Abstract
Evidence is accumulating regarding the benefits of exercise in people who are more susceptible to injury, such as the elderly, or those with a neuromuscular disease, for example Duchenne muscular dystrophy (DMD). There appears to be a consensus that exercise can be safely performed in aging and diseased muscles, but the role of eccentric exercise is not as clear. Eccentric (lengthening) contractions have risks and benefits. Eccentric contractions are commonly performed on a daily basis, and high-force voluntary eccentric contractions are often employed in strength training paradigms with excellent results; however, high-force eccentric contractions are also linked to muscle damage. This minireview examines the benefits and safety issues of using eccentric exercise in at-risk populations. A common recommendation for all individuals is difficult to achieve, and guidelines are still being established. Some form of exercise is generally recommended with aging and even with diseased muscles, but the prescription (frequency, intensity, and duration) and type (resistance vs. aerobic) of exercise requires personal attention, as there is great diversity in the functional level and comorbidities in the elderly and those with neuromuscular disease.
Keywords: eccentric, muscular dystrophy, aging, Duchenne muscular dystrophy
when the external load on an activated muscle exceeds the tension generated by the muscle contraction, the muscle lengthens during what is termed a lengthening (“eccentric”) contraction. The difficulty of explaining the force from a lengthening contraction lies in the fact that the force produced is greater than the sum of the measured active force (from an isometric contraction) and passive force at that given muscle length. When the external load is heavy enough to exceed a maximal voluntary contraction, such maximal eccentric contractions can produce high forces, which is a goal of strength training (the overload principle). This is evident in strengthening protocols that use lengthening contractions, or “negatives,” to increase strength. Not only can lengthening contractions produce more force than other types of contractions, but they can do so at a reduced oxygen requirement (47, 53). Thus the application of eccentric exercise is appealing in certain populations, where high metabolic demand is sometimes not wanted.
All of us use eccentric contractions daily without apparent detrimental effects, so why has the notion that eccentric contractions cause damage become so widely accepted? Even moderate eccentric contractions (e.g., the quadriceps while walking down stairs or transitioning from standing to sitting) that are well below maximal effort can result in damage to compromised muscles. However, the inextricable association with damage is likely due to the fact that many studies utilize maximal eccentric contractions to induce skeletal muscle injury. It is important not to confuse eccentric exercise with eccentric injury. Most studies that utilize eccentric contractions to induce skeletal muscle injury use maximal eccentric contractions without any progressive eccentric training. For humans, this is in the form of maximal voluntary contractions (MVCs) while for animals, electrical stimulation is typically used to obtain maximal stimulation of either single fibers in vitro or every fiber in a whole muscle in vivo. Supramaximal stimulation (i.e., recruitment of all motor units) is not physiological and an extreme example of eccentric contractions, but in animal studies maximal stimulation aids in obtaining reliable and reproducible injuries; it would also be difficult to assess changes in contractile function without consistency in the number of motor units recruited. Emotion, pain, or other such factors that might affect the MVC can confound results in human studies that measure contractile activity.
When activated muscles are stretched during eccentric exercise, if injury does occur, it is initiated by focal mechanical damage to sarcomeres (3, 12, 59, 74, 97). The mechanical damage triggers a more widespread injury (68, 72) that includes inflammation, disruption of the sarcolemma, and damage by reactive oxygen species (ROS) (28, 68, 82, 88). The injury culminates in degeneration of the damaged portions of fibers. Comparable protocols of damaging lengthening contractions result in more severe initial and secondary injury to muscle fibers of old compared with adult animals (9, 60, 100) and muscles of dystrophic compared with control animals (6, 24).
Much has been written about injury after eccentric contractions and the potential underlying mechanisms that contribute to the damage (1). Less has been written about eccentric exercise and the positive role it can play in rehabilitation (48). Whether or not eccentric exercise has a role in compromised muscle, such as with aging or disease, is still controversial.
MUSCULAR DYSTROPHY
The muscular dystrophies are a heterogeneous group of inherited disorders characterized by progressive weakness and degeneration of skeletal muscles. The development of molecular genetic mapping techniques has shown that a number of clinically similar conditions are linked to a variety of distinct single-gene disorders. So far, muscular dystrophies have been mapped to at least 29 different genetic loci that give rise to at least 34 different clinical disorders (21) and additional information is accumulating rapidly.
Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy; it is an X-linked disorder that affects about 1 in 3,500 newborn males worldwide (93). DMD is characterized by progressive wasting of skeletal muscles, with the limb-girdle muscles first showing weakness by the age of 5 years, followed by an inability to walk by the age of 8 to 12 years (6, 69). Death usually occurs in the second or third decade of life due to cardiac or respiratory impairment. DMD is caused by the absence of dystrophin, a 427-kDa protein found on the cytoplasmic surface of the plasma membrane of muscle fibers (the sarcolemma) in skeletal and cardiac muscle. Dystrophin provides mechanical stability to the sarcolemma and is likely involved in force transmission between the intracellular contractile apparatus and the extracellular matrix (ECM), which envelops the fiber and is connected to the tendon (78). Without dystrophin, the sarcolemma becomes fragile and more susceptible to the stress of muscle contractions. The role of dystrophin is undoubtedly more complex than simply providing mechanical stability (30, 33, 38, 39, 43, 54, 55, 81, 96), but the consequences of its absence are clear.
