Abstract
We report here the existence of anaerobic nitrogen-fixing consortia (ANFICOs) consisting of N2-fixing clostridia and diverse nondiazotrophic bacteria in nonleguminous plants; we found these ANFICOs while attempting to overcome a problem with culturing nitrogen-fixing microbes from various gramineous plants. A major feature of ANFICOs is that N2 fixation by the anaerobic clostridia is supported by the elimination of oxygen by the accompanying bacteria in the culture. In a few ANFICOs, nondiazotrophic bacteria specifically induced nitrogen fixation of the clostridia in culture. ANFICOs are widespread in wild rice species and pioneer plants, which are able to grow in unfavorable locations. These results indicate that clostridia are naturally occurring endophytes in gramineous plants and that clostridial N2 fixation arises in association with nondiazotrophic endophytes.
Microbes are not always culturable even though their biological activities may be detectable (1, 15, 20). This is true for some N2-fixing bacteria associated with plants, such as Azoarcus endophytes (16) and rhizobial bacteroids (22). Although the functional significance of microbial consortia in biofilms, for example (1, 3), has been emphasized, there are few concrete examples of their specific functions.
The availability of fixed nitrogen limits primary productivity in plant ecosystems. During their evolution, legumes have acquired a symbiotic relationship with rhizobia that fix atmospheric nitrogen. Among nonleguminous plants, several diazotrophs have been isolated and characterized as nitrogen-fixing endophytes, including Acetobacter (18), Azoarcus (11, 16), and Herbaspirillum (6, 8). Endophytes are microorganisms that spend most of their life cycles inside plant tissues without causing symptoms of plant damage (16). We still do not know whether these diazotrophic endophytes contribute substantially to the nitrogen economy of grasses (11, 12). It is possible that we have overlooked the real contributors to nitrogen fixation in nonleguminous plants. Indeed, nitrogenase transcript analysis has indicated that endophytes, such as Azoarcus sp. and others in an apparently unculturable state, fix nitrogen in plants (11).
Wild grasses can often grow in nitrogen-deficient soils, suggesting that functioning diazotrophic bacteria are associated with them. We therefore tried to isolate and characterize diazotrophic bacteria associated with wild rice species in situ and pioneer plants growing on a devastated lahar area with volcanic eruptions. For this work, we used mainly the aerial parts of plants as isolation materials to avoid bacterial contamination from soils. During efforts to isolate endophytic diazotrophs from these plants, we faced problems with unculturable diazotrophic bacteria and found an anaerobic nitrogen-fixing consortium (ANFICO) consisting of N2-fixing clostridia and diverse nondiazotrophic bacteria. The objective of this work was to clarify the members of ANFICOs and their interactions.
MATERIALS AND METHODS
Isolation of nitrogen-fixing bacteria.
Plants were surface sterilized with 70% ethanol (0.5 to 1 min) and 1 to 2% NaOCl (0.5 to 15 min) according to the age of the plant and the type of plant tissue, washed with sterilized water, and macerated with a mortar, a pestle, and sterilized quartz sand. Serial dilution series were made with sterile 0.85% saline and were inoculated onto a semisolid medium of rice extract modified Rennie (RMR) (6, 17). When it was impossible to prepare macerated plant material at sampling places, surface-sterilized stems were introduced directly into semisolid RMR medium. After 1 week of incubation at 30°C, most-probable-number (MPN) counting of diazotrophic bacteria was conducted by use of acetylene reduction assays, as described previously (6). An aliquot of acetylene reducing activity (ARA)-positive culture was spread on nutrient agar (NA) (Difco, Detroit, Mich.), Viande-Levure (VL) agar, and RMR agar (6) plates. VL medium contained the following components dissolved in 1 liter of water (pH 7.0): 8 g of nutrient broth (Difco), 5 g of yeast extract (Difco), 5 g of NaCl, 2 g of glucose, and 0.3 g of cysteine-HCl. The NA plates were incubated aerobically, whereas the VL and RMR agar plates were incubated anaerobically at 30°C for 3 days by use of the AnaeroPack system (Mitsubishi Gas Chemical, Tokyo, Japan). For the selection of spore-forming anaerobes, full growth cultures were heated at 70°C for 10 min and treated with 50% ethanol for 45 min (4). Colonies with different appearances grown on each medium were subcultured on NA or VL agar plates. Aliquots of saline suspensions of isolates were inoculated into test tubes containing semisolid RMR medium, vortexed for 5 s, incubated for 3 to 4 days at 30°C, and assayed for ARA for 24 h.
Microscopy.
Cultures were stained with 5 μg of DAPI (4′,6-diamidino-2-phenylindole) solution/ml for 10 min and then were observed by light microscopy (BX50 microscope; Olympus, Tokyo, Japan). Hucker's modified method was used to determine Gram staining characteristics, as described previously (7). Cell viability was visualized by use of a Live/Dead bac Light bacterial viability kit (L-7007; Molecular Probes, Eugene, Oreg.) and was observed by fluorescence microscopy (Axioplan 2 microscope; Carl Zeiss, Tokyo, Japan).
