Abstract
The metabolic syndrome (MetS) is associated with a threefold increase risk of cardiovascular disease (CVD) mortality partly due to increased arterial stiffening. We compared the effects of aerobic exercise training on arterial stiffening/mechanics in MetS subjects without overt CVD or type 2 diabetes. MetS and healthy control (Con) subjects underwent 8 wk of exercise training (ExT; 11 MetS and 11 Con) or remained inactive (11 MetS and 10 Con). The following measures were performed pre- and postintervention: radial pulse wave analysis (applanation tonometry) was used to measure augmentation pressure and index, central pressures, and an estimate of myocardial efficiency; arterial stiffness was assessed from carotid-femoral pulse-wave velocity (cfPWV, applanation tonometry); carotid thickness was assessed from B-mode ultrasound; and peak aerobic capacity (gas exchange) was performed in the seated position. Plasma matrix metalloproteinases (MMP) and CVD risk (Framingham risk score) were also assessed. cfPWV was reduced (P < 0.05) in MetS-ExT subjects (7.9 ± 0.6 to 7.2 ± 0.4 m/s) and Con-ExT (6.6 ± 1.8 to 5.6 ± 1.6 m/s). Exercise training reduced (P < 0.05) central systolic pressure (116 ± 5 to 110 ± 4 mmHg), augmentation pressure (9 ± 1 to 7 ± 1 mmHg), augmentation index (19 ± 3 to 15 ± 4%), and improved myocardial efficiency (155 ± 8 to 168 ± 9), but only in the MetS group. Aerobic capacity increased (P < 0.05) in MetS-ExT (16.6 ± 1.0 to 19.9 ± 1.0) and Con-ExT subjects (23.8 ± 1.6 to 26.3 ± 1.6). MMP-1 and -7 were correlated with cfPWV, and both MMP-1 and -7 were reduced post-ExT in MetS subjects. These findings suggest that some of the pathophysiological changes associated with MetS can be improved after aerobic exercise training, thereby lowering their cardiovascular risk.
Keywords: metabolic syndrome, arterial stiffness, exercise training
the metabolic syndrome (MetS) is associated with a threefold increased risk of cardiovascular disease (CVD) mortality than individuals without MetS (24). Current predictions are that, by the year 2020, as much as 40% of the population will have MetS (18). Part of this increased CVD risk in MetS is attributed to the pathophysiological changes to the arterial system, namely increased arterial stiffness, carotid thickening, and higher central arterial pressures (13, 30). A 1 m/s increase in central pulse wave velocity (PWV: a measure of arterial stiffness) corresponds to an age-, sex-, and risk factor-adjusted risk increase of 15% in cardiovascular and all-cause mortality (40). Furthermore, a 0.1-mm increase in carotid artery intima media thickness (cIMT) is associated with an 18 and 15% increased risk for a stroke and myocardial infarction, respectively (20). Therefore, it is critically important that effective interventions aimed at improving arterial health are identified.
Aerobic exercise training has been shown to be an effective intervention to improve arterial compliance/stiffness in healthy young and middle/older persons (8, 34). However, with metabolic disease, previous studies have examined exercise interventions in populations with a single metabolic risk factor (obesity, hypertension, hypercholesterolemia) (12, 28, 31), or in MetS subjects with type 2 diabetes (T2DM) (10, 21). Because the development of MetS occurs before the development of T2DM and overt CVD, it is important to know if exercise training can reverse the arterial stiffening and improve the CVD risk profile of MetS individuals before overt T2DM or CVD. Understanding the mechanisms mediating favorable changes in arterial function before the progression to T2DM is important. It is possible that the changes in the arterial expression, architecture, and/or bioactivity of structural proteins that play a major role with CVD are either reversed or ameliorated through exercise. Favorable effects of exercise on improving the balance with pro- and anti-inflammatory and oxidative stress markers could improve nitric oxide bioavailability and alter matrix metalloproteinases (MMP) and/or tissue inhibitors of MMP (TIMP) activity. MMP are regulators of tissue remodeling, and its activity is greatly affected by mechanical stretch. Chronic exercise training, which acts as a mechanical stressor to the arteries, could exert its effects by altering MMP/TIMP activity directly and/or indirectly via the anti-inflammatory and oxidative stress responses of exercise.
We examined whether aerobic exercise training can reverse arterial stiffness and improve arterial mechanics, including central pressures in individuals with MetS without overt CVD or T2DM. Furthermore, we examined the potential mechanisms (inflammation and MMP/TIMP activity) through which aerobic exercise may improve arterial health. We hypothesized that exercise training in MetS subjects would reduce central arterial stiffness compared with the nontrained MetS control group.
METHODS
Study Population
Twenty-one healthy controls (41 ± 2 yr; 71% female) and 22 MetS (45 ± 2 yr; 60% female) subjects participated in the study. The healthy controls were free from CVD, as determined by a detailed history, physical examination, and a normal resting and exercise electrocardiogram. MetS subjects were free from overt CVD and diabetes. Furthermore, none of the participants was a current or former smokers or was being treated for peripheral artery disease. MetS was defined according to the updated National Cholesterol Education Program, Adult Treatment Panel III (15) composed of three out of the following five components: 1) obesity (waist men > 102 cm, women > 88 cm); 2) low high-density lipoprotein (HDL) cholesterol (men < 40 mg/dl; women < 50 mg/dl); 3) hypertriglyceridemia (≥150 mg/dl); 4) elevated glucose (≥100 mg/dl and <126 mg/dl); and 5) elevated blood pressure (BP) (130/85 mmHg or use of hypertensive medications).
Exclusion criteria included T2DM (HbA1c ≥ 6.5% or use of diabetic medications), pulmonary disease, angina, atrial fibrillation, aortic stenosis, anemia, myocardial infarction, stroke, or coronary revascularization, as assessed by a detailed medical history physical examination, and a resting and exercise electrocardiogram. Subjects who participated in regular exercise, defined as >30 min, 3 times/wk were excluded. All subjects provided written, informed consent to participate, which was submitted to and approved by West Virginia University Institutional Review Board.
Study Design
Assessments were performed between 7:00 and 10:00 AM, in a quiet, temperature-controlled room, after a 12-h fast, and abstinence from alcohol, caffeine, and vitamins. Cardiovascular medications were withheld 24 h before assessments. On completion of the anthropometric assessments and after a minimum 15 min of quiet supine rest, subjects underwent supine measures of arterial structure/function followed by a blood draw.
Body anthropometry.
Height and weight, along with waist and hips circumference, were measured using standard laboratory procedures. Fat distribution was assessed by measuring the waist circumference at the site of the smallest circumference between the rib cage and the ileac crest, with the subjects in standing position. Hip circumference was measured at the site of the largest circumference between waist and thighs. Body composition was calculated from body volume of the BodPod (Life Measurement, Concord, CA). Subjects wore tightly fitting bathing suits and a swim cap during the volume measurements in the BodPod. Body mass index was calculated as weight (kg)/height (m)2.
