Abstract
Serotonin (5-HT), and its 5-HT1A receptor (5-HT1AR) subtype, is a powerful modulator of the cardiorespiratory system and its sensory reflexes. The nucleus tractus solitarii (nTS) serves as the first central station for visceral afferent integration and is critical for cardiorespiratory reflex responses. However, the physiological and synaptic role of 5-HT1ARs in the nTS is relatively unknown. In the present study, we examined the distribution and modulation of 5-HT1ARs on cardiorespiratory and synaptic parameters in the nTS. 5-HT1ARs were widely distributed to cell bodies within the nTS but not synaptic terminals. In anesthetized rats, activation of 5-HT1ARs by microinjection of the 5-HT1AR agonist 8-OH-DPAT into the caudal nTS decreased minute phrenic neural activity via a reduction in phrenic amplitude. In brain stem slices, 8-OH-DPAT decreased the amplitude of glutamatergic tractus solitarii-evoked excitatory postsynaptic currents, and reduced overall spontaneous excitatory nTS network activity. These effects persisted in the presence of GABAA receptor blockade and were antagonized by coapplication of 5-HT1AR blocker WAY-100135. 5-HT1AR blockade alone had no effect on tractus solitarii-evoked excitatory postsynaptic currents, but increased excitatory network activity. On the other hand, GABAergic nTS-evoked inhibitory postsynaptic currents did not change by activation of the 5-HT1ARs, but spontaneous inhibitory nTS network activity decreased. Blocking 5-HT1ARs tended to increase nTS-evoked inhibitory postsynaptic currents and inhibitory network activity. Taken together, 5-HT1ARs in the caudal nTS decrease breathing, likely via attenuation of afferent transmission, as well as overall nTS network activity.
Keywords: serotonin receptors, autonomic nervous system, patch clamp, EPSC, IPSC
within the cardiorespiratory reflex axis, the nucleus tractus solitarii (nTS) is vital in its role in integrating and processing visceral afferent signals. It is the initial central site for an effective response to physiological challenges, such as low arterial oxygen, blood pressure fluctuations, and other visceral stimuli (Andresen and Kunze 1994; Kline et al. 2010). The primary excitatory and inhibitory neurotransmitters within the nTS network are glutamate and γ-amino-butyric acid (GABA), respectively (Sapru 2004). However, numerous neuromodulators may influence their release or postsynaptic receptor function (Kline et al. 2009; Sekizawa et al. 2009).
One neuromodulator within the nTS is serotonin (5-HT), which plays an important role in the cardiorespiratory system (Gillis et al. 1989; Ling et al. 2001). 5-HT within the nTS derives from vagal afferents, raphé neurons and the nTS itself (Steinbusch 1981; Zhuo et al. 1997), where it may activate one or more 5-HT receptor (5-HTR) subtypes. The medial nTS densely expresses 5-HT1ARs (Liu and Wong-Riley 2010; Manaker and Verderame 1990; Thor et al. 1992), 1 of the 14 identified 5-HTRs. Although not all studies agree (Feldman and Galiano 1995), 5-HT1ARs in the nTS may produce tachypnea, hypotension or bradycardia (Besnard et al. 2012; Itoh and Bunag 1991). Likewise, the role of 5-HT1ARs in modulating nTS or dorsal vagal complex synaptic or neuronal activity is unclear, as increases, decreases or no change in activity has been reported (Browning and Travagli 1999; Feldman 1995; Takenaka et al. 2011; Wang et al. 1997). In other central nuclei, activation of 5-HT1ARs leads to neuronal hyperpolarization and reduced firing rate and often involves a reduction of glutamatergic and/or GABAergic transmission (Aghajanian and Sanders-Bush 2002; Ciranna 2006; Costa et al. 2012; Lalley et al. 1994; Nichols and Nichols 2008). Taken together, while these results suggest 5-HT1ARs modulate cardiorespiratory function via the nTS; the mechanisms by which this may occur remain elusive. In the present study, we sought to determine the distribution and function of 5-HT1ARs in the nTS. Our results show that their activation in the nTS profoundly influences basal respiratory parameters, likely through their modulation of excitatory and inhibitory neurotransmission.
METHODS
Ethical approval and animals.
The Animal Care and Use Committee of the University of Missouri approved all experimental protocols in accordance with National Institutes of Health guidelines (Guide for the Care and Use of Laboratory Animals). Male Sprague-Dawley rats (Harlan; n = 58, aged 4–8 wk) were maintained in the vivarium of the Dalton Cardiovascular Research Center accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The animals were held at 22°C and 40% humidity on a 12:12-h day-night cycle with water and food available ad libitum.
In vivo preparation and microinjection.
Experiments were performed as previously (Clark et al. 2011; Mueller et al. 2005) described. Briefly, rats (n = 5) were anesthetized with isoflurane (5% induction; 2–3% maintenance, in 100% O2, VetOne), and femoral venous and arterial catheters (PE-10 fused to PE-50, A-M systems) were inserted to enable drug administration and measurement of arterial pressure (AP), respectively. Mean AP (MAP) and heart rate (HR) were determined using a PowerLab data acquisition system (ADInstruments). The trachea was cannulated, and rats were mechanically ventilated with O2-enriched room air. Blood samples were taken to record arterial blood gases (Osmetech, OPTI CCA), and arterial hemoglobin oxygen saturation was monitored continuously (MouseOx, Starr Life Sciences); arterial Po2 levels were maintained at 155.6 ± 1.8 Torr, arterial Pco2 at 52.6 ± 1.8 Torr, pH at 7.35 + 0.011, and O2 saturation at 98.9 ± 0.2%. Arterial Pco2 was kept at this level to maintain phrenic nerve activity (PhrNA). Rectal temperature was monitored and maintained at ∼38°C (Tele-Thermometer, Simpson Electrics). For a measurement of central neural output, a splanchnic and phrenic nerve was isolated via a retroperitoneal or ventral cervical approach, respectively, placed on bipolar Teflon-coated silver electrodes (0.005–0.007 in., A-M Systems) and covered in silicone elastomer (Kwik-Cast, WPI). The recorded phrenic nerve was crushed distally, and the contralateral phrenic nerve was cut. Bilateral cervical vagotomy was performed to prevent entrainment of phrenic motor output with the ventilator. Nerve activity was amplified (1,000×), filtered (30–3,000 Hz, P511, Grass Technologies), rectified and integrated using a root mean square converter (time constant: phrenic = 100 ms; splanchnic = 28 ms); sympathetic nerve activity was electronically averaged. Background noise was determined from the signal between bursts of activity. The recorded nerve activity minus noise was defined as splanchnic sympathetic nerve activity (SSNA) and PhrNA.
Rats were placed in a stereotaxic apparatus (Kopf Instruments), and the brain stem was exposed via a partial occipital craniotomy, as previously described (Clark et al. 2011; Mueller and Hasser 2006). Following completion of surgery, anesthesia was gradually converted from isoflurane to inactin (100 mg/kg iv, 20 mg/kg iv supplements as required). Animals were paralyzed using gallamine (8.3 mg/kg iv, 1–2 mg/h iv maintenance). Adequate plane of anesthesia was verified regularly by lack of cardiovascular responses to tail pinch (<5-mmHg increase in MAP).
