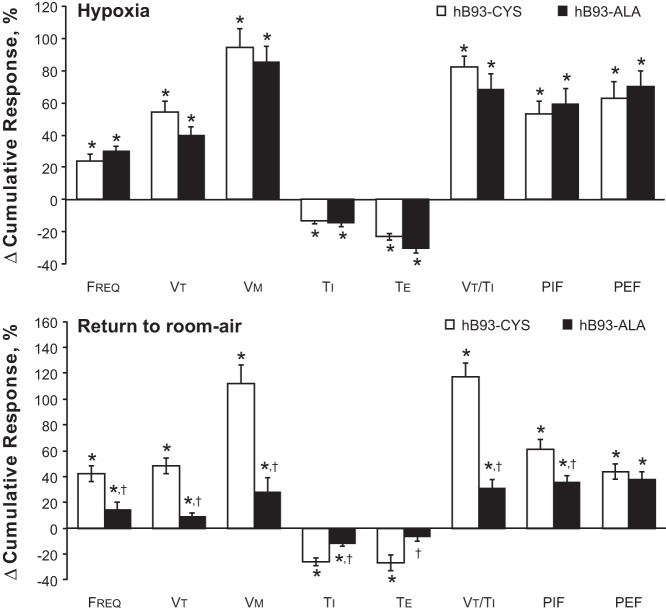
The hB93-CYS mice displayed an array of ventilatory responses upon return to room air (Figs. 1–3, Table 3), including 1) increases in fr, Vt, and Vm; 2) decreases in Ti and Te; and 3) increases in Vt/Ti, PIF, and PEF. These responses diminished over time although many had not returned to prelevels by 15 min. In contrast, many of the ventilatory responses that occurred after return to room air were markedly blunted in the hB93-ALA mice. The initial increases in fr (and the associated decreases in Ti and Te), Vt, Vm, and Vt/Ti were substantially smaller in hB93-ALA mice than in hB93-CYS mice (Figs. 1 and 2, Table 3), whereas the initial increases in PIF and PEF were similar in both groups (Fig. 3, Table 3). The cumulative %responses were considerably smaller in hB93-ALA than in hB93-CYS mice for all parameters except PEF (Fig. 4, bottom).
Fig. 2.
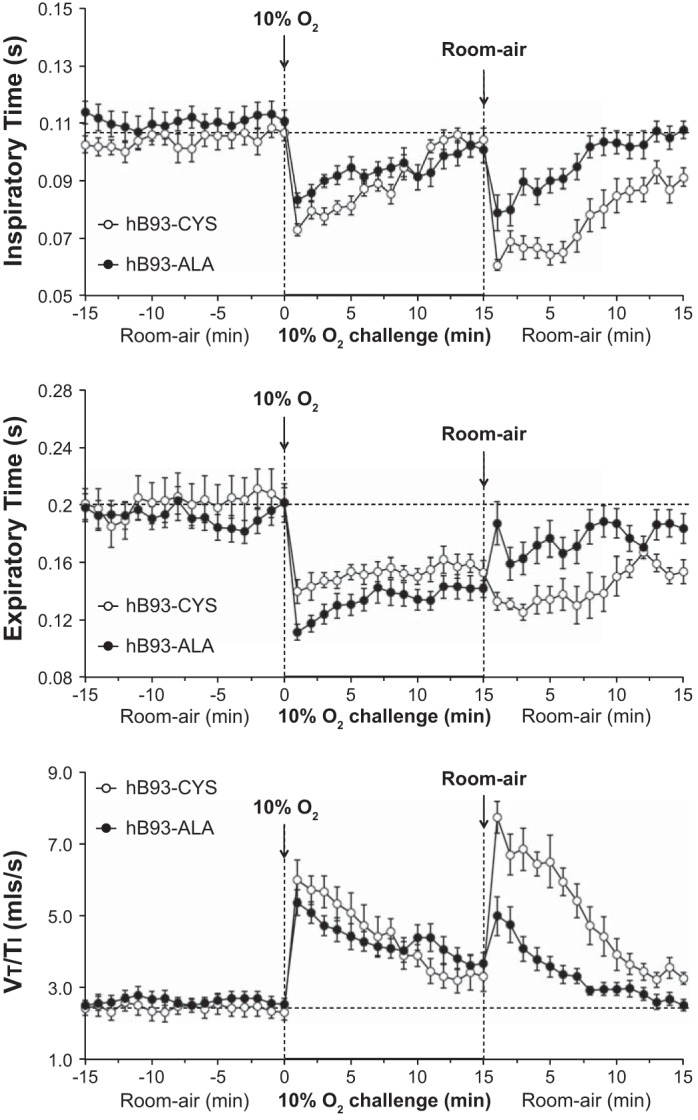
Inspiratory time (top panel), expiratory time (middle panel), and tidal volume/inspiratory time (Vt/Ti) (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) before and during hypoxic challenge (10% O2, 90% N2) and upon return to room air. The data are presented as means ± SE.