The gene for dystrophin is one of the largest known in humans (∼1.2 million base pairs) and its sheer size has presented one of many obstacles to gene therapy (55). Although DMD is still incurable, it is not untreatable. Several studies have pointed out the need to study exercise in patients with DMD (34, 62, 63), with a clear admonition against heavy resistance training and even eccentric exercise (34). Heavy resistance training is possible with neuromuscular diseases that are not severe (71); however, there is a risk of further muscle damage that could have catastrophic consequences in patients with DMD. Although there are very few randomized or controlled studies involving any strength training in patients with DMD, the data so far indicate little to no physical decline with exercise (26, 85). In fact, exercise appears to be beneficial to patients with DMD (23) or other muscular dystrophies (92).
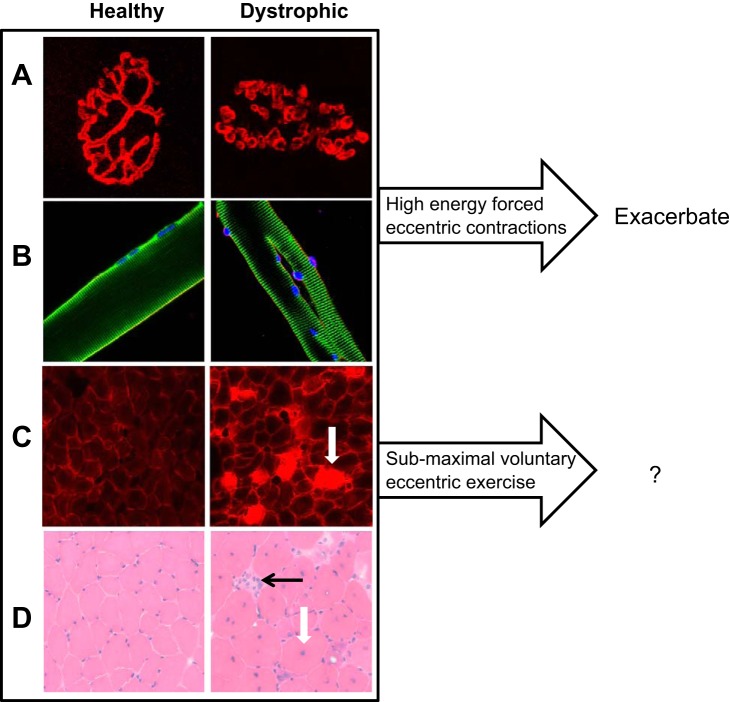
So should patients with muscular dystrophy, especially the most common form (DMD), avoid eccentric exercise? Much of what is known about muscle lacking dystrophin is derived from studies of dystrophin-deficient animals, with the most common model being the mdx mouse. The DMD and the mdx conditions are similar in that dystrophin is missing from all muscle tissues. Both DMD muscle and mdx muscle show clear changes when examined with MRI (Fig. 1). In DMD patients, pathological changes at the cell level include increased adipose and connective tissue between muscle fibers, increased variability in muscle fiber size, infiltration of inflammatory cells, alterations in myofiber shape and neuromuscular junction morphology, and centrally located nuclei, indicative of ongoing necrosis and regeneration (5, 43, 55). Except for the increase in adipose tissue (98), these same changes (Fig. 2) are found in the mdx mouse model (13, 27, 38, 54). Despite the many similarities, the absence of dystrophin is not equally damaging to patients with DMD and mdx mice. A “critical period” has been described for the mdx mouse (19), whereby there is a peak in muscle weakness and degeneration/regeneration between the 2nd and 5th weeks of life. Beyond this critical period, mdx mice still show marked susceptibility to contraction-induced injury, but not the progressive weakness observed in DMD. For this reason, some investigators have used forced exercise after the described critical period for mdx mice to continue the pathogenic process of the disease (24, 31).
Fig. 1.
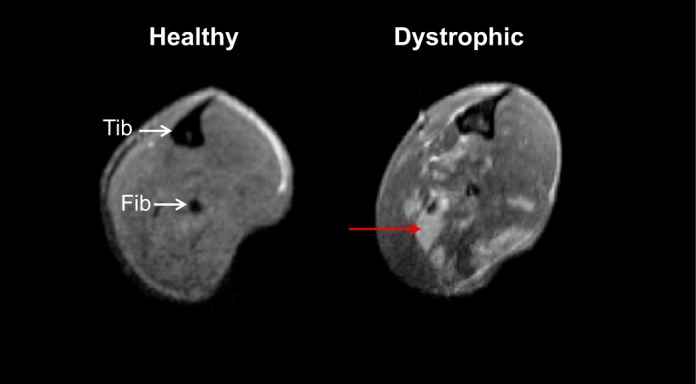
Magnetic resonance imaging (MRI) of healthy and dystrophic muscle. Representative axial MRI of hindlimb leg muscles from a healthy (wild type) and a dystrophic (mdx) mouse, both at 9 wk of age. The signal in healthy muscles is homogeneously dark, but dystrophic muscles, even without exercise, show heterogeneity, identified by unevenly distributed focal hyperintensities (red arrow) that contrast the dark signal characteristic of healthy muscle. This heterogeneity reflects muscle damage and inflammation; the amount of heterogeneity varies from muscle to muscle and limb to limb. Tib, tibia; Fib, fibula.