Phylogenetic analysis and substrate utilization.
A nearly-full-length 16S rRNA gene was amplified and sequenced as previously described (6). Multiple alignments and phylogenetic analyses were performed with the Clustal W program, as described previously (6). The utilization of substrates was examined by use of a bacterial identification kit (API20A; BioMerieux, Tokyo, Japan).
Oxygen tests.
Clostridium sp. strain B901-1b was inoculated into RMR broth and incubated, with shaking, for 21 h at 30°C with various oxygen concentrations (0.0 to 0.7% [vol/vol]) in 123-ml bottles, at a gas-to-liquid ratio of 39:1. Isolates B901-1b and B901-2 were inoculated into RMR broth in different arms of a Y-shaped test tube with a butyl-rubber stopper and were incubated at 30°C for 3 days. H2 and O2 concentrations in the headspace were determined with a gas chromatograph equipped with a thermal conductivity detector (GC-7A; Shimadzu, Kyoto, Japan). ARA was determined in the presence of 5% (vol/vol) acetylene as described previously (6).
N2 fixation of clostridia with nondiazotrophs and culture filtrate.
The clostridia and nondiazotrophs isolated in this work were coinoculated into test tubes containing semisolid RMR medium and were incubated for 3 to 4 days at 30°C in air. Culture filtrates of nondiazotrophs were prepared by centrifugation (8,000 × g for 15 min) and passaging through a sterile membrane filter (DISMIC-25; 0.20-μm pore size) (Advantec, Tokyo, Japan) after cultivation of the bacteria in RMR broth for 3 days. Subsequently, anaerobically grown cells of Clostridium sp. strain Kas107-2 were inoculated into RMR broth containing the culture filtrates and were incubated anaerobically for 72 h at 30°C. The N2-fixing activity was determined by an acetylene reduction assay performed for 24 h as described above (6).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study appear in the DDBJ database under accession numbers AB114225 to AB114271.
RESULTS AND DISCUSSION
N2-fixing consortium.
By using the MPN method and a semisolid RMR medium, we detected substantial numbers of nitrogen-fixing bacteria (7.4 × 103 to 1.5 × 105) in several surface-sterilized stems of wild rice species and in situ for the pioneer plants Miscanthus sinensis and Saccharum spontaneum (data not shown). We then performed single-colony isolation on RMR agar plates to try to isolate aerobically those diazotrophic bacteria from the MPN series and original cultures that showed N2-fixing activity (ARA). This procedure succeeded for the isolation of Herbaspirillum sp. and Azospirillum sp. from Oryza sp. reserved in Japan (6). However, the single-colony isolates were incapable of N2-fixing activity in the medium. Because there was N2-fixing activity in the original, mixed culture, we hypothesized that the microbial community supported the expression of N2 fixation by diazotrophic bacteria.
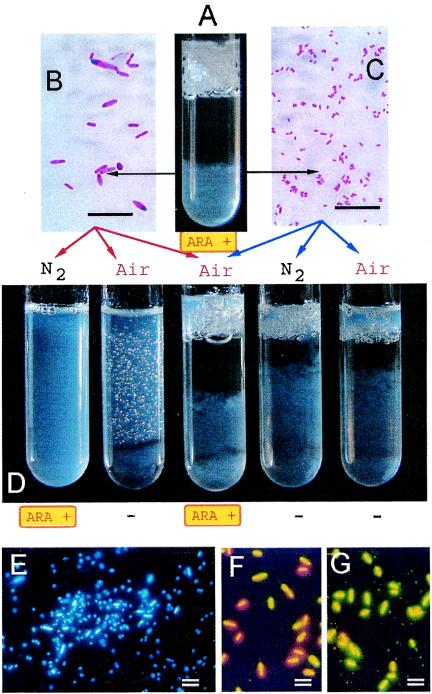
We isolated various bacteria aerobically or anaerobically from the original cultures exhibiting N2-fixing activity. After cocultivating random combinations of two different isolates in semisolid RMR medium, we found that N2-fixing activity appeared in a specific combination of an anaerobic isolate with an aerobic isolate in air. An example of this procedure with an Oryza officinalis stem is shown in Fig. 1A to D. The anaerobic isolate B901-1b (Fig. 1B) exhibited N2-fixing activity in semisolid RMR medium upon cocultivation under aerobic conditions (Fig. 1D, air) as well as upon single cultivation under anaerobic conditions (Fig. 1D, N2 gas). On the other hand, the aerobic isolate B901-2 (Fig. 1C) alone showed no N2-fixing activity in air or N2 gas (Fig. 1D). The anaerobic isolate of B901-1b alone did not grow in the oxygen-limited semisolid medium in air (Fig. 1D), suggesting that a strict anaerobic condition is required for the growth of this bacterium. This explains why we failed to isolate nitrogen-fixing bacteria from the original cultures. Because these bacteria differ morphologically (Fig. 1B and C), we observed them in cocultures. At the bottom of the test tube, the large rod-shaped cells of B901-1b and the small spherical cells of B901-2 formed a microconsortium in the culture (Fig. 1E).
