Abstract
Most of our sensory experiences are gained by active exploration of the world. While the ability to distinguish sensory inputs resulting of our own actions (termed reafference) from those produced externally (termed exafference) is well established, the neural mechanisms underlying this distinction are not fully understood. We have previously proposed that vestibular signals arising from self-generated movements are inhibited by a mechanism that compares the internal prediction of the proprioceptive consequences of self-motion to the actual feedback. Here we directly tested this proposal by recording from single neurons in monkey during vestibular stimulation that was externally produced and/or self-generated. We show for the first time that vestibular reafference is equivalently canceled for self-generated sensory stimulation produced by activation of the neck musculature (head-on-body motion), or axial musculature (combined head and body motion), when there is no discrepancy between the predicted and actual proprioceptive consequences of self-motion. However, if a discrepancy does exist, central vestibular neurons no longer preferentially encode vestibular exafference. Specifically, when simultaneous active and passive motion resulted in activation of the same muscle proprioceptors, neurons robustly encoded the total vestibular input (i.e., responses to vestibular reafference and exafference were equally strong), rather than exafference alone. Taken together, our results show that the cancellation of vestibular reafference in early vestibular processing requires an explicit match between expected and actual proprioceptive feedback. We propose that this vital neuronal computation, necessary for both accurate sensory perception and motor control, has important implications for a variety of sensory systems that suppress self-generated signals.
Keywords: sensory coding, self-motion, response selectivity efference copy, voluntary movement, vestibular, proprioception
the ability to distinguish between sensory inputs registering unexpected events (termed sensory exafference) and those resulting from our own actions (termed sensory reafference) is something we take for granted, and yet is vital for perceptual stability and accurate motor control. Over the last several decades, the dominant theory has been that the brain differentiates between these two classes of sensory input by sending a parallel “efference copy” of its motor command to sensory areas (von Holst and Mittelstaedt 1950). In turn, this anticipatory signal is subtracted from the incoming sensory signal to cancel the self-generated portion (i.e., reafference) and create a neural representation of the outside world (i.e., exafference) (reviewed in Cullen 2004; Crapse and Sommer 2008). More recent behavioral investigations have generalized this idea by proposing that an internal prediction of the sensory consequences of our actions is compared with actual sensory input (reviewed in Kawato et al. 2003; Wolpert and Ghahramani 2000; Wolpert et al. 2011).
Considerable progress has been made in understanding the mechanism underlying the brain's ability to distinguish between vestibular inputs resulting from self-generated vs. externally applied self-motion at the level of single neurons. Specifically, we and others have shown that neurons at the first central stage of vestibular processing make the distinction between reafference and exafference (McCrea et al. 1999; Roy and Cullen 2001). Neurons that respond robustly to externally applied head rotations display markedly reduced responses to the same movement when it is the result of active head motion. This selectivity is theoretically beneficial: while strong responses to unexpected (i.e., exafferent) vestibular inputs function to optimize compensatory postural responses, the cancellation of vestibular reafference serves to prevent the production of inappropriate motor commands that would oppose voluntary movements.
Our laboratory has previously provided evidence that the brain uses a mechanism that compares the internal prediction of the proprioceptive consequences of self-motion to the actual resultant feedback to cancel vestibular inputs that arise from self-generated movements (i.e., vestibular reafference; Roy and Cullen 2004). Specifically, we have proposed that the significant input carried by vestibular afferents during active movements (Cullen and Minor 2002; Jamali et al. 2009; Sadeghi et al. 2009) to the vestibular nuclei (VN) is canceled in conditions where there is a match between predicted and actual proprioceptive feedback. While this proposed mechanism accounts for all existing neurophysiological findings to date, it also makes the surprising and explicit prediction that vestibular reafference will not be canceled under certain conditions. Specifically, when simultaneously occurring active and passive motion produces activation of the same muscle proprioceptors, there will be a discrepancy between the predicted and actual proprioceptive consequences of self-motion and accordingly, in this condition, vestibular reafference would not be canceled. However, to date, this prediction had not yet been tested.
Accordingly, the goal of the present study was to directly test whether vestibular reafference is suppressed when there is a match between the predicted and actual proprioceptive consequences of self-motion, but not when there is a discrepancy. To address this question, we recorded from single neurons in monkeys trained to make both body and head movements. We found that vestibular reafference resulting from self-generated activation of the head and/or body musculature was similarly canceled. However, consistent with our prediction, vestibular reafference was not canceled when concurrent passive motion simultaneously activated the same muscle proprioceptors. Instead, we found that neurons robustly encoded both reafference and exafference. Thus our results establish that vestibular reafference is only canceled during self-motion if there is a match between expected and actual proprioceptive feedback.
METHODS
Three rhesus monkeys (Macaca mulatta) were prepared for chronic extracellular recording using aseptic surgical techniques. All experimental protocols were approved by the McGill University Animal Care Committee and were in compliance with the guidelines of the Canadian Council on Animal Care.
Surgical Procedures
The surgical techniques and anesthesia protocols were similar to those previously described by Roy and Cullen (2001). Briefly, under surgical levels of isoflurane (2–3% initially, and 0.8–1.5% for maintenance), an 18-mm-diameter eye coil (3 loops of Teflon-coated stainless steel wire) was attached to the sclera beneath the conjunctiva of one eye. In addition, a dental acrylic implant was fastened to the animal's skull using stainless steel screws. The implant held in place a stainless steel post used to restrain the animal's head, and a stainless steel recording chamber that was positioned to access the VN (posterior and lateral angles of 28° and 30°, respectively). After the surgery, buprenorphine (0.01 mg/kg im) was utilized for postoperative analgesia. Animals were given 2 wk to recover from the surgery before any experiments were performed.
Experimental Setup
Monkeys were comfortably seated in a stationary primate chair. The chair was placed in the experimental apparatus such that the animal's head was centered within a 1-m3 magnetic field coil system (CNC Engineering). The primate chair was mounted to the top of a vestibular turntable that was used to apply whole body rotations about an earth-vertical axis (see Head-restrained paradigms). Gaze, head and body position were measured using the magnetic search coil technique (Fuchs and Robinson 1966; Judge et al. 1980): 1) gaze position was recorded with the scleral coil that had been surgically implanted beneath the conjunctiva as described above, 2) head position was recorded using a second search coil that was securely fastened to the monkey's head implant, and 3) body position was recorded using a third search coil, fixed to a primate jacket worn by the monkey (Lomir Biomedical) at the level of thoracic vertebra 7 (T7). Level T7 was chosen because it is well below the lowest level of neck muscle insertion (T3), and thus recordings would not be confounded by neck movements (McCluskey and Cullen 2007). The location of T7 was verified by X-ray and/or spinal palpation. Prior to experimental sessions, the monkeys were habituated to wearing the vest. Note that monkeys quickly adapted to wearing the vest and were never observed manipulating the jacket with their feet or hands during the experiments. This was verified by online video observation of the monkey during experimental trials. Importantly, the vest was tightly securely around the monkey's body, and the coil was inserted in a pocket in the back, which was inaccessible to the monkey.
A specially designed head-holder (Roy and Cullen 1998) enabled us to either completely immobilize the animal's head (head-restrained condition) or allow the animal to rotate its head freely about exclusively the yaw (i.e., earth-vertical) axis (head-unrestrained condition). A torque motor (Kollmorgen) attached to the monkey's head-holder was used to apply head-on-body rotations about an earth-vertical axis (see Head-unrestrained paradigms below). Monkeys were trained to track a small (0.3° in diameter) visual target for a juice reward. The target was generated by a HeNe laser and projected onto a white cylindrical screen located 60 cm away from the monkey's eyes. The target was positioned on the screen by a pair of mirrors mounted on two computer-controlled galvanometers (General Scanning).
Data Acquisition
Extracellular single-unit activity was recorded using epoxy-insulated tungsten microelectrodes (7–10 MΩ impedance, Frederick-Haer, Bowdoinham, ME) as has been described elsewhere (Roy and Cullen 2001). The location of VN was determined relative to the abducens nucleus, which was identified on the basis of its stereotypical neuronal responses during eye movements (Cullen and McCrea 1993; Sylvestre and Cullen 1999). We recorded from a small region of the brain corresponding to the rostral-medial and ventral-lateral VN (Roy and Cullen 2002). Turntable velocity was measured using an angular velocity sensor (Watson Industries, Eau Claire, WI). Gaze, head and body position were measured using the magnetic search coil technique as described above (Fuchs and Robinson 1966; Judge et al. 1980). During experiments, unit activity, horizontal gaze, head, body and target positions, and table velocity were recorded on DAT tape for later playback. Action potentials were discriminated during playback using a windowing circuit (BAK) that was manually set to generate a pulse coincident with the rising phase of each action potential. Gaze, head, body, target position and table velocity signals were low-pass filtered at 250 Hz (8 pole anti-aliasing Bessel filter) and sampled at 1,000 Hz. Target, turntable motion, torque motor, and data displays were controlled on-line by a UNIX-based real-time data-acquisition system (REX) (Hayes and Optican 1982).