Fig. 2.
Findings at the cellular level in dystrophic muscle. Shown are typical differences in unexercised healthy (wild type) and dystrophic (mdx) mice. A: rhodamine-conjugated α-bungarotoxin staining of the neuromuscular junction (NMJ). NMJ morphology is often abnormal (fragmented and dispersed) in dystrophic muscle. B: immunolabeling of isolated muscle cells. Healthy skeletal myofibers are elongated tubelike structures with no branching patterns. However, a significant portion of dystrophic myofibers display altered morphology, as shown in this typical bifurcated (split) fiber. Presence of these malformed myofibers is thought to contribute to the decreased muscle specific tension and increased susceptibility to injury seen in dystrophic muscle. C: cross-sections of muscle from Evans blue dye (EBD)-injected mice. The cell membranes (sarcolemma) of dystrophic fibers often show damage, seen by cells containing EBD in the cytoplasm (white arrow), indicating lack of sarcolemmal integrity. D: cross-sections of mouse muscle stained with HE. Pathological changes such as centrally nucleated fibers (white arrow) and necrotic fibers (black arrow), both indicative of ongoing degeneration and regeneration, are found in dystrophic muscle. All of the above changes are further exacerbated by high-force eccentric contractions, but there is far less information available about changes, good or bad, occurring with progressive submaximal voluntary exercise.
Although forced maximal lengthening contractions are associated with injury, a subsequent bout of the same activity performed days or even weeks after an initial bout results in significantly less damage and protection against future injury (44); this is known as the “repeated bout effect” (RBE). Several aspects of muscle damage are ameliorated due to the RBE, including the drop in force that is used as a measure for injury (76). The factors responsible for the RBE are still being studied, both in animal models and in humans. In an effort to compare responses to injury, high-energy forced eccentric contractions have been used to cause damage in healthy (32, 40, 51, 94) and dystrophic (7, 25, 58, 70, 77, 81) mouse muscle and it is clear that dystrophic muscle is more susceptible to damage than healthy muscle (13, 19, 25, 77). Nonetheless, some animal studies now show that recovery of muscle contractile function after eccentric injury is enhanced in mdx mice (7, 14), suggesting the RBE is possible with dystrophic muscle. Interestingly, repeated bouts of eccentric exercise in mdx muscles not only lack a cumulative damaging effect, but instead they actually improved muscle strength (14).
Thus far, the role of exercise in neuromuscular disease, especially severe forms such as DMD, is still controversial; many believe that eccentric exercise should be performed with minimal resistance or avoided altogether (2, 42, 52). Some recent animal studies are challenging this doctrine. Many studies indicate a beneficial adaptation to moderate exercise in dystrophic animals (15, 18, 22, 56, 57, 89), but there is still a glaring gap in our knowledge regarding the use of exercise on populations with neuromuscular diseases. It is difficult to compare animal studies that use different species of animals, different protocols, and sometimes different outcome measures, but it can be misguided to take the findings from an animal model of a disease (e.g., the mdx mouse) and simply translate them to the human population (86). Thus there is a real need for further studies in patients with DMD and other muscular dystrophies (34, 55, 62, 63).
AGING
The age-associated condition of “frailty” (37, 91) is largely caused by loss of muscle mass and strength and increasing fatigability and susceptibility to injury (29, 61). Physical frailty, with its associated immobility and disability (17), is a major factor limiting an elderly person's chance of living independently (61). Muscle mass and strength decrease ∼10% per decade after the age of 50 (36, 73, 80, 87). Such deficits profoundly impact quality of life, even for healthy older people (99). The decrease in muscle mass results from a loss in the total number of fibers per muscle as well as a decrease in the cross-sectional area (CSA) of the remaining fibers (36, 50). The loss of muscle fibers with aging is largely irreversible, but conditioning programs that maintain, or even increase, CSA of the remaining fibers can slow the atrophy (35, 90). Eccentric exercise is generally considered a highly effective mode of conditioning for hypertrophy (75), although this is not a universal finding (64), and increased susceptibility to exercise-induced injuries and impaired or delayed recovery from injury (8, 65) raise the critical issue of the necessity to keep elderly people safe if participation in life-long physical activity is encouraged.