FIG. 1.
N2-fixing activity (ARA) during isolation steps and fluorescence micrographs showing the structure and viability of consortia of the cells. (A) Test tube that is positive for ARA during isolation from a stem of O. officinalis. (B) Anaerobic isolate B901-1b after Gram staining. (C) Aerobic isolate B901-2 after Gram staining. (D) Growth and ARA of singly and cocultured B901-1b and B901-2 in air and N2 gas. ARA was detected exclusively in a single culture of B901-1b in N2 gas and in a mixed culture in air. Gas evolution and growth occurred in test tubes, except for the single culture of B901-1b in air, which sometimes caused an accumulation of agar in the uppermost layer of the medium. ARA+, test tubes that were positive for ARA (16 to 24 nmol of ethylene produced h−1 tube−1); −, <0.1 nmol of ethylene produced h−1 tube−1. (E) Fluorescence micrograph showing a reconstructed consortium. (F and G) Living (green) and dead (red) cells of Clostridium sp. strain B901-1b (large rod) cultured in RMR broth under anaerobic conditions (F) and under aerobic conditions with the accompanying bacterium Enterobacter sp. strain B901-2 (small coccus) (G). Both preparations were exposed to air for 5 min before observation. Bar, 10 μm. According to their 16S rRNA gene sequences, B901-1b and B901-2 were identified as a Clostridium sp. and an Enterobacter sp., respectively (see Fig. 3).
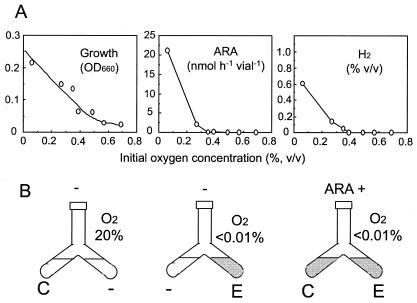
Effect of O2 on N2-fixing consortium.
Because O2 was probably a key to the formation of the N2-fixing consortium during the isolation steps (Fig. 1A to D), we examined the effects of O2 tension on growth, N2-fixing activity, and H2 production as reductant exhaust by fermentation. Anaerobic isolate B901-1b propagated, emitted H2, and showed N2 fixation with O2 concentrations of <0.4% (vol/vol) in the gas phase over the liquid culture (Fig. 2A). A separate culture experiment in a Y-shaped test tube sharing a common gas phase indicated that B901-1b was able to grow and fix nitrogen after the accompanying bacteria had eliminated the O2 by respiration (Fig. 2B). This O2-mediated growth of clostridia with the accompanying bacteria was supported by the microscopic observation that the viability of B901-1b in air varied with and without the accompanying bacteria, as shown in Fig. 1G and F, respectively. These results show that a major process that supports the growth and N2-fixing activity of the anaerobic isolate B901-1b is oxygen elimination by the accompanying bacteria, which enables the consortium to fix nitrogen in a seemingly aerobic environment (Fig. 1A to D). We therefore termed this an ANFICO. When we surveyed references describing the isolation of N2-fixing anaerobes from the environment, Line and Loutit (14) faced a phenomenon similar to the ANFICO in this work and briefly reported N2-fixing clostridia in mixed cultures derived from soils.
FIG. 2.
Effect of O2 concentration on growth, N2-fixing activity (ARA), and hydrogen evolution by Clostridium sp. strain B901-1b (A) and synergetic effects of separate cultures of B901-1b and B901-2 on N2-fixing activity (B). C and E, inoculation of Clostridium sp. strain B901-1b and Enterobacter sp. strain B901-2, respectively. Gray shading shows bacterial growth after 4 days. ARA+, positive for ARA (106 nmol of ethylene produced h−1 tube−1); −, no ARA (<0.1 nmol of ethylene produced h−1 tube−1).