Behavioral Paradigms
Head-restrained paradigms.
We focused on a well-characterized subclass of neurons in the VN [termed vestibular-only (VO) neurons], which are sensitive to passive vestibular stimulation but not eye movements (Cullen and McCrea 1993; Fuchs and Kimm 1975; Keller and Daniels 1975; Roy and Cullen 2001; Scudder and Fuchs 1992; Tomlinson and Robinson 1984).
To verify each cell's lack of sensitivity to eye movements, neuronal responses were first recorded in the head-restrained condition as monkeys made 1) saccadic eye movements and ocular fixations to follow a target stepped between horizontal positions over a range of ±30°, and 2) smooth pursuit eye movements to track sinusoidal target motion (0.5 Hz, 40°/s peak velocity). Next, to characterize neuronal responses to passive vestibular stimulation, we applied both 1) sinusoidal whole body rotations (1 Hz, 40°/s peak velocity), and 2) whole body rotations with head velocity trajectories that mimicked those made during active gaze shifts by the same monkey in the head-unrestrained condition (termed “active-like motion” profile). This latter stimulus was used to facilitate comparison of neuronal responses to passive and active (see below) head motion. Blocks of trials of 10 or more of each stimulus (i.e., cycles of sinusoidal rotation or active-like head movements) were applied in complete darkness. The sinusoidal rotation stimulus was also applied in a condition where the monkey suppressed its vestibulo-ocular reflex (VOR) by fixating a target that moved with its head. Since in this condition the monkey's eye did not move in the orbit, we could further verify each neuron's lack of eye movement sensitivity by establishing its response was comparable to that observed when stimulation was applied in darkness. We confirmed that monkeys did not generate neck torque during each whole body rotation condition by directly measuring torque via a sensor in the head-restraint system.
Head-unrestrained paradigms.
After a neuron was fully characterized in the head-restrained condition, the monkey's head was slowly and carefully released to ensure that isolation was maintained. Once released, the monkey was able to rotate its head freely about the yaw axis. Throughout this paper, we focus on the population of neurons for which we maintained isolation during active head motion (n = 42). We completed blocks of trials in which 1) monkeys oriented to light targets located 20° on either side of midline (40° eye-head gaze shifts) for a juice reward with their vest tethered to the chair (n = 26), and 2) monkeys were untethered and encouraged to orient to food targets located ∼30° on either side of midline (∼60° gaze shifts) (n = 21). In the latter case, gaze shifts were initiated by eye motion toward the target, followed by the production of head then the body motion.
In addition, for cells that remained isolated, we recorded neural responses during 1) simultaneous active head-on-body and passive whole body rotation (n = 21); 2) the application of brief (<100 ms) stereotyped perturbations of the head-on-body as the monkey made active gaze head-on-body shifts to light targets [note that single trials in which we applied unexpected head transients were randomized (∼15%) between trials in which monkeys freely made unrestrained active rotations] (n = 13); and 3) the application of resistive torque applied to the head which reduced active head-on-body movements by ∼50% [single trials in which we applied unexpected head torque were again randomized (∼15%) between trials in which monkeys freely made unrestrained active rotations] (n = 11).
Analysis of Neuronal Discharges
We recorded from a total of 42 neurons during active and passive movements from 3 monkeys (monkey R, n = 17, monkey A, n = 12 and monkey V, n = 13). Data were imported into the Matlab (The MathWorks, Natick, MA) programming environment for analysis. Recorded gaze, head and body position signals were digitally filtered with zero-phase at 60 Hz using a 51st order finite-impulse-response filter with a Hamming window. Eye position was calculated from the difference between gaze and head position signals. Head-on-body position was calculated as the difference between head and body position. Gaze, eye, head, head-on-body and body position signals were digitally differentiated to produce velocity signals. Neural firing rate was represented using a spike density function in which a Gaussian was convolved with the spike train (SD of 5 ms; Cullen et al. 1996).
To determine whether a unit could be classified as a VO neuron, we first verified that it was unresponsive to eye position and/or velocity by analyzing periods of steady fixation and saccade-free smooth pursuit using a multiple regression analysis (Roy and Cullen 1998, 2001). In addition, spike trains were assessed to confirm that neurons neither paused nor burst during saccades.
A least-squared regression analysis was then used to describe each unit's response to head motion stimulation during passive whole body rotations:
| (1) |
where f̂r is the estimated firing rate; Sv-passive and Sa-passive are coefficients representing sensitivities to passive head velocity and acceleration (i.e., passive sensitivity), respecvtively; b is a bias term; and Ḣp and Ḧp are passive head velocity and head acceleration, respectively. Similarly each unit's response to active head motion was described using the following equation:
| (2) |
where Sv-active and Sa-active are coefficients representing sensitivities to active head velocity and acceleration (i.e., active sensitivity), respectively, and Ḣa and Ḧa are active head velocity and head acceleration, respectively. Note that the same equation was used both when the active movement was generated by moving the head relative to the body and when the head and body moved together [i.e., the latter portion eye-head-body gaze shifts during which there is minimal head-on-body velocity movement (i.e., <10°/s)].
A comparable approach was next used to predict each unit's response to combined active and passive head motion using the following equation:
| (3) |
where Sv-passive and Sa-passive are the same coefficients estimated during passive rotation (Eq. 1), and İ and Ï are input velocity and acceleration, respectively. We generated two different predictions: 1) the “total motion” prediction for which the total head motion trajectory (i.e., head velocity and acceleration measured by search coil attached to the monkey's head post) was the input; and 2) the “passive-only” prediction for which only the passive head motion component of the trajectory (i.e., motion passively applied by the vestibular turntable or torque motor) was the input. As these neurons are predominantly sensitive to the velocity component of motion stimuli, and analysis of the acceleration components yielded qualitatively the same results, we focus our report on the neuronal sensitivities to the velocity component in the results section.
Next we wanted to be able to compare the sensitivity of a given neuron to active motion that occurred concurrently with a passive rotations to active motion generated in isolation. We thus estimated its sensitivity to each of the two components of motion (i.e., passive and active) during paradigms where there was concurrent passive and active motions using the following equation:
| (4) |
Where Sv-passive, Sa-passive, Sv-active, and Sa-active are coefficients estimated for data collected during combined active and passive motion.
For the first condition, in which monkeys generated active motion during whole body rotation, passive motion was measured by an angular velocity sensor attached to the table. Active motion was then computed from the difference between total head velocity (measured from the head coil) and this measurement of passive motion.
For the second condition, in which monkeys generated active motion and we applied passive head-on-body perturbations, we obtained a measurement of the passive component of motion by taking the average of the head coil signal when the perturbation was applied in isolation. Then to obtain a measurement of the active component of motion, we aligned this passive perturbation with the peak of total head velocity occurring in response to the perturbation and subtracted out the passive component. The delivered perturbation was very short (<100 ms). We specifically focused our analysis on neuronal activity during the perturbation, such that any subsequent changes in motor plan that may have occurred after the perturbation would not have affected our results.
To quantify the ability of the linear regression analyses described above in Eqs. 1–4 to model neuronal discharges, the variance accounted for (VAF) provided by each regression equation was determined. The VAF was computed as:
| (5) |
where fr represents the actual firing rate. Values are expressed as means ± SE. Note that only data for which the firing rate was greater than 20 spikes/s were included in the optimization. Statistical significance was determined using paired Student's t-tests.
RESULTS
All neurons in this study (n = 42) were classified as VO neurons on the basis of their response patterns in the head-restrained condition. First, we confirmed that each neuron was modulated in response to head velocity during passive rotation about the earth vertical axis. Because this passive rotation elicited a compensatory eye movement response (i.e., the VOR), neurons were also characterized while the monkey canceled its VOR (i.e., such that the monkey did not generate eye movement during the same vestibular stimulation) by fixating a visual target that moved with its head (VOR cancellation). Each neuron's head velocity sensitivity was the same during VOR and VOR cancellation [mean head velocity sensitivity: 0.42 ± 0.02 and 0.44 ± 0.02 (spikes/s)/(°/s), respectively; P = 0.81], confirming that it was sensitive to head, but not eye, motion. Second, we established that each neuron was unresponsive to eye position during ocular fixation and eye motion during smooth pursuit. Accordingly, each neuron responded in a manner consistent with previous characterizations of VO neurons in head-restrained monkeys (Cullen and McCrea 1993; Fuchs and Kimm 1975; Keller and Daniels 1975; Roy and Cullen 2001; Scudder and Fuchs 1992; Tomlinson and Robinson 1984). Depending on whether their activity increased during ipsilaterally (n = 23) or contralaterally (n = 19) directed passive whole body rotation, neurons were further classified as type I or II, respectively. For the purpose of this paper, type I and II neurons were considered collectively because they encoded similar signals during each behavioral task.