Cardiorespiratory parameters were allowed to stabilize at least 1 h, and baseline PhrNA (integrated amplitude and frequency) were constant at least 30 min before starting experimental protocols. Rats received unilateral nTS microinjections of either vehicle control or 8-hydroxy-2-(di-n-propylamino)tetral (8-OH-DPAT) [1 and 5 mM, 60 nl, relative to calamus scriptorius (in mm): 0.3 rostral, 0.3 lateral, 0.5 ventral; target coordinates were ascertained prior to in vivo experiments to target cardiorespiratory regions and to match in vitro recording sites]. Artificial cerebrospinal fluid (aCSF) served as vehicle and was injected prior to 8-OH-DPAT. Subsequently, MAP, HR, O2 saturation, PhrNA (peak integrated phrenic amplitude and phrenic frequency) and SSNA (mean) were recorded for 45–60 min followed by contralateral aCSF and 8-OH-DPAT microinjections. At the end of the experiment, fluorescent retrobeads (LumaFluor, 1:50, in 15–30 nl aCSF) were injected at the nTS target sites to mark microinjection sites. Rats were subsequently euthanized via inactin overdose and/or euthanasia solution (Beuthanasia-D, 0.1 ml); the brains were removed, fixed in formaldehyde, sectioned in the horizontal plane and subsequently examined.
In vitro brain stem slice preparation, electrophysiology and protocols.
As detailed previously (Kline et al. 2010), the brain stem was removed from isoflurane (VetOne)-anesthetized rats and placed in ice-cold low calcium-high magnesium aCSF (in mM: 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose, 0.4 l-ascorbic acid, 1 CaCl2 and 2 MgCl2, saturated with 95% O2/5% CO2, pH 7.4, ∼300 mosM). Horizontal slices (∼280 μm) with lengthy segments of the tractus solitarii (TS; see Fig. 5A) and the nTS were cut using a vibrating microtome (VT 1000S, Leica). Tissue sections were secured via nylon mesh in a superfusion chamber and superfused at ∼3 ml/min with standard recording aCSF (in mM: 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose and 2 CaCl2, saturated with 95% O2/5% CO2, pH 7.4, ∼300 mosM) at 31–33°C.
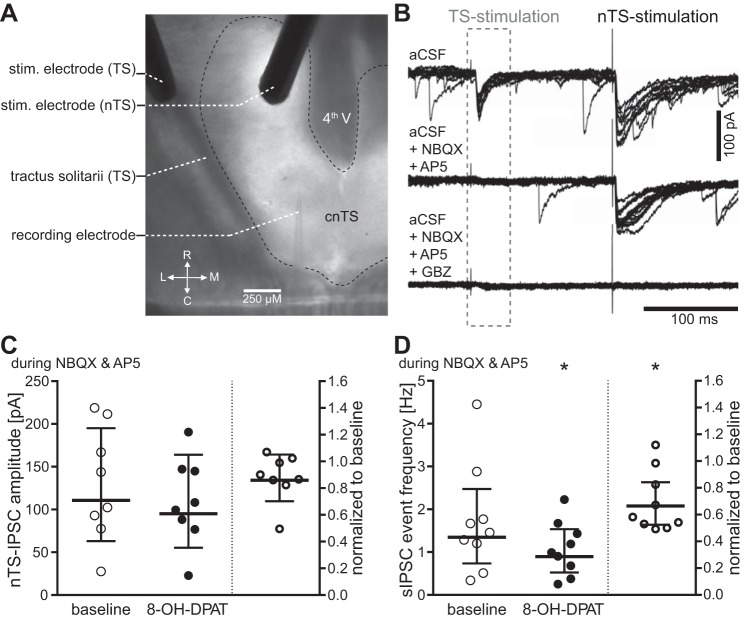
Fig. 5.
Activation of 5-HT1ARs decreases inhibitory nTS network activity. A: two bipolar stimulating electrodes were used to evoke monosynaptic glutamatergic EPSCs in the recorded neuron from visceral afferents within the TS, and monosynaptic GABAergic inhibitory postsynaptic currents (IPSCs) from the nTS network in the medial portion of the nTS. stim, stimulating. B: representative recordings showing TS-EPSCs and nTS-IPSCs upon stimulation. Note, due to our recording conditions, glutamatergic and GABAergic currents are downward (negative) deflecting. Application of 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium (NBQX) and dl-2-amino-5-phosphonovaleric acid (AP5) eliminated all excitatory currents. At the end of an experiment, IPSCs were verified by their blockade with GABAzine (GBZ) in the recording solution. C and D: individual responses to 5-HT1AR activation with 8-OH-DPAT are presented as raw values (left in each panel) and normalized to baseline (right, baseline defined as “1”) for nTS-IPSC amplitudes (C) and spontaneous IPSC (sIPSC) event frequency (D). While the group nTS-IPSC amplitude did not change (n = 9; P = 0.11), spontaneous event frequency significantly decreased with activation of 5-HT1AR (n = 9; *P < 0.05). Bars show geometric mean with 95% CI.
All recordings were made from nTS cell somas in the caudal medial-commissural nTS, a region that receives cardiorespiratory afferent information (Chitravanshi and Sapru 1995; Guyenet 2000). Electrodes (King Precision Glass, type 8250) were pulled with a Flaming/Brown micropipette puller (Sutter Instruments, model P-97). To record excitatory postsynaptic currents (EPSCs), the following recording solution was used (in mM): 10 NaCl, 130 K+-gluconate, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 2 Mg-ATP, 0.2 Na-GTP, pH 7.3, ∼280 mosM. The calculated chloride reversal potential with this pipette solution was −58.6 mV. For recordings of inhibitory postsynaptic currents (IPSCs), we used a high chloride-based solution (in mM: 140 CsCl, 5 NaCl, 10 EGTA, 10 HEPES, 1.2 MgSO4, 3 K-ATP, 0.2 Na-GTP, 5 QX314, pH 7.3, ∼280 mosM) (Chen et al. 2009). The calculated reversal potential for IPSC recordings with this high-chloride pipette was 2.7 mV. Recording pipettes were guided with a piezoelectric micromanipulator (Burleigh, PCS-6000). Neurons were recorded under voltage-clamp configuration using the patch-clamp technique. Cells were held at −60 mV. Neurons were rejected if the holding current was more negative than −50 pA upon initial rupture, or if series resistance changed more than 20% throughout the experiment. In some experiments, the series resistance was compensated. Data were recorded using a Multiclamp700B amplifier (Molecular Devices), filtered at 2 kHz and sampled at 20 kHz.
Evoked postsynaptic currents were generated with isolated stimulators (A.M.P.I., Master-8 and ISO-Flex) and concentric bipolar stimulating electrodes (F. Haer). Afferent evoked EPSCs were evoked as previously described (Kline et al. 2002) by placing a stimulating electrode on the TS containing visceral afferent fibers (see Fig. 5A). These were defined as TS-EPSCs. Evoked IPSCs were elicited in monosynaptic TS-connected neurons (see below) by placing a second bipolar electrode within the medial nTS. These were defined as nTS-IPSCs. To isolate IPSCs from EPSCs, 10 μM 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium [NBQX; non-N-methyl-d-aspartate (NMDA) receptor blocker; Tocris] and 50 μM dl-2-amino-5-phosphonovaleric acid (AP5) (NMDA receptor blocker; Tocris) were added to the recording aCSF. GABAzine (GBZ; 25 μM SR 95531 HBr, Tocris) was applied at the end of the experiment to confirm IPSC identify. For either evoked postsynaptic current stimulation, intensity was increased until a TS-EPSC and/or nTS-IPSC was evoked, after which stimulation intensity was set at 1.5 × threshold. Subsequently, the role of 5-HT1AR activation or blockade on TS-EPSCs or nTS-IPSCs was examined.
