Abstract
Increasing amino acid availability (via infusion or ingestion) at rest or postexercise enhances amino acid transport into human skeletal muscle. It is unknown whether alterations in amino acid availability, from ingesting different dietary proteins, can enhance amino acid transport rates and amino acid transporter (AAT) mRNA expression. We hypothesized that the prolonged hyperaminoacidemia from ingesting a blend of proteins with different digestion rates postexercise would enhance amino acid transport into muscle and AAT expression compared with the ingestion of a rapidly digested protein. In a double-blind, randomized clinical trial, we studied 16 young adults at rest and after acute resistance exercise coupled with postexercise (1 h) ingestion of either a (soy-dairy) protein blend or whey protein. Phenylalanine net balance and transport rate into skeletal muscle were measured using stable isotopic methods in combination with femoral arteriovenous blood sampling and muscle biopsies obtained at rest and 3 and 5 h postexercise. Phenylalanine transport into muscle and mRNA expression of select AATs [system L amino acid transporter 1/solute-linked carrier (SLC) 7A5, CD98/SLC3A2, system A amino acid transporter 2/SLC38A2, proton-assisted amino acid transporter 1/SLC36A1, cationic amino acid transporter 1/SLC7A1] increased to a similar extent in both groups (P < 0.05). However, the ingestion of the protein blend resulted in a prolonged and positive net phenylalanine balance during postexercise recovery compared with whey protein (P < 0.05). Postexercise myofibrillar protein synthesis increased similarly between groups. We conclude that, while both protein sources enhanced postexercise AAT expression, transport into muscle, and myofibrillar protein synthesis, postexercise ingestion of a protein blend results in a slightly prolonged net amino acid balance across the leg compared with whey protein.
Keywords: protein metabolism, protein anabolism, mTORC1, muscle protein synthesis, leucine
our laboratory has recently demonstrated a prolonged postexercise aminoacidemia, mixed muscle protein synthesis (MPS) rate, and mammalian/mechanistic target of rapamycin complex 1 (mTORC1) signaling response with postexercise ingestion of a soy-dairy protein blend (51). Despite a significant increase in MPS with the protein blend at 3–5 h postexercise, there was no detectible difference (P = 0.12) in mixed MPS between groups (whey vs. blend). The purpose of the present study is to determine whether different rates of digestion and subsequent prolonged changes in amino acid availability over time would create detectable differences in skeletal muscle amino acid transport kinetics, mRNA expression, and myofibrillar protein synthesis during this later recovery period.
The combination of resistance exercise and increased amino acid availability is an effective and highly practical strategy for the promotion of skeletal muscle mass and strength (5, 36, 41, 65). Resistance exercise and essential amino acids (EAA) or protein exert separate and combined effects on skeletal MPS and mTORC1 signaling (4, 5, 10, 16, 20, 30, 47, 60). Interestingly, with the use of stable isotopic methods, innovative studies demonstrated that resistance exercise in the fasted state and in combination with increased amino acid availability enhances the transport rate of amino acids from the circulation into the muscle cell (4–6).
Amino acid transporters facilitate amino acid flux across the muscle cell membrane to activate mTORC1 (15), which is thought to be essential in regulating MPS (3). Changes in amino acid availability stimulate the system A amino acid transporter 2 (SNAT2)/solute-linked carrier (SLC) 38A2, the cationic amino acid transporter 1 (CAT1)/SLC7A1(43), and the system L amino acid transporter 1 (LAT1)/SLC7A5 (which forms a heterodimer with CD98/SLC3A2) (22, 25, 67). LAT1/SLC7A5 and SNAT2/SLC38A2 function cooperatively to transport large neutral amino acids into the cell (25, 38), whereas proton-assisted transporters (PAT), such as PAT1/SLC36A, are thought to play a role in stimulating protein synthesis after amino acids such as leucine reach sufficient quantities in the cell to activate mTORC1 (33, 35).
More recently, our laboratory has demonstrated that human skeletal muscle amino acid transporter expression, transport rates, mTORC1 activation, and MPS are stimulated by the separate (21, 22) and combined (8) effects of exercise and EAA supplementation. Protein ingestion is also an effective means to increase amino acid supply and to augment the muscle protein anabolic response to exercise (29, 46, 47, 54, 58). However, proteins differ on the basis of digestion rate and composition of EAA, which together impact the metabolic fate (i.e., oxidation or incorporation into proteins) of the ingested protein source (48, 55, 63). Although many protein sources are considered to be of high quality, their varying amino acid composition may influence their amino acid transport in the gut (49) and also at the muscle membrane (56). Thus protein ingestion represents a unique means to study amino acid transporter function in humans. This is an exciting area of investigation, yet only one study has examined human skeletal muscle amino acid transporter expression following resistance exercise and dietary protein ingestion (13). Although several studies have examined muscle protein net balance with consumption of dietary protein following resistance exercise (7, 24, 53, 57–59, 64), no study has examined how the ingestion of dietary protein after resistance exercise stimulates skeletal muscle amino acid transport rates during postexercise recovery.
Amino acid transporters play a key role in muscle protein metabolism and activation of mTORC1 signaling by altering the delivery of substrate (amino acids) and/or by acting as a transporter/receptor (transceptor) of anabolic signaling (32, 37). Because of the sensitivity of skeletal muscle amino acid transporters to amino acid availability (22), we sought to examine if the prolonged hyperaminoacidemia associated with the ingestion of a blend of plant (25% soy) and dairy (50% casein, 25% whey) proteins (with varying digestion rates) would prolong the skeletal muscle net protein balance across the leg (an indicator of overall muscle protein anabolism) compared with rapidly digested whey, and whether this would influence amino acid transporter expression and amino acid transport into muscle. We hypothesized that the prolonged hyperaminoacidemia from ingesting a blend of proteins would reduce markers of protein breakdown and enhance overall skeletal muscle protein anabolism, myofibrillar protein synthesis, amino acid transport into muscle, and amino acid transporter expression compared with the ingestion of a rapidly digested protein.
MATERIALS AND METHODS
Screening of participants.
Sixteen healthy, young subjects (age range: 19–30 yr) participated in this double-blind, randomized clinical trial. Subject characteristics can be found in Table 1. The subjects were a subset of volunteers who participated in a previous study (51); however, none of the data presented herein has been previously published. The participants were recruited through locally posted flyers, newspaper advertisements, and by word of mouth. The participants were healthy and recreationally active, but were not engaged in any regular exercise-training program (<2 sessions high-intensity aerobic or resistance exercise/week) at the time of enrollment. Screening of participants was performed on 2 separate days (>7 days apart) at the Institute for Translational Sciences-Clinical Research Center (ITS-CRC). The first screening day included 1 repetition maximum (1 RM) strength testing, a clinical history, physical exam, and laboratory tests (complete blood count with differential, liver, and kidney function tests, coagulation profile, fasting blood glucose, hepatitis B and C screening, human immunodeficiency virus test, thyroid-stimulating hormone, lipid profile, urinalysis, and drug screening). The second screening day included a second 1-RM test and a dual-energy X-ray absorptiometry scan (Hologic QDR 4500W, Bedford, MA) to measure lean and fat mass. A leg extension machine (Cybex-VR2, Medway, MA) was used to establish a 1 RM, and the value was recorded as the highest weight lifted for a single repetition from the 2 testing days. All participants provided written, informed consent before enrollment in the study. The study was approved by the Institutional Review Board of the University of Texas Medical Branch and is in compliance with the Declaration of Helsinki, as revised in 1983.
Table 1.
Characteristics
| Subject |
Exercise |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age, yr | BMI, kg/m2 | Fat, % | Lean mass, kg | Leg volume, liters | Leg mass, kg | 1 RM, kg | Total weight lifted, kg | 1-RM mean, % | Time, min | |
| Blend | 8 | 22.3 ± 1.0 | 26.6 ± 0.8 | 23.9 ± 1.4 | 59.5 ± 2.5 | 10.9 ± 0.5 | 11.3 ± 0.5 | 124 ± 7 | 6,265 ± 353 | 65 ± 1 | 26 ± 1 |
| Whey | 8 | 23.6 ± 1.0 | 25.0 ± 1.3 | 25.1 ± 2.7 | 56.6 ± 3.0 | 10.4 ± 0.5 | 10.8 ± 0.6 | 126 ± 11 | 6,302 ± 527 | 63 ± 1 | 27 ± 2 |
Values are means ± SE; n, no. of subjects. Subject and exercise characteristics of participants were randomized to receive Whey (whey protein; n = 8) or Blend (soy-dairy protein blend; n = 8) at 1 h postexercise.
BMI, body mass index; 1-RM, one repetition maximum.
Study design.
