Abstract
Exposure to hypoxia elicits changes in mean arterial blood pressure (MAP), heart rate, and frequency of breathing (fr). The objective of this study was to determine the role of nitric oxide (NO) in the cardiovascular and ventilatory responses elicited by brief exposures to hypoxia in isoflurane-anesthetized rats. The rats were instrumented to record MAP, heart rate, and fr and then exposed to 90 s episodes of hypoxia (10% O2, 90% N2) before and after injection of vehicle, the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME), or the inactive enantiomer d-NAME (both at 50 μmol/kg iv). Each episode of hypoxia elicited a decrease in MAP, bidirectional changes in heart rate (initial increase and then a decrease), and an increase in fr. These responses were similar before and after injection of vehicle or d-NAME. In contrast, the hypoxia-induced decreases in MAP were attenuated after administration of l-NAME. The initial increases in heart rate during hypoxia were amplified whereas the subsequent decreases in heart rate were attenuated in l-NAME-treated rats. Finally, the hypoxia-induced increases in fr were virtually identical before and after administration of l-NAME. These findings suggest that NO factors play a vital role in the expression of the cardiovascular but not the ventilatory responses elicited by brief episodes of hypoxia in isoflurane-anesthetized rats. Based on existing evidence that NO factors play a vital role in carotid body and central responses to hypoxia in conscious rats, our findings raise the novel possibility that isoflurane blunts this NO-dependent signaling.
Keywords: hypoxia, cardiovascular, frequency of breathing, nitric oxide factors, isoflurane-anesthetized rats
exposure to a hypoxic environment elicits increases in minute ventilation in humans and animals (26, 55). This process involves activation of primary glomus cells in the carotid bodies with release of “neurotransmitters” that activate carotid body chemoafferents, which relay their information to the nucleus of the tractus solitarius (NTS) in the brain stem (26, 55). The generation of nitric oxide (NO) and/or S-nitrosothiols in the blood (29), brain (3, 14, 33), and carotid bodies (19, 26) plays vital roles in the hypoxia-induced changes in ventilation (36).
Exposure to a hypoxic challenge can reduce mean arterial blood pressure (MAP) in rats (12, 18, 44, 59), via decreases in cardiac output and regional vascular resistances (18, 44) whereas heart rate usually increases (59). Hypoxia-induced vasodilation involves direct relaxation of vascular smooth muscle via activation of ATP-sensitive K+-channels and a decrease in voltage-gated Ca2+-channel (Ca2+VS-channel) activity (10), the release of NO or related species (e.g., S-nitrosothiols) from the vascular endothelium (44, 45), and generation of plasma S-nitrosothiols via hemoglobin-based redox chemistry in red blood cells (2). Systemic injections of NO synthase (NOS) inhibitors blunt hypoxia-induced hypotension and vasodilation (44); microinjections of NOS inhibitors into the caudal NTS attenuate the ventilatory responses elicited by hypoxia (35) and peripheral chemoafferent stimulation (14) whereas they do not affect hypoxia-induced hypotension (14). Taken together, it is tempting to assume that the hypoxia-induced changes in hemodynamic function are due predominantly to peripheral mechanisms involving NO and/or S-nitrosothiols. This may also pertain to cardiac function since NOS exists in cardiac neurons and muscle cells (6), and NO and S-nitrosothiols impact virtually every facet of cardiac function (28).
Most studies examining the mechanisms underlying hypoxia-induced changes in minute ventilation used between 5–30 min of acute hypoxia (5, 37, 38). However, episodes of naturally occurring apnea in humans and animals are substantially shorter in duration (7, 26). We are interested in the mechanisms that are involved in the ventilatory and cardiovascular response to such brief episodes of hypoxia. As such, we chose to examine the responses elicited by 90-s exposures to hypoxia (10% O2, 90% N2) in rats. These brief episodes of hypoxia were chosen specifically to limit the recruitment of central pathways responsible for ventilatory roll-off, the decrease in minute ventilation observed during sustained exposure to hypoxia (13).
Despite considerable investigation into the role of NO and S-nitrosothiols in the ventilatory and cardiovascular effects of hypoxia, few have focused on their roles in the responses elicited by brief episodes of hypoxia (e.g., 12). We decided to perform our studies in isoflurane-anesthetized rats since abrupt exposure of conscious subjects to an hypoxic environment elicits a transient but strong behavioral reaction that complicates interpretation of the nonbehavioral responses and therefore the cellular mechanisms underlying them (36). Since isoflurane is widely used (37–39), understanding how it affects hypoxia-driven responses is of clinical importance. In preliminary studies, we found that exposure to brief episodes of hypoxia (90 s) elicited robust changes in respiratory frequency (fr) and cardiovascular parameters in rats anesthetized with 1.25% isoflurane. The rat is an ideal model species to investigate the effects of volatile anesthetics on ventilatory function because humans and animals show similar sensitivities to these agents with the efficacy of ventilatory depression being halothane > isoflurane > sevoflurane (23, 37). In addition, the effects of volatile anesthetics on ventilatory responses to hypoxia are species related with the rat being least susceptible (23, 30). Indeed, robust increases in minute ventilation occur in rats anesthetized with isoflurane (1.4%) or halothane (1.1%), which are accompanied by pronounced increases in carotid body chemoreceptor discharge (23, 30).
The first aim of the present study was to establish the contribution of the carotid bodies and carotid sinus nerves (CSNs) in the ventilatory and cardiovascular responses elicited by a 90-s episode of hypoxia. The second aim was to examine the role of NO/S-nitrosothiols in the initiation of these hypoxic responses. In these studies, the effects of the NOS inhibitor, NG-nitro-l-arginine methyl ester (l-NAME), on the ventilatory and cardiovascular responses elicited by exposure to four 90-s episodes of hypoxia (10% O2, 90% N2) were determined. Four episodes of hypoxia were examined to determine whether blockade of NOS elicits temporal changes in the hypoxic-induced responses. Finally, the data were recorded every second and averaged into 15-s bins to allow for detailed analyses of the rapid changes in ventilatory and cardiovascular parameters associated with the hypoxic exposures.
MATERIALS AND METHODS
Rats
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the University of Virginia Animal Care and Use Committee. Male Sprague-Dawley rats of 14–16 wk of age (study 1: 319 ± 4 g body wt; study 2: 321 ± 6 g; study 3: 326 ± 5 g) at the time of experimentation were purchased from Harlan (Madison, WI).
Surgical Procedures
Rats were anesthetized via continuous administration of 2% isoflurane in 95% O2-5% CO2 via a facemask at a flow rate of 2 l/min to help maintain the subsequent ventilatory responses to hypoxia. A rectal probe connected to a thermostatically controlled heating pad (Homeothermic Monitor, Harvard Apparatus, Holliston, MA) was used to maintain the body temperature of each rat at 37.0 ± 0.2°C. Each rat was given a 200-μl subcutaneous injection of bupivacaine (0.25%) at the site of incision for subsequent placement of the femoral artery catheter. A catheter (PE-50, Braintree Scientific, Braintree, MA) was placed in the left femoral artery to monitor MAP, and another was placed in the left femoral vein for drug injection. A 1-in., 23-gauge needle catheter (Becton Dickinson, Franklin Lakes, NJ) was placed through the thoracic wall to measure changes in thoracic cavity pressure. After surgery, each rat was maintained under anesthesia via continuous administration of 1.25% isoflurane in room air (compressed medical air) via a facemask at a flow rate of 2 l/min. This level of isoflurane was sufficient to eliminate responses to tail-pinch and audiovisual stimuli. This is important since such stimuli can antagonize the effects of isoflurane on ventilatory responses to hypoxia (23).