A series of experiments were then done to directly test whether vestibular reafference is suppressed when there is a match between the predicted and actual proprioceptive consequences of self-motion, but not when there is a discrepancy. First, we assessed whether vestibular reafference is equivalently canceled in these neurons for self-generated vestibular stimulation produced by different voluntary behaviors, specifically, active head-on-body movements (produced by activation of the neck muscles) vs. active body movements (i.e., produced by the axial musculature). Second, we determined whether vestibular reafference is not canceled in conditions where self-produced and externally applied vestibular movements simultaneously activate the proprioceptors in the same muscle, such that there is a discrepancy between the predicted and actual sensory consequences of self-motion.
Vestibular Reafference Is Similarly Suppressed for Active Head-On-Body and Whole Body Motion
To date, all previous studies of early vestibular processing have only addressed whether vestibular reafference is suppressed during self-generated movements made by turning the head relative to the body by activating the neck muscles. Thus we first investigated whether vestibular signals are also suppressed at the level of the VN during another natural behavior for which head-in-space motion is produced by activation of the axial body musculature (i.e., whole body movements relative to space). Specifically, coordinated movements of the eye, head, and body are commonly used to redirect the axis of gaze between objects of interest and, as such, head-in-space motion that is the result of body-in-space, as well as head-on-body motion (McCluskey and Cullen 2007).
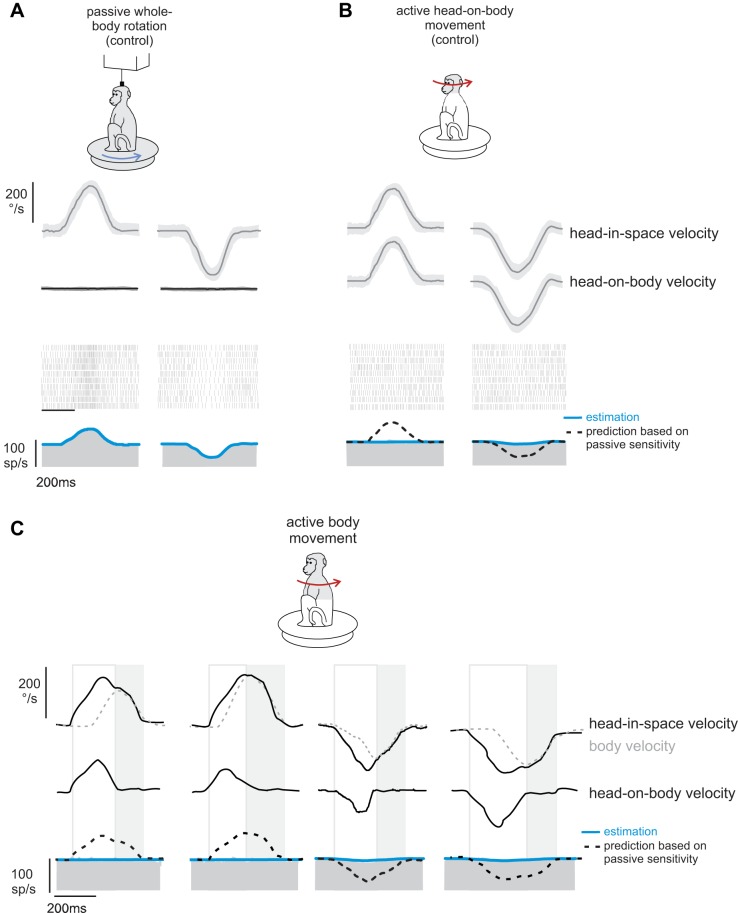
To test this proposal, we compared the vestibular sensitivities of neurons in response to passively applied motion, self-generated movements of the head relative to the body, and self-generated movements of the head produced by rotation of the whole body-in-space (termed active body movements). Figure 1 shows the response of an example neuron during each of these three paradigms. Typical of the neurons in our sample, this neuron responded robustly when the monkey experienced passively applied motion [Fig. 1A; 0.46 (spikes/s)/(°/s)] with a velocity profile corresponding to active head movements made by the same monkey (i.e., active-like movements; see methods). In contrast, and consistent with previous studies (McCrea et al. 1999; Roy and Cullen 2001), the response of the example neuron was dramatically attenuated during actively generated head-on-body motion [Fig. 1B; 0.03 (spikes/s)/(°/s)].
Fig. 1.
Responses of a typical neuron to passive vs. self-generated motion. Activity of a typical neuron to passively applied motion (A), self-generated movements of the head relative to the body (B), and self-generated movements of the head produced by rotation of the whole body-in-space (C) is shown. Blue lines overlaying the firing rate represent neuronal response estimates based on Eq. 1 (see methods; A) and Eq. 2 (B and C). Black dashed lines (B and C) represent predictions of the neuron's response sensitivity based on passive motion. The open boxes highlight epochs of active motion where head-in-space velocity is the result of the head moving relative to the body. The shaded boxes highlight epochs of active motion where head-in-space velocity is the result of the head and body moving together (i.e., active body movements).
Accordingly, having confirmed that this neuron's response to vestibular reafference was canceled when head-in-space motion was the result of active head-on-body movements, we next tested whether comparable response suppression was observed when head-in-space motion was the result of movement of the body-in-space. Indeed we found that the example neuron's response to head-in-space motion was dramatically and similarly attenuated in response to self-generated vestibular stimulation that was the result of active whole body motion [Fig. 1C; 0.04 (spikes/s)/(°/s)]. Although most neurons in our population showed substantial attenuation during active movement of both the head and the body, a small subset of neurons showed less attenuation (∼15% of the neurons in our sample showed an attenuation of 30% or less, and only 23% showed an attenuation of less than 50%). An example of such a neuron is shown in Fig. 2. Note that, compared with the example neurons shown in Fig. 1, this neuron's response to active motion (Fig. 2, B and C) was less attenuated (i.e., only 31%) relative to passive whole body motion (Fig. 2A). Nevertheless, the level of response attenuation was comparable for active motion, regardless of whether it was produced by motion of the head-on-body or body-in-space (compare Figs. 2, B and C).
Fig. 2.
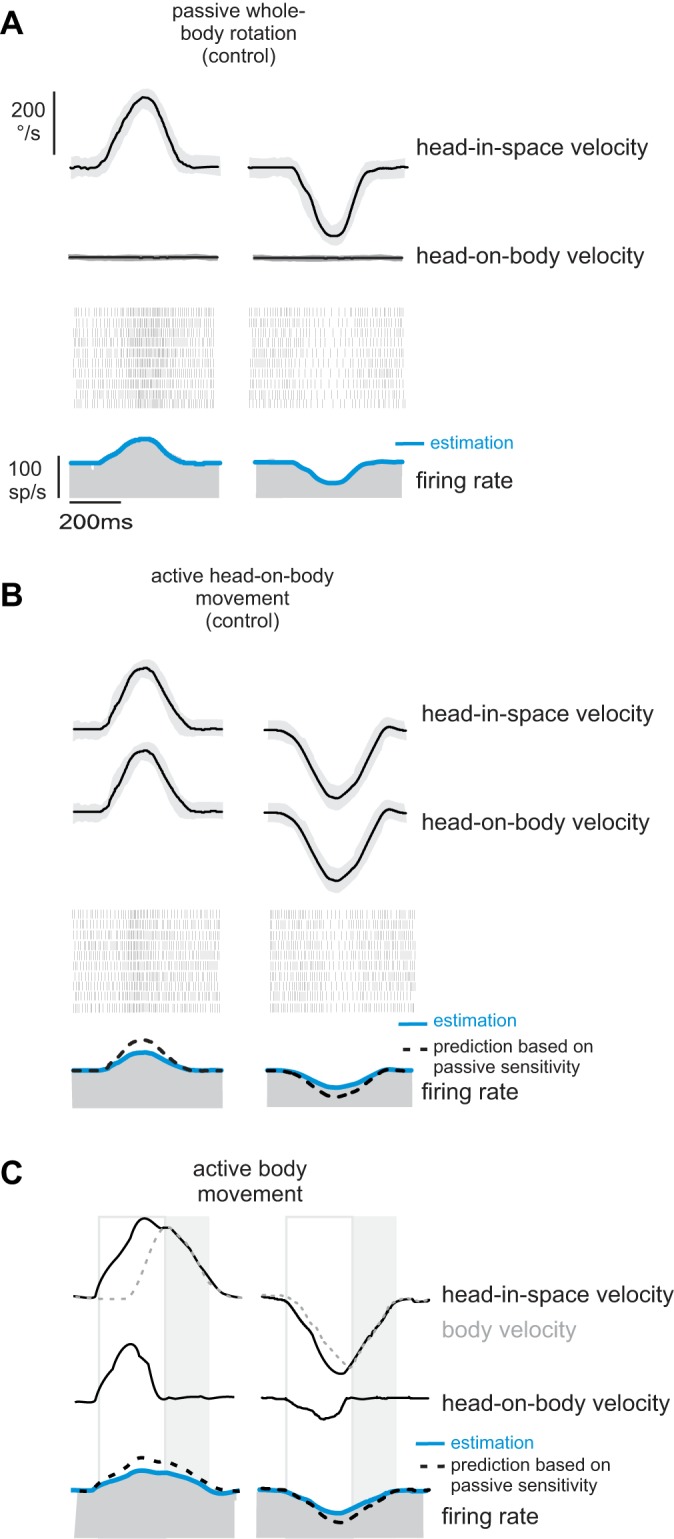
Responses of an example neuron in the minority subpopulation that were less than 50% attenuated during self-generated head motion. Activity of a neuron to passively applied motion (A), self-generated movements of the head relative to the body (B), and self-generated movements of the head produced by rotation of the whole body-in-space (C) is shown. Blue lines overlaying the firing rate represent neuronal response estimates based on Eq. 1 (see methods; A) and Eq. 2 (B and C). Black dashed lines (B and C) represent predictions of the neuron's response sensitivity based on passive motion. The open boxes highlight epochs of active motion where head-in-space velocity is the result of the head moving relative to the body. The shaded boxes highlight epochs of active motion where head-in-space velocity is the result of the head and body moving together (i.e., active body movements).