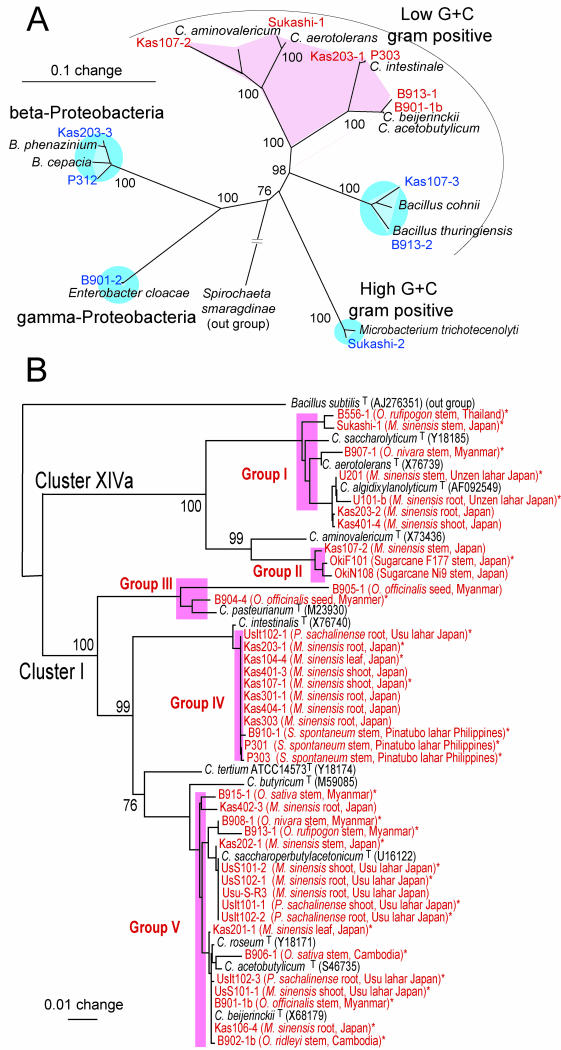
Phylogeny of members of ANFICO.
Once we had clarified the principle of ANFICOs, we were easily able to isolate them from a variety of plants. First, we determined the phylogenetic positions of 10 representative ANFICOs on the basis of their 16S rRNA gene sequences. The anaerobic nitrogen-fixing isolates fell into the cluster of the genus Clostridium, whereas the nondiazotrophic accompanying bacteria were phylogenetically dispersed in the β- and γ-Proteobacteria and the high-C+G-content and low-G+C-content gram-positive lineages (Fig. 3A). The 16S rRNA gene sequences of nondiazotrophic isolates B901-2 and Kas107-3 showed 99% homology to those of Enterobacter cloacae (Y17665) and Bacillus megaterium (AF142677) across a 1.45-kb region. Thus, B901-2 and Kas107-3 were identified as being an Enterobacter sp. and a Bacillus sp., respectively. Both bacteria were used as representatives of accompanying, nondiazotrophic bacteria for this work.
FIG.3.
Phylogenetic tree of anaerobic nitrogen-fixing bacteria (red) and accompanying bacteria (blue) from various origins and representative close relatives by 16S rRNA gene sequences. (A) Representative members of ANFICOs. Pairs of anaerobic N2-fixing bacteria and accompanying bacteria (source) are Kas107-2-Kas107-3 (Miscanthus sinensis stem), Sukashi-1-Sukashi-2 (M. sinensis stem), Kas203-1-Kas203-3 (M. sinensis root), P303-P312 (S. spontaneum stem), B913-1-B913-2 (Oryza rufipogon stem), and B901-1b-B901-2 (O. officinalis stem). (B) Tree of 40 isolates of anaerobic nitrogen-fixing bacteria from various pioneer plants and wild rice species, including M. sinensis, S. spontaneum (wild sugarcane), Polygonum sachalinense, Saccharum hybrid sp. (sugarcane), Oryza sativa (cultivated rice), and O. rufipogon, Oryza nivara, O. officinalis, and Oryza redleyi (wild rice species). In parentheses are details of the plant, tissue, and location of isolation. Clusters I and XIVa are phylogenetic clusters of the genus Clostridium (5). The trees are based on >1.2 kb of DNA sequences and were constructed by the neighbor-joining method. Bootstrap values (percentages from 1,000 replications) are indicated. The utilization of carbon sources was tested for 30 isolates, which are indicated with asterisks.
Because the genus Clostridium is heterogeneous, with many species (4, 5, 9), we performed a phylogenetic analysis of 40 anaerobic N2-fixing isolates from various origins. N2-fixing isolates fell exclusively into clusters I and XIVa among the 17 clusters of Clostridium spp. defined by Collins et al. (5), indicating that all of the anaerobic isolates in ANFICOs belong to the genus Clostridium (Fig. 3B). Indeed, the 16S rRNA gene sequences of all of the clostridial isolates, except that of isolate U101b, possessed >95% homology to those of known Clostridium species (data not shown).
The clostridial isolates were further subdivided into groups I and II in cluster XIVa and groups III, IV, and V in cluster I on the basis of a phylogenetic tree of their 16S rRNA gene sequences (Fig. 3B). Groups I, III, and V contained known species of Clostridium, such as C. saccharolyticum, C. pasteurianum, and C. acetobutylicum, respectively. In contrast, groups II and IV did not include known species of the genus Clostridium (Fig. 3B).
Four nutritional groups of clostridia have been distinguished, namely saccharolytic, proteolytic, saccharolytic and proteolytic, and specialist clostridia (9). When 30 isolates of clostridia (Fig. 3, asterisked isolates) were tested with an API20A bacterial identification kit, all isolates utilized various sugars, such as glucose, cellobiose, and mannose, but they did not utilize gelatin. These nutritional traits indicated that they are saccharolytic clostridia, which is seemingly suitable to their life in plants.