Subjects were admitted to the University of Texas Medical Branch ITS-CRC at ∼1700 the day before the study. Subjects were instructed to refrain from exercise at least 72 h before admission. The subjects were given a standardized meal at 1900 prepared by the Bionutrition Division of the ITS-CRC with a macronutrient distribution of 20% protein, 60% carbohydrate, and 20% fat at 12 kcal/kg body wt. Subjects were provided water ad libitum. The subjects were randomized to ingest a soy-dairy protein blend (n = 8; Blend) or whey protein (n = 8; Whey) at 1 h following a bout of high-intensity leg resistance exercise.
Experimental protocol.
All subjects underwent the stable isotope infusion protocol (Fig. 1) at the same time of day (0600–1600) on the day following admission. After an overnight fast (∼10 h), an 18 G polyethylene catheter was inserted into the antecubital vein, from which background blood draws for the measurement of phenylalanine concentration/enrichment and indocyanine green (ICG; Cardio-Green, Becton Dickinson, Cockeysville, MD) concentration. This was followed by initiation of a primed, constant infusion (∼10 h) of l-[ring-13C6]phenylalanine (Sigma-Aldrich, St. Louis, MO). The priming dose for the labeled phenylalanine was 2 μmol/kg, and the infusion rate was 0.05 μmol·kg−1·min−1. A retrograde catheter was inserted (0700–0800) into a hand vein on the contralateral arm, and arterialized blood was extracted with the use of a heating pad before sampling. A catheter was inserted (0900–1000) into the femoral artery and vein (retrograde) of one leg for blood sampling. The femoral arterial catheter was also used for the infusion of ICG. At ∼1030, a continuous infusion of ICG dye (0.5 mg/min) was started in the femoral artery and was maintained for 7 min to measure leg blood flow in each sampling period. Plasma ICG concentration was measured in blood samples during the resting period (Rest) and several times following protein ingestion (see below) from the femoral and wrist veins. At ∼2 and 4 h following initiation of the infusion, muscle biopsies were taken from the lateral aspect of the vastus lateralis for the determination of resting intracellular phenylalanine enrichment and concentration. All biopsies were collected with a 5-mm Bergström biopsy needle under sterile procedure and local anesthesia (1% lidocaine). Following femoral catheter placement and a series of blood draws, the participants were moved to a leg extension machine (Cybex-VR2, Medway, MA) for high-intensity resistance exercise consisting of 8 sets of 10 repetitions at 55% (set 1), 60% (set 2), 65% (set 3), and ∼70% (sets 4–8) of the participants previously determined 1 RM with 3-min rest between sets. Exercise characteristics can be found in Table 1. The nutritional supplements were ingested 1 h following exercise. Two additional muscle biopsies were collected 2 and 4 h after protein ingestion (corresponding to 3 and 5 h after exercise) to represent Early and Late postexercise periods (Fig. 1). The measurements taken during the 1 to 2 h, 2 to 3 h, 3 to 4 h, 1 to 2.5 h, 2.5 to 4 h, and 1 to 4 h postingestion were averaged to represent the 1-2 h, 2-3 h, 3-4 h, Early, Late, and Entire periods, respectively (Fig. 1). The first, second, third, and fourth muscle biopsies were sampled from two separate incisions on the same leg, respectively. To minimize multiple sampling in a given area, skin incisions were separated by ∼7 cm, while biopsies collected from the same incision were angled ∼5 cm from each other. This method has been previously utilized in our laboratory (17, 23, 26) and others (34, 47, 52). Muscle tissue was immediately blotted, frozen in liquid nitrogen, and stored at −80°C until analysis. Blood samples were collected before the infusion and during the resting and postexercise/postingestion time periods (Fig. 1) for the determination of blood enrichment (see below) and amino acid concentration. The infusion study ended following the fourth muscle biopsy, and participants were then given a standard meal.
Fig. 1.
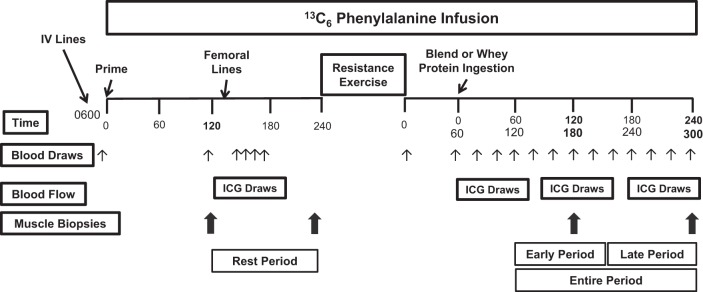
Study design. ICG, indocyanine green.
Protein supplements.
The protein beverages (Whey or Blend) were ingested at 1 h postexercise. The beverages were dissolved in 300 ml of water and enriched (8%) with l-[ring-13C6]phenylalanine in an attempt to maintain isotopic steady state in arterialized blood. The composition of the beverages is similar to that our laboratory previously reported (51). To match leucine and EAA content between the interventions, participants were given 0.305 or 0.337 g total protein/kg lean mass for Whey and Blend, respectively. The amount of protein given in each group was based on the 8.6 g of EAA in dietary (nonhydrolyzed) protein demonstrated to maximize the MPS response following resistance exercise (46). The Blend consisted of 20.1 ± 0.9 g total protein (providing 1.9 ± 0.1 g leucine, 1.0 ± 0.1 g phenylalanine, 1.3 ± 0.02 g valine, and 9.0 ± 0.4 g EAA) composed of 50% protein from sodium caseinate, 25% protein from whey protein isolate, and 25% protein from soy protein isolate. Whey consisted of 17.3 ± 0.9 g of protein (providing 1.9 ± 0.1 g leucine, 0.6 ± 0.1 g phenylalanine, 1.1 ± 0.1 g valine, and 8.7 ± 0.5 g EAA) composed of 100% whey protein isolate.
Phenylalanine amino acid concentration, ICG, lactate, glucose, and insulin.
Concentrations of phenylalanine (femoral artery and vein) were measured in the blood using gas chromatography-mass spectrometry (GCMS), as previously described using an internal standard (18, 66). Plasma glucose and lactate concentration was measured using an automated glucose and lactate analyzer (YSI, Yellow Springs, OH) at Rest, immediately postexercise, and 0 (at ingestion), 20, 40, 60, 80, 100, 120, 180, and 220 min postingestion. Serum concentrations of insulin were determined with an enzyme-linked immunosorbent assay (Millipore, St. Charles, MO), according to the manufacturer's instructions at Rest, immediately postexercise, and 0, 20, 40, 60, 80, 100, and 120 min postingestion. The serum ICG concentration to determine leg blood flow was measured spectrophotometrically (Beckman Coulter) at λ = 805 nm (40). The phenylalanine concentrations and blood flow measurements taken during the 1-2, 2-3, 3-4, 1-2.5, 2.5-4, and 1-4 h postingestion were averaged to represent the 1-2 h, 2-3 h, 3-4 h, Early, Late, and Entire periods, respectively (Fig. 1).
Amino acid parameters and transport rates.
We calculated skeletal muscle amino acid transport rates from the enrichments and concentrations of phenylalanine in the femoral artery and vein and from the enrichment of muscle tissue-free phenylalanine, using amino acid kinetics modeling, as previously described (3, 28). Phenylalanine is used in this model because it is not oxidized by muscle, which allows for the calculation and measurement of amino acid net balance across the leg and MPS. The following amino acid parameters were measured:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where CA and CV are plasma phenylalanine concentrations in the femoral artery and vein, respectively; EA, EV, and EM are phenylalanine enrichments [tracer-to-tracee ratio (TTR)] in femoral arterial and venous plasma and in muscle, respectively; BF is leg blood flow. Data are presented per 100 g leg lean mass. Similar values were obtained with correction by leg lean mass (from dual-energy X-ray absorptiometry) and leg volume (as demonstrated in Ref. 66). Leg plasma flow was calculated from the steady-state dye concentration values in the femoral and wrist vein, as previously described (39, 40). Leg blood flow was calculated by correcting the plasma flow by the hematocrit.
Muscle samples were processed as previously described (66), and muscle free tissue phenylalanine enrichments and concentrations were determined by GCMS. The intracellular concentration of phenylalanine was then calculated from the tissue value, accounting for the ratio of intracellular to extracellular water (3).
Myofibrillar and nuclear fraction isolation.