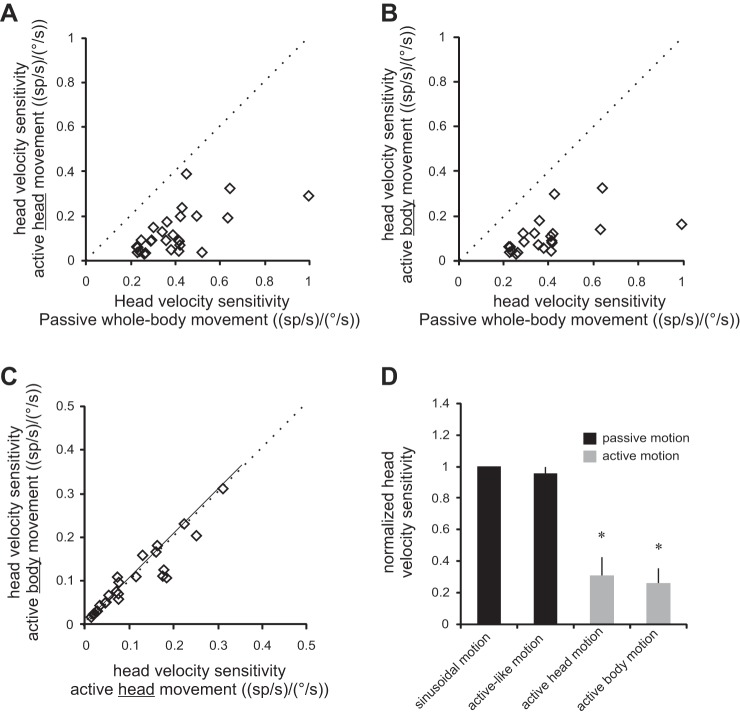
Overall, across our population, we found that neuronal responses to active head-in-space motion were significantly reduced compared with passive whole body rotation. This was true for active head-in-space motion produced by the head relative to the body (Fig. 3A, 73% attenuation, P < 0.001) and by the body-in-space (Fig. 3B, 71% attenuation, P < 0.001). Furthermore, we found that this attenuation was comparable for individual neurons in both active head motion conditions (slope = 1.02; R2 = 0.99; P = 0.74; Fig. 3, C and D). Thus, taken together, our results above show that vestibular reafference is attenuated regardless of whether it was generated by active movements of the head relative to the body (i.e., by activating neck musculature) or active movements of the whole body relative to space (i.e., by activating axial musculature). Thus our findings provide evidence that early suppression of vestibular input is a general feature of natural behaviors in which vestibular stimulation is self-generated.
Fig. 3.
Summary of neuronal sensitivities to active body motion. A and B: cell-by-cell comparison of neuronal sensitivities to active motion of the head (A) and head together with body (B) vs. passively applied head motion. Note that neuronal sensitivities to vestibular stimulation were reduced during active motion, regardless of whether it was generated by the neck (A) or axial (B) musculature. C: comparison of neuronal vestibular sensitivities to active motion produced by motion of the head vs. body. Dotted lines represent the unity line, and solid lines represent regression lines. D: histogram comparing sensitivities to passively applied motion and actively generated motion across conditions. *Significant difference (P < 0.001).
Vestibular Reafference Is Selectively Suppressed for Active Head-On-Body and Whole Body Motion When the Predicted and Actual Proprioceptive Consequences of Self-Motion Match
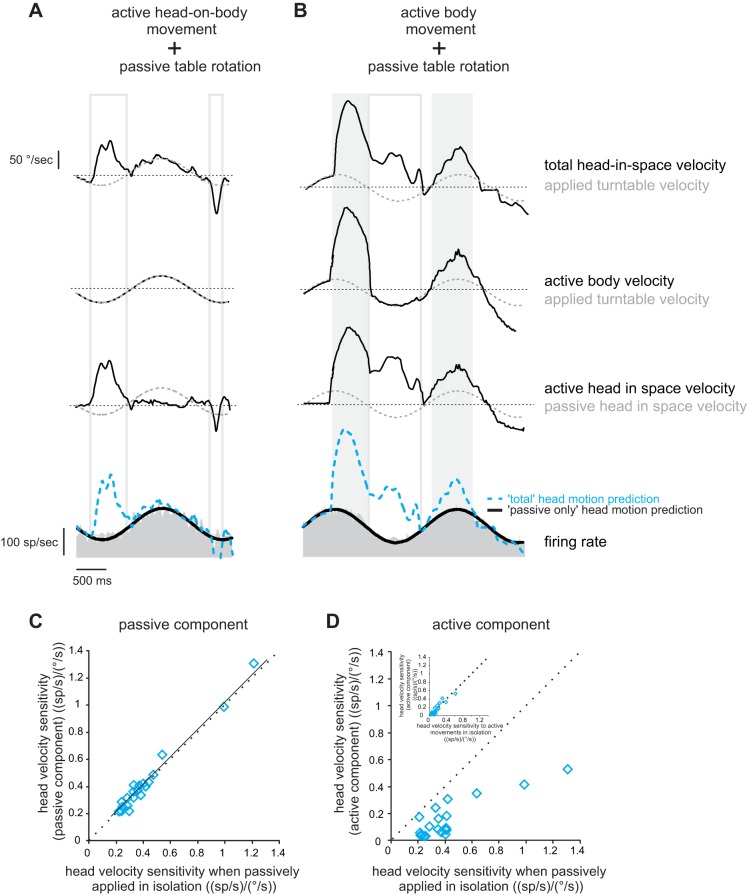
Next, we tested our prediction that vestibular reafference is suppressed when there is a match between the predicted and actual proprioceptive consequences of self-motion, but not when there is a discrepancy. Neurons were first recorded in a condition during which self-produced and externally applied motion did not activate proprioceptors in the same muscle. In this condition, neurons respond selectively to the passive head motion when monkeys generated voluntary head-on-body movements while simultaneously undergoing passive whole body sinusoidal rotations (Fig. 4A, compare dashed blue line and black line superimposed on firing rate showing total motion and passive-only predictions, respectively), consistent with previous reports (McCrea et al. 1999; Roy and Cullen 2001, 2004).
Fig. 4.
Vestibular reafference is canceled in conditions where there is no discrepancy between the predicted and actual sensory consequences of self-motion. A and B: response of a typical neuron to self-generated head-on-body (A) or body (B) movement, while passive whole body rotation was simultaneously applied. Note that head-in-space velocity equals the sum of passively applied and self-generated movement. Superimposed on the firing rates are response predictions computed based on the neuron's sensitivity to passive motion if the neuron encoded 1) the passive component of the motion-only (passive-only prediction; black trace), and 2) total head velocity (total motion prediction; dashed blue trace). C and D: cell-by-cell comparison of neuronal sensitivities to passive (C) and active (D) components of motion compared with the sensitivity to passive motion applied alone. Inset (D) shows a cell-by-cell comparison of neuronal sensitivity to active motion in isolation and when combined with passive whole body rotation. Dotted lines represent the unity line, and the solid line represents a regression line.