The effect of age on the susceptibility to the initial mechanical damage associated with lengthening contractions has been studied in mice, rats, and humans. The deficit in isometric force following single stretches of maximally activated muscles of mice was well predicted by the work input during the stretch, with the work-force deficit relationship ∼40% steeper for muscles of old compared with young or adult mice (12). In addition, when single permeabilized fibers from muscles of young and old rats were exposed to single stretches, average force deficits were ∼2-fold larger for fibers from muscles of old compared with adult rats (9). In human studies, biopsies of vastus lateralis muscles obtained immediately following a bout of exercise in which subjects resisted the backward motion of a motor-driven cycle ergometer showed at least some focal damage to sarcomeres in nearly all of the fibers examined from older subjects, compared with only 5–10% reported for young subjects (60). Similarly, biopsies of vastus lateralis muscles following the final bout of a 9-wk program of high-load resistance exercise showed ultrastructural damage in nearly 17% of fibers in older women compared with 2–5% of fibers in untrained control muscles of both young and older women and in the young subjects exposed to the exercise (83). However, when the same investigators performed this experiment in men, no difference was found between age groups (84). Although there are disparate findings, in total, the data support a greater susceptibility of muscles in old animals to injury that is due, at least in part, to a mechanically compromised sarcomeric structure that is less able to withstand stretch. One group has compared muscles of animals of different ages for damage to the sarcolemma induced by lengthening contractions (67). Resting membrane potentials were measured, with the amount of depolarization that remains in the presence of blockers of stretch-activated ion channels taken as an indicator of physical damage to the membrane (67). Despite no difference between control muscles of young and old rats for resting membrane potential, muscles of old rats showed close to 30% greater depolarization than muscles of young rats immediately following lengthening contractions (67). In addition, the portion of the depolarization that was restored through blockade of stretch-activated ion channels was less than 20% for the muscles of the old rats, compared with greater than 50% for the young rats (67), leading the authors to conclude that muscles of old animals suffer more extensive membrane damage during lengthening contractions.
Isolating the effects of age on the secondary injury is an experiment that has not been done definitively. For example, the observation by Zerba et al. (100) of a 40% greater force deficit for muscles of old compared with young or adult mice 3 days after a protocol of 75 lengthening contractions could be the result of similar secondary responses to initial mechanical injuries that varied in severity. Similarly, the threefold greater percent reduction in strength reported for older compared with young women one day following a bout of unaccustomed eccentric exercise (79) could be due to a greater susceptibility to the initial mechanical injury with a similar secondary injury response, to a more severe secondary injury in response to a given initial injury, or some combination of the two possibilities. Support for greater secondary injury in muscles of old compared with adult animals is provided by the observation that three days following a protocol of lengthening contractions that resulted in similar force deficits in adult and old mice, the number of neutrophils and macrophages, a key element of the secondary injury, was 1.5- to 2-fold greater in the muscles of the old mice (4). Inflammatory cells release reactive oxygen species (ROS). Although ROS likely play an important role in maintaining muscle health (41, 95), ROS have been proposed to promote the secondary injury, as treatment of young mice with a free radical scavenger virtually eliminated the force deficit 3 days following lengthening contractions. The same treatment in old mice only reduced the force deficit from 56% for muscles of untreated animals to 30% (100), suggesting that higher numbers of inflammatory cells in the muscles of old compared with adult mice (46) may result in greater oxygen free radical injury in old animals that was incompletely blocked by the treatment.
Recovery from injury is delayed or impaired for muscles of old animals. Following severe protocols of lengthening contractions, muscles in old mice show sustained, perhaps even irreversible force deficits and morphological evidence of damage to fibers (8, 65). For muscles of rats, force deficits following 24 lengthening contractions were eliminated in adult animals within 5 days, whereas in old animals a delay in recovery to 14 days was observed (66) and recovery of the resting membrane potential took twice as long in old compared with young animals (67). For humans, following exposure of the knee extensors to an exercise protocol with lengthening contractions, young subjects regained control levels of strength within 3–4 days, compared with 7–9 days for older subjects (79). The decreased capacity for recovery of muscles in old animals following contraction-induced injury is consistent with previous reports of impaired regeneration after whole muscle transplantation in old animals (16).
Despite the high susceptibility of muscles in old animals to injury and the decreased ability to recover, the RBE has been observed (11, 20, 66, 67, 79). After 6 wk of exposure of muscles in mice to a once per week protocol of lengthening contractions that initially resulted in a 30% force deficit and morphological evidence of injury in ∼10% of fibers in a cross section, injury was no longer observed in either adult or old animals (10). Similarly, in humans force deficits one day following an unaccustomed bout of exercise with lengthening contractions of the knee extensors was reduced from 26% to 8% following 12 wk of twice per week resistance exercise that involved both lengthening and shortening contractions (79). Even a single bout of eccentric exercise by elderly subjects reduced subsequent lengthening contraction-induced muscle soreness and serum levels of creatine kinase (20), and exposure of muscles of old rats to a single protocol of lengthening contractions reduced the force deficit and number of damaged fibers after a second protocol (66). Although damaging lengthening contractions effectively induce protective adaptations in old animals, a significant fraction of the elderly population may be unable or unwilling to engage in this form of exercise due to both perceived and real risks of severe injury. Based on this concern, Koh and his colleagues (44, 46) tested whether overt damage, degeneration, and regeneration is required to elicit protection. Muscles of mice were exposed to stretches without activation (passive stretches) prior to administering a damaging lengthening contraction protocol. Passive stretches produced no evidence of overt damage to the muscle, yet exposure to conditioning with passive stretches prior to administration of a lengthening contraction resulted in a reduction, compared with nonconditioned muscles, in the magnitude of the injury (44). For old animals, passive-stretch conditioning prior to lengthening contractions improved force production, reduced the number of damaged fibers, and reduced neutrophil and macrophage infiltration following lengthening contractions compared with nonconditioned muscles (46). Although the experiments by Koh et al. were performed in mice, the potential for conditioning with passive stretches provides an exciting alternative to lengthening contractions for a safe and effective method of protecting elderly people from injury.
