Abstract
Rationale: Obesity imposes mechanical loads on the upper airway, resulting in flow limitation and obstructive sleep apnea (OSA). In previous animal models, leptin has been considered to serve as a stimulant of ventilation and may prevent respiratory depression during sleep. We hypothesized that variations in leptin concentration among similarly obese individuals will predict differences in compensatory responses to upper airway obstruction during sleep. Methods: An observational study was conducted in 23 obese women [body mass index (BMI): 46 ± 3 kg/m2, age: 41 ± 12 yr] and 3 obese men (BMI: 46 ± 3 kg/m2, age: 43 ± 4 yr). Subjects who were candidates for bariatric surgery were recruited to determine upper airway collapsibility under hypotonic conditions [pharyngeal critical pressure (passive PCRIT)], active neuromuscular responses to upper airway obstruction during sleep, and overnight fasting serum leptin levels. Compensatory responses were defined as the differences in peak inspiratory airflow (ΔVImax), inspired minute ventilation (ΔVI), and pharyngeal critical pressure (ΔPCRIT) between the active and passive conditions. Results: Leptin concentration was not associated with sleep disordered breathing severity, passive PCRIT, or baseline ventilation. In the women, increases in serum leptin concentrations were significantly associated with increases in ΔVImax (r2 = 0.44, P < 0.001), ΔVI (r2 = 0.40, P < 0.001), and ΔPCRIT (r2 = 0.19, P < 0.04). These responses were independent of BMI, waist-to-hip ratio, neck circumference, or sagittal girth. Conclusion: Leptin may augment neural compensatory mechanisms in response to upper airway obstruction, minimizing upper airway collapse, and/or mitigating potential OSA severity. Variability in leptin concentration among similarly obese individuals may contribute to differences in OSA susceptibility.
Keywords: leptin, obesity, obstructive sleep apnea, upper airway control
obstructive sleep apnea (OSA) is characterized by repetitive upper airway obstruction during sleep, which results in recurrent oxyhemoglobin desaturations and arousals (30). Sleep apnea results from combined defects in upper airway anatomic and neuromuscular control during sleep (16). Obesity remains a predominant risk factor for OSA pathogenesis (23), although the mechanisms have not been well established.
Mechanical effects of obesity lead to elevations in the pharyngeal collapsibility (passive PCRIT) during sleep in humans (15) and rodents (25, 28). Passive PCRIT, the nasal pressure at which the pharynx collapses when neuromuscular activity is reduced (33), increases 1.0 cmH2O per 10 units increase in body mass index (BMI) in women and 1.7 cmH2O in men (15). These increases may be due to fat deposits in the pharyngeal soft tissue, which increase the extraluminal tissue pressure (14) and augment mechanical loads on the upper airway (31). Additionally, central adiposity is associated with decreased lung volumes and diminished caudal traction, further increasing pharyngeal collapsibility (8, 38).
To compensate for pharyngeal mechanical loads in obesity, airflow obstruction can elicit neuromuscular responses that restore airway patency. Adipose tissue produces humoral factors including leptin, a powerful neurohumoral ventilatory stimulant in rodents. Of note, leptin-deficient ob/ob mice exhibit depressed ventilation and reduced responses to hypercapnia, even before the development of obesity (21, 26). Independent of changes in body weight, administration of exogenous leptin to these ob/ob mice increases pulmonary ventilation, restores normal responses to hypercapnia, and reverses defects in the neuromuscular control of the upper airway (24). Leptin's actions on upper airway control and ventilatory drive may be centrally mediated by brain stem receptors in the nucleus tractus solitarius and hypoglossal motor nucleus (17). Inyushka et al. (12) demonstrated increased pulmonary ventilation, respiratory volume, and enhanced electromyography of the inspiratory muscles when leptin was directly injected into the ventrolateral solitary tract nucleus in the brain of rats. Nevertheless, the role of leptin in the regulation of upper airway neuromuscular control in sleeping humans is less well understood.
The major goal of this study is to examine the relationship between leptin and the control of upper airway patency during sleep in obesity. Previous studies have shown marked elevations in levels of circulating leptin (>100 ng/ml) in severe obesity (40) and marked variability in circulating leptin levels (4) independent of body weight. This variability may be related to differences in fat mass, fat distribution, and/or sleep apnea severity (4, 5, 13, 20, 40). We therefore hypothesized that in obese individuals, variations in individuals' circulating leptin levels will be associated with differences in compensatory responses to upper airway obstruction during sleep. To address this hypothesis, we examined the relationship between circulating leptin and upper airway passive mechanical and active neuromuscular control in a severely obese bariatric cohort.
METHODS
Subjects
This study was a retrospective analysis of subjects previously recruited from the Johns Hopkins Sleep Disorders Center and the Johns Hopkins Bariatric Surgery Clinic for a variety of physiologic protocols. Because large variations in weight may influence both ventilatory parameters and circulating leptin levels, obese subjects were recruited over a narrow range of BMI (40–50 kg/m2) to minimize potential confounds of differences in body weight on leptin concentrations (4, 40) and upper airway function (15, 32) during sleep. Subjects were excluded if they had a history of a concurrent sleep disorder (e.g., narcolepsy, restless leg syndrome, previous upper airway surgery, significant pulmonary disease or gas exchange abnormalities, or use of supplemental oxygen). Informed written consent was obtained from each participant, and the protocols were approved by the Johns Hopkins Institutional Review Board.