About 30–50 mg of frozen muscle tissue were placed in buffer (17) and homogenized (1:9 wt/vol) and centrifuged at 3,400 g for 10 min at 4°C, followed by removal of the supernatant, which was used for Western blotting for LAT1, SNAT2, and eukaryotic elongation factor 2 (eEF2). The resulting pellet was then suspended in isolation buffer (1 M sucrose, 1 M Tris·HCl, 1 M KCl, 0.5 M EDTA, pH 7.4) containing protease and phosphatase inhibitors and centrifuged for 10 min at 4°C and 700 g. After three series of PBS buffer suspensions and centrifugations at 15,000 g for 5 min at 4°C, the pellet was resuspended and agitated on ice for 2 × 20 min and in a 4°C sonication bath in high salt buffer (1:4 wt/vol). The slurry was centrifuged at 15,000 g for 10 min at 4°C, and the supernatant was taken as the nuclear extract, which was assayed for protein concentration with the BCA protein assay (Pierce, Rockford, IL) and used for Western blotting for activating transcription factor 4 (ATF4). The enrichment of nuclear protein in this isolation was verified by examination of cytoplasmic and nuclear protein fractions run on the same gel and probed for antibodies specific to histone H3 (for nuclear) and hexokinase (for cytoplasmic).
The resulting pellet was fully suspended in double distilled water and centrifuged at 15,000 g for 5 min at 4°C. To precipitate the myofibrillar proteins, 1 ml of 0.3 M NaOH was added to resuspend the pellet, and then heated at 50°C for 30 min with frequent vortexing. After centrifugation at 10,000 g for 5 min at 4°C, the supernatant was collected, an additional 1 ml of 0.3 M NaOH was added to resuspend the pellet, and then heated at 37°C for 10 min with frequent vortexing. After centrifugation at 10,000 g for 5 min at 4°C, the supernatant was collected, and the collagen pellet was discarded. Precipitate was created by addition of 1 ml perchloric acid to the collected supernatant and pelleted at 805 g for 10 min at 4°C. This pellet was washed 2× with 70% ethanol and then hydrolyzed overnight in 1.5 ml 6 M HCl.
Western blot analysis.
Western blot analysis was conducted as described previously (21). Immunoblot data were normalized to an internal loading control, which was loaded on all gels for comparison across blots, and data are adjusted to represent fold change from basal. Antibodies utilized were LAT1/SLC7A5 (ab85226, Abcam, Cambridge, MA), SNAT2/SLC38A2 (Santa Cruz Biotechnologies, Santa Cruz, CA), ATF4 (Santa Cruz Biotechnologies), histone 3H (Cell Signaling), phospho-eEF2 (Thr-56) (Cell Signaling), total-eEF2 (Cell Signaling), and monoclonal α-tubulin (Sigma-Aldrich, St. Louis, MO). LAT1/SLC7A5 and SNAT2/SLC38A2 were normalized to α-tubulin, and ATF4 was normalized to histone H3 to account for differences in loading.
Myofibrillar protein synthesis.
Bound proteins from the myofibrillar fraction and muscle intracellular free amino acids were extracted from biopsy samples as described above. GCMS (6890 Plus CG, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA) measurements were made to determine bound tracer enrichments for l-[ring-13C6]phenylalanine, as previously described (66). Using the external standard curve approach (11), muscle myofibrillar protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction (17, 62). We calculated myofibrillar protein synthesis as fractional synthesis rate (FSR) by measuring the incorporation rate of the phenylalanine tracer into the proteins [change (Δ) in protein bound enrichment over time] and using the precursor-product model to calculate the synthesis rate:
| (6) |
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM(1) + EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Due to lack of tissue, we were only able to calculate resting FSR with (n = 4) in each group. Data are expressed as percent per hour (%/h).
RNA extraction and semiquantitative real-time PCR.
RNA isolation, cDNA synthesis, and real-time quantitative PCR were performed as our laboratory has previously described (22). Total RNA was isolated by homogenizing 10- to 20-mg tissue with a hand-held homogenizing dispenser (T10 Basic Ultra Turrax, IKA, Wilmington, NC) in 1 ml of Tri reagent. The RNA was separated into an aqueous phase using 0.2 ml of chloroform and subsequently precipitated from the aqueous phase using 0.5 ml of isopropanol. RNA was washed with 1 ml of 75% ethanol, air-dried, and suspended in a known amount of nuclease-free water. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE), and RNA was DNase-treated using a commercially available kit (DNA-free, Ambion, Austin, TX). A total of 1 μg of RNA was reverse transcribed into cDNA according to the directions provided by the manufacturer (iScript, BioRad, Hercules, CA). Real-time quantitative PCR was carried out with an iQ5 Multicolor Real Time PCR cycler (BioRad). cDNA was analyzed with SYBR green fluorescence (iQ SYBR green supermix; BioRad). Primer sequences for genes of interest (LAT1/SLC7A5, CD98/SLC3A2, SNAT2/SLC38A2, PAT1/SLC36A1, CAT1/SLC7A1) have been previously published (22). β2-Microglobulin was utilized as a normalization/housekeeping gene. Relative fold changes were determined from the Ct values using the 2−ΔΔCt method (42).
Statistics.
All outcomes were assessed using standard ANOVA and analysis of covariance (ANCOVA) models. With baseline as a covariate, an ANCOVA model for each outcome was used to determine possible differences between groups at each time point. To test marginal outcomes and differences across time points, a repeated-measures ANOVA model was used in which a random-intercept model was used to account for subject-to-subject variability. Pairwise comparisons were calculated and tested using standard post hoc contrast methods. All pairwise comparisons were done using contrasts in the ANOVA model, with Tukey testing for post hoc adjustment. Assumptions of normality and homogeneity of variance were tested, and transformations were used as necessary to make all tests reliable. All calculations were done in SAS, version 9.3.
RESULTS
Subject and exercise characteristics.
The subjects were effectively randomized, as their baseline and exercise characteristics (Table 1) were not different (P > 0.05).
Plasma glucose in the femoral artery and vein (data not shown) increased from Rest only immediately postexercise and were not different (P > 0.05) between groups. Plasma lactate in the femoral artery and vein (data not shown) increased from Rest for the first hour postexercise and was not different (P > 0.05) between groups. Serum insulin (Table 2) was not different (P > 0.05) between groups and showed a time effect for an increase (P < 0.05) at 20, 40, and 60 min postingestion compared with Rest. Compared with Rest, insulin was increased (P < 0.05) at 20 and 40 min postingestion in Blend, increased at 20, 40, and 60 min postingestion in Whey, and decreased 140 min postingestion in Whey.
Table 2.
Serum insulin
| Time Postingestion |
||||||||
|---|---|---|---|---|---|---|---|---|
| Rest | 0 | 20 min | 40 min | 60 min | 80 min | 100 min | 140 min | |
| Blend | 29.1 ± 4.6 | 38.9 ± 11.5 | 66.1 ± 17.4* | 77.5 ± 10.1* | 48.9 ± 6.8* | 31.4 ± 4.4 | 27.2 ± 3.7 | 19.6 ± 2.8 |
| Whey | 24.6 ± 2.3 | 30.0 ± 4.9 | 62.4 ± 11.0* | 70.8 ± 16.8* | 51.9 ± 9.2* | 36.6 ± 6.8 | 23.9 ± 3.1 | 17.3 ± 2.7* |
Values are means ± SE of serum insulin (pmol/l) at Rest (resting period), immediately postexercise (0), and postingestion (min) for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise.
P < 0.05 vs. Rest.
Arterial, venous, and muscle intracellular phenylalanine concentration.
Phenylalanine arterial concentration increased in Blend at Early, 2 h, 3 h, and Entire and in Whey at Early and 2 h compared with Rest (P < 0.05). Phenylalanine venous concentration increased in Blend at Early, 2 h, 3 h, and Entire and in Whey at Early and 2 h compared with Rest (P < 0.05). Intracellular phenylalanine concentrations were similar between groups, and there was an overall effect for a decreased concentration at Late compared with Rest (P < 0.05). There were no differences between groups for arterial, venous, and muscle intracellular phenylalanine concentrations (Table 3).
Table 3.