Data Recording
MAP and thoracic pressure were measured by pressure transducers (SP 844) connected to a Bridge AMP (ADInstruments, Colorado Springs, CO) and ADInstruments Power Lab, and recorded with ADInstruments Lab Chart 7.0 viewing software. Heart rate was determined from the pulsatile pressure wave-form. fr was determined continuously from the intrathoracic pressure waveform. Data were collected into 15-s bins to establish one data point for analyses.
Experimental Protocol
Study 1: hypoxia-induced responses before and after CSN transection.
Each rat was given 20–25 min for MAP, heart rate, and fr to stabilize after placement of catheters before beginning the recordings. Baseline values were recorded for 5 min. Changes in MAP, heart rate, and fr elicited by a single episode of hypoxia (10% O2, 90% N2) for 90 s were given before and beginning 30 min after bilateral transection of the CSNs (n = 9 rats) or sham transection of these nerves (n = 6 rats). The principal reason for exposing rats to 90-s challenges with 10% O2 was that this allowed examination of the maximal (plateau) responses resulting from exposure to the hypoxic gas. This length of exposure to 10% O2 was not designed to have a practical medical context since patients would not be expected to have apneas of such duration (7).
Study 2: pharmacological studies.
Each rat was given 20–25 min for MAP, heart rate, and fr to stabilize following placement of all catheters. Baseline values for statistical analyses were recorded for 5 min. Each rat then received four consecutive episodes of hypoxia (10% O2, 90% N2) of 90 s in duration given 10 min apart. Ten minutes after episode 4 of hypoxia, rats were given bolus injections of vehicle (saline, n = 6 rats), l-NAME (50 μmol/kg, n = 6 rats), or the inactive enantiomer, d-NAME (50 μmol/kg, n = 5 rats). Ten minutes later, the rats again received four episodes of hypoxia (10% O2, 90% N2) of 90 s in duration given 10 min apart. This dose of l-NAME was chosen because it impairs central as well as peripheral NOS-dependent mechanisms (42), thereby allowing for the global assessment of the roles of NOS in the hypoxia-induced responses.
Study 3: repeat l-NAME study.
The above protocols left a somewhat unresolved question as to the exact temporal changes in fr following injection of vehicle or l-NAME. To resolve this issue, the temporal changes in baseline fr were determined before and after injection of vehicle (n = 6 rats) or l-NAME (50 μmol/kg, n = 6 rats). These rats were not subjected to episodes of hypoxia.
Drugs and Hypoxic Gas
l- and d-NAME were purchased from Sigma-Aldrich (St. Louis, MO). Bupivacaine was purchased from Hospira (Lake Forest, IL). Isoflurane liquid was purchased from Butler Schein (North Dublin, OH). Tanks containing compressed hypoxic gas mixture (10% O2, 90% N2) were purchased from GTS-WELCO (Charlottesville, VA).
Data Analysis
Periodically, arterial line saline flushes or intrathoracic needle dislodging caused erroneous “peaks” in the data. These were removed and replaced by the average of the two data points immediately before and after the peak. The area under the curves for the decreases in MAP recorded during the episodes of hypoxia were calculated using LabChart 7.0 (ADInstruments, Colorado Springs, CO), taking the integral relative to baseline beginning at the start of each hypoxic episode and ending exactly 4 min later. Resting values before each episode of hypoxia were determined by averaging the values recorded over the 5-min period immediately preceding exposure to hypoxia.
Statistics
All data are presented as means ± SE. Data were analyzed by one-way or repeated-measures analysis of variance (BMDP Statistical Package, Statistical Solutions, Boston, MA) followed by Student's modified t-test with Bonferroni correction for multiple comparisons between means using the error mean square terms from the analyses of variance (58). A value of P < 0.05 was taken to denote statistical significance.
RESULTS
CSN Transections
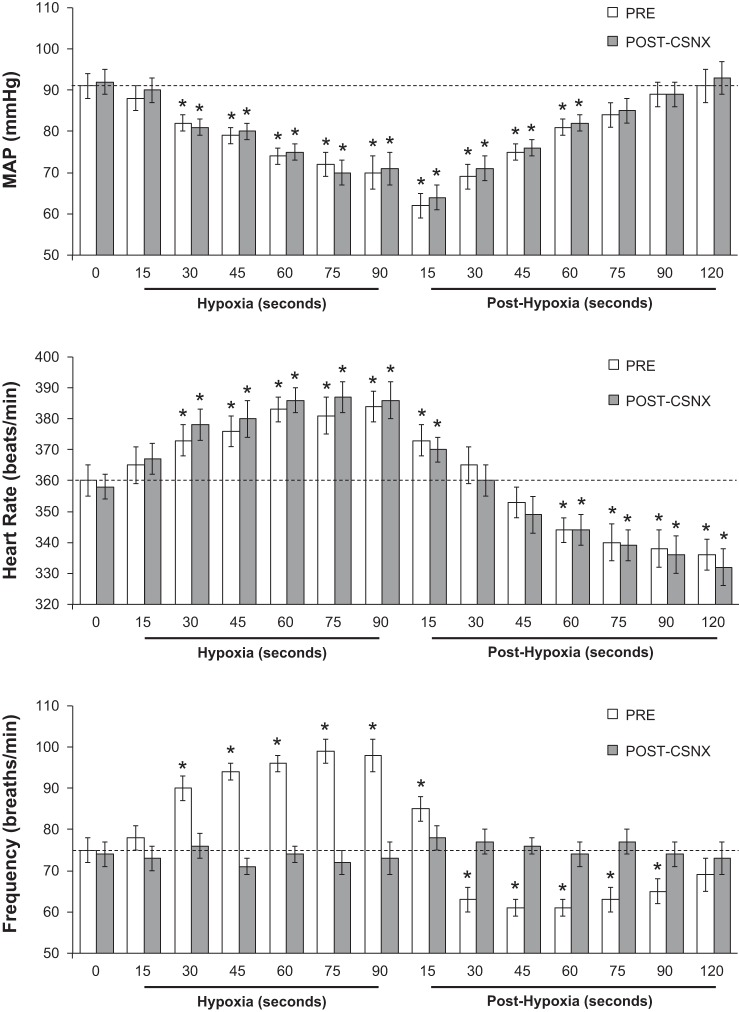
The changes in heart rate, MAP, and fr elicited by a single episode of hypoxia and upon return to room air (posthypoxia) before (Pre) and after bilateral transection of the CSNs (CSNX) are summarized in Fig. 1. Hypoxia elicited a decrease in MAP that was similar before and after CSNX. Reintroduction of room air resulted in a similar return to prehypoxia levels (within 60 s) before and after CSNX. The hypoxia-induced decreases in MAP were associated with increases in heart rate that were similar before and after CSNX. Upon return to room air, heart rate decreased to below prehypoxia levels equally before and after CSNX. This bradycardia was resolved after 5–6 min (data not shown). Prior to CSNX, hypoxia elicited increases in fr. In contrast, fr values fell below prehypoxia levels for 90 s upon reintroduction of room air. These hypoxia and posthypoxia changes in fr were absent following CSNX.
Fig. 1.