Miniature EPSCs and IPSCs (mEPSCs and mIPSCs) were recorded without external stimulation and in the presence of 1 μM tetrodotoxin (TTX) (Tocris). The lack of AP discharge to current ramps (−20 pA to +50, +100 or +200 pA; 1-s ramp in current clamp mode) at the beginning of the protocol ensured that the events were not due to action potential-driven network activity. mEPSCs were isolated from mIPSCs with GBZ to block inhibitory currents. mIPSCs were isolated from mEPSCs with NBQX and AP5. Subsequently, the role of 5-HT1AR activation or blockade on mEPSCs or mIPSCs was examined.
Drugs.
All receptor pharmacological agents were purchased from Tocris. The role of 5-HT1AR activation was tested using the prototypical agonist 8-OH-DPAT. To examine the effects of 5-HT1AR activation on cardiorespiratory parameters, 1 and 5 mM 8-OH-DPAT were microinjected in the nTS. To define the role of 5-HT1AR activation on GABAergic and glutamatergic transmission, 10 μM 8-OH-DPAT were bath applied to brain stem slices. In the latter, 5-HT1ARs were blocked by use of the specific antagonist WAY-100135 (10 μM). To eliminate the influence of the 5-HT7Rs, the 5-HT7R antagonist SB-269970 (5 μM) was used in conjunction with 8-OH-DPAT. All drug treatments were bath applied via a gravity-fed reservoir. We typically recorded neurons one to two cell layers deep and perfused drugs for 5 min to allow penetration into the slice and to compensate for dead space in the perfusion tubing. All other general compounds were purchased from Sigma (St. Louis, MO).
Immunohistochemistry.
Immunohistochemistry was performed as previously described (Austgen et al. 2009; Kline et al. 2010). Briefly, deeply anesthetized animals were transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde (Sigma). After brain stem removal, 30 μm coronal brain sections were cut using a vibratome (VT 1000S, Leica). The sections were rinsed in PBS and then blocked by 10% normal donkey serum (Millipore) in 0.3% Triton-PBS. Tissue sections were subsequently incubated with primary antibodies against 5-HT1AR (guinea pig, 1:500, 550469, BD Pharmingen) and either synaptophysin (mouse, 1:2,000; cat. no. S5768, Sigma), vGLUT-2 (vesicular glutamate transporter; rabbit, 1:1,000, cat. no. 135403, SYSY) or GAD67 (glutamic acid decarboxylase; mouse, 1:3,000, cat. no. MAB5406, Chemicon). The following day, sections were rinsed and incubated in 0.3% Triton-PBS, including the appropriate Cy-conjugated secondary antibodies from donkey (1:200; Jackson Immuno). Sections were mounted on gelatin-coated slides, air dried and coverslipped. Slides were then sealed with nail polish. One section per run was incubated without the primary antibody and served as negative control. No fluorescent staining was present on the negative control. Specificity of the antibody for 5-HT1AR was confirmed via Western blot analysis (see below), or previously for synaptophysin (Olucha-Bordonau et al. 2012), vGLUT-2 (Berube-Carriere et al. 2009) and GAD67 (Fong et al. 2005).
Immunoreactivity (IR) was examined with a conventional epifluorescent microscope (BX51, Olympus) equipped with a digital monochrome camera (ORCA-ER, Hamamatsu) and a spinning disk confocal unit (Olympus), or a confocal microscope (FluoView FV 1000, Olympus). Appropriate filter sets and excitation wavelength were used to visualize the different fluorophores. For each fluorophore used, z-stacks (0.5 μM) were taken in the same focal planes. All images were postprocessed for clarity using ImageJ (version 1.45m, National Institutes of Health) for contrast and brightness, and AutoQuant X (version X2.2.2, MediaCybernetics) for background subtraction and deconvolution of specified images.
Visceral afferent nerve labeling.
In a subset of animals (n = 3), sensory afferents originating from the nodose ganglion were identified in 30-μm horizontal nTS slices through Texas Red dextran fluorescent labeling. Similar to our laboratory's previous studies (Austgen et al. 2011; Kline et al. 2002), rats were anesthetized with isoflurane (5% induction, 2–3% maintenance), and the nodose ganglion was isolated close to the bifurcation of the common carotid artery. Texas Red dextran (∼100 nl, Life Technologies) was pressure microinjected into the ganglion using a glass capillary and Picospritzer II (2–10 psi, General Valve Cooperation, Cleveland, OH). Subsequently, the neck was sutured, and the animal allowed to recover. Postoperative treatment incorporated Baytril (0.03 ml im; Bayer, Shawnee Mission, KS) and Buprenex (0.02 ml sq; Reckitt Benckiser Pharmaceuticals, Richmond, VA). After 5 days, which is sufficient for the anterograde transport of the dye to the nTS, rats were transcardially perfused, and the nTS was isolated and immunohistochemically processed for 5-HT1ARs as above.
Western blot.
As described previously (Kline et al. 2007), frozen nTS tissue was pooled (3 rats), homogenized in RIPA buffer (1% NP-40, 0.5% deoxycholate, 0.1% SDS, 0.15 M NaCl, 50 mM Tris·HCl and 2.5 mM EDTA), and complemented with protease inhibitors (Complete, mini-EDTA-free tablets; Roche). Following 2 h of incubation on ice, samples were centrifuged at 14,000 g for 30 min at 4°C. Protein concentration of the supernatant was measured by the Micro BCA method (Pierce, Rockford, IL). Twenty micrograms of protein were separated on 4–20% Tris-glycine gel (BioRad) and transferred to a polyvinylidene difluoride membrane. Primary antibody anti-5-HT1AR (guinea pig, 1:1000, cat. no. 550469, BD Pharmingen), followed by donkey anti-guinea pig secondary antibody conjugated to horseradish peroxidase (1:10,000), was used to immunoblot the membranes. Blots were developed with Immuno-Star WesternC kid (Bio-Rad) and visualized.
Data analysis.
Cardiorespiratory responses to nTS microinjection of 8-OH-DPAT were analyzed at 5 min for 25 s and expressed relative to their individual baseline (defined as “1”). There was no significant response to aCSF when microinjected on either side of the nTS, and therefore responses were averaged. The effects of 8-OH-DPAT (1 and 5 mM) were compared with the average responses of aCSF injections analyzed at the 5-min time point. In addition, because the time course of 8-OH-DPAT effects varied among rats, we also determined the maximum change in minute PhrNA (phrenic frequency × amplitude) within the first 10 min, comparing it to aCSF at the same time point. All data are presented as means ± SE.
Electrophysiological data were analyzed with pClamp10 (Molecular Devices), MiniAnalysis (Synaptosoft) and Microsoft Excel software. Only nTS cells monosynaptically connected to TS-afferents were analyzed for this study. A direct connection was determined based on a low variability of TS-EPSC onset (i.e., jitter, <300 μs; SD of 20 TS-EPSC latencies from shock artifact, 20-kHz sampling rate) (Accorsi-Mendonca et al. 2011; Doyle and Andresen 2001; Hisadome et al. 2010; Kline et al. 2002). Reported TS-EPSC properties were an average of 20 events. Spontaneous current (sEPSC, sIPSC, mEPSC and mIPSC) detection was set at 5 × the root-mean-square noise level, and events were manually confirmed. All electrophysiology data are presented as geometric means ± 95% confidence interval due to the variability of baseline values and to allow description of a general response (Carter et al. 2007; Lando and Zucker 1994; Sceniak and Maciver 2008). Responses were also normalized and expressed relative to their individual baseline or vehicle (defined as “1”).