We characterized upper airway function in 61 bariatric candidates (50 women, 11 men), as reported in a previous publication (3). We selected the upper airway physiological data from 31 of those subjects (26 women, 5 men) whose BMI lay in the range targeted for our current study. Of these, active upper airway responses could not be determined during sleep because of repeated arousals (n = 2), inability to maintain stable breathing patterns at cycling threshold (n = 2), or excessive mouth breathing (n = 1), leaving 26 subjects (23 women, 3 men) in the final study group. Circulating leptin levels were assayed in this subgroup. The current report examines the relationship between measures of upper airway function during sleep and circulating leptin concentrations.
Study Procedures
Baseline polysomnography.
A standard full-night baseline nocturnal polysomnography was performed in a sleep laboratory. Physiological signals were digitized (Windaq, Dataq, Akron, OH, or Somnologica, Medcare, Buffalo, NY), including left and right electrooculogram, submental electromyogram, electroencephalogram, arterial oxygen saturation, nasal pressure, chest and abdominal plethysmography, and video monitoring for body position. Patients were instructed to sleep in the supine position if possible, and video monitoring was used to observe the sleep position for the duration of the night. Sleep staging and respiratory events were scored according to the American Academy of Sleep Medicine criteria (10).
Serum leptin levels.
A fasting blood sample was drawn the morning after the completion of the baseline sleep study. Serum was stored at −80°C until assay was conducted. Enzyme-linked immunosorbent assay (ELISA) was used for measurements of serum leptin (R&D Systems, Minneapolis, MN).
Upper airway assessment of nasal pressure and airflow.
On a separate night, patients returned for a physiological polysomnography study to determine their passive critical collapsing pressure by assessing the pressure-flow relationship during sleep as previously described (22). Patients returned for the physiological polysomnography no later than 4 wk from their original baseline polysomngraphy. In addition to the standard polysomngraphic procedures described above, airflow was monitored with a pneumotachograph (No. 5, Hans Rudolph, Kansas City, MO) attached to a differential pressure transducer placed between a tight-fitting nasal mask (Comfort Classic, Respironics, Murrysville, PA) and a continuous positive airway pressure (CPAP) unit designed to apply pressures between −20 and +20 cmH2O. Respiratory effort was monitored via a Hyatt-type esophageal balloon (Ackrad Laboratories, Cranford, NJ) placed perinasally for monitoring esophageal pressure and/or a piezoelectrode abdominal strain gauge. Patients were instructed to sleep in the supine position, and efforts were made to maintain constant position between measurements of active and passive responses. All monitored parameters on this night were recorded directly on a computer software system.
Experimental Protocols
Experimental protocols were performed during stable non-rapid eye movement (NREM) sleep to assess compensatory responses to upper airway obstruction. Our experimental approach is detailed below and in Fig. 1 in a prior publication (3).
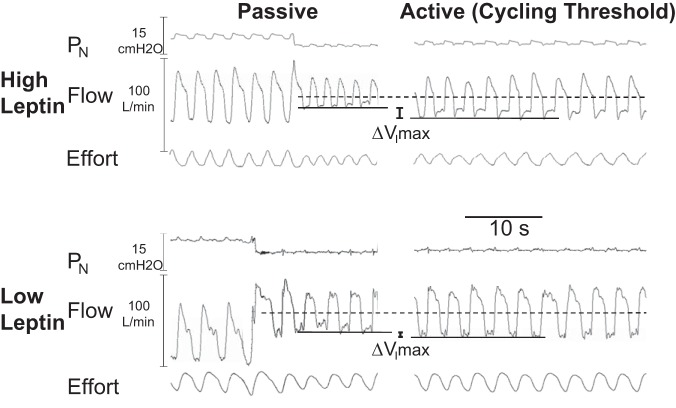
Fig. 1.
Compensatory differences in peak inspiratory airflow (ΔVImax) responses in a subject with a high circulating leptin (top) compared with one with a low circulating leptin (bottom) concentration (156 vs. 35 ng/ml, respectively). In each subject, an abrupt drop in nasal pressure was associated with the onset of inspiratory flow limitation acutely (left) in the passive state. Maintaining nasal pressure near the cycling threshold (right), however, resulted in an increase in VImax (top right) or little change in VImax (bottom right), indicating a greater compensatory response in the subject with high compared with low leptin concentration.
Passive condition—brief periods of upper airway obstruction.
We first titrated nasal CPAP to a holding pressure that eliminated flow limitation and attenuated neuromuscular activity (36). Thereafter, nasal pressure was repeatedly acutely reduced from the holding pressure in 1- to 2-cmH2O increment to lower levels each for five breaths; this stepwise reduction in nasal pressure occurred until complete upper airway closure (zero airflow) was induced, as previously described (17, 36, 37). If arousals occurred, the protocol was resumed after subjects returned to stable stage 2 NREM sleep for at least 3 min.
Active condition—sustained periods of upper airway obstruction.
