Abstract
Central-pattern-generating neural circuits function reliably throughout an animal's life, despite constant molecular turnover and environmental perturbations. Fluctuations in temperature pose a problem to the nervous systems of poikilotherms because their body temperature follows the ambient temperature, thus affecting the temperature-dependent dynamics of various subcellular components that constitute neuronal circuits. In the crustacean stomatogastric nervous system, the pyloric circuit produces a triphasic rhythm comprising the output of the pyloric dilator, lateral pyloric, and pyloric constrictor neurons. In vitro, the phase relationships of these neurons are maintained over a fourfold change in pyloric frequency as temperature increases from 7°C to 23°C. To determine whether these temperature effects are also found in intact crabs, in the presence of sensory feedback and neuromodulator-rich environments, we measured the temperature dependence of the pyloric frequency and phases in vivo by implanting extracellular electrodes into Cancer borealis and Cancer pagurus and shifting tank water temperature from 11°C to 26°C. Pyloric frequency in the intact crab increased significantly with temperature (Q10 = 2–2.5), while pyloric phases were generally conserved. For a subset of the C. borealis experiments, animals were subsequently dissected and the stomatogastric ganglion subjected to a similar temperature ramp in vitro. We found that the maximal frequency attained at high temperatures in vivo is lower than it is under in vitro conditions. Our results demonstrate that, over a wide temperature range, the phases of the pyloric rhythm in vivo are generally preserved, but that the frequency range is more restricted than it is in vitro.
Keywords: central pattern generator, pyloric rhythm, stomatogastric ganglion, robustness
every animal's nervous system must be able to function throughout life, despite constant molecular turnover, developmental changes, and environmental perturbations. A notable challenge to the nervous systems of poikilotherms is the fluctuation in the ambient temperature, which causes corresponding fluctuations in body temperature. The voltage-dependent gating dynamics of the ion channels controlling synaptic and intrinsic membrane currents exhibit varying degrees of temperature dependence (Cao and Oertel 2005; Moran et al. 2004; Myers et al. 2009; Thompson et al. 1985), potentially leading to large variations in neuronal activities. Yet the many components of poikilotherm nervous systems work in concert and produce functional activity at a wide range of temperatures (Robertson and Money 2012; Tang et al. 2010).
The poikilotherm Cancer borealis inhabits the waters stretching from Florida to Nova Scotia (Haefner 1977; Rathbun 1930). It is most commonly found at depths of 61–400 m (Haefner 1977) and at temperatures between 8 and 14°C (Haefner 1977; Stehlik et al. 1991), but it also frequents the intertidal and subtidal zones (Donahue et al. 2009; Rathbun 1930) to forage for food (Krediet and Donahue 2009). In these shallower waters, temperatures can reach nearly 24°C (Krediet and Donahue 2009). Cancer pagurus, a species closely related to C. borealis, is ordinarily found in the North Sea at temperatures between 4°C and 15°C; however, it will regularly recover from exposure to temperatures of up to 19°C in open air during commercial shipment (Barrento et al. 2010; Metzger et al. 2007). In order for the animals to feed properly, their nervous systems must retain functionality at temperatures that span these ranges.
Central pattern generators (CPGs) are a class of neuronal circuits that generate cyclic patterns controlling rhythmic behaviors such as breathing, locomotion, saccadic eye movements, vocalizations, and chewing (Berkowitz et al. 2010; Büschges et al. 2011; Chevallier et al. 2008; Doi and Ramirez 2008; El Manira et al. 2010; Harris-Warrick 2011; Katz 2007; Kiehn 2006; Marder and Calabrese 1996; Yamaguchi et al. 2008). Due to the predictability of their output under control conditions, CPGs are a convenient test bed for studying the effects of temperature perturbations on neuronal motor output. Certain features of CPG output, such as the cycle frequency, may vary widely (Tang et al. 2010); other features, such as the relative timing (i.e., phase relationships) of bursts or spikes within the rhythm, remain rather constant (Bucher et al. 2005; Cohen et al. 1992; Hooper 1997a; Skinner and Mulloney 1998; Soofi et al. 2012). Presumably, constraining these features is necessary for retaining functional behavior. The stomachs of decapod crustaceans such as C. borealis and C. pagurus are controlled by two CPGs, one of which is the pyloric circuit, which produces the pyloric rhythm and drives the musculature of the pyloric filter. In vitro, the phase relationships of the neurons in the pyloric rhythm remain remarkably constant as the pyloric frequency changes (Bucher et al. 2005; Hooper 1997a, 1997b), indicating that phase maintenance is important for the filtering of food. Accomplishing this phase maintenance is nontrivial, requiring precise compensation of active conductances, synaptic dynamics, and other processes in the network (Greenberg and Manor 2005; Hooper 1997a, 1998; Manor et al. 2003; Nadim et al. 2003).
A recent study demonstrated that, when saline temperatures are varied from 7°C to 23°C in vitro, the pyloric frequency increases fourfold while the phases remain largely constant (Tang et al. 2010), despite the fact that many of the underlying intrinsic neuron properties and synaptic dynamics change dramatically with temperature (Johnson et al. 1991, 1992; Tang et al. 2010). However, this work raises a question: Are the temperature-dependent changes in the neuronal activity we observe in vitro comparable to those that would be observed in vivo? While stomatogastric ganglion (STG) fictive motor patterns are clearly present in isolated ganglion preparations, functional behavior also requires the activation of muscles and sensory feedback, as well as interactions with other neuronal circuits (Stein 2009). The pyloric rhythm is also heavily regulated by neuromodulators, which arise both from sensory-driven descending input fibers to the STG and from the hemolymph (Nusbaum and Beenhakker 2002; Stein 2009). In the in vitro environment, neuromodulators from descending inputs are present (in non-decentralized preparations), but the hemolymph and sensory feedback are absent. Additionally, these influences are themselves temperature dependent and may influence CPG activity in vivo. We hypothesize that phase constancy is important for functional behavior and should, therefore, be present in vivo as well as in vitro. To test this, we introduced a controlled temperature perturbation while measuring the pyloric rhythm in intact C. borealis and C. pagurus, and, subsequently, we exposed a subset of the C. borealis specimens to a similar temperature perturbation in vitro.
METHODS
Dissection and Experiments
In vivo experiments.
All C. borealis experiments were performed at the Marine Biological Laboratory in Woods Hole, MA. All C. pagurus experiments were performed at the University of Ulm in Ulm, Germany.
ANIMALS.
Live C. borealis were obtained from commercial sources through the Marine Resources Center in Woods Hole and were acclimated at 11°C for at least 4 wk in circulating seawater tanks. All C. borealis were fed three times per week before and after electrode implantation. Live C. pagurus were obtained from a commercial provider (Feinfisch GmbH, Neu-Ulm, Germany) and kept in filtered, aerated, artificial seawater at 10–12°C.
ELECTRODE IMPLANTATION.
Crabs were immobilized with rubber bands and anesthetized on ice for 30–45 min. In the case of C. pagurus, animals were restrained in a custom-built holder (courtesy of H. G. Heinzel, University of Bonn, Germany). Application of a thin layer of super glue at the implantation site dried the carapace. A wall of dental cement (Protemp, ESPE) was built around the surgery site. Using a Dremel tool, an ∼2 × 2 cm (for C. borealis) or 3 × 3 cm (for C. pagurus) piece of carapace was removed from inside the wall, and a slow saline drip [440 mM NaCl, 11 mM KCl, 26 mM MgCl2, 13 mM CaCl2, 10 (for C. pagurus) or 11 mM (for C. borealis) Trizma base, 5 mM maleic acid, pH 7.4–7.6] and suction were set up to prevent excess hemolymph spilling and coagulation. The lateral ventricular nerve (lvn), dorsal ventricular nerve (dvn), and/or the median ventricular nerve (mvn) were exposed, and a home-built hook electrode was cemented onto the carapace and placed around the nerve. The contact between the hook electrode and the nerve was then insulated by encasing the contact point in a Vaseline-filled (9 parts Vaseline and 1 part mineral oil) plastic or silicone tube around the hook and wire. The surgery site was closed with a piece of Parafilm fixed with dental cement to the wall on the carapace (Hedrich and Stein 2008). In the case of C. borealis, the surgery site and the wire connections were waterproofed with marine adhesive sealant (3M Marine Fast Cure 5200) and/or DeKhotinsky cement (gift from Dr. Harvey Fishman). A Styrofoam float was used to keep the ends of the wires out of the water. During recovery from surgery and subsequent experiments, C. borealis were placed into isolated circulating seawater tanks (11°C) to recover for at least 1 day, while C. pagurus were kept in a holding tank filled with artificial seawater (9°C). C. pagurus were allowed to recover from surgery for at least 5 h before beginning experiments.