Our findings above (Figs. 1–3) established that vestibular reafference is similarly canceled for head-in-space motion is the result of body-in-space or head-on-body motion. Accordingly, we hypothesized that neurons should also respond selectively to passive motion if the active component of the motion is generated by the axial musculature (i.e., by active body motion). Figure 4B illustrates the response of the same neuron illustrated in Fig. 4A during concurrent active body motion and passively applied sinusoidal motion. Consistent with our prediction, the passive component of head motion was selectively encoded (Fig. 4B, compare dashed blue line and black line superimposed on firing rate showing total motion and passive-only predictions, respectively). Thus, irrespective of whether the active component of head motion was generated by moving the head alone or the head and body together, the responses of these vestibular neurons selectively encoded vestibular reafference.
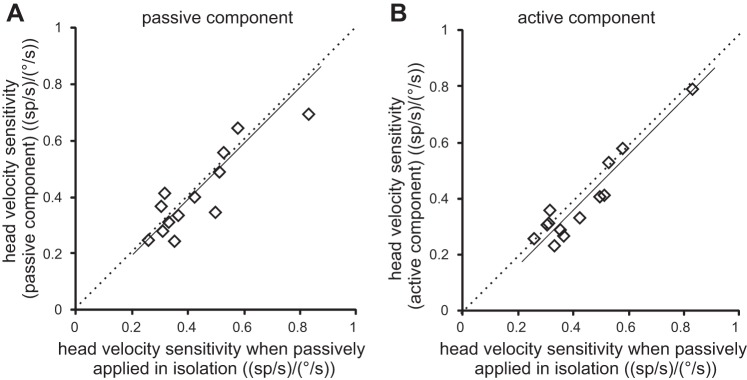
A comparison of vestibular responses to the active and passive components of motion over our population of neurons revealed that responses to vestibular reafference produced by body motion were selectively and consistently suppressed (Fig. 4, C and D). In contrast, neuronal responses to the passive component of motion were comparable when the same passive motion was applied alone (Fig. 4C, all data points fall along the unity line; P = 0.65). Moreover, neurons were less sensitive to the active component of body motion (Fig. 4D, all points fell below the unit line; P < 0.001). Note that this selective encoding of reafference cannot be explained by the predictability of the passive whole body rotations since these same neurons will continue to selectively encode the passive motion of the turntable, even when it is unpredictable (Roy and Cullen 2004). Thus, taken together, these finding are consistent with our initial hypothesis. Specifically, passive motion applied by vestibular turntable rotation did not alter the proprioceptive feedback resulting from the monkey's active body movement. Accordingly, vestibular reafference is canceled in this condition, because there is no apparent discrepancy between the predicted and actual sensory consequences of self-motion; proprioceptive feedback matches the motor-generated expectation.
Vestibular Reafference Is Not Canceled in Conditions Where There Is a Discrepancy Between the Predicted and Actual Sensory Consequences of Self-Motion
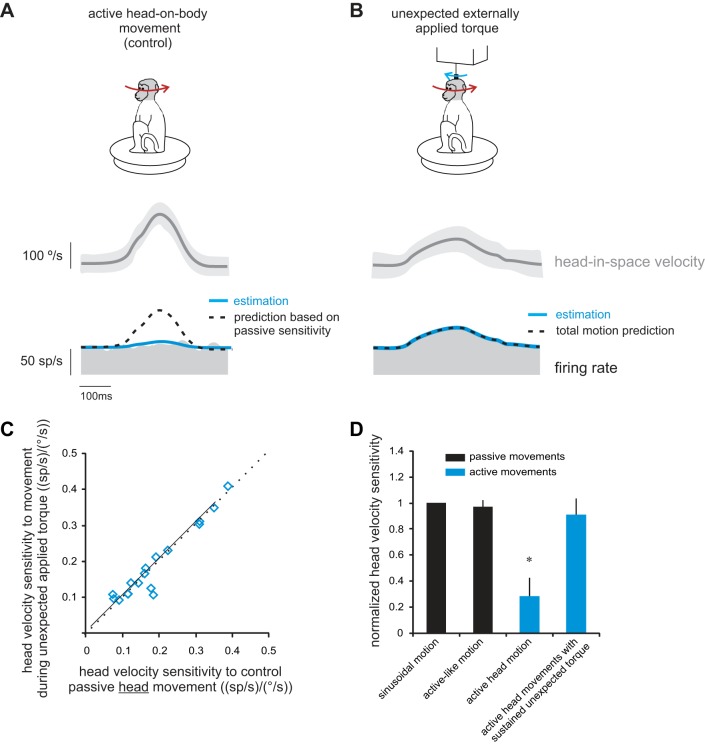
Finally, we determined whether vestibular reafference is canceled in conditions where there is a discrepancy between the predicted and actual sensory consequences of self-motion. To test this proposal, we applied unexpected, transient head-on-body perturbations using a small torque motor attached to the monkey's head (see methods), while the monkey simultaneously generated its own active head-on-body motion. We hypothesize that neurons should no longer selectively encode vestibular exafference in this condition, since there is a discrepancy between expected and actual proprioceptive sensory feedback. Specifically, the self-produced motion and the externally applied motion would both concurrently activate proprioceptors in the neck muscles.
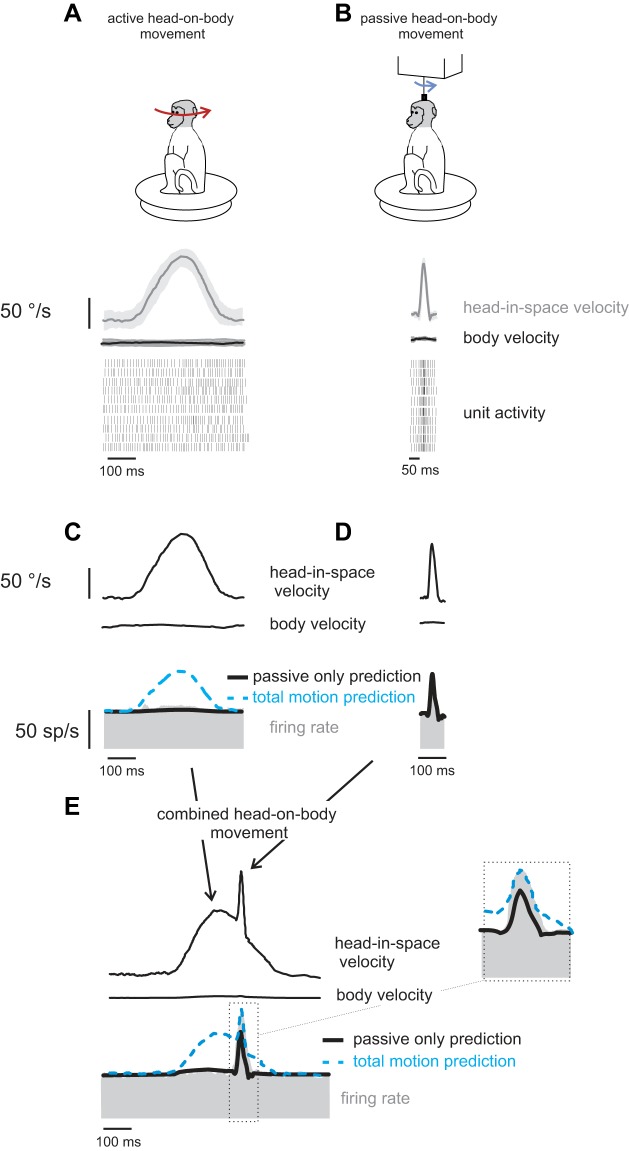
Figure 5 illustrates neuronal responses during active movements (Fig. 5A), transient passive perturbations of the head-on-body (Fig. 5B) and a condition where passive transient perturbations were applied during active head-on-body movements (Fig. 5E). Typical of our sample of neurons, the example neuron was relatively insensitive to active head-on-body motion [Fig. 5C; 0.01 (spikes/s)/(°/s)], but robustly responded to the externally applied perturbations of the head-on-body [Fig. 5D; 0.45 (spikes/s)/(°/s)]. Moreover, when the same passive perturbations were applied concurrently with active head-on-body movements, neurons no longer distinguished between vestibular exafference and reafference. Instead, neurons encoded the total vestibular input (i.e., the sum of exafference and reafference). This is illustrated by the model fits superimposed on the example neuron's firing rate in Fig. 5E (and inset). Notably, in this condition, the neuron's firing rate was underestimated by a prediction based on the passive component of motion (Fig. 5E; black line; passive-only prediction; see methods). In contrast, a model based on total head motion input (total motion prediction; dashed blue line; see methods) provided a good fit of the firing rate during the perturbation interval.
Fig. 5.