Nasal pressure was reduced stepwise from holding pressure by 1–2 cmH2O for periods of at least 10 min during NREM sleep as previously described (17, 36, 37). Stepwise reductions in nasal pressure were associated with progressive decreases in maximal inspiratory airflow (VImax), as previously described.
Data Analyses
Assessment of compensatory responses.
We utilized two methods for assessing compensatory upper airway responses. First, we measured baseline ventilatory characteristics during active and passive periods of airflow obstruction, as previously described (3). Specifically, we identified periods of non-flow-limited breathing during stable NREM sleep when nasal pressure was set to holding CPAP levels. We then determined ventilatory parameters in the passive condition and measured ventilatory parameters during prolonged periods of partial airway obstruction (active condition). These parameters were determined at the cycling threshold pressure, the nasal pressure below which active ventilatory responses could no longer maintain stable ventilation during sleep. This threshold defined the limits of subjects' ability to actively compensate for passive mechanical loads on the airway while stabilizing breathing patterns during sleep and represented the greatest degree of neuromuscular compensation during sleep (16). The degree of neuromuscular compensation was defined by the difference between VImax during the active and passive condition at the cycling threshold pressure (Fig. 1, see differences in compensatory responses, ΔVImax, between a subject with high compared with low leptin concentration). Specifically, passive and active flow-limited breaths were selected for analysis at the cycling threshold, as previously described (9).
Second, pressure-flow curves were derived for the passive and active conditions from previously established methods (22). In each condition, pressure-flow measurements at the cycling threshold pressure and standardized values for upstream resistance (22) were utilized to derive passive and active critical pressure (PCRIT). The compensatory response was given by the difference between the active and passive PCRIT for each subject (ΔPCRIT).
In addition, we examined the association between leptin concentrations and ventilatory drive, as reflected by inspiratory swings in esophageal pressure. We compared inspiratory swings in esophageal pressure for each subject during the active condition, when drive was maximal, to the corresponding passive state, when neuromuscular activity was minimal. The difference in esophageal pressure swings between these two conditions was taken as a measure of ventilatory compensation to airflow obstruction.
Statistical analysis.
Our analyses focused on testing the associations between measures of passive and active upper airway control and circulating leptin concentration, as well as other measures of adiposity and sleep apnea severity (STATA 11, College Station, TX). The primary outcome variables were passive PCRIT and compensatory neuromuscular responses, as represented by the differences in inspired minute ventilation (ΔVI), maximum inspiratory airflow (ΔVImax), and pharyngeal collapsibility (ΔPCRIT) between active and passive conditions. Predictors of upper airway function included serum leptin concentration, BMI, waist-to-hip ratio (WHR), sagittal girth (girth), and neck circumference (neck). The strength of the associations between predictors and outcome variables was analyzed with categorical and linear regression models. Multivariable regression models were used to assess the associations between compensatory neuromuscular responses and leptin concentration while adjusting for measures of adiposity (BMI, WHR, girth, neck). Finally, our sample contained relatively few men (n = 3), which limited our ability to investigate sex differences in the associations between compensatory responses and leptin concentration. We therefore provide data from the men but exclude them from our analysis, because the sample size limited our ability to discern sex-related differences in compensatory responses. Values were expressed as means ± SD unless otherwise stated, and P < 0.05 was considered significant.
RESULTS
Subject Characteristics
The anthropometric data, baseline sleep characteristics, and serum leptin concentration of the 26 subjects (23 women, 3 men) who comprised our study group are displayed in Table 1. By design, subjects were severely obese with an average BMI of 46 kg/m2 for both sexes. They had moderate to severe sleep apnea (AHI = 25 ± 26/69 ± 33; women/men) and elevated leptin concentrations, with women exhibiting a roughly two-fold elevation over men. Spirometry demonstrated that subjects' vital capacity was relatively well preserved and none had evidence of an obstructive ventilatory defect (i.e., the FEV1/FVC ratio was >70%). Arterial blood gases did not demonstrate evidence of alveolar hypoventilation in any of the women (i.e., PaCO2 was <45 mmHg), although one of the men had evidence of a mild well-compensated respiratory acidosis with a PaCO2 of 49 mmHg and a pH of 7.38. Thus spirometry and arterial blood gas results did not suggest the presence of underlying structural lung disease or the obesity-hypoventilation syndrome in the women, who comprised the study group of interest.
Table 1.