To statistically evaluate the effects of 5-HT1AR activation on cardiorespiratory or synaptic parameters, SigmaPlot 12.0 (Systat Software) and GraphPad Prism 6 (GraphPad Software) were used. In vivo data were analyzed using one-way repeated-measures ANOVA, followed by Fisher least significant difference post hoc test where appropriate to identify individual differences among groups. For in vitro protocols, the effect of a drug on EPSC amplitude, rise slope, decay slope and group spontaneous and miniature PSCs was compared within treatments using paired t-tests, or the Wilcoxon signed rank test if the data were not normally distributed. Spontaneous current amplitude and interevent intervals within an individual cell between baseline and drug condition were tested with Kolmogorov-Smirnov two-sample test. Results were considered significantly different at P values ≤ 0.05.
RESULTS
5-HT1ARs are located somatodendritically in the NTS.
The presence of 5-HT1ARs in the nTS was examined by immunohistochemistry. The specificity of the 5-HT1AR antibody was verified via Western blot analysis, where it showed a single band at the expected ∼46 kDa (Fig. 1A). 5-HT1AR-IR was found throughout the nTS (Fig. 1B) with punctate staining on cell bodies and processes (Fig. 1, C–F). To examine whether this punctate staining represented 5-HT1AR-IR within synaptic terminals, we costained sections with the presynaptic marker synaptophysin (Fig. 1C); no colocalization was observed. Labeled visceral afferents and terminals from the nodose ganglion were also devoid of 5-HT1AR-IR (Fig. 1D).
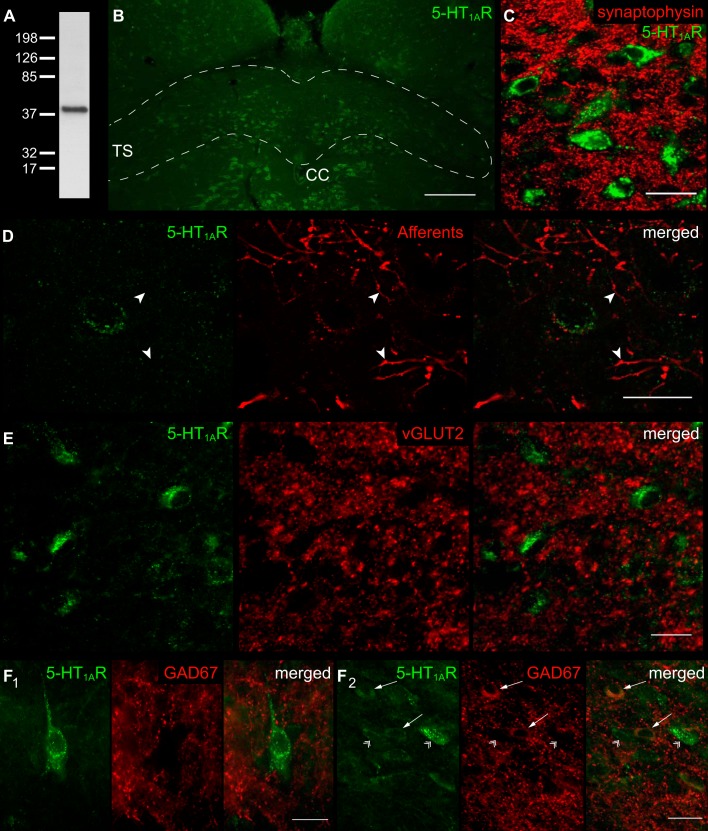
Fig. 1.
Localization of serotonin 1A receptor (5-HT1AR) within the nucleus tractus solitarii (nTS). A: immunoblot verifying antibody specificity for 50 μg of protein from the nTS. A single band for 5-HT1AR was confirmed at the appropriate size of ∼46 kDa. B: coronal section of the medial-commissural nTS (enclosed area) labeled with 5-HT1AR antibody. Scale 200 μm. TS, tractus solitarii; CC, central canal. C: nTS neurons colabeled for 5-HT1AR and synaptophysin. Note that both stainings are clearly separated, indicating that 5-HT1AR are localized somatodenritic within the nTS. Scale 25 μm. D: neuron expressing 5-HT1AR immunoreactivity with attached fluorescent Texas Red dextran labeled visceral afferent terminals. Note, afferent terminals or fibers (arrowheads) do not colabel with 5-HT1AR. The image has been background subtracted and deconvolved for clarity. Scale 20 μm. E: section showing absent colocalization of 5-HT1AR and vesicular glutamate transporter (vGLUT-2), a marker for glutamatergic terminals. Scale 20 μm. F: 5-HT1AR immunoreactivity in close apposition to glutamic acid decarboxylase (GAD67)-identified GABAergic cell terminals (F1), and colocalization with GABAergic cell bodies (F2, arrows). Note the absent colocalization of 5-HT1AR and GAD67-negative cells (arrowheads). Scale 20 μm.
In the nTS, the major excitatory and inhibitory transmitters are glutamate and GABA, respectively. Double labeling of 5-HT1ARs with vGLUT-2, an antibody targeted toward glutamatergic presynaptic terminals, showed no overlap (Fig. 1E), although the punctate staining of vGLUT-2 was closely apposed to 5-HT1AR-IR. Likewise, the punctate staining of GAD67-IR, which depicts GABAergic terminals, did not colocalize with 5-HT1AR (Fig. 1F1). However, cell bodies containing GAD67-IR (Austgen et al. 2009; Fong et al. 2005) colabeled with a subset of 5-HT1AR-IR (Fig. 1F2, arrows). All together, these data suggest that 5-HT1AR is somatodendritically located in cells of the nTS.
5-HT1AR activation decreases the amplitude and increases the frequency of phrenic nerve discharge.
To explore the functional role of 5-HT1AR in the nTS, we microinjected the prototypical 5-HT1AR agonist 8-OH-DPAT (1 and 5 mM, 60 nl) into the medial-commissural nTS, the primary central site for cardiorespiratory afferent termination. Histological analysis of the injection sites verified micropipette placement within the caudal nTS (Fig. 2C). Baseline cardiorespiratory variables are shown in Table 1. A representative recording of AP, HR, SSNA and PhrNA in response to 5 mM 8-OH-DPAT is shown in Fig. 2A. As shown, microinjection of 8-OH-DPAT produced minimal changes in AP, HR or SSNA. By contrast, 8-OH-DPAT increased PhrNA rate, but decreased PhrNA amplitude and minute PhrNA (Fig. 2, A and B). The changes in cardiorespiratory parameters 5 min (open bar in Fig. 2A) after aCSF or 8-OH-DPAT injection were normalized to their baseline preinjection activity and shown in Fig. 2B (baseline = 1). Neither 1 nor 5 mM 8-OH-DPAT in the nTS significantly altered AP, HR or SSNA compared with aCSF controls (PAP = 0.657; PHR = 0.171; PSSNA = 0.323). However, both 1 and 5 mM 8-OH-DPAT significantly decreased PhrNA amplitude and significantly increased PhrNA rate (Fig. 2B). The combination of a decrease in PhrNA amplitude and increase in PhrNA rate 5 min after 8-OH-DPAT injection resulted in minute PhrNA that was not significantly different from control. However, evaluation of maximum minute PhrNA change within each individual rat (closed bar Fig. 2A) revealed significant decreases for both concentrations of 8-OH-DPAT [P ≤ 0.01; 1 mM: 0.78 ± 0.06 normalized to baseline (time point = 4.4 ± 1.4 min); 5 mM: 0.8 ± 0.1 normalized to baseline (time point = 3.4 ± 2.0 min); aCSF: 1.0 ± 0.1]. Overall, our results suggest that activation of 5-HT1ARs in the nTS decreases phrenic motor output with no changes in cardiovascular variables (AP, HR) or sympathetic nerve activity.