ELECTROPHYSIOLOGY AND DATA ACQUISITION.
In the C. borealis experiments, animals were placed into an isolated tank of seawater and exposed to a series of increasing temperatures from 10°C to 26°C. To alter the tank temperature, chilled or preheated seawater was added, and excess water was siphoned out in between temperature steps. Before each recording, C. borealis were generally allowed to acclimate 5–10 min to the new temperature. When this was not possible, we analyzed data only after the temperature had settled and did not change by more than 1°C for at least 60 s. Temperature was recorded with a thermistor probe (Warner Instruments) and a manual thermometer. The thermistor probe and the manual thermometer were both placed in the tank water. Typically, the thermistor probe was fixed to a wall at the bottom of the tank, at eye level of the crabs. In addition, one manual probe was used at the same location, and a second thermistor probe was intermittently used at different locations in the tank to ensure the absence of a horizontal temperature gradient. We used multiple temperature measuring devices to ensure a redundant record of the tank temperature as probes have a tendency to fail in high salinity environments (over the course of the experiments, we recorded four instances of probe failure).
To determine whether the tank temperature is an accurate proxy for the temperature of the stomatogastric nervous system (STNS), we separately recorded the internal temperature of a C. borealis with a temperature probe while stepwise altering the tank temperature from 10 to 13°C and from 13 to 19°C, comparable to the in vivo C. borealis experiments. We found that the lag between the tank temperature and the internal temperature of the animal was small, and the internal temperature equilibrated with the surrounding tank water at a rate of ∼1°C/min. The slow rate of temperature change in the in vivo experiments thus allowed adequate time for the internal temperature of the animal to equilibrate to the recorded tank temperature.
In the C. pagurus experiments, the animal was placed in a small tank (∼40 cm × 30 cm × 20 cm) of artificial seawater (baseline temperature 9°C). Warm artificial seawater (∼30°C) was piped into the small tank so that the temperature of the water in the tank continuously changed by ∼0.5–1.3°C/min. Cold seawater (9°C) was then piped into the tank in a similar fashion to bring the temperature back down to baseline. Between experiments, the animal was kept in the holding tank at 9°C. Temperature was continuously measured using a USB TEMPer1 thermometer (PCsensor).
Data from seven C. borealis were analyzed and averaged across trials, with one animal undergoing four trials, two animals undergoing two trials, and four animals undergoing one trial (12 trials total). Data from five C. pagurus were analyzed and averaged across trials, with two animals undergoing three trials, one animal undergoing two trials, and two animals undergoing one trial each (10 trials total). One C. pagurus trial was removed from the study due to high irregularity of the rhythm at all temperatures, leaving nine trials from five animals.
For both species, data were filtered and amplified through an A-M Systems amplifier (model 1700, Carlsborg, WA), then recorded with a micro 1401 AD board (Cambridge Electronic Design, Cambridge, UK) and Spike2, version 6 (CED) on a Windows PC.
In vitro experiments.
In a subset of the C. borealis animals, we recorded pyloric and gastric activity in vivo and then subsequently also in vitro. Dissections were performed as previously described in chilled physiological saline (Gutierrez and Grashow 2009). The STNS was pinned down in a Sylgard-coated dish. Extracellular activity was first recorded with stainless steel pin electrodes that were placed into petroleum jelly wells on the motor nerves, then amplified and filtered with a differential amplifier (A-M Systems). During the recording, the STNS was continuously superfused with (11–26°C) saline. The temperature was monitored and controlled with a bipolar temperature controller (Warner Instruments, model CL-100). Data were acquired using a Digidata 1200 data acquisition board (Axon Instruments).
Data Analysis
C. borealis and C. pagurus were analyzed in a similar fashion. At tank temperatures of 11°C, 15°C, 19°C, 23°C, and 26°C, we extracted the average frequency, pyloric dilator (PD) offset phase, lateral pyloric (LP) onset phase, and LP offset phase from lvn or dvn recordings of five animals from each species. Pyloric frequency was defined as the reciprocal of the pyloric cycle period, which was calculated as the elapsed time between the start of one PD burst and the start of the subsequent PD burst. PD offset phase was defined as the elapsed time between the beginning and the end of a single PD burst, normalized by the current cycle period. Similarly, LP onset phase and offset phase were defined as the elapsed time between the beginning of a PD burst and the beginning and end (respectively) of the next LP burst, normalized by the cycle period. The pyloric constrictor (PY) neuron phases were not examined due to difficulty in extracting the smaller spikes from the lvn/dvn recordings. From two additional specimens of C. borealis, the mvn activity was recorded, and the inferior cardiac neuron, which fires in phase with LP, and the ventricular dilator neuron, which fires in phase with PY (Weigeldt et al. 2002), were used to calculate pyloric frequency.
For analysis, segments of data were chosen for which the average temperature was within ±1° of the target temperature. We then calculated the Q10 of the frequencies and phases to determine their relationship with temperature (Tang et al. 2010). The Q10 is the factor by which the rate of a process changes with a 10°C increase in temperature. By plotting the parameter of interest (frequency or phase) against temperature in a semi-log format, performing a linear regression analysis, and finding the slope m of the best-fit line, the Q10 can be calculated with the following formula:
All data were analyzed using Clampex and Clampfit (Axon Instruments), Spike2 (Cambridge Electronic Design) and/or MATLAB (Mathworks). Statistical analyses were performed using the SigmaPlot 10 and SigmaStat software packages (Jandel Scientific) and Excel (Microsoft). Figures were generated with Adobe Illustrator (Adobe), Inkscape, CorelDRAW (Corel), and Excel (Microsoft).
Additional data analysis was done in MATLAB on in vivo data from five C. borealis animals. LP onset times were extracted from 60-s stretches of recordings from each animal at each temperature (11, 15, 19, 23, 26°C). From these trains we computed mean and median frequency and coefficient of variation (CV). Spectrograms were plotted using built-in MATLAB functions using a 6-s time window with 90% overlap between windows. We used a frequency resolution of 0.3 Hz, ranging from 0.1 Hz to 4 Hz. Significance of the mean frequencies was tested with a one-way ANOVA.
RESULTS
Recording the Pyloric Rhythm In Vivo
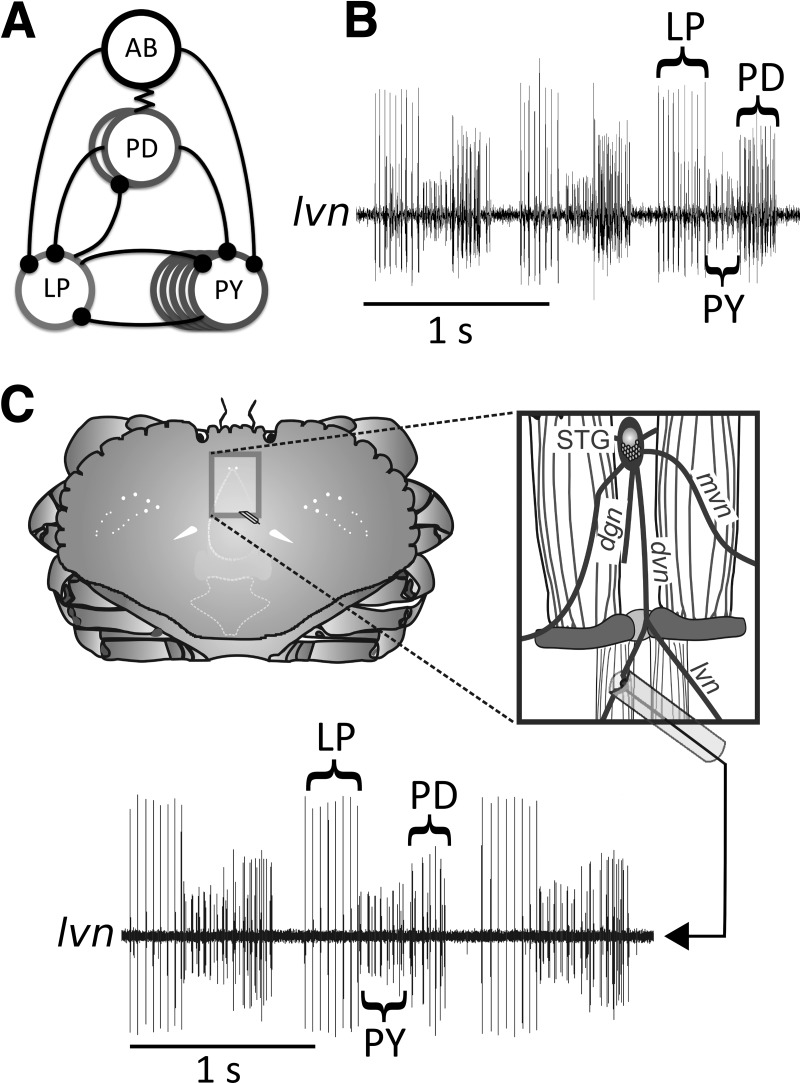
The pyloric rhythm, which controls food filtration in the stomach, is one of two patterns generated by neuronal circuits found in the STG of the STNS (Marder and Bucher 2007; Maynard and Dando 1974; Maynard and Selverston 1975; Selverston and Miller 1980). It is driven by a pacemaker kernel consisting of the electrically coupled anterior burster (AB) and two PD neurons (Fig. 1A). The pacemaker kernel neurons inhibit the single LP and the four to five electrically coupled PY neurons, which then rebound to produce a triphasic burst pattern (Fig. 1B). The pyloric cycle starts with the burst of the PD neuron (midsized spikes), followed by bursts of LP (large spikes) and PY (small spikes). The recordings of the pyloric rhythm from the intact, freely behaving crab (in vivo) are similar in appearance to those obtained from in vitro recordings (Fig. 1, B and C; Hedrich et al. 2011).