Heart rate, mean arterial blood pressure (MAP), and frequency of breathing (Frequency) values before (time 0), during one episode of hypoxia (10% O2, 90% N2 for 90 s), and following return to room air (posthypoxia) before and after bilateral transection of the carotid sinus nerves (post-CSNX). There were 6 rats in the group. Data are presented as means ± SE. *P < 0.05, significant change from time 0 based on arithmetic differences.
Pharmacology Studies: MAP
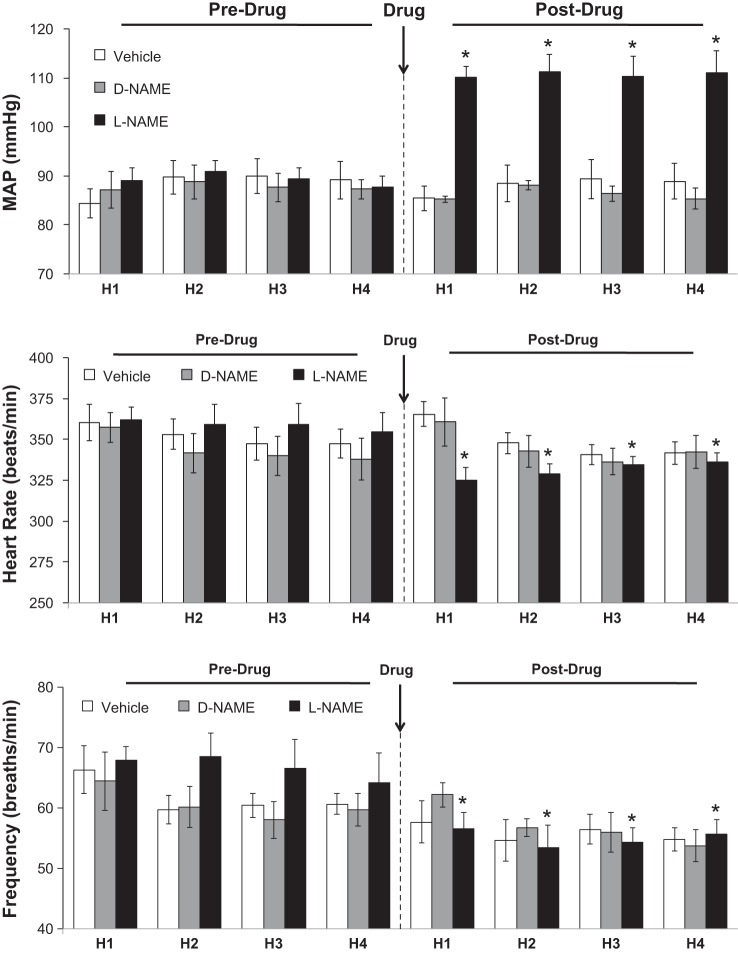
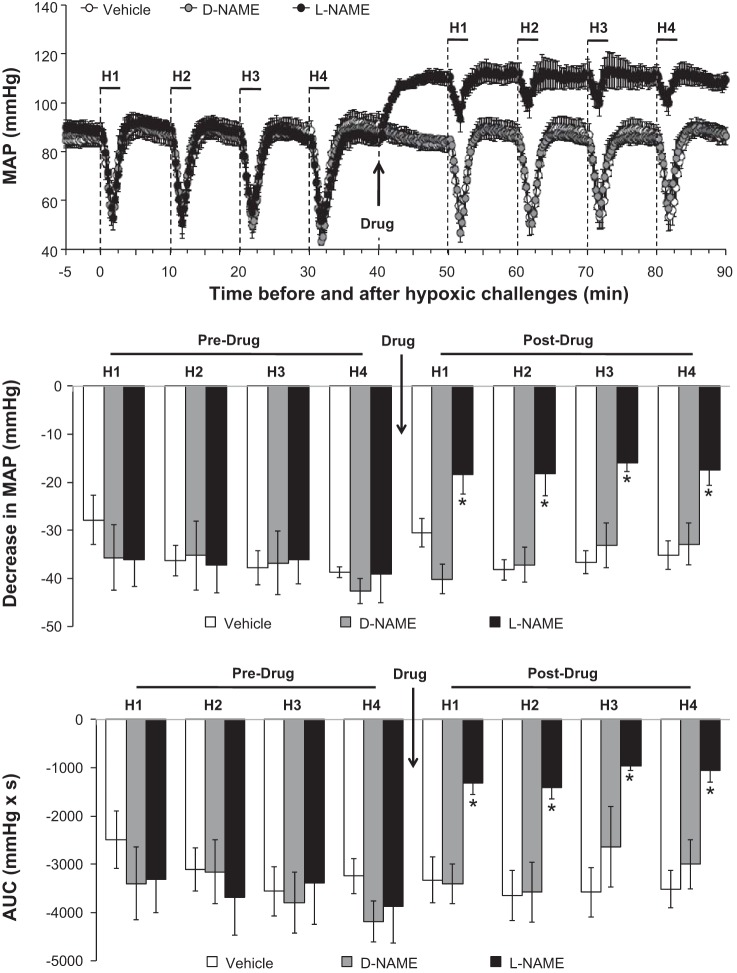
The changes in baseline MAP elicited by the drug injections are summarized in Fig. 2, top. Neither vehicle nor d-NAME affected MAP whereas l-NAME elicited a sustained hypertension. The effects of hypoxia on MAP before and after injection of vehicle, d-NAME, or l-NAME are summarized in Fig. 3. Prior to drug injection, each episode of hypoxia elicited decreases in MAP that were similar in magnitude and duration in all three groups. Neither saline nor d-NAME affected the hypoxia responses whereas l-NAME markedly diminished the decreases in MAP. The maximal (Fig. 3, middle) and total decreases in MAP (Fig. 3, bottom) elicited by hypoxia were attenuated in l-NAME-treated rats but not in vehicle- or d-NAME-treated rats.
Fig. 2.
Baseline mean arterial blood pressure (MAP, top), heart rate (middle), and frequency of breathing (bottom) values recorded prior to each exposure to hypoxia (H1–H4; 10% O2, 90% N2) before and after administration of vehicle (n = 6 rats), NG-nitro-d-arginine methyl ester (d-NAME; 50 μmol/kg iv, n = 5 rats) or NG-nitro-l-arginine methyl ester (l-NAME; 50 μmol/kg iv, n = 6 rats). Data are presented as means ± SE of the average of values recorded 5 min prior to exposure to hypoxia. *P < 0.05, significant difference from average of Pre values.
Fig. 3.
Actual mean arterial blood pressure (MAP) values (top), maximal arithmetic changes (middle), and total changes (AUC; bottom) in MAP before, during, and after 4 episodes of hypoxia (H1–H4; 10% O2, 90% N2 for 90 s) prior to and following injections of vehicle (n = 6 rats), d-NAME (50 μmol/kg iv, n = 5 rats), or l-NAME (50 μmol/kg iv, n = 6 rats). Data are presented as means ± SE of the average of every 15 s of recorded mean arterial pressure. *P < 0.05, l-NAME vs. vehicle or d-NAME.