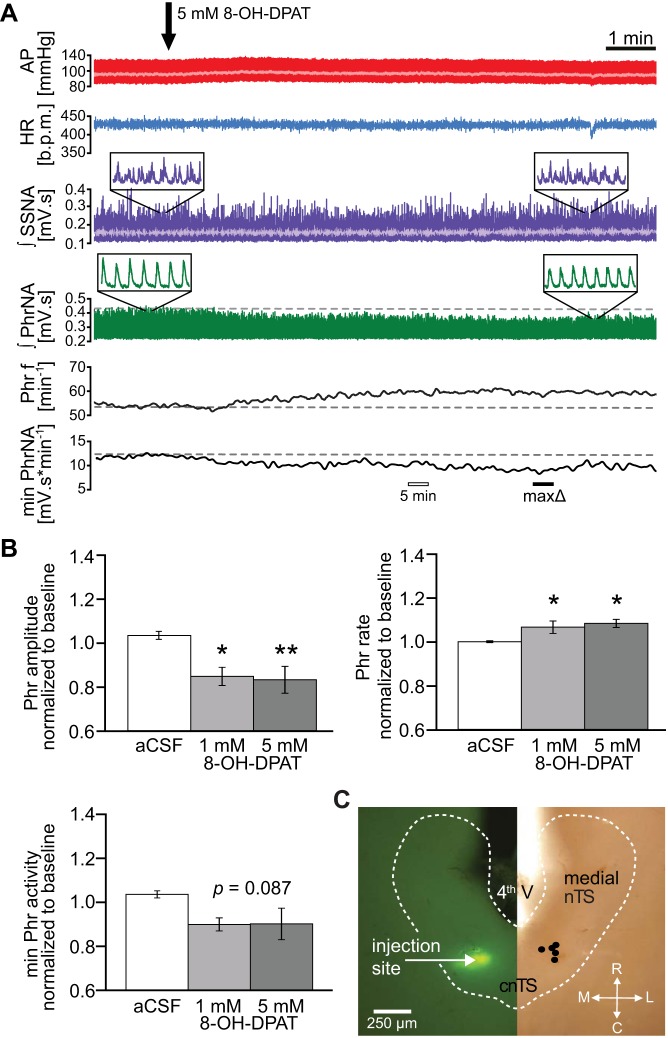
Fig. 2.
Activation of 5-HT1ARs decreases phrenic (Phr) motor output. A: representative example of pulsatile arterial pressure (AP; red), mean AP (light red trace superimposed on AP), heart rate (HR; blue), integrated splanchnic sympathetic nerve activity (∫SSNA; purple; mean SSNA in light purple) and integrated phrenic nerve activity (∫PhrNA, green). Phrenic frequency (Phr f) and minute activity (min PhrNA) are shown in the bottom panels. Note the decrease in PhrNA amplitude and increase in PhrNA rate after 8-hydroxy-2-(di-n-propylamino)tetral (8-OH-DPAT) injection (arrow), resulting in an overall decrease of minute PhrNA. Open bar depicts the point of the “5 min” measurement used for B (25-s average). Solid bar indicates time of maximum change in minute PhrNA of this individual example. B: group data (n = 5) describing the 8-OH-DPAT-induced decrease in PhrNA amplitude, increase in rate and the resulting minute nerve activity (measured 5 min after injections; open bar in A) compared with artificial cerebrospinal fluid (aCSF) control. *P ≤ 0.05; **P < 0.01 vs. aCSF. C: verification of 8-OH-DPAT injection sites. Horizontal slice of the medial-cnTS shows an example of a fluorescent bead injection (left) and the schematic summary of all injection sites (right). 4th V, fourth ventricle; bpm, beats/min; L, lateral; M, medial; R, rostral; C, caudal; cnTS, commissural nTS.
Table 1.
Baseline cardiorespiratory variables
| Parameter | Baseline |
|---|---|
| MAP, mmHg | 107 ± 3 |
| HR, beats/min | 312 ± 16 |
| O2 saturation, % | 98.9 ± 0.2 |
| SSNA, mV·s | 0.0147 ± 0.001 |
| PhrNA rate, bursts/min | 41.7 ± 4.1 |
| PhrNA amplitude, mV·s | 0.18 ± 0.04 |
| Minute PhrNA, mV·s·min−1 | 7.7 ± 2.2 |
Values are means ± SE; n = 5. MAP, mean arterial pressure; HR, heart rate; SSNA, splanchnic sympathetic nerve activity; PhrNA, phrenic nerve activity.
Activation of 5-HT1AR decreases afferent excitatory synaptic transmission.
5-HT1ARs may influence respiratory function through modulation of glutamatergic neurotransmission. To examine the contribution of 5-HT1ARs to nTS synaptic signaling, we stimulated the TS (0.5 Hz) to evoke glutamatergic EPSCs in nTS neurons that are monosynaptically connected to visceral afferents. Across the cells tested, TS-EPSCs had a mean amplitude of 69.2 pA (lower-upper 95% confidence interval, 58–82 pA), latency of 4.8 (4.4–5.2) ms and jitter of 147.4 (133–163) μs (n = 83, 45 rats), confirming such cells are monosynaptically connected to TS afferents (Accorsi-Mendonca et al. 2011; Bailey et al. 2006; Hisadome et al. 2010; Kline et al. 2002). As shown in the example from one cell (Fig. 3A), perfusion of 10 μM 8-OH-DPAT reduced the amplitude of TS-EPSCs from its aCSF baseline. This was a significant decrease for the whole sample tested (Fig. 3B). TS-EPSC rise time (n = 9; P = 1.00) or decay time (n = 9; P = 0.80) was not altered with 8-OH-DPAT. Furthermore, failure rate or mean2 variance of EPSC amplitude, indicators of presynaptic alterations, were not altered with 5-HT1AR activation (n = 10; Pfailure = 1.00, Pmean-var = 0.92). The reduction in TS-EPSC amplitude persisted throughout the 5-min washout period, consistent with prolonged effects of 8-OH-DPAT in other central tissue (Chen et al. 2008).
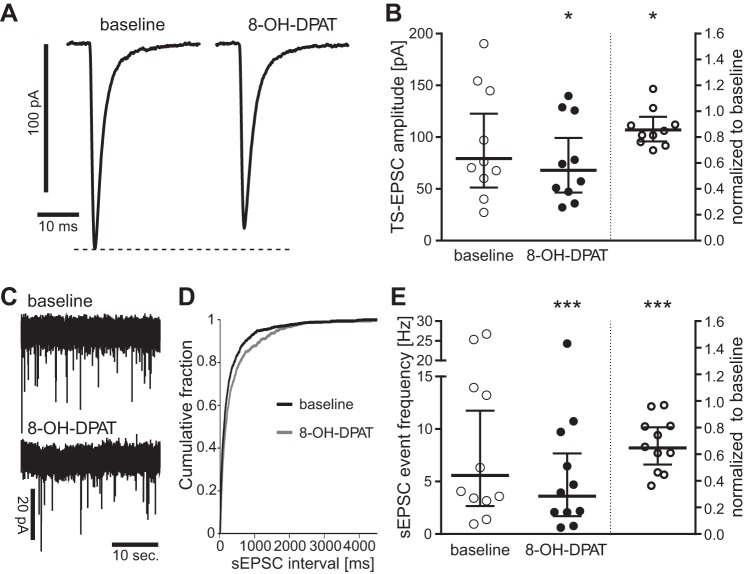
Fig. 3.