Fig. 1.
The pyloric circuit produces a triphasic rhythm. A: schematic diagram of the pyloric circuit. Inhibitory synapses are indicated by a line and black dot, and a zigzag line indicates electrical couplings. The electrically coupled anterior burster (AB) and pyloric dilator (PD) neurons together serve as the pacemaker complex, inhibiting the lateral pyloric (LP) and pyloric constrictor (PY) neurons. B: sample in vitro extracellular trace of the triphasic pyloric rhythm, with PD, LP, and PY bursts indicated. C: the part of the carapace and hypodermis surrounded by the box on the diagram of the crab (left) was cut away, revealing the major afferent nerves of the stomatogastric nervous system and the gastric mill muscles (right). A single hook electrode was anchored around the dorsal ventricular nerve (dvn) or lateral ventricular nerve (lvn) (shown), and the nerve and hook were gently pulled into a polythene tube filled with Vaseline to insulate the contact point between the nerve and electrode from the surrounding hemolymph. A reference electrode was anchored within the body cavity. The signal from the dvn or lvn was then recorded and analyzed. mvn, Median ventricular nerve; STG, stomatogastric ganglion.
Effects of Temperature on the In Vivo Pyloric Frequency
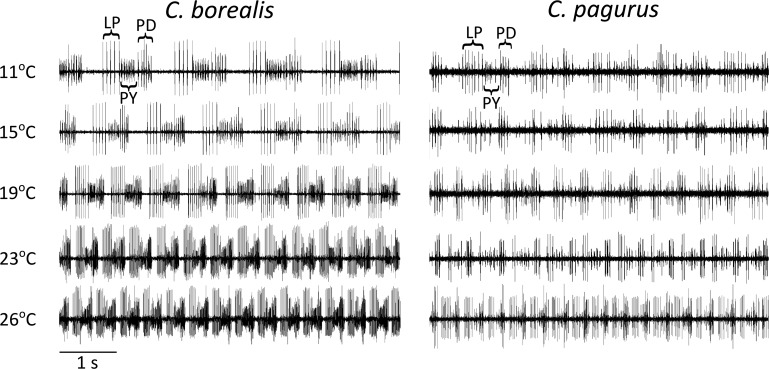
We studied the effect of increasing tank water temperature on the pyloric rhythm in intact C. borealis and C. pagurus. All animals (n = 12) responded with an increase in pyloric frequency to acutely raised tank temperatures. Figure 2 shows sample traces of the pyloric rhythm from each species as the temperature was increased. In this example, the frequency in C. borealis increased from 0.8 Hz at 11°C to 2.3 Hz at 26°C, and in C. pagurus from 0.9 Hz to 2.5 Hz.
Fig. 2.
Sample extracellular traces from Cancer borealis and C. pagurus at 11, 15, 19, 23 and 26°C. In both species, recordings were made from either the dvn or lvn.
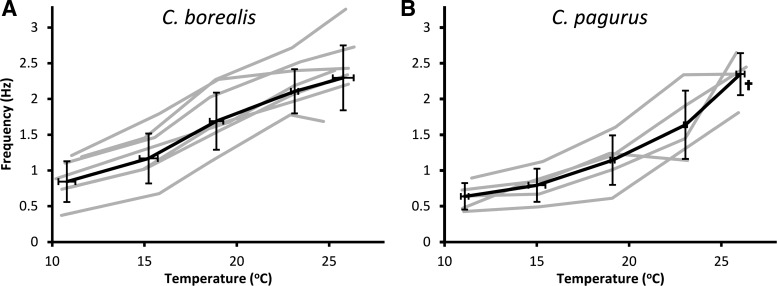
Figure 3 summarizes the effect of temperature on pyloric frequency for both species. Q10 analysis (Table 1) revealed the temperature dependence of the pyloric frequency between 11°C and 26°C. C. borealis and C. pagurus exhibited a Q10 of 2.08 and 2.41, respectively; these numbers fall near the same range as those of many biological processes (Lenz et al. 2005). The average maximum frequencies at 26°C were 2.3 ± 0.29 Hz (SD) for C. pagurus and 2.4 ± 0.48 Hz for C. borealis.
Fig. 3.
Pyloric frequency increases with temperature in C. borealis and C. pagurus. Gray lines indicate individual animals [n = 7 for C. borealis at all temperatures (A), and n = 5 for C. pagurus for all temperatures, except where indicated by a single dagger, where n = 4 (B)]. Thick black lines indicate the mean for all animals. Error bars indicate standard deviation.
Table 1.
Temperature dependence of pyloric frequencies and phases
| Species | Pyloric Rhythm Characteristic | Q10 | Standard Error of Q10 | m |
|---|---|---|---|---|
| Cancer borealis | Frequency | 2.08 | 0.18 | pos. (P < 0.001) |
| PD offset | 1.02 | 0.042 | 0 (P = 0.57) | |
| LP onset | 0.96 | 0.022 | 0 (P = 0.064) | |
| LP offset | 0.95 | 0.019 | neg. (P = 0.018) | |
| C. pagurus | Frequency | 2.41 | 0.25 | pos. (P < 0.001) |
| PD offset | 1.02 | 0.066 | 0 (P = 0.76) | |
| LP onset | 1.06 | 0.054 | 0 (P = 0.29) | |
| LP offset | 0.94 | 0.059 | 0 (P = 0.31) |
The slope of the best-fit line associated with the Q10 (m) is indicated as positive (pos.), negative (neg.), or not significantly different from zero (0) at a significance level of α = 0.05. Positive and negative values indicate that the characteristic has a positive or negative relationship (respectively) with temperature. Slope values marked with 0 indicate that the associated characteristic has no temperature dependence. PD, pyloric dilator; LP, lateral pyloric.