Pharmacology Studies: Heart Rate
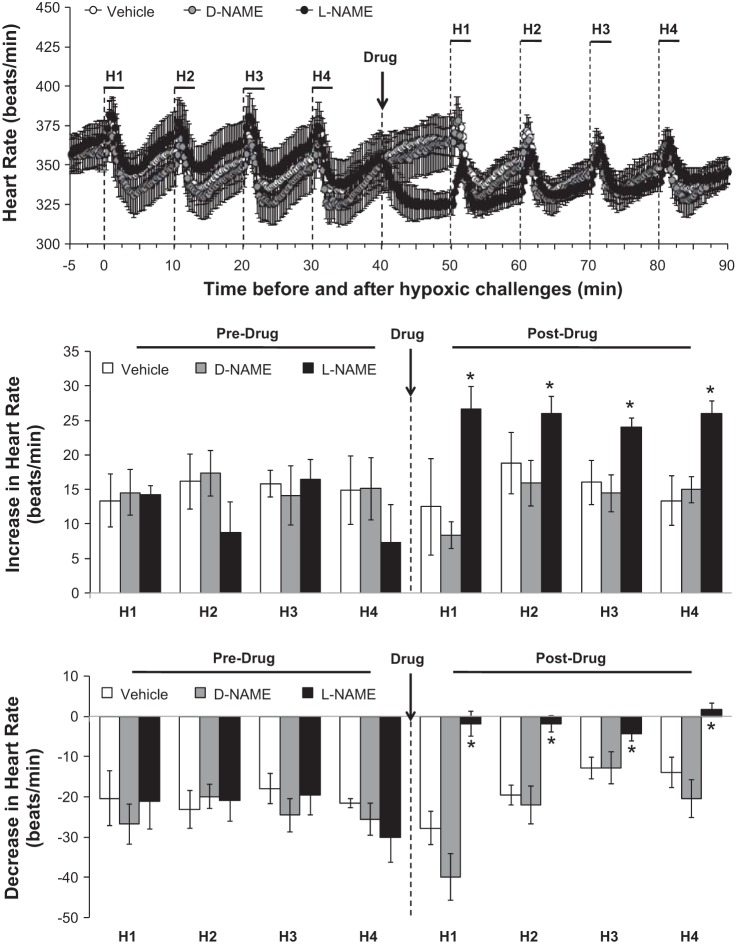
The changes in baseline heart rates (as measured before each exposure to hypoxia) elicited by the drug injections are summarized in Fig. 2. Heart rates were similar before and after administration of vehicle or d-NAME. In contrast, l-NAME elicited sustained decreases in heart rate. The effects of hypoxia on heart rate before and after injection of vehicle, d-NAME or l-NAME are summarized in Fig. 4. Prior to drug injection, each episode of hypoxia elicited similar initial increases in heart rate that upon return to room air were followed by decreases that resolved before the next episode of hypoxia was given. These hypoxia-induced changes in heart rate were similar before and after administration of vehicle or d-NAME. In contrast, the maximal increases in heart rate (Fig. 4, middle) were exaggerated whereas the maximal decreases in heart rate (Fig. 4, bottom) were diminished by l-NAME.
Fig. 4.
Actual heart rate values (top), maximal arithmetic increases (middle), and maximal arithmetic decreases (bottom) in heart rate before, during, and after 4 episodes of hypoxia (H1–H4; 10% O2, 90% N2 for 90 s) prior to and following injections of vehicle (n = 6 rats), d-NAME (50 μmol/kg iv, n = 5 rats), or l-NAME (50 μmol/kg iv, n = 6 rats). Data are presented as means ± SE. Statistics pertaining to middle and bottom panels: *P < 0.05, significant difference from average of Pre values.
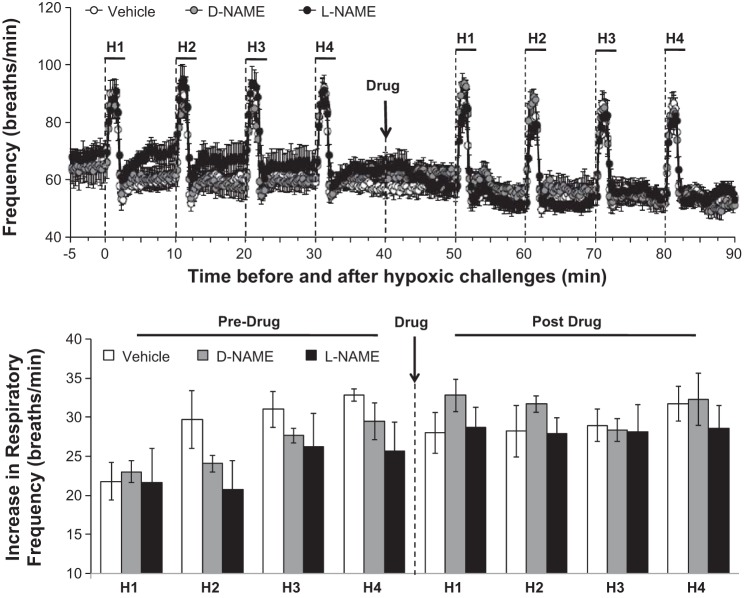
Pharmacology Studies: fr
The changes in baseline fr values elicited by the drug injections are summarized in Fig. 2. fr values after vehicle or d-NAME were slightly below but not significantly less than those before injection. Resting fr values were slightly diminished after injection of l-NAME. However, the possibility that l-NAME diminishes baseline fr was not confirmed in other groups of rats. More specifically, resting fr values recorded before and after injection of vehicle (plateau value at 30 min postinjection) were 66 ± 3 vs. 61 ± 4 breaths/min (−5 ± 2 breaths/min, P < 0.05, n = 6 rats) whereas resting fr values recorded before and after l-NAME (50 μmol/kg iv) were 64 ± 3 vs. 58 ± 4, arithmetic difference of −6 ± 2 breaths/min (P < 0.05, n = 6 rats). The arithmetic decreases in both groups were similar to one another (P > 0.05) and the actual postvehicle and post-l-NAME values were equal throughout the 60-min recording period (P > 0.05) and to those in the initial studies summarized above. These findings suggest that baseline fr decays gradually but minimally in rats breathing isoflurane (1.25%) and room air. The effects of hypoxia on fr before and after injection of vehicle, d-NAME, or l-NAME are summarized in Fig. 5. Prior to drug injection, each episode of hypoxia elicited similar initial increases in fr that resolved before the next episode of hypoxia was given (Fig. 5, top). The maximum hypoxia-induced increases in fr were similar before and after administration of vehicle, d-NAME, or l-NAME (Fig. 5, bottom).
Fig. 5.
Actual frequency of breathing values (Frequency, top) and maximal arithmetic increases in Frequency before, during, and after 4 episodes of hypoxia (H1–H4; 10% O2, 90% N2 for 90 s) prior to and following injections of vehicle (n = 6 rats), d-NAME (50 μmol/kg iv, n = 5 rats), or l-NAME (50 μmol/kg iv, n = 6 rats). Data are presented as means ± SE of the average of every 15 s of recorded mean arterial pressure. Note that there were no between-episode or between-group differences in these responses (P > 0.05 for all comparisons).
DISCUSSION
Baseline Parameters in Isoflurane-Anesthetized Rats
The rats maintained on 1.25% isoflurane presented with a stable ventilatory and cardiovascular profile. Resting MAP values were 10–15 mmHg below those usually observed in conscious rats whereas resting heart rates were similar to those of conscious rats (15). The mechanisms by which isoflurane and related volatile anesthetics affect hemodynamic status have been investigated (9, 61). These anesthetics lower MAP by reducing peripheral vascular resistance due principally to diminished sympathetic outflow (9, 61) although an increase in vascular NOS activity and thereby enhanced NO-mediated vasodilation has also been suggested to be involved in the early but not later phase of isoflurane-induced hypotension (9). The direct effects of ISO on the vasculature are complicated since it enhances the contractile activity of the major sympathetic neurotransmitter, norepinephrine, in endothelium-intact arteries whereas it suppresses the contractile effects in endothelium-denuded arteries (61). The resting fr of these isoflurane-anesthetized rats were also less (∼10 breaths/min) than those usually seen in conscious rats (62). The mechanisms by which isoflurane and related volatile anesthetics affect ventilatory function have also been widely investigated. These agents suppress ventilation by actions within the central nervous system and peripheral structures including the carotid bodies (5, 37–39).