Phenylalanine concentrations, leg blood flow, and phenylalanine net balance
| Time Postingestion |
|||||||
|---|---|---|---|---|---|---|---|
| Rest | 1–2.5 h (Early) | 2.5–4 h (Late) | 1–2 h | 2–3 h | 3–4 h | 1–4 h (Entire) | |
| Femoral arterial blood phenylalanine concentration, nmol/ml | |||||||
| Blend | 57.2 ± 1.3 | 70.8 ± 2.0a,c | 60.0 ± 2.7 | 74.4 ± 2.4a,d,e | 63.8 ± 2.6a,f | 58.5 ± 3.0 | 65.6 ± 1.9a |
| Whey | 59.0 ± 2.6 | 64.0 ± 3.0a,c | 56.6 ± 2.9 | 68.1 ± 3.0a,d,e | 57.5 ± 2.8 | 56.0 ± 3.1 | 60.6 ± 2.9 |
| Femoral venous blood phenylalanine concentration, nmol/ml | |||||||
| Blend | 62.6 ± 1.7 | 71.2 ± 1.8a,c | 63.0 ± 2.0 | 72.7 ± 2.2a,d,e | 67.2 ± 1.8a,f | 61.3 ± 2.4 | 67.1 ± 1.3a |
| Whey | 63.0 ± 2.5 | 66.3 ± 3.1a,c | 60.0 ± 2.7 | 70.0 ± 3.3a,d,e | 60.8 ± 2.9 | 59.0 ± 2.7 | 63.6 ± 2.9 |
| Leg blood flow, ml·min−1·100 g leg muscle−1 | |||||||
| Blend | 2.41 ± 0.22 | 3.47 ± 0.98 | 3.23 ± 0.49 | 3.57 ± 1.23 | 3.26 ± 0.59 | 3.27 ± 0.46 | 3.37 ± 0.73 |
| Whey | 2.66 ± 0.41 | 3.23 ± 0.52 | 3.79 ± 0.55 | 3.11 ± 0.45 | 3.65 ± 0.64 | 3.75 ± 0.57 | 3.50 ± 0.53 |
| Phenylalanine net balance, nmol·min−1·100 g lean leg mass−1 | |||||||
| Blend | −12.3 ± 1.5 | 2.3 ± 4.1a,c | −8.0 ± 2.4 | 9.3 ± 5.7a,d,e | −8.6 ± 3.7 | −8.1 ± 2.4 | −2.3 ± 2.7a |
| Whey | −10.5 ± 1.8 | −6.5 ± 2.8 | −12.0 ± 4.3 | −3.5 ± 4.4 | −12.7 ± 4.5 | −11.7 ± 4.5 | −9.1 ± 2.8 |
|
2 h |
4 h |
||||||
| Intracellular muscle phenylalanine concentration, nmol/ml | |||||||
| Blend | 68.7 ± 2.8 | 77.0 ± 4.9 | 61.5 ± 3.0b | 69.3 ± 3.2 | |||
| Whey | 70.5 ± 5.7 | 74.6 ± 9.7 | 60.4 ± 4.7b | 67.5 ± 6.3 | |||
Values are means ± SE. Femoral artery and vein blood and intracellular muscle phenylalanine concentration, leg blood flow, and phenylalanine net balance across the leg at rest and postingestion for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise are shown. Comparisons are as follows: Rest vs. Early, Late, Entire, 2 h, 3 h, and 4 h; Early vs. Late; and 2 h vs. 3 h vs. 4 h.
P < 0.05 vs. Rest.
P < 0.05, main effect of time.
P < 0.05, Early vs. Late.
P < 0.05, 2 h vs. 3 h.
P < 0.05, 2 h vs. 4 h.
P < 0.05, 3 h vs. 4 h.
Phenylalanine enrichment.
Arterial TTR was elevated across both groups at Late, 3 h, 4 h, and Entire compared with at Rest (effect of time: P < 0.05; Table 4). This effect was driven largely through an increased arterial TTR in Whey at all postingestion time points compared with Rest (P < 0.05). Venous TTR was elevated across both groups at all postexercise time points compared with Rest (effect of time: P < 0.05). There was a group difference in venous TTR at 3 h (P < 0.05). Muscle TTR was increased across both groups at all postexercise time points compared with Rest (effect of time: P < 0.05) (Table 4). Overall, these data only show minor perturbations in the steady-state conditions at Rest and postexercise conditions, which permitted us to calculate amino acid transport into and out of leg muscle.
Table 4.
Phenylalanine enrichments
| Time Postingestion |
|||||||
|---|---|---|---|---|---|---|---|
| Rest | 1–2.5 h (Early) | 2.5–4 h (Late) | 1–2 h | 2–3 h | 3–4 h | 1–4 h (Entire) | |
| Femoral artery | |||||||
| Blend | 7.92 ± 0.10 | 7.80 ± 0.19 | 8.02 ± 0.21 | 7.77 ± 0.16 | 7.87 ± 0.32 | 7.99 ± 0.23 | 7.88 ± 0.16 |
| Whey | 7.74 ± 0.15 | 8.30 ± 0.28* | 8.42 ± 0.24* | 8.18 ± 0.26* | 8.45 ± 0.24* | 8.42 ± 0.25* | 8.35 ± 0.26* |
| Femoral vein | |||||||
| Blend† | 5.78 ± 0.17 | 6.65 ± 0.18a | 6.62 ± 0.11 | 6.75 ± 0.18 | 6.50 ± 0.18 | 6.68 ± 0.12 | 6.64 ± 0.1 |
| Whey† | 5.97 ± 0.20 | 7.00 ± 0.15a | 7.09 ± 0.21 | 6.92 ± 0.16 | 7.14 ± 0.21‡ | 7.06 ± 0.20 | 7.04 ± 0.18 |
| Intracellular muscle | |||||||
| Blend† | 4.50 ± 0.22 | 5.95 ± 0.14* | 5.98 ± 0.07* | 5.93 ± 0.11* | 5.95 ± 0.06* | 6.02 ± 0.11* | 6.00 ± 0.06* |
| Whey† | 4.69 ± 0.21 | 6.18 ± 0.18* | 5.95 ± 0.28* | 6.13 ± 0.20* | 6.01 ± 0.25* | 5.88 ± 0.32* | 6.10 ± 0.29* |
Values are means ± SE of phenylalanine enrichments as tracer-to-tracee ratio (%) at rest and postingestion for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise. Comparisons are as follows: Rest vs. Early, Late, Entire, 2 h, 3 h, and 4 h.
P < 0.05 vs. Rest.
P < 0.05, main effect of time.
P < 0.05, group effect at time point.
Amino acid transport rates, net balance, and transporter mRNA expression.
Blood flow was not different between groups or across time (P > 0.05; Table 3). Phenylalanine net balance across the entire leg became positive at Early and 2 h and was less negative at 3 h in the Blend compared with Rest (P < 0.05). With the period analysis (Table 3), there was no change in net balance with Whey. In the point analysis (Fig. 2), the net balance became less negative at 0 min in Whey and was positive at only 20 and 40 min postingestion compared with Rest (P < 0.05). With the Blend, the net balance became positive at 20, 40, 60, 80, 100, and 120 min postingestion compared with Rest (P < 0.05). This positive net balance caused an overall time effect at 20, 40, 60, 80, 100, and 120 min postingestion compared with Rest (P < 0.05) in the Blend. There was a group difference and a more positive net balance in Blend than Whey at 60 and 120 min postingestion and a group difference and a more positive net balance in Whey than Blend at 20 min postingestion (P < 0.05). Phenylalanine delivery to the leg was not different between the groups, and there was an overall time effect at Early (P = 0.058), Late, 3 h, 4 h, and Entire compared with Rest (P < 0.05) (data not shown). Phenylalanine release from the leg was not different between the groups, and there was an overall time effect at Late, 3 h (P = 0.054), 4 h, and Entire (P = 0.053) compared with Rest (P < 0.05) (data not shown).
Fig. 2.
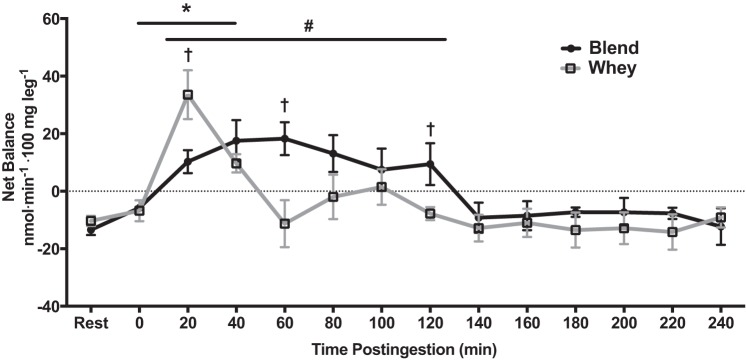
Phenylalanine net balance at Rest (resting period) and during 4 h postingestion for subjects given Whey (whey protein; n = 8) and a Blend (soy-dairy protein blend; n = 8) at 1 h postexercise. Values are means ± SE. *Whey vs. Rest, P < 0.05. #Blend vs. Rest, P < 0.05. †Blend vs. Whey, P < 0.05.
For both groups combined, inward transport of phenylalanine into leg muscle increased at Early, Late, 2 h, and 3 h compared with Rest (effect of time: P < 0.05). Phenylalanine inward transport increased in Blend during Early, 3 h, and Entire and in Whey during Early, 2 h, and Entire compared with Rest (P < 0.05). There were no group differences (P > 0.05). For Blend, outward transport of phenylalanine increased at Early (P = 0.050), Late, 2 h, and 3 h (P = 0.056) compared with Rest (effect of time: P < 0.05). Phenylalanine outward transport increased in Blend during the Early, 3 h, and Entire and in Whey during Early, 2 h, and Entire compared with Rest (P < 0.05). There were no group differences (Table 5 and Fig. 3).
Table 5.