Ubiquitous distribution of clostridia in plants.
On the basis of our examination of the phylogenetic tree, the clostridial isolates were divided into two clusters (clusters XIVa and I) and five groups (groups I, II, III, IV, and V) (Fig. 3B). These clusters and groups were not clearly correlated with the plant species (Oryza sp., M. sinensis, S. spontaneum, or Polygonum sachalinense), plant tissue (stem, seed, leaf, or root), or location of isolation (Japan, Thailand, Myanmar, Philippines, or Cambodia). In contrast, the mosaic distribution of clostridial isolates from diverse origins on the phylogenetic tree excluded the possibility of spontaneous contamination. Clostridia were generally isolated from the most diluted tube showing N2 fixation in the MPN counting series under conditions of strong surface sterilization. For example, the clostridial population was estimated to be at least 104 cells/g of fresh weight in M. sinensis sampled in June 2001 at Kashimadai, Miyagi, Japan. These results strongly suggest that clostridia are naturally occurring bacteria in the shoots and roots of pioneer plants and wild rice species.
Table 1 summarizes the N2-fixing activities in semisolid RMR medium of cocultures of random combinations of two different isolates from various origins. Since we sought diazotrophic microbes and their consortia by using ARA, the mixed cultures of clostridial isolates and appropriate nondiazotrophs always exhibited the capability for N2 fixation. In contrast, single cultures of clostridia showed almost no N2- fixing activity in semisolid RMR medium in air. These results demonstrate the dependence of the N2-fixing activity of clostridia on nondiazotrophs in culture and their unculturability by conventional methodologies. Nevertheless, six clostridial isolates showed weak activities of N2 fixation in single cultures in semisolid RMR medium in air. Interestingly, they were confined to group IV (Table 1 and Fig. 3). It is possible that the clostridia in group IV are more tolerant to O2 to some extent than are the other clostridia.
TABLE 1.
N2-fixing activities of single cultures and cocultures of clostridial isolates with accompanying bacteria in semisolid RMR medium
| Phylogenetic groupb | Clostridial isolate | ARA (nmol of ethylene produced h−1 tube−1)a
|
||
|---|---|---|---|---|
| Single culturec
|
Coculture with aerobic, nondiazotrophic isolate in aird | |||
| Air | Anoxic | |||
| I | U201 | ND | ++ | + (U221) |
| U101 | ND | ++ | +++ (U111) | |
| B907-1 | ND | + | +++ (B907-2) | |
| B556-1 | ND | ++ | +++ (B556-2), ND (B556-3) | |
| Kas401-4 | ND | ++ | +++ (Kas403-3), +++ (Kas401-2), +++ (Kas401-1), ++ (Kas403-2) | |
| Sukash-1 | ND | ++ | ++ (Sukash-2) | |
| II | Kas107-2 | ND | ND* | ++ (Kas107-3), + (Kas103-2), ND (Kas105-5), ND (Kas105-6), ND (Kas107-4) |
| OkiF101 | ND | ND* | ++ (OkiF105), ND (OkiF102), ND (OKiF103), ND (OKiF104) | |
| OkiN108 | ND | ND* | ++ (OkiN104), ++ (OkiN106), ++ (OkiN102), ++ (OkiN105), ++ (OKiN103), ND (OKiN107) | |
| III | B904-4 | ND | ++ | ++ (B904-3), ++ (B904-1), ++ (B904-2) |
| IV | Kas107-1 | ++ | +++ | +++ (Kas105-5), ++ (Kas107-4), ++ (Kas107-3), ++ (Kas105-6), + (Kas103-2) |
| UsIt102-1 | + | ++ | ++ (UsuIR-1), ++ (UsuIR-2) | |
| Kas301-1 | ++ | ++ | +++ (Kas302) | |
| Kas203-1 | ND | +++ | ++ (Kas203-3), ++ (Kas203-4), + (Kas203-5) | |
| Kas104-4 | + | ++ | +++ (Kas102-2), + (Kas104-5) | |
| Kas401-3 | + | +++ | ++ (Kas403-3), ++ (Kas401-2), + (Kas401-1), +(Kas403-2) | |
| Kas404-1 | + | ++ | ++ (Kas404-3), ++ (Kas404-4), ++ (Kas404-2), ND (Kas402-1), ND (Kas402-2) | |
| B910-1 | ND | ++ | ++ (B910-5), ++ (B910-4M), ++ (B910-3T), ++ (B910-3W), ++ (B910-4S) | |
| P303 | ND | ++ | +++ (P312), ++ (P311) | |