Hypoxic Responses in Isoflurane Rats
The changes in MAP and heart rate elicited by the hypoxic challenge (10% O2, 90% N2) in the isoflurane-anesthetized rats were substantially different from those observed in conscious rats (12, 48, 59). For example, the hypoxic challenge in isoflurane-anesthetized rats elicited a prompt and robust fall in MAP (e.g., episode 1 of hypoxia in vehicle-treated rats of −27 ± 3 mmHg) whereas falls of less than 5 mmHg occur in conscious rats (12, 48, 59). Moreover, the hypoxic challenge in isoflurane-anesthetized rats elicited a relatively minor increase in heart rate that was followed by relatively minor decrease (e.g., episode 1 of hypoxia in vehicle-treated rats of +17 ± 2 beats/min and −16 ± 3 beats/min, respectively) compared with a robust and sustained tachycardia of at least 60 beats/min in conscious rats (12, 48). In our own laboratory (unpublished observations), we have found that hypoxic challenge elicits a minor change in MAP (−3 ± 2 mmHg) in conscious Sprague-Dawley rats (n = 8) that was accompanied by a robust increase in heart rate (+68 ± 8 beats/min) as measured at 90 s, the time the peak changes in isoflurane-anesthetized rats were observed. These findings are consistent with evidence that isoflurane disturbs the mechanisms by which the heart responds to the challenges (37–39). Studies in humans (5) and animals (37–39) have demonstrated that volatile anesthetics blunt the ventilatory responses to hypoxia by actions within the carotid bodies. Studies in carotid body preparations have also provided compelling evidence that volatile anesthetics suppress the hypoxia-induced increases in intracellular Ca2+-concentrations in primary glomus cells by activation of background TASK-like K+ channels (39) although isoflurane was substantially weaker than halothane in suppressing the hypoxic response. This latter observation is supported by in vivo studies, which demonstrated that isoflurane is substantially weaker than halothane with respect to inhibition of the hypoxic ventilatory response in humans (37) and rats (23) and that 1.0% end-tidal concentration of isoflurane does not depress the hypoxic chemosensitivity of peripheral chemoreceptors (21). Moreover, Teppema et al. (54) provided evidence that the generation of free radicals may also play an essential role in depression of the acute hypoxic ventilatory response by subanesthetic doses of halothane in humans. The brief episodes of hypoxia elicited rapid and robust increases in fr that were substantially smaller in magnitude than those observed in conscious rats (12, 31, 48). For example, we reported that acute exposure to a hypoxic challenge elicited a maximal increase in fr of +123 ± 15 breaths/min in conscious Sprague-Dawley rats (31), which is substantially greater than the maximal responses (e.g., episode 1 of hypoxia in vehicle-treated rats of +24 ± 3 breaths/min) observed in the isoflurane-anesthetized Sprague-Dawley rats.
Carotid Sinus Nerve Transection Studies
The finding that the hypoxia-induced decreases in MAP and presumably baroreceptor reflex-mediated increases in heart rate were similar before and after bilateral CSNX suggests that CSN chemoafferents/baroafferents are not essential for the expression of these responses. Indeed, increases in heart rate in response to the hypoxia-induced falls in MAP in the CSNX rats would be expected because of the presence of aortic baroafferents, which travel in the aortic depressor nerves (42), the integrity of which was not affected by the CSNX procedure. Upon reintroduction to room air, MAP recovered to prehypoxia levels. A bradycardia, which was sustained for several minutes, was also observed. That these post-hypoxia changes in MAP and heart rate were similar before and after CSNX suggests that the recovery of MAP is due to the loss of the hypoxic-vasodilator stimulus, and that the bradycardia is independent of the carotid body complex. Our findings that this bradycardia was absent in l-NAME-treated rats suggests a primary role of NOS in this phenomenon.
The ventilatory responses to hypoxia are mediated principally via carotid body chemoafferent input to the NTS, which elicits signaling cascades that increase motor output to the chest wall and diaphragm (55). The vital role of the carotid body complex in tonic regulation of ventilation is supported by evidence that upon recovery from surgery, fr in conscious rats is depressed for several days following bilateral CSNX (46). In contrast, bilateral CSNX does usually suppress ventilation in animals anesthetized with volatile anesthetics (in which ventilation is already suppressed) possibly because the anesthetics inhibit resting carotid body chemoafferent activity (37–39). Our finding that bilateral CSNX did not depress fr in our isoflurane-anesthetized rats is consistent with the above possibility. That the hypoxia increases in fr were eliminated after bilateral CSNX in these isoflurane rats is consistent with evidence that hypoxia stimulates ventilation via activation of the carotid body/chemoafferent complex in humans (56) and rats (46).
Minute ventilation falls below resting levels following recovery from brief hypoxia (i.e., upon return to room air) via reductions in tidal volume and fr. This posthypoxia depression of fr, which is referred to as posthypoxia frequency decline (PHFD), is an active neural process that depends on the integrity of the ventrolateral pons (16). Two key findings of the present study were that the isoflurane rats displayed PHFD, and that this decline was absent following bilateral CSNX. These novel findings suggest that CSN input is essential for the expression of PHFD and specifically that CSN activity falls below normal activity, thereby promoting hypoventilation. This possibility is directly supported by studies in an in vitro rat carotid body preparation, which found that carotid body chemoafferent activity was transiently but substantially diminished (sensory posthypoxia decline) after recovery from mild hypoxia challenge (4). A perhaps equally compelling possibility is that input from carotid body chemoafferents (or indeed baroafferents) within the CSN play an obligatory role in allowing neurons, and especially those within the ventrolateral pons, to promote the decrease in fr (enhanced expiratory duration). This would be analogous to the permissive role of the carotid body chemoafferent input in the direct depressive effects of hypoxia on central neuronal activity (4, 16).
Pharmacology Studies: MAP
Exposure to 90-s episodes of hypoxia (10% O2, 90% N2) elicited decreases in MAP of about 40 mmHg in our rats. Although we did not determine the mechanisms responsible for the decreases in MAP, Huang et al. (18) found that the hypotensive responses elicited by brief episodes of hypoxia (10% O2, 90% N2 for 5 min) in pentobarbital-anesthetized rats were due to reductions in total peripheral resistance and in cardiac output. The hypoxia-induced changes in MAP during and following each hypoxia episode in our isoflurane-anesthetized rats were similar before and after injection of vehicle or d-NAME. This suggests that there were no substantial time-dependent impairments in the mechanisms by which hypoxia elicited its hemodynamic effects. The sustained increases in MAP elicited by l-NAME (50 μmol/kg iv) in our isoflurane-anesthetized rats are probably due to sustained increases in peripheral vascular resistance that are offset by a decrease in cardiac output (15, 18, 42). The robust and sustained nature of the l-NAME-induced hypertension in these rats certainly suggests that the volatile anesthetic did not obviously impair the activity of NOS or the dependence of the vascular system on NO factors. Our findings contrast substantially from those of Sigmon et al. (51), who reported that halothane markedly attenuated the hemodynamic effects of l-NAME. This difference between isoflurane and halothane is another example of the differing pharmacology of these two volatile anesthetics (37–39).