While muscles can be conditioned for increased resistance to injury at all ages, the protective adaptations associated with eccentric exercise appear to be impaired somewhat in old age. For young men, repeated bouts of eccentric exercise of the elbow flexors resulted in significant reductions in strength, range of motion, upper arm circumference, plasma creatine kinase and myoglobin levels, and soreness, but only in range of motion, myoglobin, and soreness for old men, and the effects were smaller in the old compared with young subjects (49). Similarly, exposure to lengthening contractions provided complete protection in adult rats against the force deficit induced by an identical subsequent contraction protocol 2 wk later, whereas only partial protection was observed for muscles of old rats (66). Finally, dorsiflexor muscles of adult mice exposed to weekly bouts of lengthening contractions showed progressive reductions in the force deficit until week 4 when the force deficit was eliminated, whereas in old mice a similar level of protection was not demonstrated until week 5 (11).
DISCUSSION
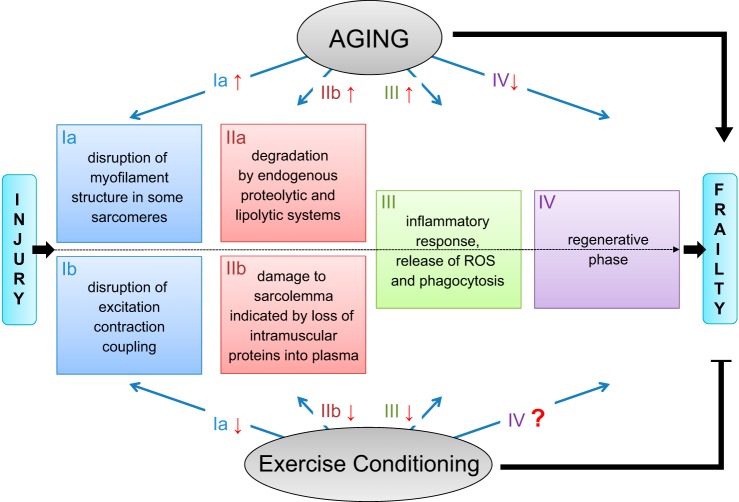
It is indisputable that repeated high-force eccentric contractions are associated with muscle damage, regardless of the initial health or age of the muscle. However, the risks and benefits of eccentric exercise involving submaximal contractions are still under investigation with regard to the elderly and those with neuromuscular disease. In a recent paper studying eccentric exercise in dystrophic animals, Call et al. state “...the dystrophic phenotype in mdx mice is not worsened when eccentric contractions are performed on a regular basis. In fact, muscle torque and force improved after multiple bouts of eccentric contractions, showing that substantial strength gains are possible without the presence of dystrophin” (14). This is one of a cadre of animal studies proclaiming the benefits of exercise in dystrophic muscle (7, 14, 15). However, there are far fewer human studies regarding the effects of eccentric exercise on muscular dystrophy, in particular DMD. Well-controlled rigorous studies are needed to determine not only the dose (frequency, intensity, and duration) of exercise appropriate for those with a neuromuscular disease, but also the type of exercise. For healthy elderly people, exercise conditioning can clearly protect muscles from injury, despite their high susceptibility to injury and an impaired ability to recover (Fig. 3). Moreover, although the adaptations are less robust than those observed following repeated bouts of damaging contractions, the exercise conditioning need not induce injury, degeneration, and/or regeneration to invoke the protective adaptations. Thus the maintenance of “conditioned” fibers in the muscles of old people has the potential to decrease the likelihood of injury, which along with skeletal muscle atrophy, weakness, and fatigability contribute to physical frailty and may actually contribute to its development and progression. Physical frailty impairs performance of the activities of daily living, increases the incidence of falls, and impacts negatively on the quality of life of old people. Despite the lack of definitive answers to many questions regarding the development of frailty and the progression of neuromuscular diseases, the longer people can be motivated to maintain a physically active life style, including both endurance and strength training, the higher will be the quality of their life.
Fig. 3.
Phases of lengthening contraction-induced injury and the effects of aging and exercise conditioning. Lengthening (eccentric) contractions can injure skeletal muscle. The injury process involves multiple phases. The sequence of the phases of injury is indicated by numerals I–IV, with events that happen more or less simultaneously designated by the same numeral. With aging, muscle structure and function deteriorates, contributing to physical frailty. Aging also results in an increased susceptibility to injury coupled with impaired regeneration, effects that may exacerbate frailty. Despite age-associated declines in muscle structure and function and increases in the likelihood of injury, exercise conditioning can provide protection from contraction-induced injury and potentially slow or delay the progression of frailty. Red arrows indicate the effects of aging or exercise conditioning on each phase of the injury process. In cases where there is no arrow, the effect has not been studied.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.M.L. and S.V.B. prepared figures; R.M.L. and S.V.B. drafted manuscript; R.M.L. and S.V.B. edited and revised manuscript; R.M.L. and S.V.B. approved final version of manuscript.