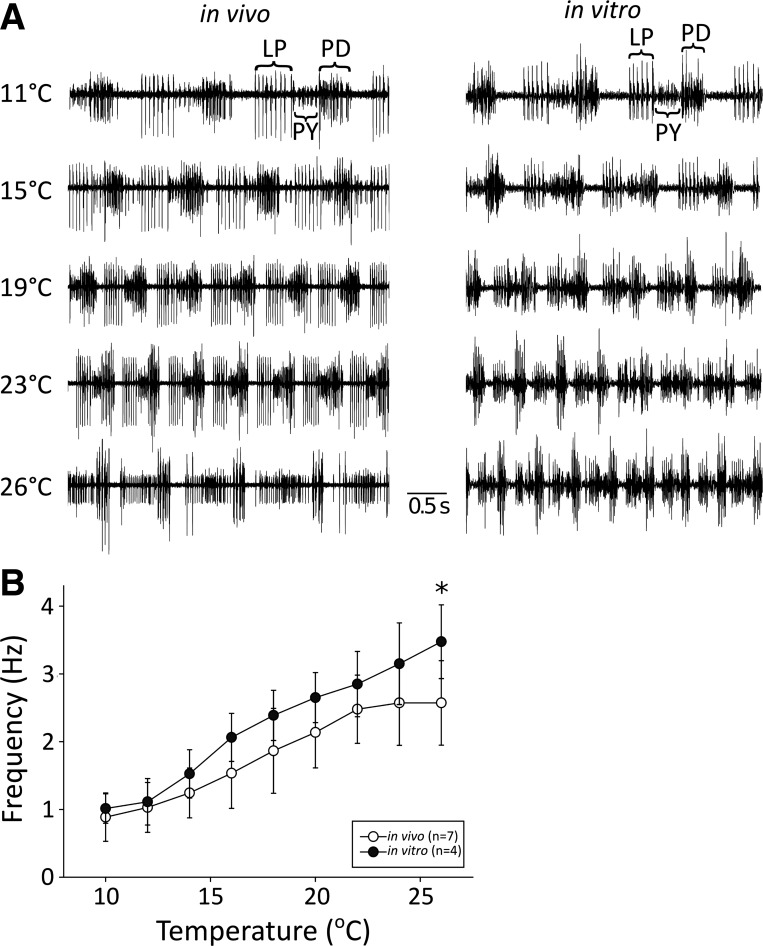
The In Vitro and In Vivo Pyloric Frequencies at High Temperatures Are Different in C. Borealis
The in vivo frequencies in Fig. 3 were generally slower than those previously reported for in vitro preparations of C. borealis (Tang et al. 2010). To examine this difference more closely, the STNS of four of the seven C. borealis whose pyloric rhythms were recorded in vivo were subsequently subjected to a similar temperature ramp in vitro. Sample traces in Fig. 4A show the increase in pyloric frequency both in vivo and in vitro. Comparison of the temperature dependence of the pyloric frequencies in vivo and in vitro across all four animals revealed a trend of higher in vitro frequencies compared with data from the same animals in vivo and was statistically significant at 26°C (Fig. 4B). The average maximum frequency reached a value of 3.5 Hz in vitro, or about 0.9 Hz faster than the frequency in vivo.
Fig. 4.
In vivo preparations exhibit slightly lower pyloric cycle frequencies than in vitro. A: sample traces of the in vivo and in vitro pyloric rhythms at 11, 15, 19, 23, and 26°C. B: average frequencies of the pyloric rhythm over a range of temperatures from 10°C to 26°C for both in vivo (n = 7) and in vitro (n = 4) preparations. Frequencies were divided into bins with widths of 2°C. Open circles indicate data from in vivo preparations, and solid circles indicate data from in vitro preparations. The x-values of the data points represent the average temperature for each bin. Bars indicate standard deviation. *Significantly different groups at a significance level of α = 0.05.
The Pyloric Rhythm Is Less Reliable at Higher Temperatures
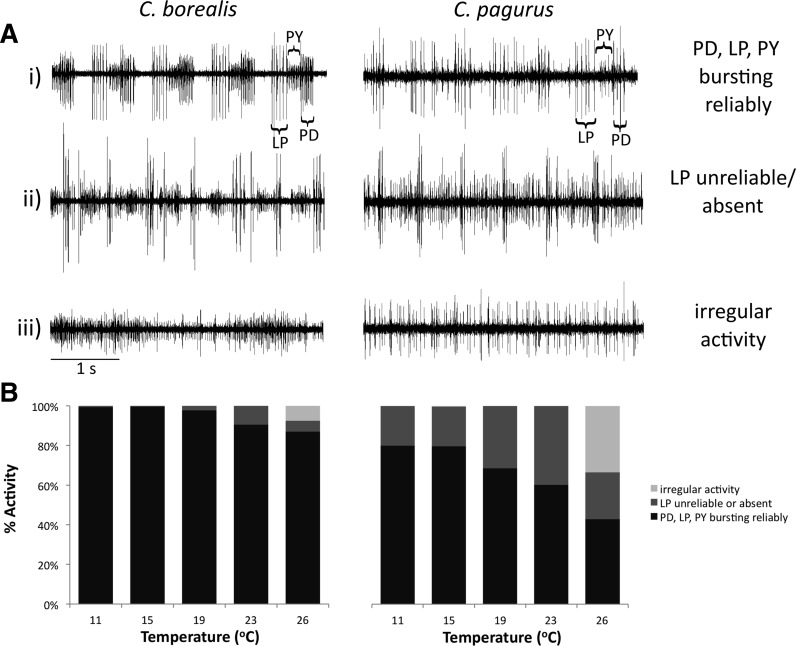
As had been seen previously in vitro (Tang et al. 2012), at higher temperatures the rhythm often became irregular. For a 100-s window of time at each temperature, network activity was visually classified as follows: 1) LP, PD, and PY are bursting; 2) LP is bursting unreliably or not at all; and 3) activity is arrhythmic (Fig. 5). While the activity of C. pagurus was generally less robust than that of C. borealis, increasing temperatures led to more disrupted motor patterns in both species.
Fig. 5.
Burst reliability decreased at high temperatures. A: example recordings from C. borealis and C. pagurus, demonstrating bursting reliability, or the absence thereof, of LP. B: qualitative in vivo pyloric activity of C. borealis and C. pagurus at 11, 15, 19, 23, and 26°C. At each temperature, a 100-s window of activity was visually analyzed, and the pyloric rhythm classified as having all three neurons bursting reliably (shown in black), having unreliable or absent LP activity (shown in dark gray), or having irregular or arrhythmic activity (shown in light gray).
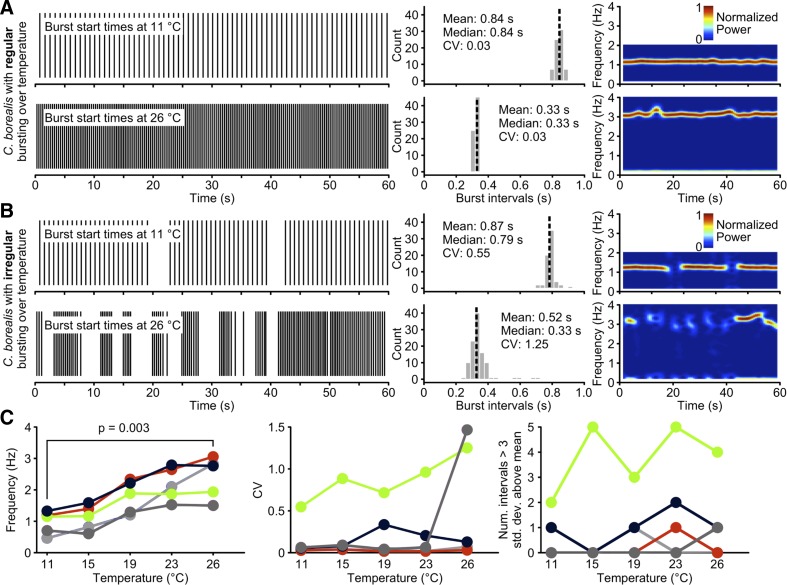
To more explicitly quantify changes in the variability of pyloric motor output across temperature, we extracted burst times from the five C. borealis animals for which 60-s-long lvn recordings at each temperature were available. The start time of each LP burst is plotted over time for two of the five animals in Fig. 6, A and B (left panels). It is immediately obvious that the LP bursts of the animal shown in Fig. 6A occurred at regular intervals, whereas those in the animal shown in Fig. 6B occurred at more irregular intervals. This was particularly apparent at 26°C, as seen in the large, intermittent gaps between LP bursts.
Fig. 6.
LP burst intervals show diverse behaviors across animals and at higher temperatures. A: plot of LP burst onset times. An example C. borealis in vivo recording in which LP bursts occurred at regular intervals at 11°C (left top panel, black lines indicate the times at which bursts occurred) and increased in frequency while retaining regular intervals when temperature was changed to 26°C (left bottom panel). Histograms (middle panels, black dashed line indicates median interval value, bin width is 0.3 s) show the distribution of the LP burst intervals and that the coefficients of variation (CVs) of the burst intervals remained constant and low in this animal, suggesting regular LP bursting. Spectrograms (right panels, hotter colors indicate more spectral power) highlight the consistency of the LP bursting over time. Plots were cut off above the fundamental frequency band to avoid plotting harmonics for clarity. B: example with irregular LP bursting (left panels). At 26°C the LP burst intervals were more variable and occurred more frequently than at 11°C. Histograms show that this animal had similar median burst intervals compared with the animal shown in A, but had much higher CVs due to the irregular bursting of LP. The broken bands in the spectrograms (right panels) show the variability of the burst intervals. C: the frequency (left panel), CV (middle panel), and number of interburst intervals three standard deviations (or more) longer than the mean interval (right panel) are shown for all five animals tested. The animal from A is shown in red, and the animal from B is shown in green. Data from the other three animals are shown in gray scale. Frequency increased significantly (P = 0.003, n = 5, one-way ANOVA) between 11°C and 26°C. No other measure had a statistically significant change over this temperature range.