Vestibular reafference is not canceled in conditions where there is a discrepancy between the predicted and actual sensory consequences of self-motion. Activity of a typical neuron during self-generated head movements (A and C) and during short perturbations applied via a torque motor attached to the head (B and D) are shown. Note that neurons respond robustly to the short-duration passive perturbation, but not the active head movement. E: perturbation of the head-on-body (blue arrow on monkey cartoon) applied during ongoing self-generated head-on-body movements (red arrow on monkey cartoon). Superimposed on the firing rates are responses predictions computed based on the same neuron's sensitivity to passive motion for the passive component of the motion (passive-only prediction; black trace), and total head velocity (total motion prediction; dashed blue trace). Inset shows the area in the box magnified.
The results shown in Fig. 5 were quantified for our population of neurons. We compared, on a neuron-by-neuron basis, each neuron's sensitivities to the passive and active components of head motion (Fig. 5E) to its sensitivity for passive head motion when applied alone (Fig. 5B). If our initial proposal is true, then a given neuron should 1) have the same sensitivity to externally applied head motion, regardless of whether it occurs alone or in combination with active motion, but 2) not have reduced sensitivity to active motion whenever it is experienced in combination with unexpected passive motion that disrupts sensory feedback. Indeed, we found evidence for both of these predictions. First, sensitivities to passive motion (Fig. 6A) were comparable when applied alone and when applied in combination with active movements (R2 = 0.59; slope of regression line through the data not different from 1; P = 0.37). Second, responses to vestibular reafference were not reduced in the combined condition; a given neuron's sensitivity to vestibular reafference was comparable to its sensitivity to vestibular exafference (Fig. 6B, R2 = 0.51; slope of regression line through the data not different from 1; P = 0.41). Thus, when self-produced and externally applied motions both concurrently activate neck proprioceptors, neurons robustly encode both active and passive components of motion.
Fig. 6.
Sensitivities to concurrent active and passive head-on-body motion. A and B: cell-by cell comparison of neuronal sensitivities to passive motion when applied alone and passively applied perturbation (A), or the actively generated component of motion in the condition where both were applied concurrently (B). Note that, in this combined condition, sensitivities to both the passive and active components of motion were comparable to those to passive motion applied alone; all points fall close to the unity line (dotted). Solid lines represent a regression line.
Thus far we have established that vestibular reafference is not canceled if a transient unexpected head-on-body motion (e.g., Fig. 5E) produces a discrepancy between actual and expected proprioceptive feedback. Accordingly, we investigated whether a more sustained discrepancy between proprioceptive feedback and the motor-derived expectation also results in the robust encoding of vestibular reafference. To test this, we controlled the correspondence between the actual and expected sensory feedback, by applying a resistive torque to the head throughout the entire duration of the active movement. The unexpected application of resistive torque during active head-on-body movements (i.e., 10% trials) reduced the monkey's peak head velocity by ∼50%.
Figure 7 illustrates the response of an example neuron to active head movements made in the natural condition (control; Fig. 7A) and during the application of sustained unexpected resistive torque (Fig. 7B). As expected, the example neuron's response during active head motion was reduced relative to that observed for passive motion [Fig. 7A, sensitivity 0.1 (spikes/s)/(°/s), compare dotted blue and black lines superimposed on the firing rate, showing the prediction based on passive sensitivity and estimation, respectively]. In contrast, the neuron showed strong modulation for active head movements during which sustained torque was unexpectedly applied [Fig. 7B, sensitivity 0.41 (spikes/s)/(°/s), superimposed dashed blue and black lines show the total motion prediction and estimation, respectively]. Overall, for our population of neurons, we found that a given neuron's sensitivity to head movements produced in the unexpected presence of sustained resistive torque was comparable to its sensitivity to passive whole body rotation (Fig. 7C). Accordingly, average sensitivities in these two behavioral conditions were similar [Fig. 7D; mean sensitivity = 0.41 ± 0.04 and 0.38 ± 0.05 (spikes/s)/(°/s); P < 0.001]. Thus our data establish that sustained as well as transient discrepancies between proprioceptive feedback and the motor-derived expectation result in robust encoding of vestibular reafference produced by active self-motion. Furthermore, it is noteworthy that the torque applied in this paradigm was resistive, while the torque applied in the transient perturbation experiment (Fig. 5) assisted the movement. Nevertheless, the result was the same in both conditions; specifically, regardless of the direction of the computed mismatch between expected and actual motion, neurons no longer distinguished active from passive rotations. Taken together, our results suggest that early vestibular central processing robustly encodes both vestibular reafference and exafference, if there is an inconsistency between the predicted and actual sensory consequences of self-motion.
Fig. 7.
Vestibular reafference is also not canceled when there was sustained discrepancy between the predicted and actual sensory consequences of self-motion. A: response of a typical neuron during active head motion under control (i.e., natural) conditions. Superimposed on the firing rates are the best fits to the data (estimation; black trace), as well as the response predictions computed based on the same neuron's sensitivity to passive motion (prediction based on passive sensitivity; blue trace). B: response of the same neuron to head movements during which unexpected resistive torque was applied throughout the active movement. Superimposed on the firing rates are the best fits to the data (estimation; black trace), as well as the responses predicted based on the total head velocity (total motion prediction; dashed blue trace). C: cell-by-cell comparison of neuronal sensitivities to head movements during which unexpected resistive torque was applied vs. during the condition where passive motion was applied alone. Dotted lines represent the unity line, and the solid line represents a regression line. D: histogram comparing sensitivities to passively applied motion and actively generated motion across conditions. *Significant difference (P < 0.001).
DISCUSSION
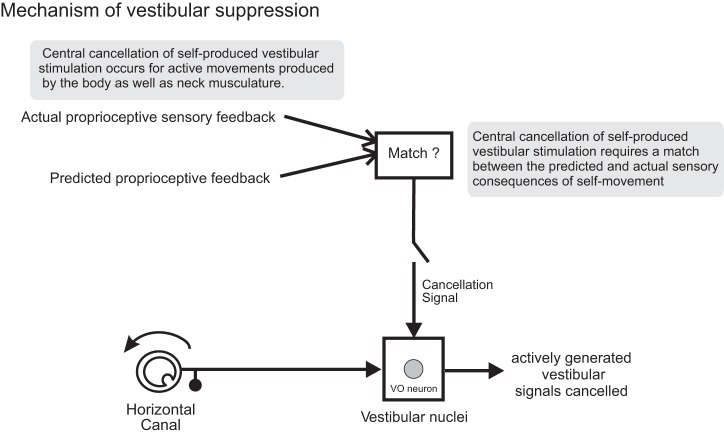
This study provides new insight into the mechanism that the brain uses to cancel self-generated vestibular sensory signals. First, by recording and comparing the activity of single central vestibular neurons during passive and self-generated movements of the head and/or body, we show for the first time that vestibular reafference is equivalently suppressed for head-in-space motion resulting from activation of the neck musculature (i.e., active head-on-body movement), and from activation of the axial muscles (i.e., active body movements). Thus our data establish that cancellation of vestibular reafference at first central stage of vestibular processing is a general feature of natural behaviors in which vestibular stimulation is self-generated (Fig. 8). Second, our experiments revealed that when simultaneous active and passive motion results in the activation the same muscle(s), vestibular reafference is not suppressed. Specifically, when externally applied passive motion caused proprioceptive sensory feedback to differ from that expected based on the motor command, neurons encoded total vestibular input (i.e., the sum of exafference and reafference) (Fig. 8). Thus our findings also show for the first time that, at its earliest stage of central processing, the vestibular system will robustly and simultaneously encode reafference as well as exafference when there is discrepancy between the predicted and actual proprioceptive consequences of self-generated motion.
Fig. 8.
Proposed mechanism underlying vestibular reafference suppression. In the model, the brain generates an expectation of sensory feedback based on an efference copy of the motor command to move the head and compares this expectation to the actual sensory feedback; if these two signals match, an inhibitory signal is sent to vestibular-only (VO) neurons to cancel the incoming reafferent vestibular signal.