ACKNOWLEDGMENTS
R. M. Lovering receives support from National Institutes of Health (NIH) Grants K01-AR-053235 and 1R01-AR-059179, and S. V. Brooks is supported by NIH Grants AG-020591 and AR-055624.
REFERENCES
- 1.Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand 171: 311–319, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Ansved T. Muscle training in muscular dystrophies. Acta Physiol Scand 171: 359–366, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong RB, Duan C, Delp MD, Hayes DA, Glenn GM, Allen GD. Elevations in rat soleus muscle [Ca2+] with passive stretch. J Appl Physiol 74: 2990–2997, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Bachinski LL, Udd B, Meola G, Sansone V, Bassez G, Eymard B, Thornton CA, Moxley RT, Harper PS, Rogers MT, Jurkat-Rott K, Lehmann-Horn F, Wieser T, Gamez J, Navarro C, Bottani A, Kohler A, Shriver MD, Sallinen R, Wessman M, Zhang S, Wright FA, Krahe R. Confirmation of the type 2 myotonic dystrophy (CCTG)n expansion mutation in patients with proximal myotonic myopathy/proximal myotonic dystrophy of different European origins: a single shared haplotype indicates an ancestral founder effect. Am J Hum Genet 73: 835–848, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks GB, Chamberlain JS, Froehner SC. Truncated dystrophins can influence neuromuscular synapse structure. Mol Cell Neurosci 40: 433–441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, King WM, Pandya S, Robison J, Schierbecker J. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology 39: 475–481, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Brooks SV. Rapid recovery following contraction-induced injury to in situ skeletal muscles in mdx mice. J Muscle Res Cell Motil 19: 179–187, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol Cell Physiol 258: C436–C442, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol 497: 573–580, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks SV, Faulkner JA. Severity of contraction-induced injury is affected by velocity only during stretches of large strain. J Appl Physiol 91: 661–666, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Brooks SV, Opiteck JA, Faulkner JA. Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. J Gerontol A Biol Sci Med Sci 56: B163–B171, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol 488: 459–469, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brussee V, Tardif F, Tremblay JP. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord 7: 487–492, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Call JA, Eckhoff MD, Baltgalvis KA, Warren GL, Lowe DA. Adaptive strength gains in dystrophic muscle exposed to repeated bouts of eccentric contraction. J Appl Physiol 111: 1768–1777, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, Talmadge RJ, Grange RW. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol 105: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Cell Physiol 256: C1262–C1266, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med 1: 381–391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter GT, Abresch RT, Fowler WM., Jr Adaptations to exercise training and contraction-induced muscle injury in animal models of muscular dystrophy. Am J Phys Med Rehabil 81: S151–S161, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J 21: 2195–2204, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Clarkson PM, Dedrick ME. Exercise-induced muscle damage, repair, and adaptation in old and young subjects. J Gerontol 43: M91–M96, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Dalkilic I, Kunkel LM. Muscular dystrophies: genes to pathogenesis. Curr Opin Genet Dev 13: 231–238, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Dangain J, Vrbova G. Long term effect of low frequency chronic electrical stimulation on the fast hind limb muscles of dystrophic mice. J Neurol Neurosurg Psychiatry 52: 1382–1389, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lateur BJ, Giaconi RM. Effect on maximal strength of submaximal exercise in Duchenne muscular dystrophy. Am J Phys Med 58: 26–36, 1979 [PubMed] [Google Scholar]
- 24.De LA, Pierno S, Liantonio A, Cetrone M, Camerino C, Fraysse B, Mirabella M, Servidei S, Ruegg UT, Conte CD. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J Pharmacol Exp Ther 304: 453–463, 2003 [DOI] [PubMed] [Google Scholar]
- 25.DelloRusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil 22: 467–475, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Eagle M. Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases. Newcastle, January 2002. Neuromuscul Disord 12: 975–983, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Ervasti JM, Campbell KP. Dystrophin and the membrane skeleton. Curr Opin Cell Biol 5: 82–87, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Evans WJ, Cannon JG. The metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev 19: 99–125, 1991 [PubMed] [Google Scholar]