The finding that the arithmetic and percent changes in MAP elicited by hypoxia were markedly attenuated following injection of l-NAME contrasts somewhat with those of Huang et al. (18), who found that the arithmetic decreases in MAP elicited by hypoxia were not decreased in l-NAME-treated rats, but because of the elevated baseline, the arithmetic response computed to a smaller percent decrease. Although this may argue against a role of NO in hypoxia-induced hypotension, Huang et al. (18) found that the hypoxia-induced reductions in peripheral vascular resistance were diminished after administration of l-NAME. Moreover, other studies have certainly provided evidence that the maintenance of hypoxia-induced decreases in MAP are attenuated by NOS inhibitors. For example, Ray and Marshall (45) found that hypoxia elicited hypotension, a decrease in femoral artery resistance (i.e., vasodilation), and a substantial increase in plasma NO (based on conversion of NO3− to NO2− and conversion of NO2− to NO) in anesthetized rats. The hypoxia-induced decreases in MAP and femoral artery vasodilation were attenuated after blockade of NOS (45). As such, it is tempting to assume that a principal mechanism by which hypoxia exerts its hypotensive and vasodilator effects is via the release of endothelium-derived NO and/or S-nitrosothiols (15). However, it is also distinctly possible that the vasodilator effects of hypoxia involve neurogenic (NO factor-mediated) vasodilator mechanisms (42), and the direct hypoxia-induced formation of blood-borne S-nitrosothiols (2). The question therefore arises as to whether NO and/or S-nitrosothiols are involved in mediating the hypotensive/vasodilator actions of hypoxia. The possible role of S-nitrosothiols is supported by evidence that exposure to hypoxia increases the S-nitrosylation status and activities of many functional proteins including Ca2+VS-channels (36).
Pharmacology Studies: Heart Rate
NOS is also localized to cardiac neurons and muscle cells (6). The primary role of NO appears to include inhibition of the positive inotropic and chronotropic responses elicited by β-adrenergic receptor stimulation, and facilitation of parasympathetic (vagal) nerve-mediated slowing of heart rate and inhibition of β-adrenergic receptor-mediated increases in cardiac contractility (6). In addition, S-nitrosylation is a ubiquitous signaling modality impacting virtually every facet of cardiac function (28). The precise effects of S-nitrosylation are complex but include inhibition of Ca2+VS-channel activity and sarcoplasmic-localized Ca2+-ATPase (28), effects that would promote bradycardia and a decrease in cardiac contractility. In agreement with a variety of studies (12), hypoxia elicited an increase in heart rate, most likely due to activation of the baroreflex system in response to the decrease in MAP (12). Upon reintroduction of room air, heart rate fell to below prehypoxia levels. This bradycardia was unaffected by prior transection of the CSNs and could not have been due to activation of the baroreflex system since MAP remained depressed. Although a variety of mechanisms could be involved, it is tempting to assume that the generation of NO/S-nitrosothiols in the heart during hypoxia limited the initial presumably baroreflex-mediated increase in heart rate and as time progresses directly suppresses cardiac function. Indeed, a key finding was that the initial tachycardia elicited by hypoxia was augmented in l-NAME-treated rats whereas the subsequent bradycardia upon return to room air was virtually absent in these rats. These findings suggest that hypoxia directly generated NO factors that suppress pacemaker activity/conductance in the heart and that blockade of NOS allows for fuller baroreceptor reflex-mediated changes in autonomic nerve activity to affect heart rate.
Pharmacology Studies: fr
The systemic administration of l-NAME elicits a pronounced increase in fr in conscious rats (12, 52). Moreover, application of NOS inhibitors to carotid body preparations increases resting activity of chemoafferents and augments the sensitivity of these afferents to hypoxia (43). These and other data suggest that NO is primarily inhibitory in the carotid body complex whereas S-nitrosothiols may have a facilitatory role (19, 20, 26, 36). In contrast to conscious animals, systemic injection of these NOS inhibitors elicit minimal effects on ventilatory parameters in anesthetized animals (17, 53), including fr in rats (40). As such, it appears that anesthetics interfere with NOS-dependent regulation of ventilatory drive in the carotid bodies and/or brain. The finding that l-NAME did not elicit an increase in fr in isoflurane-anesthetized rats suggests that the potential decrease in cerebral blood flow is not sufficient to drive ventilation or more likely because NOS inhibitors depress ventilatory responses to hypercapnia by actions in the brain stem (53). Moreover, McPherson et al. (32) demonstrated that whereas l-NAME diminished cerebral blood flow it did not interfere with hypoxia-induced vasodilation in isoflurane-anesthetized dogs. As such, an appropriate cerebral vasodilation in response to hypoxia in l-NAME-treated animals would not represent a ventilatory stimulus.
A key finding of this study was that the increases in fr elicited by hypoxia were unaffected by l-NAME despite the propensity of NOS inhibitors to promote the depth of anesthesia (and possibly respiratory depression) elicited by volatile anesthetics including isoflurane (22). l-NAME did not affect the peak magnitude of the ventilatory response during hypoxic challenge nor did it affect the posthypoxia changes in fr. These findings contrast to those in conscious rats in which blockade of endothelial NOS, the form of NOS in the primary glomus cells of the carotid bodies (19, 26, 60), inhibited the increases in fr (12), although the substantial increase in baseline fr in the l-NAME-treated rats makes interpretation somewhat difficult. Our data also contrast with evidence that the ventilatory responses to hypoxia are augmented in mice deficient in neuronal NOS (24) whereas mice deficient in endothelial NOS display enhanced ventilatory responses to hypoxia challenge (25). Taken together, it appears that despite displaying a robust increase in fr to hypoxia, isoflurane-anesthetized rats are devoid of a central and/or peripheral NOS-dependent component in the hypoxia response.
Despite direct biochemical evidence that NOS activity decreases under hypoxia (1), it appears that (in the carotid body at least) NOS activity increases during brief hypoxia challenges because carotid body O2 tension does not reach levels that inhibit NOS activity and because hypoxia increases intracellular Ca2+ levels, which promotes NOS activity (33, 60). There is also substantial evidence that hypoxic challenges increase NOS activity in other tissues with concomitant increases in NO and S-nitrosothiol bioavailability (2, 11, 29, 36). Although inhibition of NOS activity and blockade of NO neurotransmitter function are thought to be key steps in the mechanism of action of volatile anesthetics, there is evidence that these anesthetics have little effect or markedly increase NOS activity and NO production (27, 50). The interaction of isoflurane-induced and hypoxia-induced changes in NOS activity are likely to be complex although our results raise the possibilities that 1) isoflurane prevents the hypoxia-induced activation of NOS and therefore the de novo synthesis of NO factors in the brain, carotid body, or blood; and/or 2) isoflurane impairs the mechanisms by which NO and especially S-nitrosothiols exert their effects on these structures. Indeed, numerous studies have demonstrated potential mechanisms by which volatile anesthetics could impair the ability of hypoxia to stimulate NOS, including activation of TASK-like K+-channels, which would lead to diminished Ca2+VS-channel activity and therefore less Ca2+ entry into primary glomus cells and inhibition of a variety of other Ca2+ channels and intracellular Ca2+-mobilizing processes (39, 41).