We next plotted histograms of the LP burst intervals, which we define as the time difference between subsequent LP burst starts (Fig. 6, A and B, middle panels). These plots show that the LP burst intervals decreased with temperature (for example, the mean interval decreased from 0.84 s to 0.33 s in Fig. 6A), consistent with our previous analysis (Fig. 3A). Furthermore, when LP bursts already occurred at irregular intervals at low temperature (Fig. 6B), i.e., when the CV of the burst intervals was already larger than during regular bursting, the CV increased substantially with temperature (Fig. 6B, middle panels, from 0.55 to 1.25). To further highlight this point, we computed the medians of the interval distributions (Fig. 6, A and B, middle panels, black dashed lines). Since the median is less sensitive to outliers, the means and medians of the interval distributions were nearly the same when bursts occurred at regular intervals. In contrast to this, with irregular bursting (Fig. 6B), mean and median values were clearly different. In this case, the interval distribution is right skewed, and 9/155 total intervals (∼5.8% overall, maximum interval is 3.75 s) are larger than 1 s and thus not visible in the figure.
Next, to assess the stability of the pyloric rhythm over time, we plotted spectrograms, which measure the frequency content of the LP burst intervals over time. The animal shown in Fig. 6A showed regular burst intervals, and consistently there were solid bands of hotter colors in the power spectrum (Fig. 6A, right panels). In contrast, during irregular bursting (Fig. 6B), bands were interrupted, matching the time periods when bursting ceases. These power spectra give a compact picture of the frequency content of the burst train, as well as the level of stability of LP bursting over time.
Finally, we plotted the frequency of the LP burst interval, CV, and the number of particularly long gaps between LP bursts for all animals included in this analysis. Frequency increased significantly for all of these animals (Fig. 6C, P = 0.003, n = 5, one-way ANOVA comparing 11°C with 26°C). The CV showed that some animals maintain regular burst intervals over the temperature range tested, while other animals are inherently more variable (Fig. 6C, middle panel). Still other animals were highly regular through the lower temperature range with high variability emerging at only high temperatures, suggesting that responses to temperature changes are variable between animals. We counted the number of intervals three standard deviations (or more) longer than the mean interval (Fig. 6C, right panel) per 60-s recording and observed frequent interruptions through either the entire temperature range, only at high temperature, or none at all. These analyses thus show that, while LP bursts were more frequent with higher temperature, there is individual variability between animals at a given temperature in the regularity of the LP bursts.
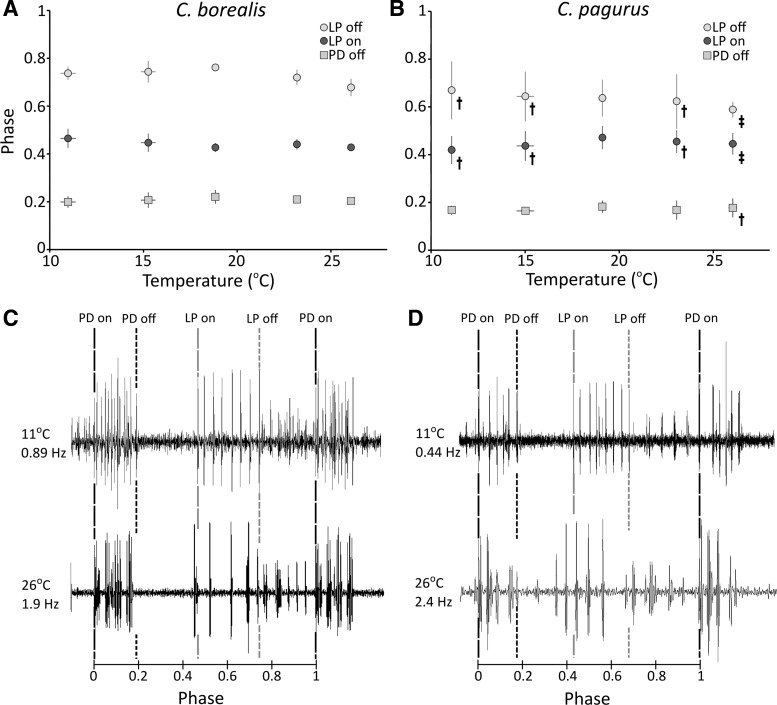
Motor Neuron Phase Relationships Are Maintained During Temperature Changes
Despite clear changes in pyloric cycle frequency with increasing temperature, the PD offset, LP onset, and LP offset phases were relatively well preserved across temperatures (Fig. 7). None of the burst phases in C. pagurus exhibited a Q10 significantly different from 1, suggesting that they are independent of temperature. Similarly, the PD offset and LP onset phases of C. borealis showed no significant temperature dependence. The LP offset phase in C. borealis, however, exhibited a slightly negative relationship with temperature (Fig. 7A). Previous in vitro findings saw a similar decrease in LP offset phase with temperature, although the effects were not statistically significant (Tang et al. 2010).
Fig. 7.
Pyloric phase relationships are maintained across temperatures. Squares, dark circles, and light circles indicate the mean PD offset phases, LP onset phases, and LP offset phases (respectively) at 11, 15, 19, 23, and 26°C for C. borealis (A) and C. pagurus (B). n = 5 for C. borealis at all temperatures, and n = 5 for C. pagurus, except where indicated by a single or double dagger. Single daggers indicate data points obtained using an n of 4, and double daggers indicate data points obtained using an n of 3. Error bars (not always visible) indicate standard deviation. Sample traces at 11°C and 26°C (not from the same trials) are shown for C. borealis (C) and C. pagurus (D), normalized by period to indicate the motor neuron phases at different temperatures.
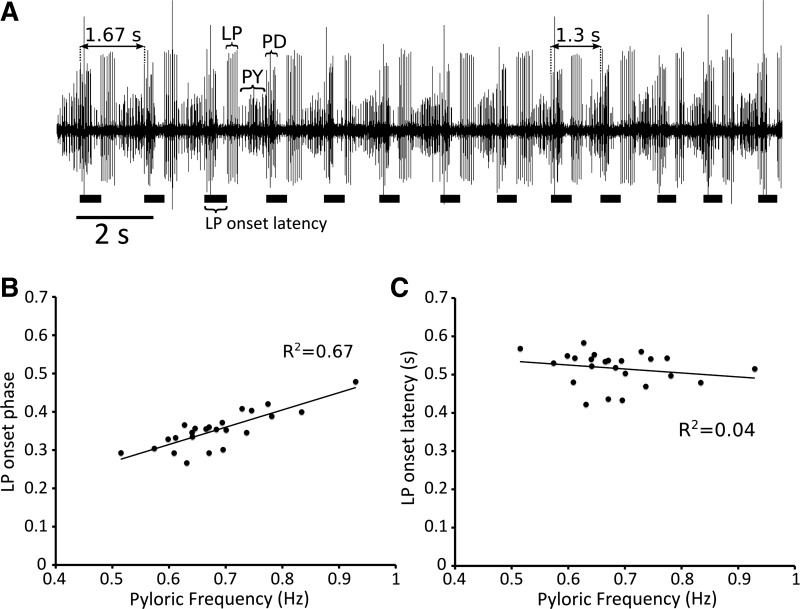
While in general, motor neuron phasing was well maintained during slow-frequency changes, limits to this phase maintenance were unmasked during rapid changes in frequency. The studied temperature changes were rather slow, and the observed changes in frequency were correspondingly slow as well. As shown in Fig. 7, most phases were well conserved during these temperature perturbations. However, this was not true on occasions where we saw rather sudden bouts of increased pyloric frequency, seemingly independent of temperature changes. Figure 8 shows an instance in C. pagurus in which the frequency rapidly increased from 0.6 Hz to 0.9 Hz within 20 s. Interestingly, during this increase in frequency, the phases were not well maintained. This lack of phase maintenance has been documented previously in vitro (Bucher et al. 2005).
Fig. 8.
Example of a lack of phase maintenance during a rapid change in frequency. A: excerpt from a C. pagurus dvn recording during which the frequency rapidly increases without concurrent phase compensation. The lack of phase maintenance in this segment of the recording is relatively pronounced. Black bars indicate length of LP onset latency. B: LP onset phase vs. frequency during the time over which pyloric frequency changed rapidly and the phase was not maintained. The phase-frequency relationship is relatively strong, suggesting that phase is not independent of frequency. C: LP onset latency vs. frequency during the same time period as in B. The latency-frequency relationship is relatively weak. Each pyloric cycle provides one data point. A linear fit of the data is indicated with a solid black line.