- 29.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 34: 1091–1096, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Franco A, Jr, Lansman JB. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature 344: 670–673, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Fraysse B, Liantonio A, Cetrone M, Burdi R, Pierno S, Frigeri A, Pisoni M, Camerino C, De LA. The alteration of calcium homeostasis in adult dystrophic mdx muscle fibers is worsened by a chronic exercise in vivo. Neurobiol Dis 17: 144–154, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Friden J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand 171: 321–326, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Gailly P, Hermans E, Octave JN, Gillis JM. Specific increase of genetic expression of parvalbumin in fast skeletal muscles of mdx mice. FEBS Lett 326: 272–274, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Grange RW, Call JA. Recommendations to define exercise prescription for Duchenne muscular dystrophy. Exerc Sport Sci Rev 35: 12–17, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Grimby G. Muscle performance and structure in the elderly as studied cross-sectionally and longitudinally. J Gerontol A Biol Sci Med Sci 50: 17–22, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Grimby G, Saltin B. The ageing muscle. Clin Physiol 3: 209–218, 1983 [DOI] [PubMed] [Google Scholar]
- 37.Hadley EC, Ory MG, Suzman R, Weindruch R, Fried L. Physical frailty: a treatable cause of dependence in old age. J Gerontol 48: 1–88, 1993 [Google Scholar]
- 38.Head SI, Williams DA, Stephenson DG. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci 248: 163–169, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Hollingworth S, Zeiger U, Baylor SM. Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J Physiol 586: 5063–5075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol 85: 58–67, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal 15: 2477–2486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen M, de GI, van AN, Geurts AC. Physical training in boys with Duchenne Muscular Dystrophy: the protocol of the No Use is Disuse study. BMC Pediatr 10: 55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG, Ward CW. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci Signal 5: ra56, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 281: R155–R161, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Koh TJ, Peterson JM, Pizza FX, Brooks SV. Passive stretches protect skeletal muscle of adult and old mice from lengthening contraction-induced injury. J Gerontol A Biol Sci Med Sci 58: 592–597, 2003 [DOI] [PubMed] [Google Scholar]
- 47.LaStayo PC, Reich TE, Urquhart M, Hoppeler H, Lindstedt SL. Chronic eccentric exercise: improvements in muscle strength can occur with little demand for oxygen. Am J Physiol Regul Integr Comp Physiol 276: R611–R615, 1999 [DOI] [PubMed] [Google Scholar]
- 48.LaStayo PC, Woolf JM, Lewek MD, Snyder-Mackler L, Reich T, Lindstedt SL. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther 33: 557–571, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Lavender AP, Nosaka K. Responses of old men to repeated bouts of eccentric exercise of the elbow flexors in comparison with young men. Eur J Appl Physiol 97: 619–626, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50, Spec No.: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Lieber RL, Thornell LE, Friden J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol 80: 278–284, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Lim JH, Kim DY, Bang MS. Effects of exercise and steroid on skeletal muscle apoptosis in the mdx mouse. Muscle Nerve 30: 456–462, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Lindstedt SL, LaStayo PC, Reich TE. When active muscles lengthen: properties and consequences of eccentric contractions. News Physiol Sci 16: 256–261, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Lovering RM, Michaelson L, Ward CW. Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am J Physiol Cell Physiol 297: C571–C580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lovering RM, Porter NC, Bloch RJ. The muscular dystrophies: from genes to therapies. Phys Ther 85: 1372–1388, 2005 [PMC free article] [PubMed] [Google Scholar]
- 56.Luthert P, Vrbova G, Ward KM. Functional improvement of skeletal muscles of dystrophic mice following electrical stimulation [proceedings]. J Physiol 291: 31P, 1979 [PubMed] [Google Scholar]
- 57.Luthert P, Vrbova G, Ward KM. Effects of slow frequency electrical stimulation on muscles of dystrophic mice. J Neurol Neurosurg Psychiatry 43: 803–809, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA. Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am J Physiol Cell Physiol 279: C1290–C1294, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Macpherson PC, Schork MA, Faulkner JA. Contraction-induced injury to single fiber segments from fast and slow muscles of rats by single stretches. Am J Physiol Cell Physiol 271: C1438–C1446, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Manfredi TG, Fielding RA, O'Reilly KP, Meredith CN, Lee HY, Evans WJ. Plasma creatine kinase activity and exercise-induced muscle damage in older men. Med Sci Sports Exerc 23: 1028–1034, 1991 [PubMed] [Google Scholar]
- 61.Marcell TJ. Sarcopenia: causes, consequences, preventions. J Gerontol A Biol Sci Med Sci 58: M911–M916, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Markert CD, Ambrosio F, Call JA, Grange RW. Exercise and Duchenne muscular dystrophy: toward evidence-based exercise prescription. Muscle Nerve 43: 464–478, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Markert CD, Case LE, Carter GT, Furlong PA, Grange RW. Exercise and Duchenne muscular dystrophy: where we have been and where we need to go. Muscle Nerve 45: 746–751, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Mayhew TP, Rothstein JM, Finucane SD, Lamb RL. Muscular adaptation to concentric and eccentric exercise at equal power levels. Med Sci Sports Exerc 27: 868–873, 1995 [PubMed] [Google Scholar]