Arterial geometry.
Ultrasound (GE Vivid i) two-dimensional images of the right common carotid artery (CCA) were obtained 1–2 cm proximal to the carotid bifurcation to measure maximal lumen diameter, and intima-medial thickness (cIMT). Cross-sectional area of the carotid artery was calculated as [(maximal lumen diameter/2)2 × π] − [(maximal lumen diameter/2 − cIMT)2 × π]. The wall-to-lumen ratio (W/L) of the right CCA was calculated as 2 × cIMT/lumen diameter in diastole (13).
Arterial function.
Brachial systolic (bSBP) and diastolic (bDBP) were measured with an automated, oscillometric, sphygmomanometer (Critikon Dinamap Compact BP monitor, GE Medical, Tampa, FL), and pulse pressure (bPP) was calculated from systolic bSBP − bDBP. Pulse wave analysis was performed noninvasively on the radial artery (SphygmoCor system, AtCor Medical, Sydney, NSW, Australia). All measurements were made in triplicate, and the mean values were used for subsequent analysis. The SphygmoCor system synthesizes a central (ascending aortic) pressure waveform from the radial pressure waveform that does not differ from that of an intra-arterially recorded wave (6) using a validated generalized transfer function (5) that has good reproducibility under major hemodynamic changes (32). These waveforms were calibrated against brachial mean arterial (MAP) and DBP to estimate aortic pressures.
The characteristics of the aortic pulse wave (Fig. 1) were determined as follows using established guidelines (18). Forward wave pressure was defined as the difference between pressure at the waveform foot and the pressure at the inflection point and used as an index of peak left ventricular (LV) ejection velocity (P1, Fig. 1). LV systolic ejection duration was taken as the time from the foot of the pressure wave upstroke to the incisura of the dicrotic notch. Augmented pressure (AP), a measure of the contribution of wave reflections to SBP, was defined as the difference between aortic cSBP and the pressure at the forward wave peak. Augmentation index (AGI) provides a measure of the contribution of wave reflection pressure (i.e., AP) to cSBP relative to total PP. AGI was calculated as the ratio of amplitude of the pressure wave above its systolic shoulder (i.e., the difference between the early and late systolic peaks of the arterial waveform), to the cPP expressed as a percentage (P2 − P1/PP × 100) (6). Since AGI varies with heart rate, AGI is commonly adjusted to a “standard heart rate” of 75 beats/min (AGI@75HR). Travel time of the forward pressure wave from the aorta to the peripheral reflection site and back was determined from the time from the initial upstroke of the pressure wave to the foot of the reflection wave and used as a proxy of aortic PWV. SBP-to-DBP shifts were assessed by the SBP (shaded area, Fig. 1) and DBP (open area, Fig. 1) pressure-time integrals. The Buckberg subendocardial viability ratio (SEVR) index, which correlates with LV subendocardial-to-subepicardial flow ratio (an apparent marker of subendocardial ischemia) (4), was calculated as the percentage ratio of the DBP (diastolic-time index)-to-SBP (tension-time index) pressure-time integral.
Fig. 1.
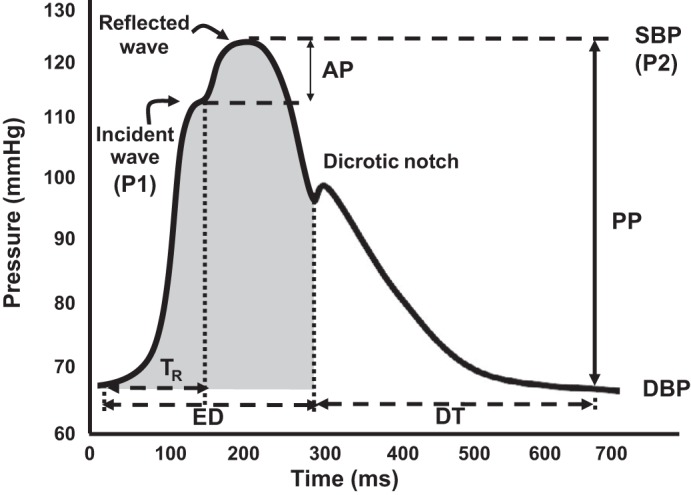
Example of central pressure waveform. The systolic (SBP) and diastolic blood pressures (DBP) are the peak and trough of the pressure waveform. Augmentation pressure (AP) is the pressure added to the forward wave by the reflected wave (P1-P2), whereas augmentation index is the ratio between AP and central pulse pressure (PP = SBP − DBP). The dicrotic notch represents closure of the aortic valve and is used to calculate ejection duration (ED). Time to wave reflection is calculated at the point of rise in the initial ejection wave to the onset of the reflected wave. Travel time (Tr) represents the time taken for the forward pressure wave from the aorta to the reflection site and back. SBP-to-DBP shifts were assessed by the SBP (shaded area) and DBP (open area) pressure-time integrals. DT, diastolic time interval.
Carotid-to-femoral PWV (cfPWV; central arterial stiffness) and carotid-to-radial PWV (crPWV: peripheral arterial stiffness) were measured by applanation tonometry (AtCor Medical, Sydney, NSW, Australia). ECG-gated waveforms were sequentially recorded. Aortic distance (D) was calculated as the difference in the distances from the carotid to the suprasternal notch and from the suprasternal notch to the femoral artery or radial artery. Time delay was calculated using a foot-of-the-wave method.
Using B-mode ultrasound (GE Vivid i), the right CCA strain was calculated as (ΔDc)/D × 100, where ΔDc is the difference between systolic and diastolic carotid diameter, and D is the diastolic diameter. CCA circumferential wall stress = mean BP × CCA W/L. Peak CCA circumferential wall tension was calculated as aortic SBP × (Dc in systole/2).
Blood analysis.
Venous blood sampling was obtained in the morning after a 12-h overnight fast. Posttraining blood samples were collected at least 48 h after the last exercise session. Serum and plasma were obtained from blood samples collected in either serum separation tubes with polymer gel/silica activator (BD Vacutainers) or plasma separation tubes (BD Vacutainers). Using standard procedures, total cholesterol, HDL cholesterol, triglycerides, glucose, and insulin were processed at West Virginia University Hospital's central laboratory in Morgantown, West Virginia. Homeostasis model assessment of insulin resistance was estimated with the following formula: insulin resistance = fasting plasma insulin (in μU/ml) × fasting plasma glucose (in mmol/l)/22.5. Homeostasis model assessment β-cell function was also assessed from 360 × fasting insulin/(fasting glucose − 63).