Subject demographics
| Demographics | Women (n = 22) | Men (n = 3) |
|---|---|---|
| Age, yr | 41.2 ± 11.7 (33.9–51) | 43.1 ± 3.7 (38.9–45.7) |
| Anthropometrics | ||
| BMI, kg/m2 | 45.9 ± 3.0 (44.0–48.4) | 46.1 ± 2.6 (44.2–49.1) |
| Neck, cm | 39.4 ± 2.8 (38.0–40.7) | 46.4 ± 1.8 (44.5–48.1) |
| Waist, cm | 128.6 ± 24.2 (118.4–133.3) | 139.4 ± 11.1 (126.8–147.7) |
| Waist-to hip ratio | 0.9 ± 0.1 (0.9–0.9) | 1.0 ± 0.0 (1.0–1.0) |
| Girth, cm | 34.0 ± 20.1 (28.0–32.0) | 33.3 ± 4.0 (29.0–37.0) |
| Sleep architecture | ||
| TST, min | 394.4 ± 65.5 (367.5–442.4) | 425.3 ± 100.0 (358.0–540.0) |
| Sleep efficiency, % | 85.2 ± 12.7 (82.0–93.0) | 94.8 ± 2.4 (92.0–96.5) |
| Stage N1, %TST | 15.0 ± 11.0 (9.5–16.7) | 21.6 ± 13.8 (5.7–30.6) |
| Stage N2, %TST | 56.8 ± 7.6 (51.1–62.0) | 55.1 ± 10.0 (46.1–65.8) |
| Stage N3, %TST | 11.5 ± 10.3 (1.3–19.2) | 3.3 ± 5.7 (0.0–9.8) |
| REM, %TST | 16.7 ± 8.3 (11.2–23.7) | 20.0 ± 4.9 (15.9–25.4) |
| Sleep-Disordered Breathing | ||
| Non-REM | ||
| AHI events/h | 22 ± 28 (5–23) | 65.4 ± 39.4 (30.7–108.2) |
| Baseline SaO2 | 96.0 ± 1.9 (94.7–97.5) | 95.1 ± 0.9 (94.3–96.0) |
| Average low SaO2 | 91.7 ± 2.4 (89.4–93.6) | 87.4 ± 3.9 (83.4–91.1) |
| REM | ||
| AHI events/h | 32 ± 23 (10–47)* | 80 ± 12 (68–92) |
| Baseline SaO2 | 95.7 ± 1.9 (95.0–97.4)* | 93.8 ± 1.8 (91.8–95.2) |
| Average low SaO2 | 90.0 ± 2.9 (88.6–91.4)* | 82.5 ± 5.3 (79.4–88.6) |
| Total | ||
| AHI events/h | 24.8 ± 26.2 (6.4–31.6) | 69 ± 33 (43–106) |
| Baseline SaO2 | 95.9 ± 1.9 (95.0–97.3) | 94.7 ± 0.8 (94.3–95.6) |
| Average low SaO2 | 90.9 ± 2.2 (89.7–92.8) | 86.2 ± 3.5 (83.0–89.9) |
| Arterial Blood Gases (19 woment/2 men) | ||
| PaO2 | 86.6 ± 13.2 (77.5–94.5) | 73.5 ± 4.95 (70.0–77.0) |
| PaCO2 | 38.9 ± 5.12 (37.0–42.5) | 45.5 ± 4.95 (42.0–49.0) |
| pH | 7.42 ± 0.04 (7.40–7.44) | 7.40 ± 0.02 (7.38–7.41) |
| Pulmonary Function Tests (21 women, 3 men) | ||
| FVC, liters | 3.42 ± 0.71 (2.88–3.92) | 5.09 ± 0.12 (5.05–5.16) |
| FVC predicted, % | 96.4 ± 16.4 (88.0–112.9) | 104 ± 3.90 (102.9–106.3) |
| FEV1, liters | 2.77 ± 0.55 (2.40–3.09) | 3.90 ± 0.04 (3.89–3.93) |
| FEV1 predicted, % | 93.5 ± 14.6 (82.0–101.8) | 96.2 ± 1.72 (95.3–97.0) |
| FEV1/FVC, % | 81.4 ± 4.70 (77.8–84.6) | 86.6 ± 13.2 (75.4–77.8) |
| Serum Leptin, ng/ml | 95.2 ± 32.6 (74.2–114.2) | 43.4 ± 10.3 (31.6–50.2) |
| Passive PCRIT, cmH2O | 0.2 ± 3.3 (−2.5–1.7) | −1.1 ± 2.2 (−3.2–1.2) |
| Active PCRIT, cmH2O | −2.9 ± 0.9 (−5.5–−0.7) | −1.9 ± 1.1 (−3.1–−3.1) |
All values are presented as mean ± SD (25–75th percentile).
AHI, apnea-hypopnea index; BMI, body mass index; SaO2, oxygen saturation; TST, total sleep time.
Three women had no rapid eye movement (REM) sleep.
Leptin, Adiposity, and Sleep-Disordered Breathing
As expected, increasing levels of obesity were associated with increased concentrations of leptin. In women, serum leptin was positively associated with BMI (r2 = 0.24, P < 0.02, β = 5.3 ± 2.0 ng/ml per kg/m2). However, leptin concentration was not associated with measures of body composition, including sagittal girth (r2 = 0.09, P < 0.19, β = 2.5 ± 2.1 ng/ml per cm), neck (r2 = 0.10, P < 0.16, β = −3.6 ± 2.4 ng/ml per cm), and WHR [r2 = 0.07, P < 0.24, β = −94 ± 77 (ng/ml)]. In multiple regression models, the association between serum leptin concentration and BMI remained significant after adjusting for WHR (P < 0.009, β = 5.7 ± 2.0 0 ng/ml per kg/m2), neck (P < 0.034, β = 4.8 ± 2.4 ng/ml per kg/m2), and girth (P < 0.028, β = 5.0 ± 2.1 ng/ml per kg/m2).