Fig. 4.
Total responses (cumulative %change from pre values) in ventilatory parameters in hB93-CYS mice (n = 15) and hB93-ALA mice (n = 16) during hypoxic challenge (top panel) and upon return to room air (bottom panel). The data are presented as means ± SE. Freq, breathing frequency; Vt, tidal volume; Vm, minute ventilation; Ti, inspiratory time; Te, expiratory time; PIF, peak inspiratory flow; PEF, peak expiratory flow; *P < 0.05, significant response. †P < 0.05, hB93-ALA mice vs. hB93-CYS mice.
Comparison of ventilatory responses in hB93-CYS and C57BL6 mice.
The changes in fr, Vt, and Vm during and following hypoxic challenge in the hB93-CYS mice described above and control C57BL6 mice (n = 13) are summarized in Fig. 5. The responses during hypoxic challenge were similar in both groups with respect to maximal and total responses and values at the 15 min time point of the challenge (Table 4, P > 0.05, for all comparisons). After return to room air, the changes in fr and Vt were similar in the hB93-CYS and C57BL6 mice except that the total responses for Vt and Vm were somewhat greater in the hB93-CYS mice (Table 4).
Fig. 5.
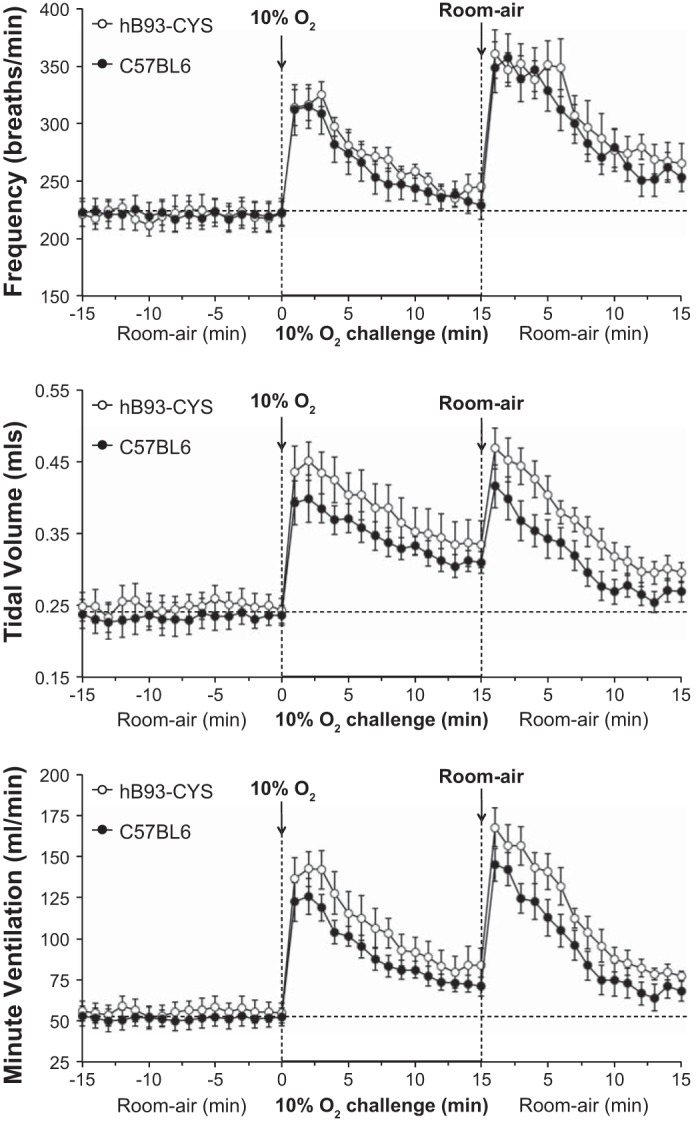
Frequency of breathing (top panel), tidal volume (middle panel), and minute ventilation (bottom panel) in conscious freely moving hB93-CYS mice (n = 15) and C57BL6 mice (n = 13) before and during hypoxic challenge (10% O2, 90% N2) and upon subsequent return to room air. The data are presented as means ± SE.
Table 4.