Plasma MMP (1, 2, 7, 9, and 10) and TIMP (1 and 2) activity, serum adhesion molecules (serum endothelial leukocyte adhesion molecule-1, soluble cell adhesion molecules-1, vascular cell adhesion molecule-1, and tissue plasminogen activator inhibitor-1), and serum inflammatory markers (IL-6, IL-8, and TNF-α) were run on a Luminex 100 Bioanalyzer (Luminex, Austin, TX), according to the kit manufacturer's (Milliplex ELISA kits, Millipore, MA) instructions.
CVD risk.
The Framingham risk score (FRS) was calculated based on sex, age, bSBP, treatment for hypertension, smoking and diabetes status, HDL, and total cholesterol to establish an individual's general 10-yr CVD risk, along with their estimated Framingham vascular age (9). Furthermore, we also estimated vascular age based on published sex-specific algorithms for cfPWV (PWVage) (26).
Intervention.
Individuals were assigned into either an 8-wk aerobic exercise intervention group (ExT) or an 8-wk nonexercise (Non-ExT) control group. Initially, 11 controls were assigned to the Non-ExT group; however, one dropped out for personal reasons. Data were only analyzed on subjects who completed the study. The Non-ExT groups (controls: n = 10, 80% female; MetS: n = 11; 55% female) were instructed to maintain their normal lifestyle activities. The ExT groups (controls: n = 11, 64% female; MetS: n = 11; 73% female) performed 8 wk of supervised aerobic exercise, 3 days/wk for 60 min/day at a fixed exercise intensity performed in the Human Performance Lab at West Virginia University. The intensity of prescribed exercise was based on individual results of maximal upright bicycle cardiopulmonary exercise tests with the resistance to peddling increasing by 25 W every 3 min until subjects reached exhaustion. Subjects were required to pedal at 50 revolutions/min. We deployed a ramp exercise protocol, whereby the exercise intensity started at 60% of heart rate reserve (heart rate range determined during exercise stress test) and increased every 2 wk by 10%; from weeks 6–8, heart rate reserve was set at 85%. Adherence to the exercise prescription was documented through the use of portable heart rate monitors (E600, Polar Electro OY, Oulu, Finland) and physical activity logs. Approved modalities included treadmills, elliptical machines, and cycle ergometers. The participants were instructed to maintain current eating behaviors for the duration of the 8-wk intervention. All posttraining measurements were performed at least 48 h after the last exercise session to avoid the immediate effects of a single bout of exercise. In addition, measurements before and after intervention periods were obtained at the same time of day for each subject.
Statistical Analysis
Our sample size calculation for our primary outcome measures (cfPWV) assumed a power of 90% and an α-error probability of 0.05. We found that we required a sample size of at least 11 subjects to detect a clinically significant effect of exercise training on arterial stiffness, such as a difference in cfPWV of 1 ± 1 (SD) m/s. Normality was evaluated by the Kolmogorov-Smirnov test. Categorical variables were compared by the χ2 test. Continuous variables were log transformed as necessary, and preexercise variables were compared between groups with a one-way ANOVA with Tukey's post hoc test. To evaluate the effects of exercise training, paired t-tests and two-way repeated-measures ANOVA were used. All analyses were performed with the statistical package SPSS version 21 (SPSS, Chicago, IL). Values shown in Tables 1–3 represent means ± SE, unless otherwise stated. P ≤ 0.05 was defined as significant.
Table 1.
Clinical characteristics of the subject cohorts
| Controls |
MetS |
|||
|---|---|---|---|---|
| Non-ExT | ExT | Non-ExT | ExT | |
| Age, yr | 40 ± 4 | 41 ± 4 | 44 ± 3 | 46 ± 4 |
| Sex, %female | 80 | 64 | 64 | 73 |
| Height, cm | 165 ± 3 | 168 ± 2 | 171 ± 3 | 168 ± 3 |
| Weight, kg | 68 ± 4 | 69 ± 4 | 102 ± 7*† | 106 ± 5* |
| Body fat, % | 25 ± 4 | 24 ± 2 | 36 ± 2*† | 45 ± 2* |
| BSA, m2 | 1.75 ± 0.06 | 1.78 ± 0.06 | 2.13 ± 0.08*† | 2.13 ± 0.06* |
| BMI, kg/m2 | 25 ± 1 | 24 ± 1 | 34 ± 2*† | 38 ± 2* |
| Waist circumference, cm | 83 ± 3 | 86 ± 6 | 107 ± 3*† | 124 ± 9* |
| Total cholesterol, mg/dl | 191 ± 11 | 177 ± 12 | 189 ± 12 | 191 ± 12 |
| Triglycerides, mg/dl | 93 ± 14 | 81 ± 14 | 161 ± 23*† | 115 ± 16 |
| HDL, mg/dl | 56 ± 5 | 57 ± 4 | 40 ± 4*† | 50 ± 5 |
| Glucose, mg/dl | 93 ± 3 | 92 ± 3 | 97 ± 1 | 98 ± 2 |
| HbA1c, % | 4.9 ± 0.3 | 5.4 ± 0.1 | 5.7 ± 0.1* | 5.6 ± 0.1* |
| Insulin, μIU/ml | 5 ± 1 | 5 ± 1 | 11 ± 2*† | 10 ± 1*† |
| Beta cell function | 62 ± 10 | 79 ± 37 | 111 ± 20*† | 105 ± 16*† |
| HOMA-IR | 1.3 ± 0.2 | 1.0 ± 0.3 | 2.7 ± 0.5*† | 2.4 ± 0.3*† |
| Framingham risk score, % | 2 ± 1 | 2 ± 1 | 8 ± 2*† | 8 ± 4*† |
| Framingham vascular age, yr | 36 ± 3 | 34 ± 4 | 54 ± 4*† | 47 ± 7*† |
| Hypertensive, % | 0 | 0 | 73 | 82 |
| Hypertension category | ||||
| Normal, % | 40 | 36 | 18 | 18 |
| Prehypertension, % | 60 | 64 | 0 | 0 |
| Stage 1, % | 0 | 0 | 82 | 73 |
| Stage 2, % | 0 | 0 | 0 | 9 |
| Diabetes mellitus, % | 0 | 0 | 0 | 0 |
| Smokers, % | 0 | 0 | 0 | 0 |
| Medications | ||||
| Diuretics, % | 0 | 0 | 10 | 18 |
| Β-blockers, % | 0 | 0 | 0 | 18 |
| ACEI/ARB, % | 0 | 0 | 36 | 18 |
| CA2+ blockers, % | 0 | 0 | 0 | 0 |
| Statins, % | 0 | 0 | 0 | 18 |
Values are means ± SE.
BSA, body surface area; BMI, body mass index; HDL, high density lipoprotein; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ExT, exercise trained; Non-ExT, non-exercise trained; MetS, metabolic syndrome.
P < 0.05 compared with healthy control Non-ExT group.
P < 0.05 compared with healthy control ExT group.
Table 3.