Study Limitations
A limitation of this study is that we did not verify that the isoflurane-anesthetized rats had comparable blood gases at baseline, and that they achieved comparable changes in blood oxygen during the acute hypoxic challenges. However, our findings that each hypoxic episode elicited similar temporal increases in fr in the two groups of rats prior to administration of vehicle or l-NAME suggests that each hypoxic challenge elicited similar changes in arterial blood-gas chemistry. Exposure to hypoxic challenges elicits profound falls in Po2 and Pco2 in isoflurane-anesthetized rats (8, 49). These reductions in Pco2 are in stark contrast to the increases in Pco2 that occur during episodes of apnea (7). As such, our study does not address the potent effects of blood Pco2 on ventilatory drive and therefore cannot be considered as a model of apnea in which to examine the effects of isoflurane or the roles of NOS. Another limitation of the present study is that we did not determine the effects of hypoxia on tidal volumes or the effects of l-NAME on these hypoxia-induced responses. Although we determined that the increase in fr elicited by hypoxic gas challenge is markedly suppressed in isoflurane-anesthetized rats and that these responses were not blunted by l-NAME, it is possible these effects on fr may not be mirrored by the effects on tidal volume. Nonetheless, the clear dependency of the hypoxia-induced changes in fr on the integrity of the carotid sinus nerves in the isoflurane-anesthetized rats lends strength to the argument that isoflurane diminishes NOS-dependent processes within the carotid bodies.
Summary and Clinical Implications
Our key findings were that whereas l-NAME markedly attenuated the falls in MAP during hypoxia challenges in isoflurane-anesthetized rats, it had minimal impact on the ventilatory responses. As discussed above, there is substantial evidence that NO and S-nitrosothiols play pivotal roles in the ventilatory responses to hypoxia challenges in conscious animals. Our findings raise the possibility that isoflurane blocks the actions of NO-containing factors within the carotid bodies or brain sites processing ventilatory information rather than the de novo formation of NO and S-nitrosothiols in these structures (26, 28, 29) or the blood (2, 57) or from the release of preformed pools of NO-containing factors (15). Considering evidence that NO itself plays an inhibitory role in the carotid body (34), the findings that l-NAME diminishes the hypoxic ventilatory response in conscious rats raises the possibility that other products of NOS, namely S-nitrosothiols, may be involved in hypoxic signaling in the brain and carotid bodies as well as gas exchange. Indeed, hypoxic challenge elicits increases in circulating S-nitrosothiols in conscious rats (29); the microinjection of S-nitrosothiols such as l-S-nitrosoglutathione and l-S-nitrosocysteine into the NTS of conscious rats increases minute ventilation (29); S-nitroso-N-acetyl-penicillamine increases CSN chemoafferent activity in isolated carotid body-CSN preparations from cats (19, 20, 34), and S-nitrosothiols exert positive effects on ventilatory function and pulmonary gas-exchange mechanisms in a variety of species (11). As suggested by Rubanyi and Vanhoutte (47), decreased O2 availability in tissues could increase NO radical bioactivity by decreasing tissue superoxide anions, which directly interact with NO. This could be a mechanism involved in the vascular effects of hypoxia, and may be relevant to the ventilatory effects with increased NO bioavailability being inhibitory but enhanced S-nitrosothiol bioavailability being excitatory (29). Although isoflurane is likely to attenuate the hypoxic ventilatory response by a multiplicity of mechanisms including blockade of hypoxia-induced increases in NOS activity, we are currently examining whether this anesthetic inhibits the hypoxic ventilatory response via alterations in the biological activities of NO and S-nitrosothiols within the carotid body and brain stem.
GRANTS
This study was supported by a National Heart, Lung, and Blood Institute (NHLBI) Program Project Grant (1P01-HL-101871; B. Gaston, S. J. Lewis) and individual grants from Galleon Pharmaceuticals (S. J. Lewis), and the NHLBI (R01-HL-59337; B. Gaston).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.P.M., S.M.B., A.P.y., J.N.B., B.G., and S.J.L. conception and design of research; J.P.M., R.J.P., and A.P.Y. performed experiments; J.P.M., R.J.P., S.M.B., A.P.Y., J.N.B., B.G., and S.J.L. approved final version of manuscript; S.M.B., J.N.B., B.G., and S.J.L. interpreted results of experiments; J.N.B. and S.J.L. analyzed data; J.N.B., B.G., and S.J.L. drafted manuscript; S.J.L. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Erin Whalen for suggestions regarding experimental protocols and in the writing of this manuscript.
REFERENCES
- 1.Abu-Soud HM, Rousseau DL, Stuehr DJ. Nitric oxide binding to the heme of neuronal nitric oxide links its activity to changes in oxygen tension. J Biol Chem 271: 32515–32518, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 15: 452–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiueh CC. S-nitrosoglutathione (GSNO) mediates brain response to hypoxia. Pediatr Res 51: 414, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cummings KJ, Wilson RJ. Time-dependent modulation of carotid body afferent activity during and after intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 288: R1571–R1580, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Dahan A, Van Den Elsen MJLJ, Berkenbosch A, Degoede J, Olievier ICW, Van Kleef J, Bovill JG. Effects of subanesthetic halothane on the ventilatory responses to hypercapnia and acute hypoxia in healthy volunteers. Anesthesiology 80: 727–738, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Danson EJ, Paterson DJ. Cardiac neurobiology of nitric oxide synthases. Ann NY Acad Sci 1047: 183–196, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res 1135: 186–194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellenberger EA, Lucas HL, Mueller JL, Barrington PL, Chung E, Ohgami Y, Quock RM. Possible involvement of neuronal nitric oxide synthase enzyme in early-phase isoflurane-induced hypotension in rats. Life Sci 76: 499–507, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Gauthier KM. Hypoxia-induced vascular smooth muscle relaxation: increased ATP-sensitive K+ efflux or decreased voltage-sensitive Ca2+ influx? Am J Physiol Heart Circ Physiol 291: H24–H25, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 73: 1186–1193, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gozal D, Torres JE, Gozal YM, Littwin SM. Effect of nitric oxide synthase inhibition on cardiorespiratory responses in the conscious rat. J Appl Physiol 81: 2068–2077, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Gozal D, Gozal E, Simakajornboon N. Signaling pathways of the acute hypoxic ventilatory response in the nucleus tractus solitarius. Respir Physiol 121: 209–221, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Granjeiro EM, Machado BH. NO in the caudal NTS modulates the increase in respiratory frequency in response to chemoreflex activation in awake rats. Respir Physiol Neurobiol 166: 32–40, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hashmi-Hill MP, Sandock K, Bates JN, Robertson TP, Lewis SJ. Flavin adenine dinucleotide may release preformed stores of nitrosyl factors from the vascular endothelium of conscious rats. J Cardiovasc Pharmacol 50: 142–154, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hayashi F, Fukuda Y. Neuronal mechanisms mediating the integration of respiratory responses to hypoxia. Jpn J Physiol 50: 15–24, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Haxhiu MA, Chang CH, Dreshaj IA, Erokwu B, Prabhakar NR, Cherniack NS. Nitric oxide and ventilatory response to hypoxia. Respir Physiol 101: 257–266, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Huang KL, Wu CP, Kang BH, Lin YC. Chronic hypoxia attenuates nitric oxide-dependent hemodynamic responses to acute hypoxia. J Biomed Sci 9: 206–212, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Rev 47: 46–53, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Iturriaga R, Villanueva S, Mosqueira M. Dual effects of nitric oxide on cat carotid body chemoreception. J Appl Physiol 89: 1005–1012, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Joensen H, Sadler CL, Ponte J, Yamamoto Y, Lindahl SG, Eriksson LI. Isoflurane does not depress the hypoxic response of rabbit carotid body chemoreceptors. Anesth Analg 91: 480–485, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Johns RA, Moscicki JC, DiFazio CA. Nitric oxide synthase inhibitor dose-dependently and reversibly reduces the threshold for halothane anesthesia. A role for nitric oxide in mediating consciousness? Anesthesiology 77: 779–784, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Karanovic N, Pecotic R, Valic M, Jeroncic A, Carev M, Karanovic S, Ujevic A, Dogas Z. The acute hypoxic ventilatory response under halothane, isoflurane, and sevoflurane anaesthesia in rats. Anaesthesia 65: 227–234, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Kline DD, Yang T, Huang PL, Prabhakar NR. Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. J Physiol 511: 273–287, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kline DD, Yang T, Premkumar DR, Thomas AJ, Prabhakar NR. Blunted respiratory responses to hypoxia in mutant mice deficient in nitric oxide synthase-3. J Appl Physiol 88: 1496–1508, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol 91: 249–286, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Lang XE, Wang X, Jin JH. Mechanisms of cardioprotection by isoflurane against I/R injury. Front Biosci 18: 387–393, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature 413: 171–174, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Maruyama R, Fukuda Y. Ventilation- and carotid chemoreceptor discharge-response to hypoxia during induced hypothermia in halothane anesthetized rat. Jpn J Physiol 50: 91–99, 2000 [DOI] [PubMed] [Google Scholar]
- 31.May WJ, Gruber RB, Discala JF, Puskovic V, Henderson F, Palmer LA, Lewis SJ. Morphine has latent deleterious effects on the ventilatory responses to a hypoxic challenge. Open J Mol Integ Physiol 3: 166–180, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPherson RW, Koehler RC, Traystman RJ. Hypoxia, alpha 2-adrenergic, and nitric oxide-dependent interactions on canine cerebral blood flow. Am J Physiol Heart Circ Physiol 266: H476–H482, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Mironov SL, Langohr K. Modulation of synaptic and channel activities in the respiratory network of the mice by NO/cGMP signalling pathways. Brain Res 1130: 73–82, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Mosqueira M, Iturriaga R. Carotid body chemosensory excitation induced by nitric oxide: involvement of oxidative metabolism. Respir Physiol Neurobiol 131: 175–187, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Ogawa H, Mizusawa A, Kikuchi Y, Hida W, Miki H, Shirato K. Nitric oxide as a retrograde messenger in the nucleus tractus solitarii of rats during hypoxia. J Physiol 486: 495–504, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer LA, May WJ, deRonde K, Brown-Steinke K, Gaston B, Bates JN, Lewis SJ. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Resp Physiol Neurobiol 185: 571–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandit JJ. The variable effect of low-dose volatile anaesthetics on the acute ventilatory response to hypoxia in humans: a quantitative review. Anaesthesia 57: 632–643, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Pandit JJ, O'Gallagher K. Effects of volatile anesthetics on carotid body response to hypoxia in animals. Adv Exp Med Biol 605: 46–50, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Pandit JJ, Winter V, Bayliss R, Buckler KJ. Differential effects of halothane and isoflurane on carotid body glomus cell intracellular Ca2+ and background K+ channel responses to hypoxia. Adv Exp Med Biol 669: 205–208, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Patel GM, Horstman DJ, Adams JM, Rich GF. Nitric oxide synthase inhibitors alter ventilation in isoflurane anesthetized rats. Anesthesiology 88: 1240–1248, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Phillis JW, DeLong RE, Towner JK. The effects of nifedipine and felodipine on cerebral blood flow during anoxic episodes. Stroke 17: 229–234, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Possas O, Johnson AK, Lewis SJ. Role of nitrosyl factors in the hindlimb vasodilation produced by baroreceptor afferent nerve stimulation. Am J Physiol Regul Integr Comp Physiol 290: R741–R748, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Prabhakar NR, Kumar GK, Chang CH, Agani FH, Haxhiu MA. Nitric oxide in the sensory function of the carotid body. Brain Res 625: 16–22, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544: 195–209, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray CJ, Marshall JM. Measurement of nitric oxide release evoked by systemic hypoxia and adenosine from rat skeletal muscle in vivo. J Physiol 568: 967–978, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol 522: 493–501, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H822–H827, 1986 [DOI] [PubMed] [Google Scholar]
- 48.Sabino JP, Oliveira Md, Giusti H, Glass ML, Salgado HC, Fazan R., Jr Hemodynamic and ventilatory response to different levels of hypoxia and hypercapnia in carotid body-denervated rats. Clinics (Sao Paulo) 68: 395–399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shingu K, Eger E, 3rd, Johnson BH, Lurz FW, Taber V. Effects of halothane, isoflurane, enflurane, thiopental, and fentanyl on blood gas values in rats exposed to hypoxia. Anesth Analg 62: 155–159, 1983 [PubMed] [Google Scholar]
- 50.Sjakste N, Dzintare M, Baumane L, Meirena D, Lauberte L, Kalvinś I. Role of nitric oxide (NO) in the mechanism of anesthetic action. Anesteziol Reanimatol 3: 61–65, 2001 [PubMed] [Google Scholar]
- 51.Sigmon DH, Florentino-Pineda I, Van Dyke RA, Beierwaltes WH. Halothane impairs the hemodynamic influence of endothelium-derived nitric oxide. Anesthesiology 82: 135–143, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Subramanian S, Erokwu B, Han F, Dick TE, Strohl KP. l-NAME differentially alters ventilatory behavior in Sprague-Dawley and Brown Norway rats. J Appl Physiol 93: 984–989, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Teppema L, Berkenbosch A, Olievier C. Effect of NG-nitro-l-arginine on ventilatory response to hypercapnia in anesthetized cats. J Appl Physiol 82: 292–297, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Teppema LJ, Nieuwenhuijs D, Sarton E, Romberg R, Olievier CN, Ward DS, Dahan A. Antioxidants prevent depression of the acute hypoxic ventilatory response by subanaesthetic halothane in men. J Physiol 544: 931–938, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. J Physiol 553: 3–11, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation 126: 325–334, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Travis MD, Hoque A, Bates JN, Lewis SJ. Blockade of voltage-sensitive Ca2+-channels markedly diminishes nitric oxide- but not l-S-nitrosocysteine- or endothelium-dependent vasodilation in vivo. Eur J Pharmacol 408: 289–298, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Walker BR, Brizzee BL. Renal vascular response to combined hypoxia and hypercapnia in conscious rats. Am J Physiol Regul Integr Comp Physiol 254: R552–R558, 1988 [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto Y, König P, Henrich M, Dedio J, Kummer W. Hypoxia induces production of nitric oxide and reactive oxygen species in glomus cells of rat carotid body. Cell Tissue Res 325: 3–11, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Yoshino J, Akata T, Shirozu K, Izumi K, Hoka S. Diabetes-associated alterations in volatile anesthetic actions on contractile response to norepinephrine in isolated mesenteric resistance arteries. Anesthesiology 112: 595–606, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Young AP, Gruber RB, Discala JF, May WJ, Palmer LA, Lewis SJ. Co-activation of μ- and δ-opioid receptors elicits tolerance to morphine-induced ventilatory depression via generation of peroxynitrite. Resp Physiol Neurobiol 186: 255–264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]