| P301 | ND | ++ | +++ (P312), +++ (P311) | |
| V | B915-1 | ND | +++ | +++ (B915-2) |
| Kas202-1 | ND | ++ | +++ (Kas202-3), ++ (Kas202-2), ++ (Kas202-4), + (Kas202-5) | |
| UsIt102-2 | ND | +++ | +++ (UsuIR-1), +++ (UsuIR-2) | |
| UsIt101-1 | ND | +++ | +++ (UsuIS-1), +++ (UsuIS-2) | |
| UsS102-1 | ND | +++ | +++ (UsuSR-2), +++ (UsuSR-1) | |
| UsS101-2 | ND | +++ | +++ (UsuSS-2), +++ (UsuSS-1) | |
| B908-1 | ND | +++ | +++ (B908-2), ++ (B908-3) | |
| B913-1 | ND | +++ | +++ (B913-2) | |
| Kas201-1 | ND | +++ | +++ (Kas201-4), ND (Kas201-3) | |
| B906-1 | ND | ++ | +++ (B906-2) | |
| UsIt102-3 | ND | ++ | +++ (UsuIR-1), +++ (UsuIR-2) | |
| Kas106-4 | ND | ++ | ++ (Kas101-2), +++ (Kas106-5) | |
| Kas402-3 | ND | ++ | ++ (Kas404-3), ++ (Kas404-2), ++ (Kas404-4), + (Kas402-1), ND (Kas402-2) | |
| UsS101-1 | ND | +++ | +++ (UsuSS-1), +++ (UsuSS-2) | |
| B902-1 | ND | ++ | +++ (B902-2) | |
| B901-1 | ND | ++ | +++ (B901-2) | |
+, ++, and +++, 0.2 to 2, 2 to 20, and 20 to 200 nmol h−1 tube−1, respectively. ND, not detected (<0.1 nmol h−1 tube−1).
Phylogenetic groups were defined as shown in Fig. 3 according to 16S ribosomal DNA sequences.
ARA of the respective clostridial isolate in semisolid RMR medium in air and under anoxic conditions in an AnaeroPack system (Mitsubishi, Tokyo, Japan). Asterisk, no ARA under anoxic conditions but positive ARA when cocultured with the appropriate accompanying bacteria.
Isolates in the same row originated from identical plant materials.
ANFICO interaction for N2 fixation by clostridia.
During our examination of the N2-fixing activity profiles of the clostridial isolates with accompanying bacteria, we found another interaction of a few ANFICOs (Table 1). Three clostridial isolates (Kas107-2, OkiF101, and OkiN108) expressed no N2- fixing activity in single cultures, even in an anoxic environment (Table 1). However, they showed N2-fixing activities in coculture with some isolates of accompanying bacteria from identical plant tissues (Table 1). All of the clostridial isolates from group II displayed the accompanying bacterium-dependent N2 fixation (Table 1). When we cross-assayed the N2-fixing activities of cocultures with isolates from stems of M. sinensis and sugarcane (Table 2), the induction of N2-fixing activity occurred in more than just the original combinations (Table 2). This suggests the presence of exchangeable, specific ANFICO relationships for the expression of N2 fixation. Thus, we examined whether the accompanying bacteria produced specific metabolites that induced the N2 fixation of the clostridial isolate.
TABLE 2.
N2-fixing activity of clostridial isolates cocultured with accompanying bacteria of different origins in semisolid RMR medium
| Culture condition | Acompanying bacterium | ARA (nmol of ethylene produced h−1 tube−1) of clostridial isolatea
|
||
|---|---|---|---|---|
| Kas107-2 | OkiF101 | OkiN108 | ||
| Cocultures | ||||
| Air | Kas107-3 | 15.1 ± 1.1* | 18.4 ± 1.1 | 24.1 ± 1.5 |
| Kas107-4 | ND | ND | ND | |
| OkiF105 | 9.0 ± 1.1 | 13.3 ± 2.2* | 14.7 ± 0.5 | |
| OkiF102 | ND | ND | ND | |
| OkiN104 | 5.9 ± 0.2 | 7.4 ± 0.9 | 6.7 ± 0.5* | |
| OkiN107 | ND | ND | ND | |
| Single cultures | ||||
| Air | None | ND | ND | ND |
| Anoxic | None | ND | ND | ND |
ND, not detected (<0.3 nmol of ethylene h−1 tube−1). ARAs of clostridial isolates (group II) in semisolid RMR medium in air and under anoxic conditions in an AnaeroPack system (Mitsubishi) are shown. Bacteria with Kas107, OkiF, and OkiN prefixes were isolated from stems of M. sinensis and Saccharum hybrid sp. (sugarcane cultivars F177 and Ni9), respectively. Asterisks, original combinations of ANFICOs. Values are means with standard deviations for triplicate determinations.