Differences in blood biomarkers pre- and postintervention in MetS and controls
| Controls |
MetS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-ExT |
ExT |
Non-ExT |
ExT |
|||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Total cholesterol, mg/dl | 191 ± 11 | 178 ± 13 | 177 ± 12 | 173 ± 11 | 189 ± 12 | 194 ± 15 | 191 ± 12 | 183 ± 9 |
| Triglycerides, mg/dl | 93 ± 14 | 86 ± 10 | 81 ± 17 | 90 ± 17 | 161 ± 23*‡ | 173 ± 18 | 115 ± 16 | 130 ± 17 |
| HDL, mg/dl | 56 ± 5 | 52 ± 5 | 57 ± 4 | 53 ± 4 | 40 ± 4*‡ | 38 ± 3 | 50 ± 5 | 47 ± 4 |
| Glucose, mg/dl | 94 ± 3 | 89 ± 3 | 92 ± 3 | 94 ± 2 | 97 ± 1 | 96 ± 2 | 98 ± 2 | 96 ± 3 |
| Insulin, μIU/ml | 5.2 ± 1.0 | 5.3 ± 1.0 | 4.5 ± 1.4 | 4.4 ± 1.1 | 11.1 ± 2.3* | 11.8 ± 2.3 | 9.8 ± 1.4‡ | 11.5 ± 2.5 |
| HOMA-IR | 1.25 ± 0.27 | 1.21 ± 0.24 | 1.02 ± 0.33 | 1.00 ± 0.24 | 2.71 ± 0.61*‡ | 2.83 ± 0.58 | 2.37 ± 0.34‡ | 2.68 ± 0.57 |
| sE-selectin, ng/ml | 0.78 ± 0.09 | 0.75 ± 0.07 | 0.57 ± 0.06 | 0.56 ± 0.02 | 0.91 ± 0.10 | 0.87 ± 0.09 | 0.95 ± 0.01 | 0.83 ± 0.01 |
| sVCAM-1, ng/ml | 7.7 ± 1.8 | 8.6 ± 1.9 | 6.4 ± 1.4 | 5.5 ± 1.2 | 7.3 ± 1.4 | 7.2 ± 1.3 | 6.0 ± 1.5 | 5.5 ± 1.3 |
| sICAM-1, ng/ml | 3.5 ± 0.9 | 3.4 ± 0.8 | 4.4 ± 0.7 | 4.2 ± 0.7 | 4.0 ± 0.6 | 3.8 ± 0.6 | 5.0 ± 0.7 | 5.1 ± 0.9 |
| tPAI-1, ng/ml | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 |
| IL-6, pg/ml | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.0 ± 0.3 | 4.3 ± 1.9 | 2.3 ± 0.3 | 3.1 ± 1.1 | 3.0 ± 0.8 |
| IL-8, pg/ml | 3.2 ± 1.0 | 3.7 ± 1.0 | 2.8 ± 0.6 | 2.4 ± 0.5 | 5.1 ± 0.8 | 4.8 ± 1.2 | 4.1 ± 0.9 | 4.0 ± 0.6 |
| TNF-α, pg/ml | 5.3 ± 1.1 | 5.5 ± 1.5 | 3.2 ± 0.4 | 2.8 ± 0.3 | 5.3 ± 0.9 | 5.0 ± 0.9 | 5.5 ± 0.6 | 6.0 ± 0.8 |
| MMP-1, pg/ml | 21 ± 3 | 32 ± 10 | 23 ± 3 | 24 ± 5 | 24 ± 3 | 24 ± 4 | 27 ± 5 | 22 ± 5† |
| MMP-2, pg/ml | 3,452 ± 429 | 3,484 ± 508 | 3,962 ± 202 | 3,962 ± 442 | 4,263 ± 427 | 4,260 ± 326 | 4,383 ± 405 | 3,793 ± 143 |
| MMP-7, pg/ml | 648 ± 61 | 629 ± 60 | 705 ± 139 | 674 ± 138 | 543 ± 48 | 571 ± 64 | 893 ± 215 | 789 ± 202§ |
| MMP-9, pg/ml | 849 ± 145 | 656 ± 111 | 751 ± 77 | 635 ± 100 | 853 ± 121 | 741 ± 55 | 858 ± 95 | 879 ± 141 |
| MMP-10, pg/ml | 20 ± 2 | 30 ± 8 | 25 ± 3 | 24 ± 1 | 25 ± 3 | 24 ± 2 | 27 ± 4 | 27 ± 5 |
| TIMP-1, pg/ml | 1,459 ± 711 | 1,173 ± 456 | 1,533 ± 544 | 1,569 ± 555 | 1,245 ± 205 | 1,323 ± 222 | 2,273 ± 581 | 2,302 ± 664 |
| TIMP-2, pg/ml | 1,585 ± 836 | 1,314 ± 607 | 1,794 ± 655 | 1,781 ± 694 | 1,290 ± 233 | 1,305 ± 239 | 1,824 ± 470 | 1,888 ± 575 |
Values are means ± SE. Sample size for examining the effects of exercise training on blood biomarkers are as follows: adhesion molecules (Control Non-ExT n = 6; Control ExT, n = 9; MetS Non-ExT n = 9; MetS ExT, n = 9), inflammatory markers (Control Non-ExT n = 6; Con ExT, n = 10; MetS Non-ExT n = 9; MetS ExT, n = 9), and MMP (Control Non-ExT n = 7; Control ExT, n = 10; MetS Non-ExT n = 8; MetS ExT, n = 10). s, Soluble; tPAI-1, tissue plasminogen activator inhibitor-1; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of MMP.
P < 0.05 compared with healthy control Non-ExT group.
P < 0.05 compared with healthy control ExT group.
P ≤ 0.05 compared with Pre values within a group (Controls Non-ExT, Controls ExT, MetS Non-ExT, MetS-ExT).
Significant (P ≤ 0.05) group (e.g., MetS-Non-ExT vs MetS-ExT) by time (Pre to Post) interaction.
RESULTS
Age and anthropometric and metabolic characteristics of the healthy controls and MetS groups are shown in Table 1. MetS and controls groups did not differ by age or sex; however, by study design, the MetS groups had significantly higher anthropometric and metabolic characteristics. Additionally, preexercise peak aerobic capacity (V̇o2peak) was lower (P < 0.05) in MetS Non-ExT (18 ± 2 ml·min−1·kg−1) and MetS ExT (16 ± 1 ml·min−1·kg−1) groups vs. healthy Non-ExT (25 ± 2 ml·min−1·kg−1) and healthy ExT (24 ± 2 ml·min−1·kg−1) controls. However, within each group (i.e., MetS Non-ExT vs. ExT, and controls Non-ExT vs. ExT), individuals were well matched with no significant differences in age and baseline metabolic and arterial parameters.