Theoretical Implications and Evidence for an Internal Model
Most of our sensory experiences are gained by active exploration of the world, and the importance of our ability to distinguish sensory inputs that are a consequence of our own actions (i.e., reafference) from those that result from changes in the external world (i.e., exafference) has long been appreciated. For instance, in the 19th century, von Helmholtz (1867) made the salient observation that tapping on the canthus of the eye results in an illusionary shift of the visual world. However, we never see the world “shift” when we make saccades. To further this, von Holst and Mittelstaedt (1950) and Sperry (1950) proposed that the brain must use its knowledge about the movement that was produced to avoid responding to sensory inputs that arise from self-generated actions. Von Holst and Mittelstaedt formalized this idea and proposed the “principle of reafference.” In this model, the brain subtracts a copy of the expected sensory results of a motor command, termed “efference copy” (or “corollary discharge” by Sperry 1950), from the sensory signal to eliminate reafferent signals. Indeed, numerous behavioral studies have demonstrated that stimuli are perceived as less intense when caused by a self-generated action, for example, visual (Bridgeman and Nardello 1994; Haarmeier et al. 2001); auditory (Aliu et al. 2009; Martikainen et al. 2005; Sato 2008), and tactile (Bays et al. 2005; Blakemore et al. 1998; Hesse et al. 2009; Shergill et al. 2003; Tsakiris and Haggard 2003; Weiskrantz et al. 1971). Moreover, when sensory feedback arising from a given motor command is experimentally altered such that it does not match what is expected based on the issued motor command (e.g., Blakemore et al. 1999), the perception of self-generated sensory stimulation is not attenuated. These results led to the proposal that the brain computes an internal estimate of the sensory consequence of voluntary actions, based on the efference copy signal, which is then compared with the actual sensory input (reviewed in Blakemore et al. 2000; Cullen 2011).
Single-unit studies at the first central stage of vestibular processing have provided evidence for this proposal. Notably, the suppression of vestibular signals that arise from self-generated head movements is consistent with a mechanism that compares an expectation of sensory reafference, based on the efference copy of the motor command to move the head, with the actual sensory feedback originating from muscle proprioceptors (Roy and Cullen 2004). However, the proposed mechanism that accounts for neurophysiological findings to date also makes the explicit prediction that vestibular reafference will not be canceled under certain conditions (e.g., Roy and Cullen 2004). Indeed, our present findings provide strong support for this prediction. The analysis of vestibular coding when both proprioceptive reafference and exafference resulted from movement of the same muscle revealed the inability of VN neurons to dissociate between these two categories of sensory input. Thus the algorithm used by the brain to cancel self-generated vestibular signals computes the discrepancy between the predicted and actual sensory consequences of movements, rather than sensory exafference, per se. Importantly, this algorithm is fundamentally different from the mechanism proposed by von Holst and Mittelstaedt (1950), which does not explicitly require an agreement between expected and actual sensory input. We speculate that the mechanism that we have discovered in primate vestibular system is potentially behaviorally advantageous for more than only the suppression of vestibular reafference. It could also provide the neural substrate for learning. For example, the mechanical characteristics of our motor apparatus (e.g., neck/body muscle type, muscle strength) change over time. Thus it is essential that the brain can fine-tune its prediction of the sensory consequences of self-generated movements. We speculate that, when a mismatch between the predicted and actual sensory consequences of movements is persistent and consistent, this mismatch drives learning to update the prediction of the sensory consequences of movement to be current and accurate.
Neural Mechanisms Underlying the Cancellation of Vestibular Reafference
The mechanism underlying the cancellation of vestibular reafference in primates also appears to differ from those that govern the suppression of self-produced stimulation in the mormyrid fish electrosensory system (Bell 1981; Mohr et al. 2003; Sawtell 2010; Sawtell and Williams 2008), a system which has provided considerable insight into the mechanisms underlying reafference suppression. Notably, the mormyrid brain computes a negative image of the organism's predicted reafference, which is then used at the first central stage of sensory processing to suppress electroreceptor reafference. However, in this sensory system, a cancellation signal is produced to eliminate self-generated inputs, regardless of whether or not the fish's motor command to elicit an electric organ discharge is actually executed (Bell 1981; Requarth and Sawtell 2011; Sawtell et al. 2007). Thus, because a match is not required between expected and actual sensory feedback, cancellation of reafference in this sensory system appears consistent with von Holst and Mittelstadt's (1950) original proposal. In contrast, our present results demonstrate that, in the primate vestibular system, reafference cancellation is accomplished via a more sophisticated, but more behaviorally relevant, algorithm, which selectively modulates sensory processing of self-motion by computing the difference between expected and actual sensory inputs.
Interestingly, we found that, although there was little variation in a given neuron's sensitivity to active head motion across trials, the level of attenuation for self-generated head motion varied across our population. Although the reason for this variability is still unknown, it is likely the result of differing degrees of precision in the match between the inhibitory reafference cancellation input (most likely of cerebellar origin, see Brooks and Cullen 2013) and the afferent input to a given VN neuron.
Implications for Function
Our findings further confirm the generality of reafference cancellation for self-motion produced by different effectors. Specifically, we observed comparable suppression of vestibular reafference resulting from either activation of the neck musculature (head-on-body motion), or axial muscles that move the head and body (e.g., orienting body movements; Anastasopoulos et al. 2009; McCluskey and Cullen 2007). Because the neurons that were the focus of our study likely play a central role in many aspects of vestibular processing, our findings have important functional implications. Specifically, through their ascending projections to the thalamus (Shiroyama et al. 1999; Wild 1988; Zwergal et al. 2009), these neurons provide vestibular signals to cortical areas involved in the computation of self-motion perception and orientation (Deecke et al. 1977; Marlinski and McCrea 2008; Meng et al. 2007; Meng and Angelaki 2010). Additionally, through their projections to the spinal cord, these neurons mediate the vestibulo-collic reflex to stabilize the head during self-motion (Boyle et al. 1996; Boyle and Johanson 2003) or potentially other vestibulo-spinal reflexes to ensure the maintenance of posture (Abzug et al. 1974; Shinoda et al. 1988). Finally, via reciprocal connections to regions of the cerebellum vital for the control of posture and spatial orientation, including the rostral fastigial nucleus (Batton et al. 1977; Carleton and Carpenter 1984; Homma et al. 1995; Shimazu and Smith 1971) and the nodulus/uvula (Walberg and Dietrichs 1988; Xiong and Matsushita 2000), these same neurons likely contribute to the fine-tuning of motor commands.
Accordingly, the behaviorally dependent gating of sensory responses observed in the present study for early vestibular processing has specific implications for understanding how the brain ensures stable perception and accurate posture and motor control. When combined active and passive movements do not result in disrupted sensory feedback from the active movement, the neurons described in the present study respond such that only passive vestibular signals are encoded to signal motion and adjust postural tone in response to any unexpected head movement. This preferential selectivity to vestibular exafference is behaviorally advantageous (e.g., recovery from tripping over an obstacle requires a selective but robust response to the unexpected vestibular stimulation). However, when proprioceptive feedback resulting from self-generated motion is disrupted, as is the case when the externally applied motion activates proprioceptors in the same muscle, neurons cannot distinguish between the two types of motion, and thus both the passive and active sensory components are robustly encoded. This result parallels findings for other voluntary behaviors, for instance studies of self-produced tactile stimulation (Blakemore et al. 1999, 2000) and perceived force during tapping (Bays et al. 2005) and lifting tasks (Diedrichsen et al. 2003, 2005) have shown that a match between sensory feedback and the causal motor command is required for accurate sensation. What we have shown here suggests a common neural mechanism that explains the suppression of self-generated sensory inputs among sensory systems. We speculate that the extent to which self-produced vestibular stimulation is attenuated depends on the magnitude of the discrepancy between the sensory feedback predicted by an internal forward model of the motor system and the actual sensory feedback produced by the movement. Such discrepancies are commonly encountered in conditions that drive motor learning (reviewed in Lackner and DiZio 2005; Shadmehr et al. 2010), for instance changes in the motor apparatus or external environment, ultimately requiring that the reafferent cancellation mechanism learns a new “match” to resolve the ensuing systematic discrepancy.
GRANTS
This research was supported by Fonds Québécois de la Recherche sur la Nature et les Technologies (J. X. Brooks), Canadian Institutes of Health Research (K. E. Cullen), as well as National Institute of Deafness and Other Communications Disorders Grant DC-002390 (K. E. Cullen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.X.B. and K.E.C. conception and design of research; J.X.B. performed experiments; J.X.B. analyzed data; J.X.B. and K.E.C. interpreted results of experiments; J.X.B. prepared figures; J.X.B. and K.E.C. drafted manuscript; J.X.B. and K.E.C. edited and revised manuscript; K.E.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Jamali, D. Mitchell, and A. Dale for critically reading this manuscript, and S. Nuara and W. Kucharski for excellent technical assistance.