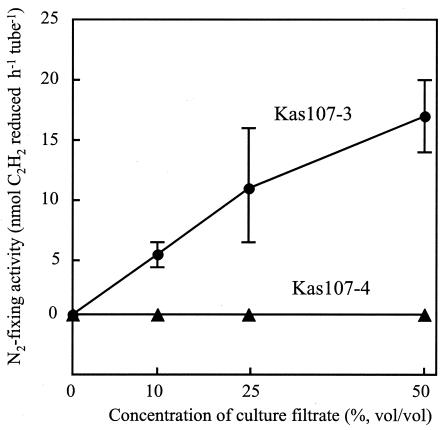
The addition of a specific culture filtrate of Bacillus sp. strain Kas107-3 caused the expression of N2-fixing activity in Clostridium sp. strain Kas107-2, whereas a Kas107-4 filtrate did not (Fig. 4). These data suggest that N2 fixation in ANFICOs is dependent on at least two factors, the presence of unknown metabolites and low O2 concentrations. The concept of ANFICOs enables us to recognize and isolate N2-fixing clostridia from plants. Interestingly, this type of ANFICO includes two clostridial isolates (OkiF101 and OkiN108) from sugarcane. Sugarcane has been intensively studied for biological N2 fixation by bacterial endophytes (2, 12, 18).
FIG. 4.
N2-fixing activity of Clostridium sp. strain Kas107-2 culture with filtrates of accompanying bacterial isolates Kas107-3 and Kas107-4. Clostridium sp. strain Kas107-2 was anaerobically grown in RMR broth without rice extract at 30°C for 72 h with shaking in the presence of various concentrations (percentages [vol/vol]) of the culture filtrates of the accompanying bacteria Kas107-3 and Kas107-4. After the 72-h incubation, the cell densities of Kas107-2 in medium supplemented with the filtrates reached 3 × 107 to 5 × 107 CFU ml−1. The ARAs of cultures of Clostridium sp. strain Kas107-2 were determined in triplicate. Error bars indicate standard deviations. Kas107-3 was identified as a Bacillus sp. by its 16S rRNA gene sequence (Fig. 3). The cell morphology of Kas107-4 was very similar to that of Kas107-3 in terms of its rod shape, gram-positive staining, and endospore formation (data not shown).
Implications of ANFICOs in microbial ecology and nitrogen fixation.
Their sensitivity to molecular oxygen generally restricts Clostridium spp. to anaerobic areas such as water, submerged soil, rumina, and intestines (4, 9). Anoxic microsites existing in soil particles (19) and litter (21) often provide a habitat for clostridia because they have been isolated from nonsubmerged soil and litter (13). Interestingly, early works suggested the presence of N2 fixation by strictly anaerobic organisms and clostridia in the rice rhizosphere (10) and by the soil microbial community in aerobic cultures (14). Therefore, it is not surprising that clostridia reside in the aerial parts of plant tissues, which are exposed to the air and to O2 produced by photosynthesis. The plant-dwelling clostridia probably sometimes proliferate in anoxic microzones produced by ANFICOs or plant respiration, while they survive in spore forms under higher O2 concentrations.
To our knowledge, this is the first report on the ubiquitous distribution and phylogenetic characterization of clostridia from living plants, including their aerial parts. The existence of these organisms has not been suspected from prior works on plant endophytes, since most studies have not employed culturing techniques for the isolation of obligate anaerobes. We have clearly shown that the presence of ANFICOs explains the apparent unculturability of N2-fixing microbes by the conventional procedure of single-colony isolation. Indeed, a survey work on the purification of N2-fixing microbes from pasture grasses in Southeast Asia had failed due to the problem of the apparent unculturability of diazotrophs (M. Araragi, personal communication).
If clostridia and aerobic diazotrophs are mixed during aerobic isolation steps, the aerobic diazotrophs should be selectively purified because of no growth of strictly anaerobic clostridia under aerobic conditions. The discovery of ANFICOs is thus probably attributable to the fact that the in situ plant materials (mainly shoots) used in this work were not contaminated by conventional diazotrophic endophytes, such as Herbaspirillum sp. (6). Therefore, the results of this work do not contradict the existence of conventional diazotrophic endophytes but reveal the existence of ANFICOs that have been hidden by them.
This work indicates that clostridia are naturally occurring endophytes in gramineous plants and that N2 fixation by the clostridia arises in association with nondiazotrophic endophytes in culture. We still do not know whether ANFICOs really fix nitrogen in planta. However, the finding of ANFICOs in plants indicates that clostridia should be candidates as real diazotrophic endophytes in grass for future studies (12). This work also demonstrates a new principle in environmental microbiology, that consortia of bacteria, rather than monocultures, may stand for a particular activity in a complex environment.
Acknowledgments
We thank Y.-I. Sato, H. Urairong, M. Abe, and S. Tajima for their support in the sampling of wild rice species.