5-HT1AR activation decreases afferent synaptic transmission and excitatory nTS network activity. A: representative example (average of 20 sweeps) of the reduction in TS-excitatory postsynaptic current (EPSC) amplitude induced by the 5-HT1AR agonist 8-OH-DPAT (10 μM). B: individual data for the response to 8-OH-DPAT displayed as raw values (left) and normalized to baseline (right, baseline defined as “1”). The summary bars show the geometric mean with 95% confidence interval (CI). n = 10. *P < 0.05. C: representative example of the reduction in spontaneous EPSC (sEPSC) event frequency induced by 8-OH-DPAT. D: cumulative fraction of sEPSC intervals shows a significant shift (n = 11; P < 0.05, Kolmogorov-Smirnov two-sample test) to longer intervals in the presence of 8-OH-DPAT. E: individual data for the sEPSC frequency response to 8-OH-DPAT displayed as raw values (left) and normalized to baseline (right, baseline defined as “1”). Bars show geometric mean with 95% CI; n = 11. ***P < 0.001.
To examine whether the 5-HT1AR-induced decrease in TS-EPSC amplitude resulted from increased GABAergic transmission that would subsequently inhibit glutamate release and/or induce postsynaptic shunting, we examined synaptic currents in the presence of 8-OH-DPAT and GBZ to eliminate GABAA receptor-mediated inhibition within the nTS. GBZ alone (25 μM) did not change the amplitude of TS-EPSCs (n = 7; P = 0.17). In the presence of GBZ, activation of 5-HT1AR by 8-OH-DPAT (10 μM) significantly decreased TS-EPSC amplitude (Table 2). These results suggest that the 5-HT1AR-mediated decrease in TS-EPSCs does not occur via an increase in inhibitory GABAergic transmission.
Table 2.
Effects of 5-HT1AR agonist 8-OH-DPAT in the presence of various blockers
| GBZ | 8-OH-DPAT + GBZ | n | P Value | |
|---|---|---|---|---|
| TS-EPSC amplitude, pA | 106.5 (73–155) | 92.3 (61–140) | 7 | <0.05 |
| sEPSC frequency, Hz | 4.8 (2–11) | 3.5 (1–10) | 7 | <0.05 |
| sEPSC amplitude, pA | 30.4 (21–44) | 33.2 (23–47) | 7 | 0.2 |
| SB | 8-OH-DPAT + SB | |||
|---|---|---|---|---|
| TS-EPSC amplitude, pA | 123.3 (92–165) | 112.7 (84–152) | 10 | <0.05 |
| sEPSC frequency, Hz | 6.6 (3–14) | 5.3 (3–11) | 13 | <0.05 |
| sEPSC amplitude, pA | 31.5 (22–46) | 31.1 (22–45) | 13 | 0.95 |
| WAY | 8-OH-DPAT + WAY | |||
|---|---|---|---|---|
| TS-EPSC amplitude, pA | 72.4 (42–126) | 66.4 (37–117) | 7 | 0.18 |
| sEPSC frequency, Hz | 2.7 (0.9–7.9) | 2.4 (0.8–6.7) | 7 | 0.22 |
| sEPSC amplitude, pA | 23.9 (19–30) | 24.6 (19–31) | 7 | 0.25 |
| TTX + GBZ | 8-OH-DPAT + TTX + GBZ | |||
|---|---|---|---|---|
| mEPSC frequency, Hz | 4.3 (2.1–8.5) | 3.9 (1.9–8.1) | 11 | 0.47 |
| mEPSC amplitude, pA | 22.1 (16–30) | 22.1 (16–30) | 11 | 0.90 |
Values are geometric means (with lower and upper 95% confidence intervals in parentheses). Group values summarize the continued effect of 8-hydroxy-2-(di-n-propylamino)tetral (8-OH-DPAT) during GABAzine (GBZ) (GABAA receptor blocker) and SB-269970 (SB) [serotonin 7 receptor (5-HT7R) blocker]. These effects were ablated by 5-HT1AR-antagonist WAY-100135 (WAY). Miniature excitatory postsynaptic currents (mEPSCs) were unaltered in the presence of 8-OH-DPAT. TS, tractus solitarii; sEPSC, spontaneous excitatory postsynaptic currents.
While 8-OH-DPAT is a specific 5-HT1AR agonist, it may exhibit some affinity for the 5-HT7R (Lovenberg et al. 1993). To exclude a potential 5-HT7R contribution, we examined the effect of 8-OH-DPAT (10 μM) on TS-EPSCs in the presence of the 5-HT7R blocker SB-269970 (5 μM). SB-269970 alone did not change TS-EPSCs amplitude (n = 9; P = 0.15). Adding 8-OH-DPAT to the SB-269970 perfusion solution significantly decreased TS-EPSC amplitude (Table 2). These results show that 8-OH-DPAT decreases glutamatergic EPSCs, even in the presence of 5-HT7R blockade, suggesting its actions occur through 5-HT1ARs.
5-HT1AR-activation reduces excitatory network activity independent of GABAAR and 5-HT7R.
Recordings of sEPSCs in the absence of stimulation allows evaluation of the nTS network activity within the available circuitry of the slice (Fortin and Champagnat 1993). As shown in the representative cell (Fig. 3C), 5-HT1AR activation with 10 μM 8-OH-DPAT significantly reduced sEPSC event frequency compared with aCSF baseline. Overall, 5-HT1AR activation significantly shifted the cumulative fraction of sEPSC intervals to the right (Fig. 3D; P < 0.05, Kolmogorov-Smirnov, two-sample test) and decreased mean sEPSC frequency (Fig. 3E, n = 9). By contrast, 5-HT1AR activation did not change the cumulative fraction of sEPSC amplitudes (not shown) nor their mean amplitude [aCSF baseline, 22.4 (19–27) pA vs. 8-OH-DPAT, 23.1 (18–29) pA; n = 11; P = 1.00].
Similar to TS-EPSCs, the 5-HT1AR-mediated decrease in spontaneous currents was not influenced by GABAergic or 5-HT7R inhibition. Individually, GBZ did not alter sEPSC frequency (n = 7; P = 0.47) or amplitude (n = 7; P = 0.25). In the presence of GBZ, 5-HT1AR activation by 8-OH-DPAT significantly decreased sEPSC frequency, but did not change sEPSC amplitude (Table 2). Furthermore, 5-HT7R blockade alone did not alter sEPSC frequency (n = 13; P = 0.27) nor amplitude (n = 13; P = 0.38). Adding 8-OH-DPAT during SB-269970 perfusion significantly decreased sEPSC frequency but not amplitude (Table 2). Such data show that 8-OH-DPAT decreases sEPSCs in the presence of GABAergic and 5-HT7R blockade, suggesting its actions occur through 5-HT1ARs.