Arterial Parameters
It is well known that MetS patients have higher central and brachial pressures, an increased arterial stiffness, and arterial remodeling compared with healthy controls. Similar differences were evident in our population (Table 2). In healthy controls, 8 wk of exercise training significantly reduced cfPWV and AGI, and there was a significant time [preintervention (Pre) vs. postintervention (Post)] by group (Non-ExT vs. ExT) interaction for cfPWV. However, no other significant differences in arterial structure/function were found pre- and postexercise in healthy controls. Importantly, 8 wk of exercise training in MetS patients reduced bSBP and cSBP (cSBP), although only a significant reduction was identified in the cSBP (P < 0.05). Furthermore, AP was significantly reduced (20%, P < 0.05) in the MetS-ExT group. Although AGI was not affected by exercise training, a reduction (21%, P = 0.06) in AGI@75HR was identified. Indeed, resting HR was reduced (6%, P < 0.05) in MetS-ExT group. Due to the reduction in cSBP, the systolic region of the pressure wave was reduced (9%, P < 0.05), along with an improvement in SEVR (8%, P < 0.05), in the MetS-ExT group, and there was a significant time (Pre vs. Post) by group (Non-ExT vs. ExT) interaction for SEVR. Importantly, aerobic training lowered cfPWV in MetS individuals by 9% (P < 0.05), which remained significant after adjusting for differences in MAP. Furthermore, there was a significant time (Pre vs. Post) by group (Non-ExT vs. ExT) interaction for cfPWV. In contrast, 8 wk of exercise training had no effect on improving crPWV, cIMT, carotid cross-sectional area, carotid lumen-to-wall ratio, carotid wall stress, or tension in MetS-ExT subjects.
Table 2.
Differences in arterial parameters pre- and postintervention in MetS and controls
| Controls |
MetS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-ExT (n = 10) |
ExT (n = 11) |
Non-ExT (n = 11) |
ExT (n = 11) |
|||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| bSBP, mmHg | 117 ± 3 | 115 ± 4 | 111 ± 3 | 110 ± 3 | 128 ± 4‡ | 126 ± 4 | 125 ± 5‡ | 120 ± 4 |
| bDBP, mmHg | 74 ± 2 | 75 ± 3 | 73 ± 2 | 71 ± 2 | 84 ± 3* | 81 ± 2 | 80 ± 2* | 78 ± 2 |
| bPP, mmHg | 42 ± 3 | 41 ± 2 | 38 ± 2 | 39 ± 2 | 43 ± 3 | 45 ± 3 | 46 ± 5 | 42 ± 3 |
| bMAP, mmHg | 91 ± 3 | 88 ± 3 | 87 ± 2 | 85 ± 2 | 100 ± 3* | 97 ± 2 | 96 ± 2*‡ | 93 ± 3 |
| cSBP, mmHg | 107 ± 3 | 104 ± 4 | 103 ± 3 | 101 ± 2 | 117 ± 4‡ | 114 ± 3 | 116 ± 5‡ | 110 ± 4† |
| cPP, mmHg | 31 ± 2 | 30 ± 2 | 29 ± 2 | 28 ± 2 | 32 ± 2*‡ | 33 ± 2 | 36 ± 5 | 32 ± 3 |
| P1 H, mmHg | 24 ± 2 | 23 ± 1 | 22 ± 2 | 23 ± 1 | 25 ± 2 | 26 ± 2 | 27 ± 3 | 25 ± 2 |
| AP, mmHg | 7.2 ± 1.7 | 6.5 ± 1.8 | 6.6 ± 1.8 | 5.6 ± 1.6 | 6.9 ± 1.4 | 6.7 ± 1.3 | 8.9 ± 1.4 | 7.1 ± 1.4† |
| AGI, % | 22 ± 5 | 20 ± 5 | 21 ± 5 | 18 ± 5† | 21 ± 4 | 20 ± 3 | 24 ± 2 | 23 ± 4 |
| AGI@75HR, % | 18 ± 4 | 16 ± 5 | 14 ± 5 | 11 ± 4 | 17 ± 4 | 16 ± 3 | 19 ± 3 | 15 ± 4† |
| HR, beats/min | 67 ± 3 | 67 ± 4 | 61 ± 2 | 61 ± 2 | 68 ± 2 | 66 ± 2 | 65 ± 2 | 61 ± 3† |
| SEVR, % | 153 ± 7 | 156 ± 8 | 170 ± 6 | 169 ± 6 | 149 ± 6 | 150 ± 7 | 155 ± 8 | 168 ± 8†§ |
| TTI, mmHg·s−1·min−1 | 2,137 ± 91 | 2,090 ± 87 | 1,945 ± 71 | 1,904 ± 78 | 2,430 ± 84‡ | 2,345 ± 93 | 2,297 ± 112‡ | 2,098 ± 93† |
| DTI, mmHg·s−1·min−1 | 3,278 ± 114 | 3,179 ± 136 | 3,267 ± 84 | 3,183 ± 103 | 3,581 ± 122 | 3,457 ± 97 | 3,460 ± 88 | 3,465 ± 116 |
| AoTr, ms | 141 ± 4 | 146 ± 5 | 151 ± 6 | 152 ± 4 | 143 ± 4 | 148 ± 3 | 148 ± 3 | 155 ± 5 |
| cfPWV, m/s | 6.59 ± 0.35 | 6.86 ± 0.45 | 6.34 ± 0.25 | 6.06 ± 0.27†§ | 7.45 ± 0.44*‡ | 7.44 ± 0.36 | 7.93 ± 0.58*‡ | 7.24 ± 0.44†§ |
| crPWV, m/s | 8.29 ± 0.32 | 7.97 ± 0.37 | 8.33 ± 0.32 | 7.65 ± 0.39 | 7.76 ± 0.22 | 7.64 ± 0.24 | 7.93 ± 0.40 | 7.98 ± 0.36 |
| cIMT, mm | 0.61 ± 0.03 | 0.63 ± 0.03 | 0.58 ± 0.02 | 0.58 ± 0.02 | 0.69 ± 0.06‡ | 0.67 ± 0.04 | 0.70 ± 0.04*‡ | 0.67 ± 0.04 |
| Dc, mm | 5.70 ± 0.16 | 5.85 ± 0.15 | 5.83 ± 0.18 | 5.86 ± 0.19 | 5.93 ± 0.14 | 5.88 ± 0.20 | 6.18 ± 0.14 | 6.09 ± 0.17 |
| cCSA, mm2 | 10 ± 1 | 11 ± 1 | 10 ± 1 | 10 ± 1 | 12 ± 1*‡ | 12 ± 1 | 12 ± 1*‡ | 12 ± 1 |
| CCA W/L ratio | 0.21 ± 0.02 | 0.21 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.22 ± 0.01 | 0.22 ± 0.01 |
| CCA strain, % | 8.0 ± 0.9 | 7.9 ± 0.7 | 7.2 ± 0.9 | 7.5 ± 0.6 | 6.7 ± 0.5 | 7.0 ± 0.5 | 7.3 ± 0.6 | 7.9 ± 0.8 |
| CCA WS, mmHg | 20 ± 1 | 19 ± 1 | 17 ± 1 | 17 ± 1 | 24 ± 3‡ | 23 ± 2 | 22 ± 2‡ | 21 ± 2 |
| CCA WT, kPa | 33 ± 1 | 33 ± 1 | 32 ± 1 | 32 ± 1 | 37 ± 1‡ | 37 ± 1 | 39 ± 2‡ | 37 ± 2 |
Values are mean ± SE;
n, no. of subjects. b, brachial; c, central; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure; P1 H, aortic incident pressure wave; AP, aortic augmentation pressure; AGI, aortic augmentation index; AGI@75HR, aortic augmentation adjusted for heart rate; HR, heart rate; SEVR, subendocardial viability ratio; TTI, tension time index; DTI, diastolic time index; AoTr, return of aortic reflective wave; cfPWV, carotid-femoral pulse wave velocity (PWV); crPWV, carotid-redial PWV; Dc, carotid diameter in diastole; CSA, cross-sectional area; CCA, common carotid artery; W/L wall-to-lumen ratio; WS, circumferential wall stress, WT, circumferential wall tension; Pre, preintervention; Post, postintervention.