DISCUSSION
Temperature compensation is found in many oscillatory and highly diverse systems (Izumo et al. 2003; Rinberg et al. 2013; Thuma et al. 2013; Zhurov and Brezina 2005). In poikilotherms, which have little or no control over their body temperature, temperature-dependent components of the neural circuitry are particularly affected by changing environmental conditions. These animals must thus compensate for temperature-dependent changes to prevent disruption of vital functions, such as breathing and chewing.
Temperature Influences on the Pyloric Frequency
Increasing temperature elicited a significant increase in the in vivo pyloric frequency of both C. pagurus and C. borealis. This effect of temperature on pyloric frequency was previously shown in vitro (Tang et al. 2010). Our results show a similar temperature dependence of the pyloric rhythm in the intact animal and that this effect is thus robust to sensory feedback, neuromodulators, and input from other components of crustacean neuronal circuitry that are not present in the in vitro preparation.
Two interesting observations arise from the experiments that directly compared the in vivo and in vitro pyloric response in C. borealis to temperature perturbations. First, at low temperatures, the pyloric network activity in vivo was virtually indistinguishable from in vitro. These results are consistent with previous findings that recordings from STG neurons in intact crustaceans are generally similar to those from in vitro preparations at control temperatures (Hartline and Maynard 1975) and respond similarly to neuromodulator application (Heinzel et al. 1993) and, to an extent, neuromodulatory release from projection neurons (Diehl et al. 2013; Hedrich et al. 2011). The present experiments demonstrate that, to a point, the in vitro and in vivo preparations also respond similarly to temperature perturbations.
Our second observation, however, is that, at higher temperatures, the pyloric network oscillates more slowly in vivo than in vitro. This trend became apparent at temperatures of 15°C and higher, although it was only statistically significant at the highest temperature tested. One reason for the lower frequencies in vivo could be restrictions imposed by the musculoskeletal system. At typical experimental temperatures of 10–13°C, the pyloric musculature in crustaceans exhibits pyloric-timed phasic contractions (Hooper et al. 1986; Jorge-Rivera and Marder 1996; Morris and Hooper 2001; Thuma et al. 2003), and muscle activity patterns recorded in vivo are consistent with the in vitro pyloric rhythm (Morris and Maynard 1970; Rezer and Moulins 1983). Small-amplitude tooth movements in crabs are timed to the pyloric rhythm (Diehl et al. 2013; Weigeldt et al. 2002), indicating that the muscular and mechanical systems are well-tuned to the neuronal signals they receive. This makes sense because it is energetically favorable for the rhythmicity of a neuronal system to be similar to the resonant frequency of the mechanical body that it controls (Goodman et al. 2000). Studies in lobsters, however, suggest that, at faster pyloric frequencies (above ∼1 Hz), the temporal dynamics of certain pyloric muscles are too slow to allow them to fully relax between cycles (Morris et al. 2000). Similarly, the pyloric musculature in the shrimp is unable to follow rhythmic motor neuron stimulation at all frequencies without the presence of certain neuromodulators (Meyrand and Marder 1991). Temperature perturbation in isolated muscle preparations in the spiny lobster Panulirus interruptus also demonstrated that muscle contractions at higher temperatures are reduced and virtually absent at 16°C (Thuma et al. 2013), suggesting that the upper limit of the speed of the pyloric musculature is lower than that of the underlying neuronal circuitry, and the in vivo pyloric rhythm may be restricted to lower frequencies at high temperatures.
What mechanism could lead to reduced pyloric cycle frequency in vivo? The STNS is sensitive to sensory feedback and neuromodulators (both of which are largely absent in vitro) that may be involved in modifying pyloric frequencies in vivo at high temperatures. The effects of sensory feedback in in vivo closed-loop conditions on CPG activity are not easily generalizable. In biological CPGs, the removal of sensory feedback can either increase or decrease cycle frequency, depending on the system in question. Stimulating afferent nerves in cats reduces stepping frequency during walking (Whelan and Pearson 1997). Conversely, the frequency of the locust flight pattern generator is reduced when sensory afferents are removed (Pearson and Wolf 1987), and feedback strength can modulate wing beat frequency (Ausborn et al. 2007). There are myriad complex interactions between sensorimotor pathways in the intact animal, making it difficult to discern the specific effect of any particular pathway on CPG output.
The frequency and phase relationships of STG motor patterns are influenced by several types of sensory and neuromodulatory inputs (Daur et al. 2009; Eisen and Marder 1984; Flamm and Harris-Warrick 1986; Harris-Warrick et al. 1998; Katz et al. 1989; Marder and Weimann 1992; Rezer and Moulins 1992; Smarandache et al. 2008; Weimann et al. 1993). While the effects of neuromodulators and sensory feedback have been closely examined under typical control temperatures, their intersection with temperature effects has not been closely examined. Preliminary in vitro studies indicate that the influence of neuromodulators on the pyloric frequency and phases is temperature dependent (Haddad and Marder 2013). Potentially, temperature-related changes in neuromodulatory input may modify motor neuron activity at high temperatures. As discussed earlier, the presence of sensory feedback is another potential cause for reduced maximal pyloric frequencies in vivo. Further studies are necessary to precisely determine which aspects of the in vivo environment are responsible for producing the divergence of the in vivo and in vitro frequencies at elevated temperatures.
Recent modeling studies have shown that varying the underlying parameters of a neuronal oscillator can result in large differences in pyloric rhythm output at extreme temperatures (Caplan et al. 2014; Rinberg et al. 2013). Our findings indicate that greater variability in the pyloric rhythm at high temperature is also seen in vivo (Figs. 5 and 6). It is thus likely that the intrinsic cellular properties of the pyloric rhythm, sensory feedback, and presence of neuromodulators all significantly contribute to determining the pyloric activity across a large range of temperatures.
Temperature Influences on the Phasing of the Pyloric Motor Neurons
Between temperatures of 11°C and 26°C, phases were generally temperature independent, as indicated by Q10 values, which were not significantly different from 1. These results indicate that phase constancy, widely known to be present in vitro, is also present in the intact animal over a wide temperature and frequency range and is, therefore, robust to the presence of sensory feedback and neuromodulators. Previous in vitro studies have shown that the LP neuron is particularly susceptible to a failure to fire at temperatures approaching 27°C (Tang et al. 2012), even as the PD and PY neurons remain active. We see a similar occurrence in vivo; the LP offset phase advances at temperatures nearing 26°C.
At the temperatures analyzed, pyloric frequency generally increased at a steady rate with temperature (Fig. 3). However, we also witnessed more than one instance in C. pagurus in which the frequency changed rapidly, but the phases were not well maintained. A prominent example of this lack of phase maintenance is shown in Fig. 8. Interestingly, previous in vitro studies show that, when the pyloric frequency is altered via current injection (Hooper 1997a), or undergoes natural cycle-to-cycle variability (Bucher et al. 2005), phases are largely, but not perfectly, compensated. Transient phase changes have previously been seen under in vitro decentralized conditions when the sensory gastropyloric receptors were stimulated, inducing a nearly threefold increase in pyloric frequency within 15 s (Katz and Harris-Warrick 1990). Serotonin directly excites the AB neuron (Eisen and Marder 1984; Marder and Eisen 1984); Katz and Harris-Warrick (1990) suggested that gastropyloric receptors influence the pyloric network via an enhancement of bursting in AB, but not in the PDs to which AB is electrically coupled. Transient increases in frequency without concomitant phase compensation may happen because the neuromodulator-induced increase in AB cycle frequency occurs with faster dynamics than do the changes in synaptic and membrane properties necessary for phase compensation. More generally, it may be that the upper physiological limit of the rate of change in pyloric frequency is greater than the upper physiological limit of the rate of phase adaptation, and this difference in the time scale between frequency change and phase compensation is only unmasked during rapid changes in frequency.
Temperature dependence of cellular and network properties also has implications for the behaviors controlled by those networks. Our present results raise the question of how temperature affects the stomatogastric musculature. Proper phasing of the motor neurons is crucial for the pyloric filter to function; our results demonstrate that, at temperatures in the range at which crabs may feed in the wild, a proper pyloric rhythm is often present. These findings align with recent results demonstrating that the ability of the lobster (Panuliris interruptus) to digest food is not impaired at higher temperatures (Thuma et al. 2013). Evidence is also mounting that previous exposure to extreme temperatures improves the ability of neuronal circuits to withstand wide temperature ranges (Robertson and Money 2012; Tang et al. 2012). Many rhythmic behaviors in other poikilotherms are significantly affected by temperature, including the goldfish startle-escape response (Preuss and Faber 2003) and the chirping frequency of cricket song (Doherty 1985). Other motor behaviors, such as the flight CPG in the deafferented locust, display robust temperature compensation (Foster and Robertson 1992; Robertson et al. 1996; Robertson and Money 2012), which may result from underlying processes with similar Q10 values but converse effects (Robertson and Money 2012).