Phenylalanine transport
| Time Postingestion |
||||
|---|---|---|---|---|
| Rest | 1–2 h | 2–3 h | 3–4 h | |
| Inward transport | ||||
| Blend | 83.0 ± 12.1 | 115.2 ± 17.5 | 178.4 ± 53.5* | 124.1 ± 23.2 |
| Whey | 82.1 ± 12.9 | 124.8 ± 14.2* | 104.6 ± 18.4 | 117.8 ± 30.1 |
| Outward transport | ||||
| Blend | 92.3 ± 13.1 | 105.9 ± 15.5 | 187.0 ± 52.7* | 132.2 ± 23.8 |
| Whey | 92.7 ± 14.0 | 128.3 ± 14.3* | 117.3 ± 20.2 | 129.4 ± 29.3 |
Values are means ± SE of phenylalanine transport rates (nmol·min−1·100 g lean leg mass−1) across the muscle membrane at rest and postingestion for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise. Comparisons are as follows: Rest vs. Early, Late, Entire, 2 h, 3 h, and 4 h.
P < 0.05 vs. Rest.
Fig. 3.
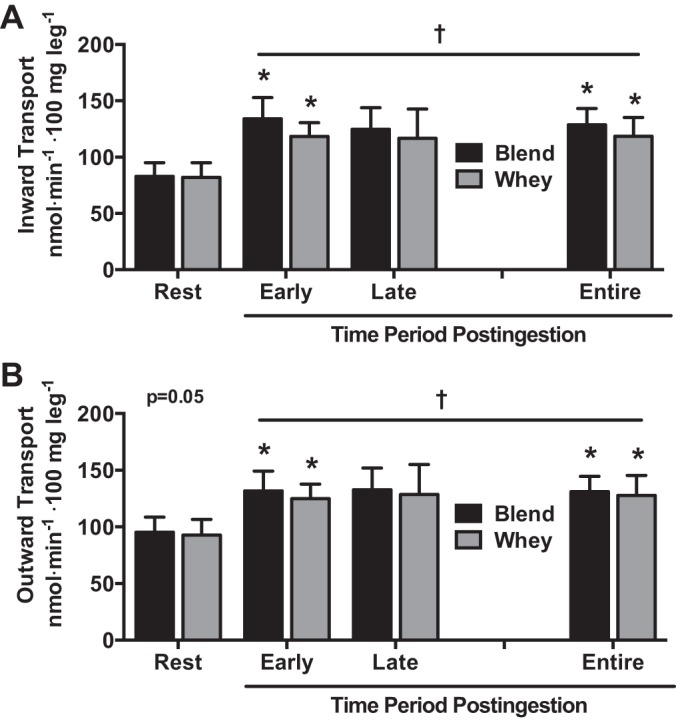
Phenylalanine inward (A) and outward (B) transport averages during Rest, Early (1–2.5 h postingestion), Late (2.5–4 h postingestion), and Entire (1–4 h postingestion) periods for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise. Values are means ± SE. †Effect of time, P < 0.05. *Different from rest, P < 0.05.
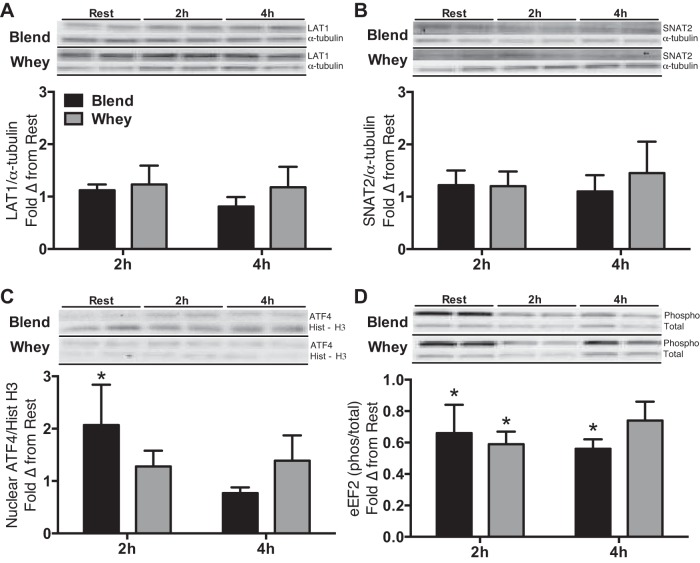
CD98/SLC3A2, PAT1/SLC36A1, and CAT1/SLC7A1 mRNA expression were elevated at 2 and 4 h postingestion compared with Rest for both groups (P < 0.05). LAT1/SLC7A5 mRNA expression was elevated (P < 0.05) at 2 and 4 h postingestion compared with Rest for Blend and only at 2 h for Whey. However, there was a trend (P = 0.06) for LAT1/SLC7A5 mRNA expression to be elevated 4 h postingestion compared with Rest in Whey. SNAT2/SLC38A2 mRNA expression was elevated at 2 h postingestion compared with Rest for both groups (P < 0.05). CAT1/SLC7A1 mRNA expression was greater at 4 h than at 2 h postingestion for both groups (P < 0.05). With Whey only, PAT1/SLC36A1 mRNA expression was greater at 4 h than at 2 h postingestion (P < 0.05; Fig. 4). LAT1 and SNAT2 protein expression was not different (P > 0.05) from Rest at any time point or between groups (Fig. 5). Nuclear ATF4 protein expression (a known regulator of amino acid transporter expression) was not different (P > 0.05) between groups and was only elevated (P < 0.05) from Rest in the Blend at 2 h postingestion. Representative immunoblots for protein expression data are shown in Fig. 5.
Fig. 4.
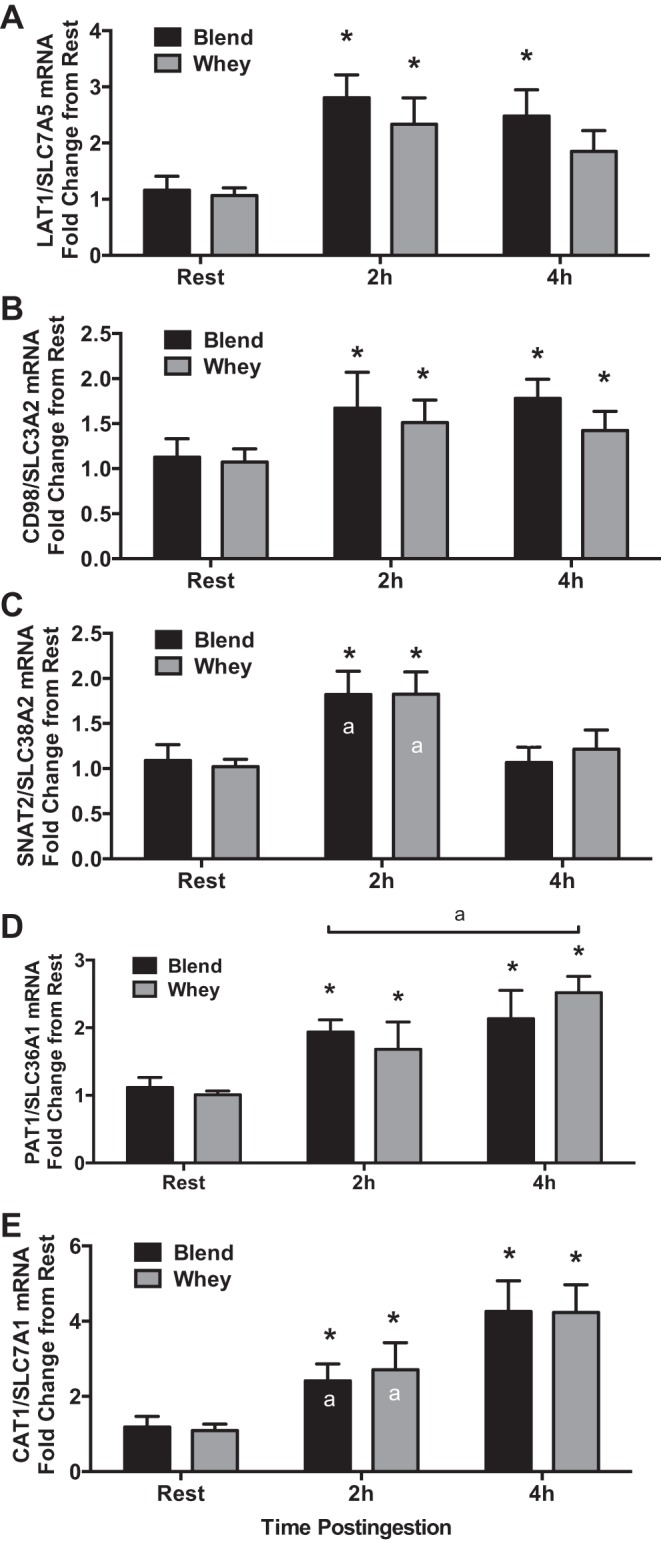
mRNA expression of system L amino acid transporter 1 (LAT1)/solute-linked carrier (SLC) 7A5 (A), CD98/SLC3A2 (B), system A amino acid transporter 2 (SNAT2)/SLC38A2 (C), proton-assisted transporter 1 (PAT1)/SLC36A1 (D), and cationic amino acid transporter 1 (CAT1)/SLC7A1 (E) during Rest, Early (2h), and Late (4h) periods for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise. Values are means ± SE. *Different from rest, P < 0.05. a2 h vs. 4 h, P < 0.05.