P < 0.05 compared with healthy control Non-ExT group.
P < 0.05 compared with healthy control ExT group.
P ≤ 0.05 compared with Pre values within a group (Controls Non-ExT, Controls ExT, MetS Non-ExT, MetS-ExT).
Significant (P ≤ 0.05) group (e.g., MetS-Non-ExT vs. MetS-ExT) by time (Pre to Post) interaction.
In the Non-ExT controls and MetS subjects, no differences in arterial structure and function were found 8 wk Post compared with baseline values (Table 2).
Blood Biomarkers
We also examined the effects of exercise training on improving metabolic markers, inflammation, and vascular proteins circulating in the blood. No differences in metabolic markers, adhesion, or the inflammation markers were evident in either the healthy controls or MetS group after the intervention (Table 3). However, a reduction in MMP-1 (P < 0.05), and to a lesser extent MMP-7 (P = 0.06), was evident after exercise training in the MetS group, but not in the healthy control group. A significant time (Pre and Post) by group (MetS Non-ExT vs. MetS ExT) interaction was identified for MMP-7.
Aerobic Capacity and CVD risk
Aerobic capacity was increased (P < 0.05) in MetS (16.6 ± 1.0 to 19.9 ± 1.0) and healthy controls (23.8 ± 1.6 to 26.3 ± 1.6) after exercise training, whereas no changes were found in MetS (18.5 ± 1.6 vs. 18.2 ± 1.2) and healthy controls (25.6 ± 2.8 vs. 25.8 ± 2.5) who remained inactive. As expected, the FRS and the vascular age calculated from the Framingham risk algorithm were higher (P < 0.05) in both MetS groups compared with healthy controls (Table 1). Exercise training did not affect the FRS in MetS (8 ± 4 vs. 6 ± 3%) or healthy controls (2 ± 1 vs. 2 ± 1%). However, in MetS subjects, exercise training significantly reduced vascular age calculated from the Framingham risk algorithm (from 47 ± 8 to 44 ± 7 yr, P = 0.02) and from cfPWV (from 55 ± 5 to 50 ± 4 yr, P = 0.04). Adding PWVage to the FRS tended to reduce the calculated CVD risk (10 ± 5 vs. 6 ± 3%), but statistical significance was not reached (P = 0.15). In contrast, neither vascular age calculated from the FRS (34 ± 4 vs. 33 ± 4 yr, P = 0.6) or from cfPWV (40 ± 3 vs. 37 ± 4 yr, P = 0.1) was altered in the healthy control ExT group. Similarly, adding PWV to the FRS did not alter their calculated CVD risk (3 ± 1 vs. 2 ± 1%).
Relationship Between Aerobic Capacity and Arterial Health
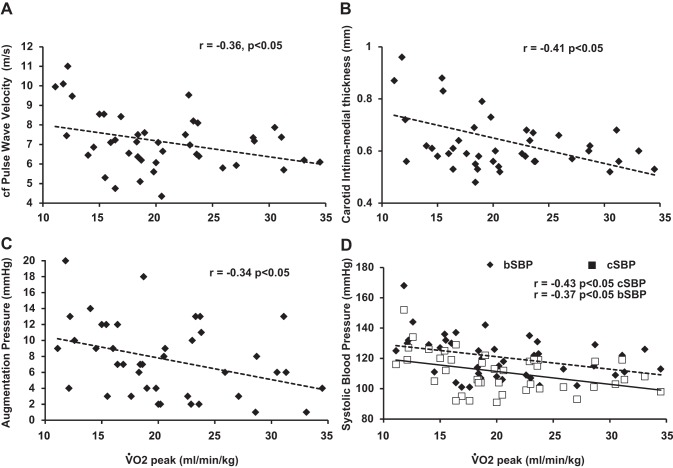
An inverse relationship (P < 0.05) was identified between V̇o2peak and cfPWV (Fig. 2A), cIMT (Fig. 2B), AP (Fig. 2C), and both cSBP and bSBP (Fig. 2D). We also calculated the change (Post-Pre values) in cfPWV, cIMT, AP, AGI, AGI@75HR, and both bSBP and cSBP, and compared that to the change in V̇o2peak. A significant and inverse relationship was found only between V̇o2peak and cfPWV (Fig. 3).
Fig. 2.
Relationship between peak aerobic capacity (V̇o2peak) and arterial markers, namely, carotid femoral pulse wave velocity (A), carotid intima medial thickness (B), augmentation pressure (C), and brachial (bSBP) and central SBP (cSBP; D).
Fig. 3.
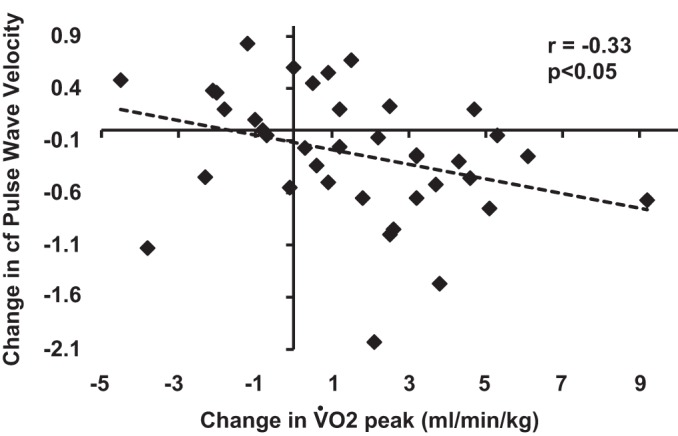
Relationship between the change (postintervention-preintervention values) in V̇o2peak and carotid femoral pulse wave velocity.