It is difficult to predict a priori the effect of temperature on any particular neuronal circuit, since each has its own unique set of temperature-sensitive subcellular elements. However, temperature modulation has been used as a tool to trace the mechanisms of temperature dependence of complex behaviors in both poikilotherms and warm-blooded animals. Selective cooling of specific brain structures in the songbird (Fee and Long 2011; Long and Fee 2008) and frog (Yamaguchi et al. 2008), for example, has aided in locating brain regions that are involved in vocalization. Precise temperature control can thus serve as a method for dissecting a neuronal network and gaining further insight into its function (Robertson and Money 2012).
Conclusion
In this study, we found that the pyloric cycle frequencies in intact C. borealis and C. pagurus exhibited a significantly positive relationship with temperature, while phases were generally maintained. Additionally, at elevated temperatures, the pyloric frequency of C. borealis was higher in vitro than it was in vivo.
Proper motor neuron phasing is necessary for rhythmic motor systems to function correctly. One strategy to ensure proper phasing at all times during an animal's lifetime could be to restrict CPG frequencies to a narrow range, so that motor neuron phasing is maintained by the uniform delays between bursts from each neuron type. An alternative strategy, employed by the STG, is to allow the frequency and burst delays to vary while keeping the phases of each neuron type constant. Such a strategy may provide greater versatility to the nervous system, allowing it to function properly in a wide range of environmental conditions.
GRANTS
This work was supported by National Science Foundation Graduate Research Fellowship and Integrative Graduate Education and Research Traineeship (to W. Soofi), National Institutes of Health Grant NS-81013 (to E. Marder), a Grass Fellowship (to M. L. Goeritz), and Deutsche Forschungsgemeinschaft STE 937/7-1 and STE 937/9-1 (to W. Stein). We thank Ulm University for financial support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W. Soofi, M.L.G., E.M., and W. Stein conception and design of research; W. Soofi and M.L.G. performed experiments; W. Soofi, M.L.G., and T.J.K. analyzed data; W. Soofi, M.L.G., A.A.P., E.M., and W. Stein interpreted results of experiments; W. Soofi, M.L.G., T.J.K., and W. Stein prepared figures; W. Soofi drafted manuscript; W. Soofi, M.L.G., T.J.K., A.A.P., E.M., and W. Stein edited and revised manuscript; W. Soofi, M.L.G., T.J.K., A.A.P., E.M., and W. Stein approved final version of manuscript.
REFERENCES
- Ausborn J, Stein W, Wolf H. Frequency control of motor patterning by negative sensory feedback. J Neurosci 27: 9319–9328, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrento S, Marques A, Vaz-Pires P, Nunes ML. Live shipment of immersed crabs Cancer pagurus from England to Portugal and recovery in stocking tanks: stress parameter characterization. ICES J Mar Sci 67: 435–443, 2010 [Google Scholar]
- Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Front Behav Neurosci 4: 36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D, Prinz AA, Marder E. Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci 25: 1611–1619, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büschges A, Scholz H, El Manira A. New moves in motor control. Curr Biol 21: R513–R524, 2011 [DOI] [PubMed] [Google Scholar]
- Cao XJ, Oertel D. Temperature affects voltage-sensitive conductances differentially in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 94: 821–832, 2005 [DOI] [PubMed] [Google Scholar]
- Caplan J, Williams AH, Marder E. Many parameter sets in a multicompartment model oscillator are robust to temperature perturbations. J Neurosci 34: 4963–4975, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier S, Jan Ijspeert A, Ryczko D, Nagy F, Cabelguen JM. Organisation of the spinal central pattern generators for locomotion in the salamander: biology and modelling. Brain Res Rev 57: 147–161, 2008 [DOI] [PubMed] [Google Scholar]
- Cohen AH, Ermentrout GB, Kiemel T, Kopell N, Sigvardt KA, Williams TL. Modelling of intersegmental coordination in the lamprey central pattern generator for locomotion. Trends Neurosci 15: 434–438, 1992 [DOI] [PubMed] [Google Scholar]
- Daur N, Nadim F, Stein W. Regulation of motor patterns by the central spike-initiation zone of a sensory neuron. Eur J Neurosci 30: 808–822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl F, White RS, Stein W, Nusbaum MP. Motor circuit-specific burst patterns drive different muscle and behavior patterns. J Neurosci 33: 12013–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JA. Temperature coupling and “trade-off” phenomena in the acoustic communication system of the cricket, Gryllus bimaculatus De Geer (Gryllidae). J Exp Biol 114: 17–35, 1985 [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Nichols A, Santamaria CA, League-Pike PE, Krediet CJ, Perez KO, Shulman MJ. Predation risk, prey abundance, and the vertical distribution of three brachyuran crabs on gulf of Maine shores. J Crustacean Biol 29: 523–531, 2009 [Google Scholar]
- Eisen JS, Marder E. A mechanism for production of phase shifts in a pattern generator. J Neurophysiol 51: 1375–1393, 1984 [DOI] [PubMed] [Google Scholar]
- El Manira A, Kyriakatos A, Nanou E. Beyond connectivity of locomotor circuitry-ionic and modulatory mechanisms. Prog Brain Res 187: 99–110, 2010 [DOI] [PubMed] [Google Scholar]
- Fee MS, Long MA. New methods for localizing and manipulating neuronal dynamics in behaving animals. Curr Opin Neurobiol 21: 693–700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. I. Effects on motor pattern and activity of neurons within the pyloric circuit. J Neurophysiol 55: 847–865, 1986 [DOI] [PubMed] [Google Scholar]
- Foster JA, Robertson RM. Temperature dependency of wing-beat frequency in intact and deafferented locusts. J Exp Biol 162: 295–312, 1992 [Google Scholar]
- Goodman L, Riley MA, Mitra S, Turvey MT. Advantages of rhythmic movements at resonance: minimal active degrees of freedom, minimal noise, and maximal predictability. J Mot Behav 32: 3–8, 2000 [DOI] [PubMed] [Google Scholar]
- Greenberg I, Manor Y. Synaptic depression in conjunction with A-current channels promote phase constancy in a rhythmic network. J Neurophysiol 93: 656–677, 2005 [DOI] [PubMed] [Google Scholar]
- Gutierrez GJ, Grashow RG. Cancer borealis stomatogastric nervous system dissection. J Vis Exp 25: 1207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad SA, Marder E. Modulator induced changes in motor patterns are temperature compensated. Program No. 559.08. In: 2013 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2013 [Google Scholar]
- Haefner PA. Aspects of the biology of the jonah crab, Cancer borealis Stimpson, 1859 in the mid-Atlantic Bight. J Nat Hist 11: 303–320, 1977 [Google Scholar]
- Harris-Warrick RM. Neuromodulation and flexibility in central pattern generator networks. Curr Opin Neurobiol 21: 685–692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, Skarbinski J. Distributed effects of dopamine modulation in the crustacean pyloric network. Ann N Y Acad Sci 860: 155–167, 1998 [DOI] [PubMed] [Google Scholar]
- Hartline DK, Maynard DM. Motor patterns in the stomatogastric ganglion of the lobster Panulirus argus. J Exp Biol 62: 405–420, 1975 [DOI] [PubMed] [Google Scholar]
- Hedrich UB, Diehl F, Stein W. Gastric and pyloric motor pattern control by a modulatory projection neuron in the intact crab Cancer pagurus. J Neurophysiol 105: 1671–1680, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich UBS, Stein W. Characterization of a descending pathway: activation and effects on motor patterns in the brachyuran crustacean stomatogastric nervous system. J Exp Biol 211: 2624–2637, 2008 [DOI] [PubMed] [Google Scholar]
- Heinzel HG, Weimann JM, Marder E. The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J Neurosci 13: 1793–1803, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SL. Phase maintenance in the pyloric pattern of the lobster (Panulirus interruptus) stomatogastric ganglion. J Comput Neurosci 4: 191–205, 1997a [DOI] [PubMed] [Google Scholar]
- Hooper SL. The pyloric pattern of the lobster (Panulirus interruptus) stomatogastric ganglion comprises two phase-maintaining subsets. J Comput Neurosci 4: 207–219, 1997b [DOI] [PubMed] [Google Scholar]
- Hooper SL. Transduction of temporal patterns by single neurons. Nat Neurosci 1: 720–726, 1998 [DOI] [PubMed] [Google Scholar]
- Hooper SL, O'Neil MB, Wagner R, Ewer J, Golowasch J, Marder E. The innervation of the pyloric region of the crab, Cancer borealis: homologous muscles in decapod species are differently innervated. J Comp Physiol A 159: 227–240, 1986 [DOI] [PubMed] [Google Scholar]
- Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A 100: 16089–16094, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM. Elevated temperature alters the ionic dependence of amine-induced pacemaker activity in a conditional burster neuron. J Comp Physiol A 170: 201–209, 1992 [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM. Temperature sensitivity of graded synaptic transmission in the lobster stomatogastric ganglion. J Exp Biol 156: 267–285, 1991 [DOI] [PubMed] [Google Scholar]
- Jorge-Rivera JC, Marder E. TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis. J Comp Physiol A 179: 741–751, 1996 [DOI] [PubMed] [Google Scholar]
- Katz PS. Evolution and development of neural circuits in invertebrates. Curr Opin Neurobiol 17: 59–64, 2007 [DOI] [PubMed] [Google Scholar]
- Katz PS, Eigg MH, Harris-Warrick RM. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol 62: 558–570, 1989 [DOI] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM. Neuromodulation of the crab pyloric central pattern generator by serotonergic/cholinergic proprioceptive afferents. J Neurosci 10: 1495–1512, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006 [DOI] [PubMed] [Google Scholar]
- Krediet CJ, Donahue MJ. Growth-mortality trade-offs along a depth gradient in Cancer borealis. J Exp Mar Bio Ecol 373: 133–139, 2009 [Google Scholar]
- Lenz PH, Hower AE, Hartline DK. Temperature compensation in the escape response of a marine copepod, Calanus finmarchicus (Crustacea). Biol Bull 209: 75–85, 2005 [DOI] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456: 189–194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y, Bose A, Booth V, Nadim F. Contribution of synaptic depression to phase maintenance in a model rhythmic network. J Neurophysiol 90: 3513–3528, 2003 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007 [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996 [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS. Electrically coupled pacemaker neurons respond differently to same physiological inputs and neurotransmitters. J Neurophysiol 51: 1362–1374, 1984 [DOI] [PubMed] [Google Scholar]
- Marder E, Weimann J. Modulatory control of multiple task processing in the stomatogastric nervous system. In: Neurobiology of Motor Programme Selection: New Approaches to the Study of Behavioural Choice, edited by Kien J, McCrohan C, Winlow W. New York: Pergamon, 1992 [Google Scholar]
- Maynard DM, Dando MR. The structure of the stomatogastric neuromuscular system in Callinectes sapidus, Homarus americanus and Panulirus argus (Decapoda Crustacea). Philos Trans R Soc Lond B Biol Sci 268: 161–220, 1974 [DOI] [PubMed] [Google Scholar]
- Maynard DM, Selverston AI. Organization of the stomatogastric ganglion of the spiny lobster IV: the pyloric system. J Comp Physiol 100: 161–182, 1975 [Google Scholar]
- Metzger R, Sartoris FJ, Langenbuch M, Portner HO. Influence of elevated CO(2) concentrations on thermal tolerance of the edible crab Cancer pagurus. J Therm Biol 32: 144–151, 2007 [Google Scholar]
- Meyrand P, Marder E. Matching neural and muscle oscillators: control by FMRFamide-like peptides. J Neurosci 11: 1150–1161, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol 14: 362–369, 2004 [DOI] [PubMed] [Google Scholar]
- Morris J, Maynard DM. Recordings from the stomatogastric nervous system in intact lobsters. Comparative Biochem and Physiol 33: 969–974, 1970 [Google Scholar]
- Morris LG, Hooper SL. Mechanisms underlying stabilization of temporally summated muscle contractions in the lobster (Panulirus) pyloric system. J Neurophysiol 85: 254–268, 2001 [DOI] [PubMed] [Google Scholar]
- Morris LG, Thuma JB, Hooper SL. Muscles express motor patterns of non-innervating neural networks by filtering broad-band input. Nat Neurosci 3: 245–250, 2000 [DOI] [PubMed] [Google Scholar]
- Myers BR, Sigal YM, Julius D. Evolution of thermal response properties in a cold-activated TRP channel. PLos One 4: e5741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Booth V, Bose A, Manor Y. Short-term synaptic dynamics promote phase maintenance in multi-phasic rhythms. Neurocomputing 52: 79–87, 2003 [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature 417: 343–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG, Wolf H. Comparison of motor patterns in the intact and deafferented flight system of the locust. J Comp Physiol A 160: 259–268, 1987 [Google Scholar]
- Preuss T, Faber DS. Central cellular mechanisms underlying temperature-dependent changes in the goldfish startle-escape behavior. J Neurosci 23: 5617–5626, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun MJ. The cancroid crabs of America of the families Euryalidae, Portunidae, Atelecyclidao, Cancridao and Xanthidae. In: US National Museum Bulletin.Washington, DC: Smithsonian Institute, 1930, p. 193 [Google Scholar]
- Rezer E, Moulins M. Expression of the crustacean pyloric pattern generator in the intact animal. J Comp Physiol 153: 17–28, 1983 [Google Scholar]
- Rezer E, Moulins M. Humoral induction of pyloric rhythmic output in lobster stomatogastric ganglion: in vivo and in vitro studies. J Exp Biol 163: 209–230, 1992 [DOI] [PubMed] [Google Scholar]
- Rinberg A, Taylor AL, Marder E. The effects of temperature on the stability of a neuronal oscillator. PLoS Comput Biol 9: e1002857, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RM, Money TGA. Temperature and neuronal circuit function: compensation, tuning and tolerance. Curr Opin Neurobiol 22: 724–734, 2012 [DOI] [PubMed] [Google Scholar]
- Robertson RM, Xu H, Shoemaker KL, Dawson-Scully K. Exposure to heat shock affects thermosensitivity of the locust flight system. J Neurobiol 29: 367–383, 1996 [DOI] [PubMed] [Google Scholar]
- Selverston AI, Miller JP. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. I. Pyloric system. J Neurophysiol 44: 1102–1121, 1980 [DOI] [PubMed] [Google Scholar]
- Skinner FK, Mulloney B. Intersegmental coordination in invertebrates and vertebrates. Curr Opin Neurobiol 8: 725–732, 1998 [DOI] [PubMed] [Google Scholar]
- Smarandache CR, Daur N, Hedrich UBS, Stein W. Regulation of motor pattern frequency by reversals in proprioceptive feedback. Eur J Neurosci 28: 460–474, 2008 [DOI] [PubMed] [Google Scholar]
- Soofi W, Archila S, Prinz AA. Co-variation of ionic conductances supports phase maintenance in stomatogastric neurons. J Comput Neurosci 33: 77–95, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik LL, Mackenzie CL, Morse WW. Distribution and abundance of four brachyuran crabs on the northwest Atlantic shelf. Fish Bull 89: 473–492, 1991 [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A 195: 989–1009, 2009 [DOI] [PubMed] [Google Scholar]
- Tang LS, Goeritz ML, Caplan JS, Taylor AL, Fisek M, Marder E. Precise temperature compensation of phase in a rhythmic motor pattern. PLoS Biol 8: e1000469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LS, Taylor AL, Rinberg A, Marder E. Robustness of a rhythmic circuit to short- and long-term temperature changes. J Neurosci 32: 10075–10085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci 5: 817–824, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuma JB, Hobbs KH, Burstein HJ, Seiter NS, Hooper SL. Temperature sensitivity of the pyloric neuromuscular system and its modulation by dopamine. PLos One 8: e67930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuma JB, Morris LG, Weaver AL, Hooper SL. Lobster (Panulirus interruptus) pyloric muscles express the motor patterns of three neural networks, only one of which innervates the muscles. J Neurosci 23: 8911–8920, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigeldt D, Böhm H, Heinzel HG. Sensory feedback in the operating stomatogastric nervous system of the crab ( Cancer pagurus). In: The Crustacean Nervous System, edited by Wiese K. Berlin: Springer-Verlag, 2002 [Google Scholar]
- Weimann JM, Marder E, Evans B, Calabrese RL. The effects of SDRNFLRFamide and TNRNFLRFamide on the motor patterns of the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol 181: 1–26, 1993 [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Pearson KG. Comparison of the effects of stimulating extensor group I afferents on cycle period during walking in conscious and decerebrate cats. Exp Brain Res 117: 444–452, 1997 [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Gooler D, Herrold A, Patel S, Pong WW. Temperature-dependent regulation of vocal pattern generator. J Neurophysiol 100: 3134–3143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurov Y, Brezina V. Temperature compensation of neuromuscular modulation in aplysia. J Neurophysiol 94: 3259–3277, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]