Fig. 5.
Fold change (Δ) from rest of protein expression of LAT1 (A), SNAT2 (B), activating transcription factor 4 (ATF4; C), and eukaryotic elongation factor 2 (eEF2; D) phosphorylation in the hours postingestion for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise. Representative immunoblots of protein expression are shown for samples at rest and in the hours postingestion for subjects given Whey and a Blend at 1 h postexercise. All samples were loaded in duplicate. Representative blots for the groups were found on separate blots, yet all samples were derived at the same time and processed in parallel. Values are means ± SE. *P < 0.05 vs. Rest. ATF4 is presented as n = 7 in each group.
Myofibrillar protein synthesis and markers of protein turnover.
Our laboratory has previously shown that both Blend and Whey increase mixed MPS and mTORC1 signaling to a similar extent following resistance exercise (51). However, to confirm that no group differences occurred during postexercise recovery, we compared postexercise myofibrillar protein synthesis rates between Blend and Whey, and whether other markers of protein synthesis (eEF2 phosphorylation) and breakdown [muscle atrophy F-box (MAFbx) and muscle RING (really interesting novel gene) finger (Murf)-1 mRNA] differed between groups. Phosphorylated eEF2 was not different (P > 0.05) between groups, but was reduced (P < 0.05) at 2 and 4 h postingestion with the Blend, but only at 2 h with Whey (Fig. 5). Resting myofibrillar protein synthesis was not different (P = 0.662) between Whey (0.035 ± 0.011%/h) and Blend (0.0413 ± 0.008%/h), so we pooled the resting data. Postexercise myofibrillar protein synthesis increased above resting values in both groups (P < 0.05) and was not different (P = 0.333) between Whey (0.093 ± 0.007%/h) and Blend (0.081 ± 0.009%/h) (Fig. 6). mRNA expression of MuRF-1 was increased at 2h in Blend and 4 h postingestion in both Whey and Blend compared with Rest (P < 0.05). There were no group differences for either MAFbx or MuRF-1 mRNA expression (Fig. 7). mRNA expression of MAFbx was unaltered compared with Rest in both groups (P < 0.05).
Fig. 6.
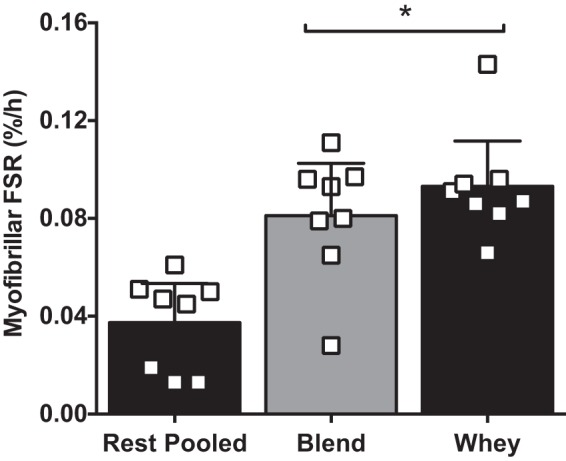
Skeletal muscle myofibrillar fractional synthetic rate (FSR) in the vastus lateralis at rest [pooled from Whey (n = 4) and a Blend (n = 4)] and during the 3–5 h postexercise recovery period for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise. Values are means ± SE. *Different from rest, P < 0.05.
Fig. 7.
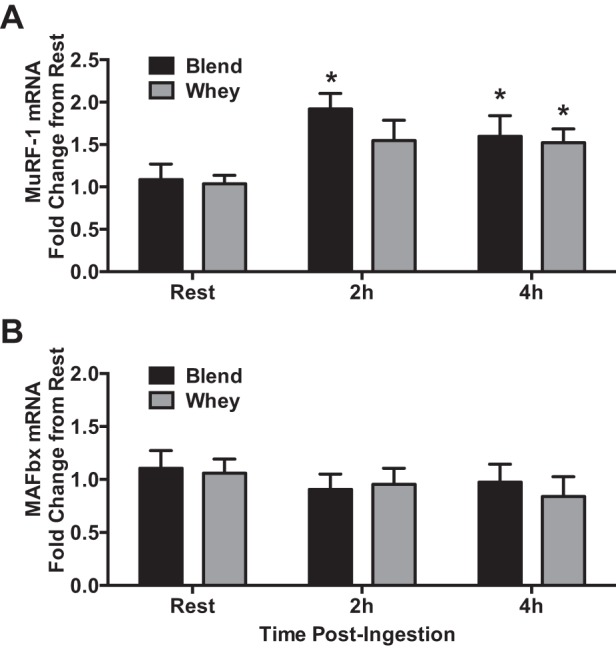
mRNA expression of muscle RING (really interesting novel gene) finger (MURF)-1 (A) and muscle atrophy F-box (MAFbx; B) during Rest, Early, and Late periods for subjects given Whey (n = 8) and a Blend (n = 8) at 1 h postexercise. Values are means ± SE. *Different from rest, P < 0.05.
DISCUSSION
In the present study, we utilized arterial and venous femoral catheterization of the leg and vastus lateralis muscle biopsies during infusion of a stable isotopically labeled amino acid tracer to comprehensively assess several measures of skeletal muscle amino acid transport and muscle protein anabolism in the postexercise recovery period following the ingestion of Blend or Whey. Importantly, we examined how postexercise protein ingestion impacts the immediate postexercise recovery transport kinetics and also the adaptive response to expand the amino acid transporter machinery (mRNA expression). We report two novel findings: 1) increased postexercise phenylalanine net balance (i.e., an indicator of overall muscle protein anabolism) across the leg was prolonged with Blend ingestion during the acute postexercise recovery phase (0–2 h postingestion) compared with Whey; and 2) dietary protein ingestion of Blend and Whey increased postexercise amino acid (phenylalanine) transport into muscle and mRNA expression of amino acid transporters associated with the regulation of mTORC1 signaling and MPS.
Similar to studies with resistance exercise and/or amino acids (4, 5, 9, 50, 60, 61), we observed increased amino acid flux across the muscle cell membrane with the postexercise ingestion of dietary protein. As predicted, the prolonged aminoacidemia in the Blend delayed the amino acid flux to its highest point, 2–3 h postingestion, whereas in Whey it was highest at 1–2 h postingestion. However, both groups experienced a similar increase when the values where averaged over the 1- to 2.5-h and 1- to 4-h periods, which is probably why we did not detect differences between groups in MPS. This transport data and the slight differences in mTORC1 signaling (51) suggest that, although the end result (MPS) could be the same, the mechanism to stimulate MPS may be different. The magnitude of amino acid transport rate was less with dietary protein compared with previous studies using crystalline amino acids (5, 9, 50, 60, 61). Interestingly, Biolo et al. (4) were not able to detect an increase in phenylalanine transport at a similar time following resistance exercise in the fasted state; however, they did demonstrate increased transport of lysine, leucine, and alanine. In a follow-up study (5), these investigators provided an infusion of amino acids in a similar postexercise recovery period and significantly increased amino acid concentrations, particularly phenylalanine, to twice the amount demonstrated in this study with ingestion of dietary protein. This suggests that phenylalanine transport is an effective means to assess the enhanced postexercise protein anabolic response of exogenous amino acids. Further support for this concept, by the same researchers, demonstrated that insulin infusion alone following resistance exercise was insufficient to stimulate phenylalanine transport (6). Although insulin is thought to independently stimulate amino acid transport (1, 45), these reports suggest that postexercise insulin action on MPS and amino acid transport requires excess amino availability in human skeletal muscle (2, 18). Even with these differences in magnitude of the response, we arrive at a similar conclusion: that the increased amino acid availability (from dietary protein) following exercise is likely driving the increased phenylalanine transport in this model.