In women, leptin concentration was not associated with sleep-disordered breathing severity as reflected by the apnea-hypopnea index (AHI) in non-REM (r2 = 0.04, P < 0.31), REM (r2 < 0.001, P < 0.94), or in non-REM and REM sleep combined (r2 = 0.03, P < 0.56). Additionally, no associations were observed between leptin concentration and either baseline oxygen saturation or average low oxygen saturation during sleep-disordered breathing episodes in either non-REM or REM sleep.
Upper Airway Characteristics and Sleep-Disordered Breathing
Sleep apnea was absent in only one patient, whose passive PCRIT was below −4 cmH2O, a previously described threshold for the development of sleep apnea (7, 22). In contrast, sleep-disordered breathing was present, but AHI varied widely in severity in patients whose passive PCRIT exceeded this threshold (n = 25), suggesting varying degrees of active neuromuscular responses. Variability in these responses can account for alterations in VImax under active conditions, which was a strong predictor of AHI (non-REM and REM combined). Specifically, subjects with low compared with high VImax at atmospheric pressure during sleep (median, 158 ml/s; low VImax, 38 ± 51 ml/s; high VImax, 278 ± 115 ml/s) had significantly higher AHI (53 ± 33 vs. 14 ± 8 episodes/h, P < 0.001).
Leptin, Ventilation, and Compensatory Responses to Upper Airway Obstruction
At holding pressure, baseline ventilatory characteristics were not associated with leptin concentration in women, including minute ventilation (VI) (r2 = 0.004, P < 0.76) and VImax at the cycling threshold (r2 < 0.001, P < 0.99). Similarly, passive PCRIT was not associated with leptin concentration (r2 < 0.002, P < 0.80), suggesting that leptin does not determine upper airway mechanical loads in severe obesity.
Compensatory responses to airflow obstruction were measured as the differences in ventilatory parameters between active and passive conditions, as shown in Fig. 1. In this figure, an augmented ΔVImax response is illustrated in a subject with a high leptin (Fig. 1, top) compared with a subject with a low leptin (Fig. 1, bottom) concentration (156 vs. 35 ng/ml, respectively). In each subject, an abrupt drop in nasal pressure was associated with the onset of inspiratory flow limitation in the passive condition (Fig. 1, left). In contrast, maintaining nasal pressure near the cycling threshold (Fig. 1, right) resulted in an increase in VImax or little change in VImax in the subject with a high compared with low leptin level (Fig. 1, right vs. bottom, respectively). The comparatively greater ΔVImax response in the subject with high compared with low leptin concentration reflects a greater compensatory upper airway response to airflow obstruction.
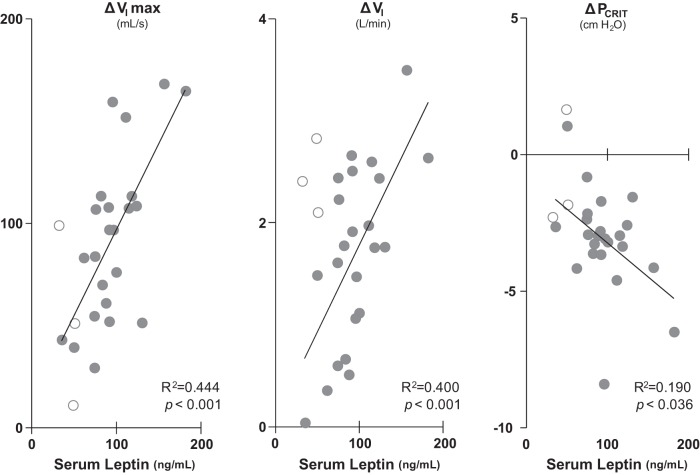
Examining compensatory responses for the group as a whole, we found that ΔVImax was highly associated with serum leptin concentrations (Table 2) but not with indices of obesity (BMI) or fat distribution (WHR, girth, neck). Compensatory responses in VImax, VI, and PCRIT were each associated with increasing leptin concentrations in the women (Fig. 2). Even after removing the two women with the highest leptin levels from these analyses as potential outliers, compensatory responses in ΔVImax and ΔVI remained positively associated with circulating leptin levels (P < 0.03 and <0.01, respectively), although the ΔPCRIT association lost significance (P = 0.27). Compared with women, we found that men demonstrated low leptin concentrations and airflow responses to upper airway obstruction (ΔVImax and ΔPCRIT). Despite reductions in upper airway responses (ΔVImax), the men's ventilatory responses (ΔVI) were elevated, consistent with alterations in respiratory pattern that compensated for upper airway obstruction (e.g., increased inspiratory duty cycle). Increases in leptin were also associated with more negative ΔPCRIT, indicating augmented compensatory responses that decreased active PCRIT below passive levels.
Table 2.