Comparison of responses between hB93-CYS and wild-type C57BL6 mice
| Parameter |
||||
|---|---|---|---|---|
| Group | Frequency | Tidal Volume | Minute Ventilation | |
| Hypoxic challenge | ||||
| Maximal response, %change from prevalues | hB93-CYS | +47 ± 5* | +81 ± 10* | +156 ± 14* |
| C57BL6 | +42 ± 5* | +71 ± 8* | +143 ± 15* | |
| Response at 15 min, %change from prevalues | hB93-CYS | +11 ± 4* | +35 ± 7* | +50 ± 6* |
| C57BL6 | +4 ± 3 | +33 ± 5* | +39 ± 5* | |
| Total response, %change from prevalues | hB93-CYS | +23 ± 3 | +54 ± 7* | +91 ± 12* |
| C57BL6 | +18 ± 2 | +48 ± 6* | +77 ± 9* | |
| Return to room air | ||||
| Maximal response, %change from prevalues | hB93-CYS | +63 ± 8* | +88 ± 9* | +201 ± 20* |
| C57BL6 | +60 ± 7* | +78 ± 9* | +181 ± 19* | |
| Response at 15 min, %change from prevalues | hB93-CYS | +20 ± 4* | +19 ± 3* | +39 ± 6* |
| C57BL6 | +15 ± 4* | +15 ± 4* | +32 ± 5* | |
| Total response, %change from prevalues | hB93-CYS | +39 ± 3* | +46 ± 4* | +105 ± 8* |
| C57BL6 | +33 ± 3* | +34 ± 3*† | +82 ± 7*† | |
Data are presented as means ± SE. There were 15 mice in the hB93-CYS group and 13 mice in the C57BL6 group.
P < 0.05, significant response.
P < 0.05, C57BL6 mice vs. hB93-CYS mice.
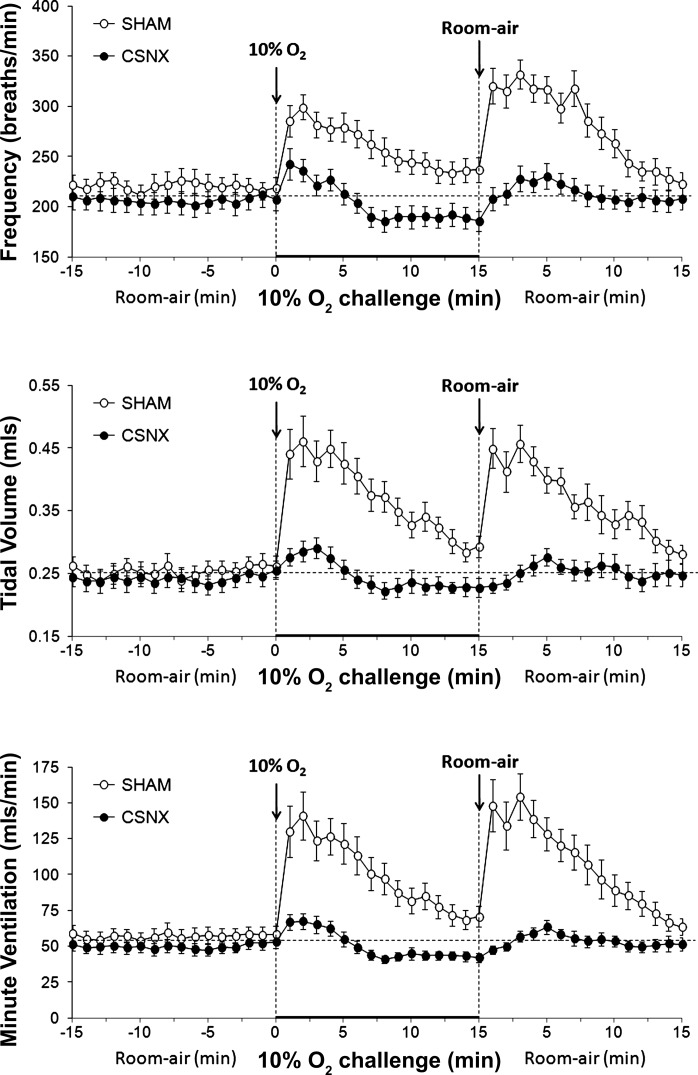
Ventilatory responses in SHAM and CSNX mice.
There were no between-group differences in age, body weight, or resting ventilatory parameters in the SHAM or CSNX mice (Table 5) (P > 0.05 for all comparisons). The changes in fr, Vt, and Vm during and after hypoxic challenge in SHAM and CSNX mice (both C57BL6) are summarized in Fig. 6 and Table 6. The ventilatory responses during hypoxic challenge and upon return to room air were markedly diminished in CSNX mice compared with SHAM mice. In contrast, the maximal responses elicited during hypercapnic challenge were similar in SHAM and CSNX mice whereas the responses upon return to room air were markedly diminished in CSNX mice (data not shown).