DISCUSSION
We have shown that MetS is associated with an increase in arterial stiffness, cIMT, carotid cross-sectional area, carotid wall stress, and tension (13). Part of this arterial dysfunction reflects the sedentary lifestyle of individuals with MetS. We examined whether exercise training would be an effective nonpharmacological therapy to lower arterial stiffness and improve other markers of arterial health in MetS patients who had yet to transition to more adverse stages of the cardiometabolic disease, such as the co-occurrence of T2DM, or the presence of overt CVD (angina, heart attack, stroke, etc.). Of particular importance, cfPWV is significantly reduced after 8 wk of aerobic exercise in MetS subjects by almost 1 m/s, which may reflect a lowering of CVD events and mortality by ∼14% (40).
Increasing arterial stiffness is regarded as a measure of premature vascular aging (29). According to normative values of cfPWV (29a), the baseline biological vascular age of our MetS group may be estimated to be 6 yr older than it would be in healthy individuals of a similar age. The reduction in cfPWV in the MetS ExT group would translate into a 5-yr reversal of age-related arterial stiffening (2) and lower the FRS (with vascular age included) CVD risk by 4%. Furthermore, the reduction in cfPWV coincided with a reduction in cSBP (6 mmHg) in MetS subjects. As cSBP predicts cardiovascular outcomes more closely than bSBP (41), the reduction in cSBP is clinically relevant, whereby a 2-mmHg reduction SBP is associated with a 7% decrease in CVD mortality (19). The reduction in cSBP can be attributed, in part, to a reduction in cfPWV and AP. A decrease in cfPWV can result in a corresponding decrease in the transmission velocity of the forward and reflected waves, causing the reflected wave to arrive later in the central aorta augmenting pressure. Thus, once AGI (an indirect measure of systemic arterial stiffness) was adjusted to a standard HR (AGI@75HR), a reduction in the reflected pressure wave was evident. The improvements in central pressure dynamics also corresponded to an increase in SEVR, an indirect marker of subendocardial perfusion (4), which may suggest an improvements in cardiac function.
Aerobic exercise training did not alter cIMT, carotid cross-sectional area, W/L ratios, or carotid dynamics in MetS subjects. This lack of change in cIMT is similar to that reported elsewhere in healthy men (35, 36), suggesting that regular exercise training typically does not alter the large conduit vessels.
Our data might suggest that exercise training exerted greater benefits to MetS subjects than healthy controls, as evident by the larger exercise-induced decreases in cSBP, AP, and tension-time index and increase in SEVR in MetS subjects compared with healthy controls. Likewise, MetS patients also exhibited a tendency to have greater reduction in cfPWV (0.7 vs. 0.3 m/s) and increase in V̇o2peak (3.3 vs. 2.5 ml·min−1·kg−1) than controls. However, it must be emphasized that no significant ANOVA interaction effects were observed between MetS and healthy controls with these Pre-Post responses, and that our study was also not originally powered to test this question. Nevertheless, it is tempting to speculate the potential for greater improvement in these parameters for MetS patients may be due to their elevated baseline compared with controls and, therefore, could provide a greater range for the exercise stimulus to act on. This is an important question that will require additional research/subjects before a clear understanding is gained.
Recent evidence suggests that any initial benefit with exercise training on arterial stiffness in persons with established MetS and T2DM is attenuated over the long term (11, 21). Whereby at 3 mo of aerobic exercise training a 14–23% reduction in cfPWV has been reported (21, 22), yet after 6 mo of exercise training the reduction in cfPWV was not maintained (21). These data suggest that the prescription of aerobic exercise on improving arterial stiffness may be more beneficial before the occurrence of T2DM in those with MetS, than starting once the patient is already at a high cardio-metabolic risk. To what extent this initial reduction in arterial stiffness through short-term exercise training in MetS subjects without T2DM is maintained for an extended period of time warrants further investigation.
Arterial stiffness contributes substantially to cardiac workload and energetics and is inversely correlated with V̇o2peak (16, 39). We have shown that the change in V̇o2peak and cfPWV with exercise training is inversely related. These data suggest that individuals with more compliant arteries may have more optimal coupling between the heart and arterial system, allowing for greater peak cardiovascular performance. Furthermore, it has been reported that MetS subjects with a high aerobic fitness have a lower risk of mortality than unfit MetS subjects (17), which may in part be explained by the lower degree of arterial stiffening (16).
Potential Mechanism Behind Improved Arterial Stiffness
The stiffness of the arterial system is dependent on the dynamic and material properties of the artery (42). Sympathetic activation of the vascular smooth muscle cells causes vasoconstriction, decreasing lumen diameter, and increasing arterial stiffness. Although we did not measure sympathetic activity, exercise training has been shown to improve autonomic control in obese women (38), which may contribute to the improved arterial stiffness in the present study. Arterial stiffness is also, in part, modulated by endothelial dysfunction. Exercise training in both obese/MetS patients has been shown to improve endothelial function (27, 37), which may improve arterial function via intermittent increases in shear stress, which stimulates nitric oxide bioavailability, and upregulating endothelial nitric oxide synthase protein expression and phosphorylation (14). Changes in distending pressure affect cfPWV; however, in our study, MAP did not change, and the decrease in cfPWV remained significant after adjusting for MAP. Thus it is unlikely that a reduction in distending pressure contributed to the improved arterial compliance.
The material properties that contribute to an increased cfPWV are increased collagen concentration, nonenzymatic glycation, resulting in the formation of collagen cross-link, and vascular smooth muscle hypertrophy (42). Although we did not measure the amount of advanced glycation end-product deposition, we measured plasma markers of tissue remodeling (MMP and TIMP) and found that exercise training lowered circulating MMP-1 and -7. Interestingly, both MMP-1 and -7 were positively associated with cfPWV. More evidence is required to identify to what extent MMPs play a role in arterial remodeling through exercise training.
Chronic low-grade inflammation, specifically IL-6, IL-8, and TNF-α, is thought to be involved in the pathogenesis of MetS (1) and cfPWV (23), and in the present these markers tended to be elevated in MetS vs. controls. However, exercise training did not alter inflammatory markers in MetS subjects. This may be due to the shorter duration of exercise we used, as 1 yr of exercise training improved TNF-α and IL-18 in MetS subjects, with or without T2DM (3, 33).
Future Directions
To what extent oxidative stress, the sympathetic nervous system, and the structure and function of resistance vessels impacts arterial stiffness after exercise training in MetS requires further examination in both human and animal models. Given the lack of change in metabolic profile of our MetS population, it would be important to identify whether exercise training of longer durations (6 mo plus), or exercise training plus diet control would exert greater benefits on both arterial function and metabolic profile of MetS patients. Furthermore, it will also be important to conduct longitudinal studies to identify whether the beneficial effects of short-term exercise training on lowering arterial stiffness is maintained, and whether persistent exercise training can delay or prevent the transition to MetS with overt CVD/diabetes.