Regression models for compensatory responses in women
| ΔVI, l/min | β | 95% CI | r2 | P Value |
|---|---|---|---|---|
| Leptin, ng/ml | 0.017 | 0.008–0.027 | 0.400 | 0.001 |
| BMI, kg/m2 | 0.011 | −0.121–0.143 | 0.001 | 0.864 |
| Girth, cm | 0.090 | −0.022–0.204 | 0.117 | 0.110 |
| Neck, cm | 0.018 | −0.125–0.161 | 0.003 | 0.795 |
| WHR | −3.790 | −7.954–0.373 | 0.146 | 0.072 |
| ΔVImax, ml/s | β | 95% CI | r2 | P value |
| Leptin, ng/ml | 0.83 | 0.41–1.26 | 0.444 | 0.001 |
| BMI, kg/m2 | 1.98 | −4.09–8.06 | 0.021 | 0.504 |
| Girth, cm | 3.92 | −1.39–9.24 | 0.101 | 0.139 |
| Neck, cm | −1.84 | −8.43–4.76 | 0.016 | 0.569 |
| WHR | −16.6 | −225–192 | 0.001 | 0.870 |
| ΔPCRIT, cmH2O | β | 95% CI | r2 | P value |
| Leptin, ng/ml | −0.02 | −0.1–0.0 | 0.190 | 0.036 |
| BMI, kg/m2 | −0.07 | −0.3–0.2 | 0.010 | 0.592 |
| Girth, cm | −0.05 | −0.3–0.2 | 0.001 | 0.681 |
| Neck, cm | 0.17 | −0.1–0.5 | 0.070 | 0.224 |
| WHR | −4.3 | −13.5–4.8 | 0.040 | 0.335 |
ΔVImax, maximum inspiratory airflow; ΔVI, minute ventilation; ΔPCRIT, pharyngeal critical pressure; Girth, sagittal girth; Neck, neck circumference; WHR, waist-to-hip ratio.
Fig. 2.
Compensatory responses ΔVImax, minute ventilation (ΔVI), and upper airway critical pressure (ΔPCRIT) were significantly associated with serum leptin concentration in 23 women (●). Statistical results represent data from women only. Data in 3 men (○) are also shown.
Circulating leptin concentrations were also associated with increases in ventilatory drive, as represented by the different esophageal pressure swings between passive and active conditions. A trend toward a significant linear relationship was found between esophageal pressure differences and leptin levels for the entire group (men and women combined, n = 19), such that progressive increases in these pressure swings were associated with increased leptin concentrations (β coefficient: 0.09 ± 0.5 cmH2O per ng/ml, P = 0.06). This relationship was largely unaltered after adjusting for concomitant increases in upper airway patency (ΔVImax) (β coefficient: 0.10 ± 0.5 cmH2O per ng/ml, P = 0.07). Restricting our analysis to the women alone (n = 17), this association did not achieve statistical significance, although the magnitude of the association was similar to that of the entire group (β coefficient: 0.09 ± 0.8 cmH2O per ng/ml, P = 0.16). These findings suggest that leptin levels might predict active responses in ventilatory drive during sustained periods of airway obstruction, although associations were less pronounced than those between compensatory upper airway neuromuscular responses and leptin levels.
In multivariable regression models, leptin concentration remained significantly associated with these compensatory responses after adjusting for specific measures of obesity and fat distribution in the obese women. In these multivariable regression models, BMI, WHR, girth, and neck were not associated with compensatory neuromuscular responses nor did they significantly alter point estimates of the β coefficient for leptin responses in VImax (7.90–9.80 ml/s per 10 ng/ml) and VI (0.15–0.22 l/min per 10 ng/ml) and PCRIT (−0.2–−0.29 cmH2O per 10 ng/ml). These findings suggest that leptin increases compensatory neuromuscular responses to upper airway obstruction during sleep, independent of measures of obesity and regional fat distribution.
DISCUSSION
In the current study, we demonstrated a significant association between upper airway neuromuscular responses and circulating leptin concentration in obese women during sleep. These responses were independent of other measures of adiposity including BMI, WHR, neck circumference, and sagittal girth. Furthermore, leptin was not associated with baseline measures of sleep-disordered breathing severity, passive PCRIT, or ventilation. These results suggest that leptin is either a marker or mediator of upper airway compensatory neuromuscular responses and that the neurohumoral actions of leptin can minimize upper airway collapse and restore ventilation during sleep.
Obesity imposes mechanical load on the pharynx that increases its collapsibility, causing airflow obstruction during sleep (34). Increases in passive PCRIT are associated with increased sleep apnea severity, independent of leptin concentration. In our obese subjects, passive PCRIT in women was atmospheric, implying that the airway would occlude completely when neuromuscular activity wanes during sleep. Airflow obstruction, however, elicited variable increases in VImax and minute ventilation that mitigated the effects of elevated mechanical loads on airflow obstruction. We found that these active responses were directly related to circulating leptin concentration rather than baseline ventilatory parameters at holding pressure or measures of adiposity and fat distribution. These findings suggest that leptin may compensate for passive mechanical loads on the upper airway by augmenting active responses to obstruction, thereby minimizing the potential severity of the disease in obese individuals. Nevertheless, these compensatory responses may not be sufficient to prevent upper airway obstruction if mechanical loads on the upper airway (passive PCRIT) are markedly elevated.