Table 5.
Resting variables in SHAM and CSNX mice (X57BL6)
| Experimental Group |
||
|---|---|---|
| Parameter | SHAM | CSNX |
| n | 9 | 9 |
| Age, days | 83 ± 3 | 84 ± 2 |
| Body weight, g | 22.6 ± 0.5 | 21.2 ± 0.6 |
| Frequency, breaths/min | 221 ± 12 | 206 ± 11 |
| Tidal volume, ml | 0.25 ± 0.02 | 0.24 ± 0.02 |
| Minute ventilation, ml/min | 57 ± 6 | 50 ± 5 |
Data are presented as means ± SE.
SHAM, sham-operated mice.
CSNX, mice with bilateral carotid sinus nerve transection. There were 9 mice in each group. There were no between-group differences in any parameter (P > 0.05, for all comparisons).
Fig. 6.
Frequency of breathing (top panel), tidal volume (middle panel), and minute ventilation (bottom panel) in conscious freely moving sham-operated C57BL6 (SHAM) mice (n = 9) or bilateral carotid sinus nerve-transected C57BL6 (CSNX) mice (n = 9) before and during hypoxic challenge (10% O2, 90% N2) and upon subsequent return to room air. The data are presented as means ± SE.
Table 6.
Comparison of responses between SHAM and CSNX mice (all C57BL6)
| Parameter |
||||
|---|---|---|---|---|
| Group | Frequency | Tidal Volume | Minute Ventilation | |
| Hypoxic challenge | ||||
| Maximal response, %change from prevalues | SHAM | +36 ± 6* | +79 ± 6* | +143 ± 8* |
| CSNX | +17 ± 2*† | +19 ± 4*† | +35 ± 6*† | |
| Response at 15 min, %change from prevalues | SHAM | +7 ± 3* | +16 ± 4* | +24 ± 4* |
| CSNX | −10 ± 2*† | −6 ± 3*† | −15 ± 3*† | |
| Total response, %change from prevalues | SHAM | +18 ± 3* | +46 ± 4* | +74 ± 6* |
| CSNX | −1 ± 2† | +3 ± 3† | +2 ± 4† | |
| Return to room air | ||||
| Maximal response, %change from prevalues | SHAM | +52 ± 7* | +80 ± 6* | +174 ± 15* |
| CSNX | +11 ± 3*† | +17 ± 7*† | +32 ± 10*† | |
| Response at 15 min, %change from prevalues | SHAM | +1 ± 2 | +11 ± 3* | +12 ± 4* |
| CSNX | +1 ± 3 | +2 ± 3 | +3 ± 3 | |
| Total response, %change from prevalues | SHAM | +27 ± 4* | +44 ± 3* | +88 ± 8* |
| CSNX | +4 ± 2† | +5 ± 4† | +10 ± 5† | |
Data are presented as means ± SE.
SHAM, sham-operated mice.
CSNX, mice with bilateral carotid sinus nerve transection. There were 9 mice in each group.
P < 0.05, significant response.
P < 0.05, CSNX mice versus SHAM mice.
Fetal hemoglobin expression in hB93-CYS and hB93-ALA mice.
Hb electrophoresis demonstrated human fetal Hb expression in hB93-CYS and hB93-ALA mice (Fig. 7). There was a trend for fetal-Hb to fall with age in B93CYS mice and to rise with age in B93-ALA mice. Percent fetal-Hb in hB93-ALA and hB93-CYS mice of 4–17 wk of age were similar to one another (hB93-ALA: 1.00 ± 0.08% vs. hB93-CYS: 0.83 ± 0.13%, P > 0.05). However, percent fetal-Hb in hB93-ALA mice of 10–17 wk of age was higher than those of 10- to 17-wk-old hB93-CYS mice (hB93-ALA: 1.07 ± 0.07% vs. hB93-CYS: 0.70 ± 0.06%, P < 0.05).
Fig. 7.
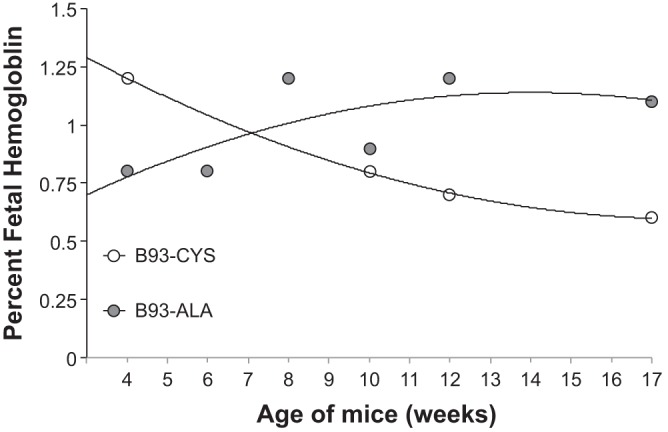
Percent fetal hemoglobin in B93-CYS and B93-ALA mice. Each point is from one mouse.