REFERENCES
- Abzug C, Maeda M, Peterson BW, Wilson VJ. Cervical branching of lumbar vestibulospinal axons. J Physiol 243: 499–522, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliu SO, Houde JF, Nagarajan SS. Motor-induced suppression of the auditory cortex. J Cogn Neurosci 21: 791–802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasopoulos D, Ziavra N, Hollands M, Bronstein A. Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp Brain Res 193: 323–336, 2009 [DOI] [PubMed] [Google Scholar]
- Batton RR, 3rd, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol 174: 281–305, 1977 [DOI] [PubMed] [Google Scholar]
- Bays PM, Wolpert DM, Flanagan JR. Perception of the consequences of self-action is temporally tuned and event driven. Curr Biol 15: 1125–1128, 2005 [DOI] [PubMed] [Google Scholar]
- Bell CC. An efference copy which is modified by reafferent input. Science 214: 450–453, 1981 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM. Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci 11: 551–559, 1999 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci 1: 635–640, 1998 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert D, Frith C. Why can't you tickle yourself? Neuroreport 11: R11–R16, 2000 [DOI] [PubMed] [Google Scholar]
- Boyle R, Belton T, McCrea RA. Responses of identified vestibulospinal neurons to voluntary eye and head movements in the squirrel monkey. Ann N Y Acad Sci 781: 244–263, 1996 [DOI] [PubMed] [Google Scholar]
- Boyle R, Johanson C. Morphological properties of vestibulospinal neurons in primates. Ann N Y Acad Sci 1004: 183–195, 2003 [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Nardello C. Conflict between aftereffects of retinal sweep and looming motion. Psychol Res 56: 78–82, 1994 [DOI] [PubMed] [Google Scholar]
- Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol 23: 947–955, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton SC, Carpenter MB. Distribution of primary vestibular fibers in the brainstem and cerebellum of the monkey. Brain Res 294: 281–298, 1984 [DOI] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci 9: 587–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE. Sensory signals during active versus passive movement. Curr Opin Neurobiol 14: 698–706, 2004 [DOI] [PubMed] [Google Scholar]
- Cullen KE. The neural encoding of self-motion. Curr Opin Neurobiol 21: 587–595, 2011 [DOI] [PubMed] [Google Scholar]
- Cullen KE, McCrea RA. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. I. Secondary vestibular neurons. J Neurophysiol 70: 828–843, 1993 [DOI] [PubMed] [Google Scholar]
- Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci 22: RC226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Rey CG, Guitton D, Galiana HL. The use of system identification techniques in the analysis of oculomotor burst neuron spike train dynamics. J Comput Neurosci 3: 347–368, 1996 [DOI] [PubMed] [Google Scholar]
- Deecke L, Schwarz DW, Fredrickson JM. Vestibular responses in the rhesus monkey ventroposterior thalamus. II. Vestibulo-proprioceptive convergence at thalamic neurons. Exp Brain Res 30: 219–232, 1977 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Hon A, Lehman SL, Ivry RB. Anticipatory adjustments in the unloading task: is an efference copy necessary for learning? Exp Brain Res 148: 272–276, 2003 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Lehman SL, Ivry RB. Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movements. J Neurophysiol 93: 801–812, 2005 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol 38: 1140–1161, 1975 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Haarmeier T, Bunjes F, Lindner A, Berret E, Thier P. Optimizing visual motion perception during eye movements. Neuron 32: 527–535, 2001 [DOI] [PubMed] [Google Scholar]
- Hayes RB, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. Western Electronic Show and Conference Proceedings 2: 1–10, 1982 [Google Scholar]
- von Helmholtz H. Handhuch der Physiologischerl Optik. Leipzig, Germany: Voss, 1867 [Google Scholar]
- Hesse MD, Nishitani N, Fink GR, Jousmaki V, Hari R. Attenuation of somatosensory responses to self-produced tactile stimulation. Cereb Cortex 20: 425–432, 2009 [DOI] [PubMed] [Google Scholar]
- Homma Y, Nonaka S, Matsuyama K, Mori S. Fastigiofugal projection to the brainstem nuclei in the cat: an anterograde PHA-L tracing study. Neurosci Res 23: 89–102, 1995 [PubMed] [Google Scholar]
- Jamali M, Sadeghi SG, Cullen KE. Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. J Neurophysiol 101: 141–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res 142: 171–188, 2003 [DOI] [PubMed] [Google Scholar]
- Keller EL, Daniels PD. Oculomotor related interaction of vestibular and visual stimulation in vestibular nucleus cells in alert monkey. Exp Neurol 46: 187–198, 1975 [DOI] [PubMed] [Google Scholar]
- Lackner JR, DiZio P. Motor control and learning in altered dynamic environments. Curr Opin Neurobiol 15: 653–659, 2005 [DOI] [PubMed] [Google Scholar]
- Marlinski V, McCrea RA. Activity of ventroposterior thalamus neurons during rotation and translation in the horizontal plane in the alert squirrel monkey. J Neurophysiol 99: 2533–2545, 2008 [DOI] [PubMed] [Google Scholar]
- Martikainen MH, Kaneko K, Hari R. Suppressed responses to self-triggered sounds in the human auditory cortex. Cereb Cortex 15: 299–302, 2005 [DOI] [PubMed] [Google Scholar]
- McCluskey MK, Cullen KE. Eye, head, and body coordination during large gaze shifts in rhesus monkeys: movement kinematics and the influence of posture. J Neurophysiol 97: 2976–2991, 2007 [DOI] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol 82: 416–428, 1999 [DOI] [PubMed] [Google Scholar]
- Meng H, Angelaki DE. Responses of ventral posterior thalamus neurons to three-dimensional vestibular and optic flow stimulation. J Neurophysiol 103: 817–826, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and origins. J Neurosci 27: 13590–13602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C, Roberts PD, Bell CC. The mormyromast region of the mormyrid electrosensory lobe. I. Responses to corollary discharge and electrosensory stimuli. J Neurophysiol 90: 1193–1210, 2003 [DOI] [PubMed] [Google Scholar]
- Requarth T, Sawtell NB. Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits. Curr Opin Neurobiol 21: 602–608, 2011 [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci 1: 404–410, 1998 [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci 21: 2131–2142, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci 24: 2102–2111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol 87: 2337–2357, 2002 [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE. Effects of canal plugging on the vestibuloocular reflex and vestibular nerve discharge during passive and active head rotations. J Neurophysiol 102: 2693–2703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A. Action observation modulates auditory perception of the consequence of others' actions. Conscious Cogn 17: 1219–1227, 2008 [DOI] [PubMed] [Google Scholar]
- Sawtell NB. Multimodal integration in granule cells as a basis for associative plasticity and sensory prediction in a cerebellum-like circuit. Neuron 66: 573–584, 2010 [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Williams A. Transformations of electrosensory encoding associated with an adaptive filter. J Neurosci 28: 1598–1612, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Williams A, Bell CC. Central control of dendritic spikes shapes the responses of Purkinje-like cells through spike timing-dependent synaptic plasticity. J Neurosci 27: 1552–1565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol 68: 244–264, 1992 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010 [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: the neuroscience of force escalation. Science 301: 187, 2003 [DOI] [PubMed] [Google Scholar]
- Shimazu H, Smith CM. Cerebellar and labyrinthine influences on single vestibular neurons identified by natural stimuli. J Neurophysiol 34: 493–508, 1971 [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Ohgaki T, Futami T, Sugiuchi Y. Vestibular projections to the spinal cord: the morphology of single vestibulospinal axons. Prog Brain Res 76: 17–27, 1988 [DOI] [PubMed] [Google Scholar]
- Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. Projections of the vestibular nuclei to the thalamus in the rat: a Phaseolus vulgaris leucoagglutinin study. J Comp Neurol 407: 318–332, 1999 [PubMed] [Google Scholar]
- Sperry RW. Neural basis of spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43: 482–489, 1950 [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J Neurophysiol 82: 2612–2632, 1999 [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Robinson DA. Signals in vestibular nucleus mediating vertical eye movements in the monkey. J Neurophysiol 51: 1121–1136, 1984 [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Haggard P. Awareness of somatic events associated with a voluntary action. Exp Brain Res 149: 439–446, 2003 [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das reafferenzprinzip. Naturwissenschaften 37: 464–476, 1950 [Google Scholar]
- Walberg F, Dietrichs E. The interconnection between the vestibular nuclei and the nodulus: a study of reciprocity. Brain Res 449: 47–53, 1988 [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Elliott J, Darlington C. Preliminary observations on tickling oneself. Nature 230: 598–599, 1971 [DOI] [PubMed] [Google Scholar]
- Wild JM. Vestibular projections to the thalamus of the pigeon: an anatomical study. J Comp Neurol 271: 451–460, 1988 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci 12: 739–751, 2011 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci 3, Suppl: 1212–1217, 2000 [DOI] [PubMed] [Google Scholar]
- Xiong G, Matsushita M. Connections of Purkinje cell axons of lobule X with vestibulospinal neurons projecting to the cervical cord in the rat. Exp Brain Res 131: 491–499, 2000 [DOI] [PubMed] [Google Scholar]
- Zwergal A, Strupp M, Brandt T, Buttner-Ennever JA. Parallel ascending vestibular pathways: anatomical localization and functional specialization. Ann N Y Acad Sci 1164: 51–59, 2009 [DOI] [PubMed] [Google Scholar]