5-HT1AR activation does not alter mEPSCs.
sEPSCs derive from action potential-dependent and independent glutamate release (Fortin and Champagnat 1993). To examine the effects of 5-HT1AR activation on EPSCs that occur locally at the synapse and independent of action potentials (i.e., mEPSCs), we added TTX (1 μM; a voltage-gated Na+-channel blocker) and GBZ to the perfusion solution. This treatment did not change EPSCs amplitude (n = 11; P = 0.32), but decreased EPSC event frequency in 5 out of 11 cells (3 did not change and 3 increased). The total sample, however, did not reach significance (n = 11; P = 0.52). The addition of 8-OH-DPAT did not change mEPSC amplitude or frequency (Table 2). These results suggest 5-HT1AR-activation alters synaptic transmission via action potential-dependent mechanisms.
Blocking 5-HT1ARs increases excitatory network activity and ablates the effect of 8-OH-DPAT.
We examined the tonic role of 5-HT1ARs on TS- and sEPSCs by blocking the receptor with 10 μM WAY-100135, a selective 5-HT1AR antagonist (Cliffe et al. 1993). Blocking 5-HT1ARs did not alter evoked TS-EPSC amplitude (Fig. 4A). Moreover, subsequent application of 8-OH-DPAT in the presence of WAY-100135 did not change TS-EPSC amplitude (Table 2). In contrast to TS-EPSCs, WAY-100135 significantly increased sEPSC frequency (Fig. 4B) but not amplitude [aCSF baseline, 21.6 (18–25) pA vs. WAY-100135, 21.3 (18–25) pA; n = 9; P = 0.73]. Concurrent 8-OH-DPAT and 5-HT1AR block did not change sEPSC frequency or amplitude (Table 2). Taken together, these data suggest that 5-HT1ARs are constitutively active within the nTS network, but not at the sensory afferent-nTS synapse.
Fig. 4.
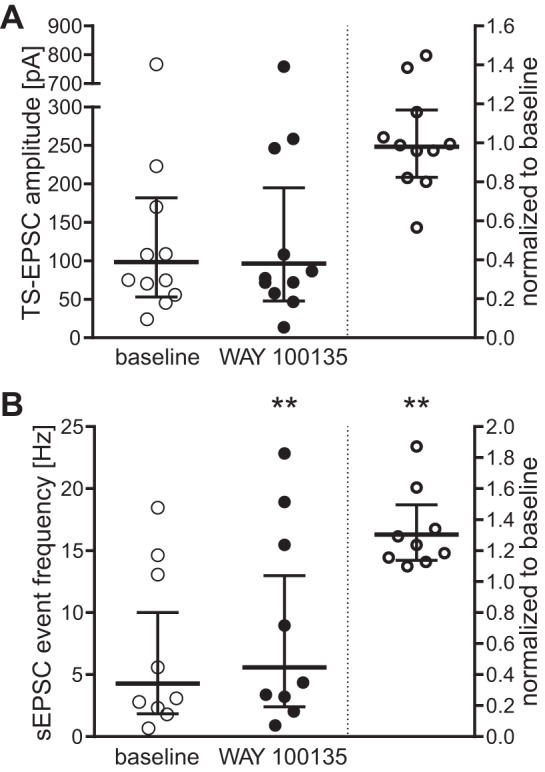
Block of 5-HT1AR increases excitatory nTS network activity. Individual responses to 5-HT1AR blockade with the specific antagonist WAY-100135 (10 μM) are displayed as raw values (left in each panel) and normalized to baseline (right, baseline defined as “1”) for TS-EPSC amplitude (A) and sEPSC event frequency (B). TS-EPSC amplitudes remain unaltered (n = 11; P = 0.97). Spontaneous event frequency, on the other hand, significantly increased, indicating a tonic role of 5-HT1AR. Bars show geometric mean with 95% CI; n = 9. **P < 0.01.
Activation of 5-HT1AR decreases GABAergic network but not evoked activity.
5-HT1ARs decrease inhibitory transmission in other brain stem nuclei, including synaptic signaling in the nucleus ambiguus originating from the nTS (Chen et al. 2008). Whether 5-HT1ARs alter evoked IPSCs or sIPSCs in the nTS is unknown. In the following protocols, IPSCs were recorded at −60 mV using a high chloride intracellular recording solution (calculated Cl− potential, 2.7 mV) and pharmacologically isolated. Sequential stimulation of the TS and nTS (Fig. 5A) elicited monosynaptic EPSCs and IPSCs, respectively (Fig. 5B, top) (Chen and Bonham 2005). Blocking glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (10 μM NBQX) and NMDA (50 μM AP5) receptors (AMPAR and NMDAR, respectively) eliminated the TS-EPSCs and isolated nTS-evoked IPSCs and sIPSCs (Fig. 5B, middle). Overall, nTS-IPSC amplitudes were not altered by glutamate receptor blockade [aCSF baseline, 73.9 (48–113) pA vs. NBQX + AP5, 62.2 (32–122) pA; n = 10; P = 0.91]. By contrast, spontaneous events, which initially comprised a mixture of sEPSCs and sIPSCs under these recording conditions, significantly decreased in frequency during NMDAR and AMPAR blockade [aCSF baseline, 3.9 (2.3–6.6) Hz vs. NBQX + AP5, 0.9 (0.6–1.3) Hz, n = 19; P < 0.001]. This illustrates the prevalence of excitatory nTS network activity. Subsequent application of GBZ eliminated all evoked IPSCs and sIPSCs, verifying that the nTS evoked IPSCs and sIPSCs were GABAergic (Fig. 5B, bottom).
Compared with baseline (during NBQX + AP5 perfusion), concurrent activation of 5-HT1ARs decreased the amplitude of nTS-IPSCs in five out of eight cells. However, this did not reach statistical significance in either the normalized or raw current values (Fig. 5C). In contrast, 5-HT1AR activation significantly decreased sIPSC frequency (Fig. 5D) but not amplitude [baseline, 55.0 (39–77) pA vs. 8-OH-DPAT, 51.2 (38–69) pA; n = 9; P = 0.65]. These data suggest a general decrease of inhibitory network activity with activation of 5-HT1AR.
Activation of 5-HT1AR alters inhibitory postsynaptic receptor function.
mIPSCs were isolated by the addition of TTX (1 μM), AP5 and NBQX to the aCSF. As expected, TTX, AP5 and NBQX significantly decreased the frequency of postsynaptic currents from aCSF baseline, which contains action potential-dependent and independent EPSCs and IPSCs [aCSF baseline, 2.6 (1.0–6.6) Hz vs. TTX + AP5 + NBQX, 0.9 (0.6–1.6) Hz; n = 8; P < 0.001]. The amplitude of the postsynaptic currents did not change (n = 8; P = 0.47). Such data suggest a decrease in excitatory and AP-dependent network activity.
Compared with TTX-AP5-NBQX baseline, 8-OH-DPAT did not alter mIPSC frequency (Table 3). However, 5-HT1AR activation significantly decreased mIPSC amplitude without affecting mIPSC rise time or decay time. This suggests that 5-HT1AR activation affects action potential independent mechanisms at the synapse for inhibitory transmission in the nTS.
Table 3.