DISCUSSION
The role of hemoglobin in short-term potentiation.
The major finding of this study was that murine STP, in which return from hypoxia to room air resulted in a dramatic increase in ventilatory drive lasting for at least 10 min, was similar in the hB93-CYS and C57BL6 mice, but was virtually absent in the hB93-ALA mice. This suggests that murine STP is dependent on erythrocytic hB93-CYS. STP was also markedly diminished in the conscious CSNX C57BL6 mice, consistent with evidence that the CB-chemoafferent complex is essential for the generation of STP in other species (39, 40). Of note, CSN activity in isolated CB-CSN preparations does not increase on reoxygenation (27, 47, 48) suggesting that mediators intrinsic to the CB complex are not responsible for the increase in CSN activity in vivo in STP, whereas the CB itself is essential for initiation of the HVR. Further, O2 tension itself was not likely responsible for different STP between the hB93-CYS and the hB93-ALA mice. Although the hB93-ALA mutation slightly increases the affinity of Hb for O2 (35), the mutant (and wild-type knock-in) mice had an identical initial HVR responses, demonstrating that dissolved O2 levels could not have been altered in the hB93-ALA mice to a degree sufficient to affect the CB response to hypoxia.
On the other hand, hB93-CYS-dependent reactions have been reported to signal a change in Hb conformation. Specifically, this cysteine is modified by NO+, and conformational change from R (oxygenated) to T (deoxygenated) results in exposure of this NO+ to transnitrosation reactions; reactions of nitrogen oxides bound to the hB93-CYS are sterically hindered when Hb is in the R conformation (3, 6, 18, 42, 45). Products of transnitrosation reactions from Hb-CYS-NO in Hb molecules in T-state Hb can include S-nitrosylated anion exchange protein-1 and low-mass S-nitrosothiols (6, 42, 45). These reactions proceed as a logarithmic function of Hb-O2 saturation at fixed temperature, pH, and Pco2 (6, 42). No other class of compounds has been identified that requires HbC93 for formation in hypoxia. Further, although somatic cell Hb regulates vascular nitrogen oxide signaling (52), this is Hb α, not Hb β: it would not be differentially affected in these β-mutant mice. We therefore speculate that an Hb-CYS-93-derived S-nitrosothiol, formed during oxyhemoglobin desaturation, signals STP downstream of the CB in the CSN. Of interest, Hb desaturation in the systemic periphery can result in gradual formation of low-mass S-nitrosothiol species that return to the central circulation (42): circulation of a Hb-derived S-nitrosothiol could permit STP by continuing to stimulate residual ventilatory activity after the Po2 increases to normal. It could be expected that the concentrations of circulating S-nitrosothiols would take time to degrade to levels that no longer exert physiological effects. Thus the appearance, magnitude, and time of decay of STP would parallel the levels of circulating S-nitrosothiols. We believe that this may represent a novel paradigm whereby blood O2 content, signaled by Hb conformation, rather than simply the tension of dissolved O2 and CO2, might signal ventilatory effects.
We cannot definitively rule out the possibility that compounds derived from the hB93-CYS during Hb desaturation reach central neurons, such as those in the nTS or the area postrema, to affect ventilatory control (10, 11). Indeed, administration of deoxyHb-derived GSNO to the nTS can affect Vm (33). Although the lipophobicity of S-nitrosothiols makes this seem unlikely, concordance of central and peripheral S-nitrosothiol-mediated ventilatory regulation has been proposed (33), and may be relevant to STP. Neuronal NOS (nNOS) activation produces S-nitrosothiols in the central nervous system (17, 28). STP is markedly diminished in nNOS knock-out mice (26) or by blockade of central serotonin receptors in cats (39, 40): central S-nitrosothiols and serotonergic pathways likely participate in STP signaling downstream from the CSN afferents.
Initial ventilatory excitant responses during hypoxic challenge.