Obstructive sleep apnea and obesity are both associated with marked increases in circulating leptin concentration (1, 4), raising the possibility that OSA may confound the relationship between leptin and active neuromuscular responses. In addition, sleep apnea (13) and nocturnal intermittent hypoxia (29) are thought to induce a leptin-resistant state (19), which if present would attenuate our association between leptin and compensatory neuromuscular responses. Instead, we found no relationship between serum leptin concentration and nocturnal hypoxemia (see results). Rather, increases in leptin concentration were associated with increases in compensatory responses, which could potentially ameliorate OSA (22). These findings are consistent with the notion that leptin contributes to active upper airway responses during sleep and that any further increases in leptin from sleep apnea would serve to mitigate the severity of airflow obstruction during sleep (35).
In severe obesity, circulating leptin concentrations can vary considerably among similarly obese individuals (4, 40). This variability has been attributed to differences in regional adiposity, because elevations in circulating leptin levels have been associated with a predominance of subcutaneous compared with visceral fat (6, 18). Women, who generally have more subcutaneous fat than men (20), exhibit significantly higher circulating leptin levels (27). Women also have far greater active neuromuscular responses to obstruction (3), thereby potentially contributing to the decreased sleep apnea prevalence and severity in women compared with men (15). These findings suggest that leptin may mediate reductions in OSA susceptibility in women by increasing neuromuscular responses to upper airway obstruction.
Several factors may limit the interpretation of our findings. First, our sample size was small and restricted to a severely obese population, limiting our ability to generalize our findings to less obese cohorts. The small sample size may have also limited our ability to discern a significant relationship between ventilatory drive and serum leptin as has been reported in murine models (11, 12, 21, 39). Nonetheless, our findings suggest that leptin's effect on ventilatory responses to airflow obstruction was less pronounced than that on compensatory upper airway neuromuscular responses during sleep. Second, the lack of men in our study prevented us from characterizing their relationship between leptin and neuromuscular compensatory responses and from conducting a direct comparison of sex-related differences. Post hoc analysis showed that statistical significance would still be observed in both primary outcome parameters (ΔVI and ΔVImax and ΔPCRIT) were men included in the overall analysis. Additional men will need to be recruited from the bariatric surgery cohort for future studies. Third, we did not control for the menstrual cycle and menopausal status in women. It is possible that hormonal differences and the inclusion of postmenopausal women may have influenced our serum leptin levels and associated compensatory responses. Likewise, we acknowledge the daily rhythmic variations in circulating leptin levels. Current literature suggests that leptin levels are influenced by the interaction of circadian rhythms, feeding patterns, and sleep time, with a nadir in the morning and a peak at night. To control for these fluctuations, patient's blood was drawn in the mornings immediately after their baseline study. This allowed us to standardize subjects' leptin measurements to a morning condition after an 8-h observed fasting period. Fourth, circulating leptin concentrations may not accurately reflect central nervous system levels. Active responses are thought to be centrally mediated; if leptin modulates these responses, it would need to be transported across the blood-brain barrier (2). Fifth, although we found that compensatory neuromuscular responses were highly associated with circulating leptin concentration rather than measures of regional fat distribution, it is certainly possible that other adipokines or measures of regional adiposity could also predict these responses. Sixth, we recognize that sleep apnea and its treatment may confound the relationship between compensatory neuromuscular responses and leptin concentration. Nevertheless, our female subjects had relatively mild sleep apnea, and their disease severity was not associated with circulating leptin concentration. Seventh, we acknowledge the positional effects of upper airway collapse. Patients were instructed to sleep supine if possible, and great efforts were made to maintain consistent position between the different sleep nights, as well as during the active and passive measurements. Given the nature of our study design, each subject served as his own baseline control, allowing us to control for positional and mechanical variability among the subjects. The observed compensatory responses were thus purely neurally derived. Eighth, it is also possible that leptin levels were associated with increases in ventilatory as well as upper airway compensatory responses. We sought to quantify differences in ventilatory responses between active and passive conditions from measurements of esophageal pressure excursions within the first tenth of a second after dropping the nasal pressure to induce airway occlusion (P0.1). These measurements, however, were difficult to standardize due to variability in the degree of airway obstruction (n = 9), signal artifacts (n = 2), and lung deflation transients immediately after abrupt decreases in nasal pressure. Measurements were not possible in other subjects, who were unable to tolerate the esophageal catheter (n = 5). Nonetheless, we did not discern any significant relationship between compensatory P0.1 responses and circulating leptin concentrations in our remaining subject sample (n = 10). Finally, we acknowledge that observed cross-sectional associations between neuromuscular responses and leptin concentration cannot establish causality. Future studies are needed to determine if leptin has a direct effect on compensatory responses to upper airway obstruction during sleep, as suggested by recent findings in a mouse model (24).