K. Minamisawa, T. Miyaki, B. Ye, and M. Saito were supported by a grant from Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
REFERENCES
- 1.Barer, M. R., and C. R. Harwood. 1999. Bacterial viability and culturability. Adv. Microb. Physiol. 41: 93-137. [DOI] [PubMed] [Google Scholar]
- 2.Boddey, R. M., O. C. de Oliveira, S. Urquiaga, V. M. Reis, F. L. de Oliveira, V. L. D. Baldani, and J. Döbereiner. 1995. Biological nitrogen fixation associated with sugar cane and rice: contributions and prospects for improvement. Plant Soil 174: 195-209. [Google Scholar]
- 3.Caldwell, D. E., G. M. Wolfaardt, D. R. Korber, and J. R. Lawrence. 1997. Do bacterial communities transcend Darwinism? Adv. Microb. Ecol. 15: 105-191. [Google Scholar]
- 4.Cato, E. P., W. L. George, and S. M. Finegold. 1986. Genus Clostridium, p. 1141-1200. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md.
- 5.Collins, M. D., et al. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44: 812-826. [DOI] [PubMed] [Google Scholar]
- 6.Elbeltagy, A., K. Nishioka, T. Sato, H. Suzuki, B. Ye, T. Hamada, T. Isawa, H. Mitsui, and K. Minamisawa. 2001. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 67: 5285-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbeltagy, A., K. Nishioka, H. Suzuki, T. Sato, Y. Sato, H. Morisaki, H. Mitsui, and K. Minamisawa. 2000. Isolation and characterization of endophytic bacteria from wild and traditionally cultivated rice varieties. Soil Sci. Plant Nutr. 46: 617-629. [Google Scholar]
- 8.Gyaneshwar, P., E. K. James, P. M. Reddy, and J. K. Ladha. 2002. Herbaspirillum colonization increases growth and nitrogen accumulation in aluminium-tolerant rice varieties. New Physiol. 154: 131-145. [Google Scholar]
- 9.Hippe, H., J. R. Andreesen, and G. Gottschalk. 1992. The genus Clostridium —nonmedical, p. 1800-1866. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. Schleifer (ed.), The prokaryotes, 2nd ed. SpringerVerlag, Heidelberg, Germany.
- 10.Hirota, Y., T. Fujii, Y. Sano, and S. Iyama. 1978. Nitrogen fixation in the rhizosphere of rice. Nature 276: 416-417. [Google Scholar]
- 11.Hurek, T., L. L. Handley, B. Reinhold-Hurek, and Y. Piche. 2001. Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol. Plant-Microbe Interact. 15: 233-242. [DOI] [PubMed] [Google Scholar]
- 12.James, E. K. 2000. Nitrogen fixation in endophytic and associative symbiosis. Field Crop Res. 65: 197-209. [Google Scholar]
- 13.Kuhner, C. H., C. Matthies, G. Acker, M. Schmittroth, A. S. Grossner, and H. L. Drake. 2000. Clostridium akagii sp. nov. and Clostridium acidisoli sp. nov.: acid-tolerant, N2-fixing clostridia isolated from acidic forest soil and litter. Int. J. Syst. Evol. Microbiol. 50: 873-881. [DOI] [PubMed] [Google Scholar]
- 14.Line, M. A., and M. W. Loutit. 1973. Nitrogen-fixation by mixed cultures of aerobic and anaerobic micro-organisms in an aerobic environment. J. Gen. Microbiol. 74: 179-180. [Google Scholar]
- 15.Pace, N. R. 1997. A molecular view of microbial diversity and the biosphere. Science 276: 734-740. [DOI] [PubMed] [Google Scholar]
- 16.Reinhold-Hurek, B., and T. Hurek. 1998. Life in grasses: diazotrophic endophytes. Trends Microbiol. 6: 139-144. [DOI] [PubMed] [Google Scholar]
- 17.Rennie, R. J. 1981. A single medium for the isolation of acetylene-reducing (dinitrogen-fixing) bacteria from soils. Can. J. Microbiol. 27: 8-14. [DOI] [PubMed] [Google Scholar]
- 18.Sevilla, M., R. H. Burris, N. Gunapala, and C. Kennedy. 2001. Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and nif− mutant strains. Mol. Plant-Microbe Interact. 14: 359-366. [DOI] [PubMed] [Google Scholar]
- 19.Sexstone, A. J., N. P. Revsbech, T. B. Parkin, and J. M. Tiedje. 1985. Direct measurement of oxygen profile and denitrification rate in soil aggregates. Soil Sci. Soc. Am. J. 49: 645-651. [Google Scholar]
- 20.Strous, M., et al. 1999. Missing lithotroph identified as new planctomycete. Nature 400: 446-449. [DOI] [PubMed] [Google Scholar]
- 21.van der Lee, G. E. M., B. de Winder, W. Bouten, and A. Tietema. 1999. Anoxic microsites in Douglas fir litter. Soil Biol. Biochem. 31: 1295-1301. [Google Scholar]
- 22.Zhou, J. C., Y. T. Tchan, and J. M. Vincent. 1985. Reproductive capability of bacteroid in nodules of Trifolium repens L. and Glycine max (L.) Merr. Planta 163: 473-482. [DOI] [PubMed] [Google Scholar]