The diminished ventilatory response during hypoxic challenge in conscious CSNX mice is consistent with the vital role of CB chemoafferents in the expression of HVR in this species (2, 25). The finding that the initial responses during hypoxic challenge were similar in B93-CYS and B93-ALA mice suggests that the initial ventilatory response, unlike STP, is not dependent on hB-CYS-93. At least three possibilities exist in this regard. First, the initial hypoxic ventilatory response may be exclusively O2-dependent, as is commonly assumed. However, ventilatory roll-off during hypoxia is completely ablated in mice lacking the ability to catabolize GSNO (44), suggesting a role for central and/or peripheral GSNO formation in the initial ventilatory response to hypoxia (see below). Thus the initial hypoxic ventilatory response may represent a combination of an acute effect of low Po2 coupled with gradual central and peripheral GSNO formation; in this scenario, the kinetics of formation and breakdown would determine the extent and timing of roll-off and STP. Second, as less than 1:1,000 Hb molecules are normally S-nitrosylated in vivo, 1% fetal Hb [likely capable of being S-nitrosylated (23)] may be sufficient to stimulate the initial hypoxic response, but not posthypoxic effects. Third, different Hb-derived and nNOS-derived S-nitrosothiols may have opposing effects: for example, GSNO may stimulate increased minute ventilation, whereas another S-nitrosothiol formed may inhibit ventilation. Of note, activation of nNOS in the CBs plays a key role in initiating HVR (29). While the nNOS-mediated generation of NO could not mediate this response because NO inhibits hypoxic chemotransduction in the CB (29, 57), nNOS generates S-nitrosothiols (17) which can increase CSN chemosensory discharges in isolated CB-CSN preparations (24, 41). Moreover, the generation of S-nitrosothiols in brain pathways downstream of CSN chemoafferent input may also be involved. More specifically, HVR is initiated by activation of N-methyl-d-aspartate (NMDA) receptors (NMDARs) in the nTS with ensuing increases in intracellular Ca2+ driving increases in nNOS activity (19). Although activation of nNOS plays a key role in initiating HVR (19), NO inhibits hypoxic chemotransduction in the brain stem (56). As such, the generation of nNOS-mediated generation of S-nitrosothiols such as GSNO and S-nitrosocysteinylglycine (CGSNO), which are readily detectable in the brain (5, 28, 49), may be involved in the HVR. Indeed, Lipton et al. (33) found that the microinjection of GSNO, CGSNO, and S-nitrosocysteine (CSNO) into the nTS of conscious rats increases Vm. Our finding that the initial ventilatory responses that occur upon exposure to hypoxia are exaggerated in conscious mice deficient in GSNO reductase (44), tentatively supports a role for central GSNO in the expression of these initial responses.
Ventilatory roll-off.
The hB93-ALA mice also had normal ventilatory roll-off. This observation is an additional argument against a central ventilatory effect of hB93-CYS-derived compounds: roll-off is a centrally mediated effect involving diminished activity of NMDARs in the nTS (19). That said, GSNO catabolism is almost certainly involved mediating roll-off: as noted above, genetic deletion of GSNO reductase completely ablates roll-off (44). S-nitroso-N-acetyl penicillamine decreases the elevated chemosensory discharges in isolated CB-CSN preparations during hypoxic challenge (24, 41). Although beyond the scope of this discussion, other S-nitrosothiols, including S-nitroso-albumin (50) and CSNO formed during hypoxia, may have opposing effects at different sites in the respiratory control pathway, including S-nitrosylation of NMDARs (4, 54), S-nitrosylation-independent effects on NMDARs (54), or effects on other ion-channels and receptors (21, 32) expressed in PGCs (29, 46) and central respiratory centers.
Baseline parameters and temperature responses to hypoxic challenge.
Our finding that resting body weights, Tc, and ventilatory parameters of hB93-ALA mice were similar to those of the wild-type C57BL6 mice and hB93-CYS mice suggests that the loss of B93-CYS did not adversely affect health (including metabolism) or baseline ventilatory physiology. It has been shown that hB93-ALA mice have nitrosylated Hb, likely reflecting fetal Hb (23, 43), and so the role of Hb in baseline physiology may not be compromised. One way or another, it is clear that the control of normoxic ventilatory physiology is a multifactorial process that is too important to be regulated by a single component, and the mice are thus likely to have compensated for loss of hB93-CYS through fetal Hb or other compensatory mechanisms. It should be noted that although hB93-ALA mice had higher percent fetal-Hb expression than hB93-CYS mice, this was not sufficient to overcome the dramatic loss of the STP responses, which clearly supports the vital role of hB93-CYS in STP. In addition, the findings that the decreases in Tc during hypoxia and gradual recovery to prehypoxia levels were similar in C57BL6, hB93-CYS, and hB93-ALA mice suggests that the marked differences in posthypoxic ventilatory responses in hB93-ALA mice compared with hB93-CYS and C57BL6 mice are not due to differences in body metabolism between the three groups.
Summary.