Changes in amino acid availability stimulate SNAT2/SLC38A2, CAT1/SLC7A1, LAT1/SLC7A5, and CD98/SLC3A2 (22, 25, 43, 67). We found an increase in mRNA expression, from Rest, of select amino acid transporters, (LAT1/SLC7A5, CD98/SLC3A2, SNAT2/SLC38A2, PAT1/SLC36A1, and CAT1/SLC7A1), concomitant with increased mTORC1 signaling and MPS at 3 and 5 h of recovery from resistance exercise, coupled with Whey or Blend ingestion 1 h postexercise. Compared with the previously examined fasted state postexercise response in young adults (21), we see increases in SNAT2/SLC38A2, PAT1/SLC36A1, CD98/SLC3A2, and CAT1/SLC7A1 at 3 h postexercise (2 h postingestion) with Whey and Blend. This further supports the sensitivity of these amino acid transporters to amino acid availability and their possible role in promoting MPS. By 5 h postexercise (4 h postingestion), we demonstrated similar values to the fasted study (21), indicating that the prolonged amino acid availability and mTORC1 signaling in the Blend or the strong initial anabolic signal from Whey did not cause further stimulation via this mechanism. Our laboratory (14) has previously reported that a combination of EAA and exercise stimulate increased expression of similar amino acid transporters (LAT1/SLC7A5, SNAT2/SLC38A2), but not increased CD98/SLC3A2 and PAT1/SLC36A1 mRNA expression as we show in this study. This may be a factor of a difference in the level (20 g EAA vs. ∼9 g) and type [EAA only vs. EAA and non-EAA (NEAA)] of amino acid availability. CAT1/SLC7A1 expression tends to be greater at 3 h postexercise with protein ingestion compared with fasting recovery (21) or 20-g EAA (14), which could be due to the NEAA in the ingested protein.
A recent study showed that 25 g of Whey protein ingestion following resistance exercise increased skeletal muscle amino acid transporter expression above Rest at similar time points (13) compared with our study; however, the fold changes reported in that study were approximately double what we found in our study. Given the sensitivity of these transporter mechanisms to amino acid availability and muscle contraction (4, 5, 14, 19), this may be a reflection of the different dose of protein (25 g Whey from Ref. 13 vs. 20.1 g Whey vs. 17.3 g Blend) or the overall content of leucine (3 g from Ref. 13 vs. 1.9 g) ingested. Preliminary data from our laboratory suggest that, when subjects ingest 10 g of EAA with low (1.8 g) vs. high (3.5 g) amounts of leucine, the high-leucine group exhibited greater stimulation of skeletal muscle amino acid transporter expression (unpublished observations). Interestingly, LAT1/SLC7A5 expression appears to be approximately one- to twofold higher 3 h postexercise (2 h postingestion) when 20-g leucine-enriched EAA are ingested following resistance exercise (14) compared with fasted conditions (21) or here with protein ingestion, suggesting the higher leucine content may be driving this response. Thus it may be that leucine content of a protein source is a key regulator of amino acid transporter expression. In addition, it is likely that increases in amino acid transporter protein expression occurred beyond the 5 h postexercise time point, as observed in our laboratory's previous resistance exercise study (22). Amino acid transporter mRNA expression and amino acid transport kinetics are loosely linked outcomes during the short time frame of our acute study. We propose the changes in mRNA expression and eventual increases in protein expression are likely to have an impact when the muscle is exposed to a subsequent increase in amino acid availability (i.e., the next meal). More research in this area is needed as very little is known regarding the kinetics and functional relevance of amino acid transporters in human muscle biology.
The molecular mechanisms driving the increase in amino acid transporter expression are poorly understood. It has been suggested, from data collected in cell culture studies, that the nuclear transcription factor, AFT4, regulates gene expression of select amino acid transporters (1, 45) in conditions of amino acid deprivation (1), overabundance (44), and presence of insulin (1, 44). However, this relationship is not as pronounced in human skeletal muscle under physiological conditions of crystalline amino acid ingestion (22) or following resistance exercise in the fasted state (21). Here we demonstrate nuclear ATF4 to slightly increase 2 h postingestion of the Blend, which may play a role in promoting the increase in amino acid transporter gene expression. We did not see this same response in Whey, which may be a factor of the biopsy sampling time. Further evidence is needed to determine the role of ATF4 or other transcription factors (e.g., general control nonderepressible 2) in regulating amino acid transporter expression in human skeletal muscle in response to muscle contraction or amino acid availability.
As with previous studies (24, 53, 57–59), we also demonstrated that Whey protein exhibits a rapid increase in amino acid net balance that is short-lived, returning to resting values around the first hour following postexercise ingestion. As a novel feature, in this study, we demonstrated that the Blend had a less rapid rise in net balance across the leg, but was able to prolong a positive net balance to 2 h postingestion. Additionally, the net balance in the Blend was greater than Whey at 60 and 120 min postingestion. This difference between groups could reflect a transient increase in the intracellular amino acid pool, potentially due to a greater reduction in breakdown, in the Blend, during 1–2 h postingestion, which we unfortunately could not accurately assess due to the confounding influences of recent exercise and amino acid flux perturbations. This prolonged net balance is likely due to the intermediate digestion of soy and the prolonged digestion of casein. This prolonged hyperaminoacidemia is not just specific to phenylalanine (which had a slightly higher content in the Blend), but also valine, as our laboratory has previously reported (51). This suggests that a similar effect on amino acid net balance could be occurring with other amino acids besides phenylalanine. As external support of our net balance data, milk (24, 64) or the slowly digested casein (53) can also prolong net balance up to 2 h postingestion. A previous study demonstrated that a blend of fast (whey) and slowly (casein) digested proteins provided as fat-free milk had a prolonged postexercise net balance compared with a single protein provided as soy milk (64). This provides further evidence that combining proteins with varying digestion rates can sustain the postexercise net protein balance.
We also examined two key markers of muscle protein breakdown, the E3 ligases MuRF-1 and MAFbx. We found similar expression patterns for both atrogenes with Blend or Whey ingestion 3 and 5 h following resistance exercise. Although MuRF-1 was upregulated in both groups, the expression level was approximately twofold less than what we have reported following resistance exercise in the fasted state (27). As mentioned earlier, in reference to early net balance differences, any potential difference in breakdown between the groups may have occurred sometime before 3 h postexercise (2 h postingestion). Thus, after this time, the mRNA data suggest that the additional amino acid supply and/or equivalent insulin stimulus in both Blend and Whey were effective in reducing markers associated with postexercise muscle protein breakdown, which may also be an important part in the overall muscle protein turnover response to exercise combined with postexercise protein intake.
Similar to our laboratory's previous report with mixed MPS (51), we found no difference in postexercise myofibrillar protein synthesis between Whey and Blend. As we have previously suggested (51), a blend of proteins with different digestion rates and prolonged aminoacidemia may have a different cellular response, but a similar effect (MPS) compared with a bolus of whey protein when matched for leucine. In the Blend (soy, whey, and casein), it seems likely that there is an initial anabolic signal generated with initial whey digestion, albeit weaker than only a whey bolus, that is prolonged with stimulation from slower released soy and casein. These postexercise rates of myofibrillar protein synthesis are comparable to those reported elsewhere for dietary protein ingestion following resistance exercise (37). Given the divergent results regarding muscle protein anabolism between net phenylalanine balance across the leg and vastus lateralis protein synthesis, it is important to note key differences in these methods: 1) the temporal differences in myofibrillar protein synthesis were assessed during the later period of recovery, 2–4 h postingestion at a time when net balance was similar between groups; and 2) net balance assesses uptake of phenylalanine in all the muscles of the leg, irrespective of the potential protein(s) being synthesized, which experience different or in some instances no activation with exercise, whereas the precursor product assessment of myofibrillar protein synthesis is only specific to the activity of that protein fraction in the vastus lateralis. Some of the mechanisms for the postexercise muscle protein anabolism with protein blend ingestion are likely increased translation initiation, as our laboratory has reported (51), but also increased translation elongation, as suggested by the decreases in eEF2 phosphorylation demonstrated in this study. These data offer further support for the hypothesis that a blend of protein with different digestion rates and prolonged aminoacidemia may have a different cellular response, but a similar effect (MPS) to that of rapidly digested whey.
When researchers supply a dose of protein well above the leucine threshold, the amount of leucine probably has little additional effect on rates of MPS, which have already been maximized (46). On the other hand, because we did not oversupply protein (∼20 g protein; 9 g EAA), we believe that matching the leucine content is essential in our investigation as leucine content plays a very important role in regulating MPS. Two recent studies have elegantly demonstrated that the leucine content in a supplement is a primary stimulator of MPS, especially when the total protein or content of other amino acids is low (12, 13). By matching the proteins according to leucine content, we ended up with a difference in total protein and calories between the ingested proteins. The difference in total protein ingested (<3 g) was very minimal and is mostly composed of NEAA, which does not stimulate MPS. We have demonstrated that adding 120 kcal does not further stimulate muscle protein anabolism when sufficient EAA are provided (31). Thus the 10- to 20-kcal difference in total energy (in this study) is unlikely to have influenced the response.