In conclusion, our study demonstrated that increased circulating leptin levels were associated with heightened ventilatory responses to upper airway obstruction. Leptin may augment compensatory neural mechanisms in response to upper airway obstruction during NREM sleep, thereby potentially minimizing upper airway collapse and mitigating potential OSA severity. Variability in leptin concentration among similarly obese individuals may be related to alterations in regional fat and can account for differences in OSA susceptibility between obese women and men. Further research is required to determine leptin's influence on ventilation and upper airway neuromuscular control over a broad range of subjects and potential treatment effects in human diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL50381.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D.S., V.Y.P., P.L.S., H.S., and A.R.S. conception and design of research; S.D.S., C.-H.C., J.P.K., S.P.P., H.S., and A.R.S. analyzed data; S.D.S., C.-H.C., J.P.K., B.M.M., S.P.P., V.Y.P., P.L.S., H.S., and A.R.S. interpreted results of experiments; S.D.S., C.-H.C., J.P.K., P.J.C.B., H.S., and A.R.S. prepared figures; S.D.S., C.-H.C., J.P.K., H.S., and A.R.S. drafted manuscript; S.D.S., C.-H.C., J.P.K., B.M.M., S.P.P., V.Y.P., P.J.C.B., P.L.S., H.S., and A.R.S. edited and revised manuscript; S.D.S., C.-H.C., J.P.K., B.M.M., S.P.P., V.Y.P., P.J.C.B., P.L.S., H.S., and A.R.S. approved final version of manuscript; J.P.K., B.M.M., S.P.P., P.L.S., H.S., and A.R.S. performed experiments.
REFERENCES
- 1.Barcelo A, Barbe F, Llompart E, de la PM, Duran-Cantolla J, Ladaria A, Bosch M, Guerra L, Agusti AG. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med 171: 183–187, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348: 159–161, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Chin CH, Kirkness JP, Patil SP, McGinley BM, Smith PL, Schwartz AR, Schneider H. Compensatory responses to upper airway obstruction in obese apneic men and women. J Appl Physiol 112: 403–410, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Fruhbeck G, Gomez-Ambrosi J. Depot-specific differences in the lipolytic effect of leptin on isolated white adipocytes. Med Sci Monit 8: BR47-BR55, 2002 [PubMed] [Google Scholar]
- 6.Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 280: E827–E847, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 143: 1300–1303, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 172: 114–117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med 157: 1461–1467, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007 [Google Scholar]
- 11.Inyushkin AN, Inyushkina EM, Merkulova NA. Respiratory responses to microinjections of leptin into the solitary tract nucleus. Neurosci Behav Physiol 39: 231–240, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Inyushkina EM, Merkulova NA, Inyushkin AN. Mechanisms of the respiratory activity of leptin at the level of the solitary tract nucleus. Neurosci Behav Physiol 40: 707–713, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 118: 580–586, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kairaitis K, Howitt L, Wheatley JR, Amis TC. Mass loading of the upper airway extraluminal tissue space in rabbits: effects on tissue pressure and pharyngeal airway lumen geometry. J Appl Physiol 106: 887–892, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 104: 1618–1624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105: 197–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer JG, Moar KM, Findlay PA, Hoggard N, Adam CL. Association of leptin receptor (OB-Rb), NPY and GLP-1 gene expression in the ovine and murine brainstem. Regul Pept 75–76: 271–278, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, Byrne CD, O'Rahilly S. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes 47: 1384–1391, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Munzberg H, Bjornholm M, Bates SH, Myers MG., Jr. Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci 62: 642–652, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res 2: 321–327, 2009 [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159: 1477–1484, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 284: 3015–3021, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Polotsky M, Elsayed-Ahmed AS, Pichard LE, Harris CC, Smith PL, Schneider H, Kirkness JP, Polotsky VY, Schwartz AR. Effects of leptin and obesity on the upper airway. J Appl Physiol 112: 1637–1643, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polotsky M, Elsayed-Ahmed AS, Pichard LE, Richardson RA, Smith PL, Schneider H, Kirkness JP, Polotsky VY, Schwartz AR. Effect of age and weight on upper airway function in a mouse model. J Appl Physiol 111: 696–703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polotsky VY, Smaldone MC, Scharf MT, Li J, Tankersley CG, Smith PL, Schwartz AR, O'Donnell CP. Impact of interrupted leptin pathways on ventilatory control. J Appl Physiol 96: 991–998, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Polotsky VY, Wilson JA, Smaldone MC, Haines AS, Hurn PD, Tankersley CG, Smith PL, Schwartz AR, O'Donnell CP. Female gender exacerbates respiratory depression in leptin-deficient obesity. Am J Respir Crit Care Med 164: 1470–1475, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Ray AD, Ogasa T, Magalang UJ, Krasney JA, Farkas GA. Aging increases upper airway collapsibility in Fischer 344 rats. J Appl Physiol 105: 1471–1476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol 111: 881–890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- 31.Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med 173: 453–463, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 144: 494–498, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: Pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 5: 185–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL. Obesity and upper airway control during sleep. J Appl Physiol 108: 430–435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol 64: 535–542, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol 64: 789–795, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Squier SB, Patil SP, Schneider H, Kirkness JP, Smith PL, Schwartz AR. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J Appl Physiol 109: 977–985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strohl KP, Thomas AJ. Ventilatory behavior and metabolism in two strains of obese rats. Respir Physiol 124: 85–93, 2001 [DOI] [PubMed] [Google Scholar]
- 40.van Dielen FM, van't Veer C, Schols AM, Soeters PB, Buurman WA, Greve JW. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obes Relat Metab Disord 25: 1759–1766, 2001 [DOI] [PubMed] [Google Scholar]
- 41.van Dielen FM, van't Veer C, Buurman WA, Greve JW. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. J Clin Endocrinol Metab 87: 1708–1716, 2002 [DOI] [PubMed] [Google Scholar]