The present study demonstrates that hB93-CYS is essential for expression of STP in conscious mice. We have provided evidence that systemic injections of CSNO activate vagal afferents in rats (30, 38). As such, it is possible that hB93-CYS-derived S-nitrosothiols may stimulate ventilation through actions on PGCs or chemoafferent terminals in the CBs. The possibility that S-nitrosothiols derived from hB93-CYS may directly activate CSN chemoafferents from the CB suggests an entirely new paradigm in understanding ventilatory regulation, a paradigm in which Hb conformation, rather than dissolved O2 tension, could signal a ventilatory response to blood O2 content. S-nitrosothiols exert their biological effects via a multiplicity of processes (20, 21, 31, 36). However, the precise mechanisms of signaling between hB93-CYS-derived S-nitrosothiols and CSN chemoafferents remain to be determined.
GRANTS
This study was supported by a National Institutes of Health (NIH) Program Project Grant (1P-01-HL-101871; B. Gaston, L. A. Palmer, S. J. Lewis) and individual grants from the Department of Defense (W81XWH-07-0134; L. A. Palmer), Galleon Pharmaceuticals (S. J. Lewis, B. Gaston), and NIH (R01-HL-59337; B. Gaston).
DISCLOSURES
B. Gaston has nonprofitable intellectual property related to the subject matter (no income).
AUTHOR CONTRIBUTIONS
Author contributions: B.G., S.Y., L.A.P., J.N.B., and S.J.L. conception and design of research; B.G., W.J.M., S.S., S.Y., N.V.M., L.A.P., J.N.B., and S.J.L. analyzed data; B.G., W.J.M., L.A.P., and S.J.L. interpreted results of experiments; B.G., L.A.P., and S.J.L. edited and revised manuscript; B.G., W.J.M., S.S., S.Y., N.V.M., L.A.P., J.N.B., and S.J.L. approved final version of manuscript; W.J.M., S.S., S.Y., N.V.M., L.A.P., and S.J.L. performed experiments; W.J.M., L.A.P., and S.J.L. prepared figures; S.J.L. drafted manuscript.
REFERENCES
- 1.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 15: 452–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biscoe TJ, Pallot DJ. The carotid body chemoreceptor: an investigation in the mouse. Q J Exp Physiol 67: 557–576, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Chan NL, Kavanugh JS, Rogers PH, Arnone A. Crystallographic analysis of the interaction of nitric oxide with quaternary-T human hemoglobin. Biochemistry 43: 118–32, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HSV, Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci 3: 15–21, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Do KQ, Benz B, Grima G, Gutteck-Amsler U, Kluge I, Salt TE. Nitric oxide precursor arginine and S-nitrosoglutathione in synaptic and glial function. Neurochem Int 29: 213–224, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, Gaston B. Hemoglobin conformation couples S-nitrosothiol content in erythrocytes to oxygen gradients. Proc Natl Acad Sci USA 102: 5709–5714, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly DF, Rigual R. Single-unit recordings of arterial chemoreceptors from mouse petrosal ganglia in vitro. J Appl Physiol 88: 1489–1495, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Epstein MA, Epstein RA. A theoretical analysis of the barometric method for measurement of tidal volume. Respir Physiol 32: 105–210, 1978 [DOI] [PubMed] [Google Scholar]
- 9.Epstein RA, Epstein MA, Haddad GG, Mellins RB. Practical implementation of the barometric method for measurement of tidal volume. J Appl Physiol 49: 1107–1115, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Ferguson AV, Beckmann LM, Fisher JT. Effects of subfornical organ stimulation on respiration in the anesthetized rat. Can J Physiol Pharmacol 67: 1097–1101, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+. J Appl Physiol 108: 989–994, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaston B, Drazen JM, Jansen A, Sugarbaker DA, Loscalzo J, Richards W, Stamler JS. Relaxation of human bronchial smooth muscle by S-nitrosothiols in vitro. J Pharmacol Exp Ther 268: 978–984, 1994 [PubMed] [Google Scholar]
- 13.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 149: 538–551, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Gaston B, Singel D, Doctor A, Stamler JS. S-Nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 73: 1186–1193, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golanov EV, Christensen JR, Reis DJ. Neurons of a limited subthalamic area mediate elevations in cortical cerebral blood flow evoked by hypoxia and excitation of neurons of the rostral ventrolateral medulla. J Neurosci 21: 4032–4041, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Gow AJ, Chen Q, Hess DT, Day BJ, Ischiropoulos H, Stamler JS. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J Biol Chem 277: 9637–9640, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature 391: 169–173, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Gozal D, Gozal E, Simakajornboon N. Signaling pathways of the acute hypoxic ventilatory response in the nucleus tractus solitarius. Respir Physiol 121: 209–221, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Haldar SM, Stamler JS. S-nitrosylation: integrator of cardiovascular performance and oxygen delivery. J Clin Invest 123: 101–110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu Rev Pharmacol Toxicol 42: 585–600, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med 14: 773–777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iturriaga R, Villanueva S, Mosqueira M. Dual effects of nitric oxide on cat carotid body chemoreception. J Appl Physiol 89: 1005–1012, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Izumizaki M, Pokorski M, Homma I. Role of the carotid bodies in chemosensory ventilatory responses in the anesthetized mouse. J Appl Physiol 97: 1401–1407, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol 539: 309–315, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. Proc Natl Acad Sci USA 99: 821–826, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluge I, Gutteck-Amsler U, Zollinger M, Do KQ. S-nitrosoglutathione in rat cerebellum: identification and quantification by liquid chromatography-mass spectrometry. J Neurochem 69: 2599–2607, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the carotid body. Prog Biophys Mol Biol 91: 249–286, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lewis SJ, Owen JR, Bates JN. S-nitrosocysteine elicits hemodynamic responses similar to those of the Bezold-Jarisch reflex via activation of stereoselective recognition sites. Eur J Pharmacol 531: 254–258, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Li S, Whorton AR. Functional characterization of two S-nitroso-l-cysteine transporters, which mediate movement of NO equivalents into vascular cells. Am J Physiol Cell Physiol 292: C1263–C1271, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipton A, Johnson M, Macdonald T, Lieberman M, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature 413: 171–174, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410: 490–494, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Mawjood AHM, Miyazaki G, Kaneko R, Wada Y, Imai K. Site-directed mutagenesis of hemoglobin: test of functional homology of the F9 amino acid residues of hemoglobin α and β chains. Protein Engineering 13: 113–120, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Marozkina NV, Gaston B. S-nitrosylation signaling regulates cellular protein interactions. Biochim Biophys Acta 1820: 722–729, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin-Body RL. Brain transections demonstrate the central origin of hypoxic ventilatory depression in carotid body-denervated rats. J Physiol 407: 41–52, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meller ST, Lewis SJ, Bates JN, Brody MJ, Gebhart GF. Is there a role for an endothelium-derived relaxing factor in nociception? Brain Res 531: 342–345, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980 [DOI] [PubMed] [Google Scholar]
- 40.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980 [DOI] [PubMed] [Google Scholar]
- 41.Mosqueira M, Iturriaga R. Carotid body chemosensory excitation induced by nitric oxide: involvement of oxidative metabolism. Respir Physiol Neurobiol 131: 175–187, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest 117: 2592–2601, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer LA, Doctor A, Gaston B. SNO-hemoglobin and hypoxic vasodilation. Nature Med 14: 1008–1009, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Palmer LA, May WJ, Deronde K, Brown-Steinke K, Bates JN, Gaston B, Lewis SJ. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Respir Physiol Neurobiol 185: 571–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature 409: 622–626, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol 174: 292–298, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA 107: 10719–10724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto-Lloret J, Donnelly DF, Rico AJ, Moratalla R, González C, Rigual RJ. Hypoxia transduction by carotid body chemoreceptors in mice lacking dopamine D2 receptors. J Appl Physiol 103: 1269–1275, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Salt TE, Zhang H, Mayer B, Benz B, Binns KE, Do KQ. Novel mode of nitric oxide neurotransmission mediated via S-nitroso-cysteinyl-glycine. Eur J Neurosci 12: 3919–3925, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Scharfstein JS, Keaney JF, Jr, Slivka A, Welch GN, Vita JA, Stamler JS, Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest 94: 1432–1439, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491: 473–477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun MK, Reis DJ. Dopamine or transmitter release from rat carotid body may not be essential to hypoxic chemoreception. Am J Physiol Regul Integr Comp Physiol 267: R1632–R1639, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Takahashi H, Shin Y, Cho SJ, Zago WM, Nakamura T, Gu Z, Ma Y, Furukawa H, Liddington R, Zhang D, Tong G, Chen HS, Lipton SA. Hypoxia enhances S-nitrosylation-mediated NMDA receptor inhibition via a thiol oxygen sensor motif. Neuron 53: 53–64, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Vitagliano S, Berrino L, D'Amico M, Maione S, De Novellis V, Rossi F. Involvement of nitric oxide in cardiorespiratory regulation in the nucleus tractus solitarii. Neuropharmacology 35: 625–631, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Wang ZZ, Stensaas LJ, Dinger BG, Fidone SJ. Nitric oxide mediates chemoreceptor inhibition in the cat carotid body. Neuroscience 65: 217–229, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 300: C385–C393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]