- 65.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J 18: 355–357, 2004 [DOI] [PubMed] [Google Scholar]
- 66.McBride TA, Gorin FA, Carlsen RC. Prolonged recovery and reduced adaptation in aged rat muscle following eccentric exercise. Mech Ageing Dev 83: 185–200, 1995 [DOI] [PubMed] [Google Scholar]
- 67.McBride TA, Stockert BW, Gorin FA, Carlsen RC. Stretch-activated ion channels contribute to membrane depolarization after eccentric contractions. J Appl Physiol 88: 91–101, 2000 [DOI] [PubMed] [Google Scholar]
- 68.McCully KK, Faulkner JA. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol 59: 119–126, 1985 [DOI] [PubMed] [Google Scholar]
- 69.McDonald CM, Abresch RT, Carter GT, Fowler WM, Jr, Johnson ER, Kilmer DD, Sigford BJ. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil 74: S70–S92, 1995 [DOI] [PubMed] [Google Scholar]
- 70.McMillan A, Shi D, Pratt SJP, Lovering RM. Diffusion tensor MRI to assess damage in healthy and dystrophic skeletal muscle after lengthening contractions. J Biomed Biotechnol EPub 2011 November 15. Article ID 970726. 10.1155/2011/970726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milner-Brown HS, Miller RG. Muscle strengthening through high-resistance weight training in patients with neuromuscular disorders. Arch Phys Med Rehabil 69: 14–19, 1988 [PubMed] [Google Scholar]
- 72.Morgan DL, Allen DG. Early events in stretch-induced muscle damage. J Appl Physiol 87: 2007–2015, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol 99: 1050–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Colch) 64: 55–62, 1983 [DOI] [PubMed] [Google Scholar]
- 75.Norrbrand L, Fluckey JD, Pozzo M, Tesch PA. Resistance training using eccentric overload induces early adaptations in skeletal muscle size. Eur J Appl Physiol 102: 271–281, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol 63: 70–76, 1991 [DOI] [PubMed] [Google Scholar]
- 77.Petrof BJ. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Mol Cell Biochem 179: 111–123, 1998 [DOI] [PubMed] [Google Scholar]
- 78.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ploutz-Snyder LL, Giamis EL, Formikell M, Rosenbaum AE. Resistance training reduces susceptibility to eccentric exercise-induced muscle dysfunction in older women. J Gerontol A Biol Sci Med Sci 56: B384–B390, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function, and adaptability. Scand J Med Sci Sports 5: 129–142, 1995 [DOI] [PubMed] [Google Scholar]
- 81.Pratt SJ, Shah SB, Ward CW, Inacio MP, Stains JP, Lovering RM. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J Physiol 591: 559–570, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rader EP, Song W, Van RH, Richardson A, Faulkner JA. Raising the antioxidant levels within mouse muscle fibres does not affect contraction-induced injury. Exp Physiol 91: 781–789, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA. High-volume, heavy-resistance strength training and muscle damage in young and older women. J Appl Physiol 88: 1112–1118, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA. Ultrastructural muscle damage in young vs. older men after high-volume, heavy-resistance strength training. J Appl Physiol 86: 1833–1840, 1999 [DOI] [PubMed] [Google Scholar]
- 85.Scott OM, Hyde SA, Goddard C, Jones R, Dubowitz V. Effect of exercise in Duchenne muscular dystrophy. Physiotherapy 67: 174–176, 1981 [PubMed] [Google Scholar]
- 86.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, Donald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23: 371–377, 1994 [DOI] [PubMed] [Google Scholar]
- 88.Stauber WT, Fritz VK, Vogelbach DW, Dahlmann B. Characterization of muscles injured by forced lengthening. I. Cellular infiltrates. Med Sci Sports Exerc 20: 345–353, 1988 [DOI] [PubMed] [Google Scholar]
- 89.Taylor RG, Fowler WM, Jr, Doerr L. Exercise effect on contractile properties of skeletal muscle in mouse muscular dystrophy. Arch Phys Med Rehabil 57: 174–180, 1976 [PubMed] [Google Scholar]
- 90.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol 89: 143–152, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Verdery RB. Failure to thrive in the elderly. Clin Geriatr Med 11: 653–659, 1995 [PubMed] [Google Scholar]
- 92.Vignos PJ, Jr, Watkins MP. The effect of exercise in muscular dystrophy. JAMA 197: 843–848, 1966 [PubMed] [Google Scholar]
- 93.Wagner KR. Genetic diseases of muscle. Neurol Clin 20: 645–678, 2002 [DOI] [PubMed] [Google Scholar]
- 94.Warren GL, Ingalls CP, Shah SJ, Armstrong RB. Uncoupling of in vivo torque production from EMG in mouse muscles injured by eccentric contractions. J Physiol 515: 609–619, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Westerblad H, Allen DG. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid Redox Signal 15: 2487–2499, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 33: 657–662, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Wood SA, Morgan DL, Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. Am J Physiol Cell Physiol 265: C792–C800, 1993 [DOI] [PubMed] [Google Scholar]
- 98.Xu S, Pratt SJ, Spangenburg EE, Lovering RM. Early metabolic changes measured by 1H MRS in healthy and dystrophic muscle after injury. J Appl Physiol 113: 808–816, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young A, Skelton DA. Applied physiology of strength and power in old age. Int J Sports Med 15: 149–151, 1994 [DOI] [PubMed] [Google Scholar]
- 100.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol Cell Physiol 258: C429–C435, 1990 [DOI] [PubMed] [Google Scholar]