Limitations
There are several limitations. There may be sex differences in the effects of exercise training on arterial stiffness that could not be detected, given the small number of male vs. female subjects. Second, more direct measures of autonomic function should be used to assess whether changes in sympathetic activity contributed to a reduction in cfPWV. Another limitation is the circulating levels of MMPs do not necessary reflect what is going on at the arterial sites. However, because the changes on the cellular level are reflected in body fluids, determination of MMPs in blood have been recommended as noninvasive tools in the diagnosis and monitoring of several diseases (25). Although we reviewed each participant's medical history and performed a physical examination, we cannot completely rule out that participants may have had some degree of peripheral artery disease. Despite this, exercise training in MetS (with or without peripheral artery disease) significantly improved arterial health. Four of our MetS patients had a body mass index >40 kg/m2 indicating they were severely obese. However, being severely obese did not limit their ability to improve arterial function, as we repeated the statistical analyses excluding these individuals and found that the main story of the paper did not change. Lastly, a potential limitation is the measurement of plasma lipids obtained from lithium heparin-coated collection tubes, given that heparin precipitates some lipoproteins. This may explain, in part, the lack of change in blood lipids postexercise training.
In conclusion, the present study indicates that aerobic exercise training is an effective approach to reduce arterial stiffening and improve CVD risk profiles in individuals with the MetS (without T2DM and overt CVD). Whether long-term aerobic exercise training maintains the beneficial effects on arterial stiffness in MetS and delays the transition to T2DM requires further study.
GRANTS
This study was supported in part by American Heart Association Grant 11CRP7370056 (P. D. Chantler), National Heart, Lung, and Blood Institute Grant T32-HL-090610 (S. B. Fournier), and National Institute of General Medical Sciences of the National Institutes of Health under Award U54-GM-104942.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A.D., S.B.F., B.L.R., E.D., D.E.B., and P.D.C. performed experiments; D.A.D., S.B.F., I.M.O., J.C.F., and P.D.C. interpreted results of experiments; D.A.D., S.B.F., and P.D.C. drafted manuscript; D.A.D., S.B.F., B.L.R., E.D., D.E.B., I.M.O., J.C.F., and P.D.C. edited and revised manuscript; D.A.D., S.B.F., B.L.R., E.D., D.E.B., I.M.O., J.C.F., and P.D.C. approved final version of manuscript; B.L.R. and P.D.C. conception and design of research; P.D.C. analyzed data; P.D.C. prepared figures.
REFERENCES
- 1.Ahonen TM, Saltevo JT, Kautiainen HJ, Kumpusalo EA, Vanhala MJ. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. Nutr Metab Cardiovasc Dis 22: 285–291, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 68: 50–58, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, Fallucca S, Alessi E, Letizia C, Jimenez A, Fallucca F, Pugliese G. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis 20: 608–617, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Buckberg GD, Fixler DE, Archie JP, Hoffman JIE. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 30: 67–81, 1972 [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Nevo E, Fetics B, Pak PH, Yin FCP, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure: validation of generalized transfer function. Circulation 95: 1827–1836, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, Yin FCP. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension 27: 168–175, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Currie KD, Thomas SG, Goodman JM. Effects of short-term endurance exercise training on vascular function in young males. Eur J Appl Physiol 107: 211–218, 2009 [DOI] [PubMed] [Google Scholar]
- 9.D'Agostino RB, Sr Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117: 743–753, 2008 [DOI] [PubMed] [Google Scholar]
- 10.da Silva CA, Ribeiro JP, Canto JC, da Silva RE, Silva Junior GB, Botura E, Malschitzky MA. High-intensity aerobic training improves endothelium-dependent vasodilation in patients with metabolic syndrome and type 2 diabetes mellitus. Diabetes Res Clin Pract 95: 237–245, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Dobrosielski D, Gibbs B, Ouyang P, Bonekamp S, Clark J, Wang NY, Silber H, Shapiro E, Stewart K. Effect of exercise on blood pressure in type 2 diabetes: a randomized controlled trial. J Gen Intern Med 27: 1453–1459, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension 38: 222–226, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Fournier SB, Reger BL, Donley DA, Bonner DE, Warden BE, Gharib W, Failinger CF, Olfert MD, Frisbee JC, Olfert IM, Chantler PD. Exercise reveals impairments in left ventricular systolic function in patients with metabolic syndrome. Exp Physiol 99: 149–163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang P. A comprehensive definition for metabolic syndrome. Dis Model Mech 2: 231–237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jae SY, Heffernan KS, Fernhall B, Oh YS, Park WH, Lee MK, Choi YH. Association between cardiorespiratory fitness and arterial stiffness in men with the metabolic syndrome. Diabetes Res Clin Pract 90: 326–332, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med 164: 1092–1097, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness. Circulation 115: 459–467, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Aerobic training-induced improvements in arterial stiffness are not sustained in older adults with multiple cardiovascular risk factors. J Hum Hypertens 27: 335–339, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care 32: 1531–1535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 46: 1118–1122, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in united states adults. Circulation 110: 1245–1250, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Mannello F, Tanus-Santos JE, Meschiari CA, Tonti GA. Differences in both matrix metalloproteinase 9 concentration and zymographic profile between plasma and serum with clot activators are due to the presence of amorphous silica or silicate salts in blood collection devices. Anal Biochem 374: 56–63, 2008 [DOI] [PubMed] [Google Scholar]
- 26.McEniery CM, Yasmin Hall IR, Qasem A, Wilkinson IB, Cockcroft JR ACCT. Investigators. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46: 1753–1760, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Regular aerobic exercise, without weight loss, improves endothelium-dependent vasodilation in overweight and obese adults. Obesity (Silver Spring) 18: 1667–1669, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, Kim MK, Nakata Y, Tanaka K, Ajisaka R. Effect of habitual aerobic exercise on body weight and arterial function in overweight and obese men. Am J Cardiol 104: 823–828, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension 54: 3–10, 2009 [DOI] [PubMed] [Google Scholar]
- 29a.Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J 31: 2338–2350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scuteri A, Najjar SS, Orru' M, Usala G, Piras MG, Ferrucci L, Cao A, Schlessinger D, Uda M, Lakatta EG. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. Eur Heart J 31: 602–613, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol 38: 506–513, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 47: 1203–1208, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Stensvold D, Slordahl SA, Wisloff U. Effect of exercise training on inflammation status among people with metabolic syndrome. Metab Syndr Relat Disord 10: 267–272, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol 92: 1458–1464, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Thijssen DHJ, De Groot PCE, Smits P, Hopman MTE. Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 190: 221–228, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 118: 346–354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrao CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol 285: H974–H982, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens 27: 461–467, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005 [DOI] [PubMed] [Google Scholar]