Limitations to the study are as follows. 1) We did not assess the kinetics of other amino acids following resistance exercise, which could be variable (4). 2) Due to the challenges of maintaining an isotopic steady state with multiple perturbations in kinetic parameters following the combination of exercise and dietary protein ingestion, we could only assess a later (2–5 h) postexercise period, not the immediate postexercise period (0–2 h), without violating the assumptions of our stable isotopic model for calculating amino acid transport rates. As such, this only allowed us to accurately calculate the transport model parameters of inward and outward transport. However, the measurement of the rate of amino acid transport into leg muscle is the focus of our study and a novel means to investigate the effects of dietary protein.
In summary, we found that the increase in postexercise phenylalanine net balance across the leg (an indicator of muscle protein anabolism) was prolonged with ingestion of a protein blend compared with whey protein. We also report that ingesting a protein blend or whey protein enhances the rate of amino acid transport into muscle, increases select amino acid transporter (LAT1/SLC7A5, CD98/SLC3A2, SNAT2/SLC38A2, PAT1/SLC36A1, CAT1/SLC7A1) mRNA expression, and increases postexercise myofibrillar protein synthesis. These results provide further support for the efficacy of ingesting a protein blend to increase and prolong postexercise muscle protein anabolism. Further research is necessary to determine the efficacy of protein blend supplementation on muscle growth and strength during chronic resistance exercise training.
GRANTS
This study was supported by grants from Solae LLC, now DuPont Nutrition & Health, and National Institutes of Health Grants T32-HD-07539, P30 AG-024832, and R01 AR-049877.
DISCLOSURES
This study was primarily funded by Solae, LLC. Two of the authors are employees of Solae, LLC (M. B. Cope and R. Mukherjea). However, the corresponding author had complete control of the study design, analyses, and interpretation of the data. The paper is objective and not biased toward industry.
AUTHOR CONTRIBUTIONS
Author contributions: P.T.R., M.B.C., R.M., K.J., E.V., and B.B.R. conception and design of research; P.T.R., D.K.W., J.M.D., D.M.G., M.J.D., K.L.T., E.V., and B.B.R. performed experiments; P.T.R., K.J., E.V., and B.B.R. analyzed data; P.T.R., J.M.D., M.J.D., M.B.C., R.M., E.V., and B.B.R. interpreted results of experiments; P.T.R. prepared figures; P.T.R. drafted manuscript; P.T.R., D.K.W., J.M.D., D.M.G., M.J.D., K.L.T., M.B.C., R.M., K.J., E.V., and B.B.R. edited and revised manuscript; P.T.R., D.K.W., J.M.D., D.M.G., M.J.D., K.L.T., M.B.C., R.M., K.J., E.V., and B.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our subjects, as well as the nurses and staff at the Institute for Translational Sciences-Clinical Research Center for assistance in screening, admitting, and assisting the subjects during data collection. Also we extend our thanks to Dr. Denis Gore for assistance with placement of central lines, Shaheen Dhanani for assistance with recruitment, and also Ming Zheng and Shelley Medina for technical assistance.
Present addresses: D. K. Walker, Center for Translational Research in Aging & Longevity, Texas A&M University, College Station, TX 77843; M. J. Drummond, Department of Physical Therapy, University of Utah, Salt Lake City, Utah, 84108; J. M. Dickinson, Department of Exercise and Wellness, Arizona State University, Phoenix, Arizona, 85004; K. L. Timmerman, Department of Kinesiology and Health, Miami University, Oxford, OH 45056.
REFERENCES
- 1.Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem 282: 16744–16753, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab 289: E999–E1006, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab 268: E75–E84, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273: E122–E129, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 48: 949–957, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab 14: 255–271, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol 96: 674–678, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab 283: E648–E657, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol 106: 1692–1701, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 6: 421–424, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99: 276–86, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 590: 2751–2765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickinson JM, Drummond MJ, Coben JR, Volpi E, Rasmussen BB. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr 32: 273–280, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickinson JM, Rasmussen BB. Essential amino acid sensing, signaling, and transport in the regulation of human muscle protein metabolism. Curr Opin Clin Nutr Metab Care 14: 83–88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond MJ, Bell JA, Fujita S, Dreyer HC, Glynn EL, Volpi E, Rasmussen BB. Amino acids are necessary for the insulin-induced activation of mTOR/S6K1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin Nutr 27: 447–456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 302: E1113–E1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106: 1374–1384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 111: 135–142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E1011–E1018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signaling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care 11: 222–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc 38: 667–674, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol 18: 1426–1436, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1: 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 68: 599–607, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab 292: E77–E83, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gislason SR, Hassenkam T, Nedel S, Bovet N, Eiriksdottir ES, Alfredsson HA, Hem CP, Balogh ZI, Dideriksen K, Oskarsson N, Sigfusson B, Larsen G, Stipp SL. Characterization of Eyjafjallajokull volcanic ash particles and a protocol for rapid risk assessment. Proc Natl Acad Sci U S A 108: 7307–7312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance exercise decreases eIF2B epsilon phosphorylation and potentiates the feeding-induced stimulation of p70S6K1 and rpS6 in young men. Am J Physiol Regul Integr Comp Physiol 295: R604–R610, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Glynn EL, Fry CS, Timmerman KL, Drummond MJ, Volpi E, Rasmussen BB. Addition of carbohydrate or alanine to an essential amino acid mixture does not enhance human skeletal muscle protein anabolism. J Nutr 143: 307–314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goberdhan DC. Intracellular amino acid sensing and mTORC1-regulated growth: new ways to block an old target? Curr Opin Investig Drugs 11: 1360–1367, 2010 [PMC free article] [PubMed] [Google Scholar]
- 33.Goberdhan DC, Meredith D, Boyd CA, Wilson C. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development 132: 2365–2375, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol 299: R1254–R1262, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene 29: 4068–4079, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 37: 297–308, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296: E603–E613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19: 461–463, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism 27: 97–106, 1978 [DOI] [PubMed] [Google Scholar]
- 40.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 41: 459–473, 1971 [DOI] [PubMed] [Google Scholar]
- 41.Kraemer WJ, Hatfield DL, Volek JS, Fragala MS, Vingren JL, Anderson JM, Spiering BA, Thomas GA, Ho JY, Quann EE, Izquierdo M, Hakkinen K, Maresh CM. Effects of amino acids supplement on physiological adaptations to resistance training. Med Sci Sports Exerc 41: 1111–1121, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Lopez AB, Wang C, Huang CC, Yaman I, Li Y, Chakravarty K, Johnson PF, Chiang CM, Snider MD, Wek RC, Hatzoglou M. A feedback transcriptional mechanism controls the level of the arginine/lysine transporter cat-1 during amino acid starvation. Biochem J 402: 163–173, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo JQ, Chen DW, Yu B. Upregulation of amino acid transporter expression induced by l-leucine availability in L6 myotubes is associated with ATF4 signaling through mTORC1-dependent mechanism. Nutrition 29: 284–290, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Malmberg SE, Adams CM. Insulin signaling and the general amino acid control response. Two distinct pathways to amino acid synthesis and uptake. J Biol Chem 283: 19229–19234, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587: 897–904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul GL. The rationale for consuming protein blends in sports nutrition. J Am Coll Nutr 28, Suppl: 464S–472S, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Poncet N, Taylor PM. The role of amino acid transporters in nutrition. Curr Opin Clin Nutr Metab Care 16: 57–65, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88: 386–392, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Fry CS, Borack MS, Cope MB, Mukherjea R, Jennings K, Volpi E, Rasmussen BB. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr 143: 410–416, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, Kjaer M, Holm L. Whey and casein labeled with l-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab 300: E231–E242, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Schjerling P, van Hall G, Kjaer M, Holm L. Positive muscle protein net balance and differential regulation of atrogene expression after resistance exercise and milk protein supplementation. Eur J Nutr 53: 321–33, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 107: 987–992, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care 12: 66–71, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Taylor PM. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr 99: 223S–230S, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tipton KD, Elliott TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab 292: E71–E76, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 36: 2073–2081, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Tipton KD, Elliott TA, Ferrando AA, Aarsland AA, Wolfe RR. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl Physiol Nutr Metab 34: 151–161, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab 276: E628–E634, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab 281: E197–E206, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78: 250–258, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, Gundermann DM, Rasmussen BB. Exercise, Amino Acids and Aging in the Control of Human Muscle Protein Synthesis. Med Sci Sports Exerc 43: 2249–2258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 85: 1031–1040, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Willoughby DS, Stout JR, Wilborn CD. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 32: 467–477, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley-Liss, 2005, p. vii, 474 [Google Scholar]
- 67.Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto K, Takeda E, Goya T, Endou H. Human l-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 1514: 291–302, 2001 [DOI] [PubMed] [Google Scholar]