Altered inhibitory postsynaptic receptor function with 5-HT1AR activation
| TTX + NBQX + AP5 | 8-OH-DPAT + TTX + NBQX + AP5 | n | P Value | |
|---|---|---|---|---|
| mIPSC frequency, Hz | 0.9 (0.5–1.6) | 0.9 (0.4–1.9) | 8 | 0.95 |
| mIPSC amplitude, pA | 33.1 (25–45) | 29.3 (21–41) | 8 | <0.05 |
| mIPSC rise time, ms | 3.2 (2.9–3.7) | 3.6 (3.2–4.1) | 8 | 0.17 |
| mIPSC decay time, ms | 4.8 (4.6–5.0) | 4.6 (4.2–5.1) | 8 | 0.84 |
Values are geometric means (with lower and upper 95% confidence intervals in parentheses). Group values depict indicators for changes occurring pre- or postsynaptically. Note, activation of 5-HT1ARs significantly decreased miniature inhibitory postsynaptic current (mIPSC) amplitude, implying a change at the postsynaptic receptor. TTX, tetrodotoxin; NBQX, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium; AP5, dl-2-amino-5-phosphonovaleric acid.
Blockade of 5-HT1AR and inhibitory network activity.
5-HT1ARs constitutively modulate EPSCs within the nTS (see above). To determine whether the same holds true for inhibitory transmission, we blocked 5-HT1AR by perfusing WAY-100135 while recording inhibitory currents. Evoked nTS-IPSC amplitude increased in four of six cells during 5-HT1AR blockade compared with baseline condition; however, as a whole, it did not reach statistical significance (Fig. 6A). sIPSCs frequency also increased by 5-HT1AR activation in 8 of 10 cells (Fig. 6B) but did not reach statistical significance in the mean data. The sIPSC amplitude did not change [baseline, 38.1 (28–53) pA vs. WAY-100135, 42.2 (32–56) pA; n = 10; P = 0.23].
Fig. 6.
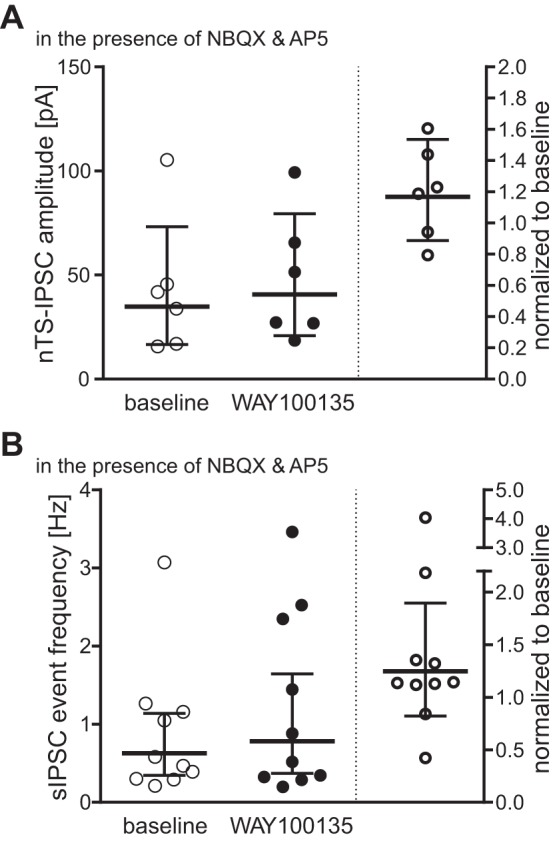
Block of 5-HT1ARs tends to increase inhibitory nTS transmission. Individual responses to 5-HT1AR blockade with WAY-100135 are displayed as raw values (left in each panel) and normalized to baseline (right, baseline defined as “1”) for nTS-IPSC amplitude (A) and sIPSC event frequency (B). Note, both nTS-IPSC amplitude and sIPSC event frequency remain statistically unaltered, although the majority of cells show a change with WAY-100135 treatment toward increased values.
DISCUSSION
In the present study, we demonstrate 5-HT1ARs are located throughout the nTS, primarily at postsynaptic neurons and apposed by glutamatergic and GABAergic terminals. 5-HT1AR activation in vivo overall decreased central respiratory output but did not alter cardiovascular parameters. Synaptically, activation of 5-HT1ARs reduced both excitatory glutamatergic and inhibitory GABAergic synaptic transmission, whereas 5-HT1AR inhibition of glutamatergic transmission was tonic at rest. These results provide insight into the functional role of this 5-HTR subtype in the nTS and respiratory system.
Previous studies reported that in vivo activation of 5-HT1ARs in the nTS excites, inhibits or does not alter neuronal activity (Oskutyte et al. 2009; Wang et al. 1997). These varying responses may result from differences in 5-HT1AR distribution, including disparity in its localization within the axon, dendrite, soma, synaptic or extrasynaptic regions, as well as differential activation of cellular phenotypes, including glutamatergic or GABAergic cells. In the present study, we show via immunohistochemistry that 5-HT1ARs were localized primarily within cell bodies and fibers throughout the nTS. Similar distributions in the nTS have been observed by others in the rat, including its protein (Liu and Wong-Riley 2010; Manaker and Verderame 1990; Thor et al. 1992) and mRNA (Pompeiano et al. 1992), as well as within humans (Spurney et al. 1997). We advance these studies by providing evidence that such 5-HT1AR labeling is apposed to, but not colabeled with, presynaptic markers, including those for presynaptic terminals in general (synaptophysin), glutamatergic terminals (vGLUT-2) and GABAergic terminals (GAD67). Moreover, labeled visceral afferents and terminals were also devoid of 5-HT1AR staining. Such results suggest 5-HT1ARs are not localized to presynaptic terminals nor sensory afferents, but rather within cells of the nTS.
We further provide functional evidence that unilateral 5-HT1AR activation within the medial-commissural nTS alters respiratory but not cardiovascular parameters. Specifically, the most prominent effect of 5-HT1AR activation was to decrease phrenic amplitude resulting in an overall reduction in minute nerve activity. Consistent with our in vitro data, this likely occurred via 5-HT1AR reduction of one or more active respiratory-related circuits, such as nTS projections to the retrotrapezoid nucleus, rostral ventrolateral medulla, or rostral ventral respiratory group (Alheid et al. 2011; Kline et al. 2010). Activation of 5-HT1ARs also increased phrenic rate similar to previous studies in which 8-OH-DPAT administered into the fourth ventricle or the dorsal motor nucleus of the vagus increases diaphragm muscular activity or phrenic rate (Besnard et al. 2012; Sporton et al. 1991). These effects may also indicate reduced glutamatergic excitatory transmission with 5-HT1AR activation and are consistent with increased phrenic rate following kynurenic acid injections into the caudal nTS (Costa-Silva et al. 2010). Of note, unilateral injection of 8-OH-DPAT decreased phrenic minute nerve activity by ∼20%; we anticipate that this response would have been even greater had the agonist been administered bilaterally. Although not likely because AP did not change, it is possible that responses would have been even greater in animals with afferent denervation to eliminate compensatory responses. Taken together, the data suggest that 5-HT, acting at 5-HT1ARs in the nTS, can have a significant impact on respiratory regulation.
5-HT1AR activation decreased afferent evoked TS-EPSC amplitude and the overall frequency of spontaneous excitatory network activity. This network 5-HT1AR modulation is tonic at rest, as evidenced by the increase in its activity following receptor blockade. In addition, it is independent of GABA, as shown by the prevailing effect of 8-OH-DPAT with coapplication of GBZ. 5-HT1AR-induced changes likely result from actions at the postsynaptic complex, as evidenced by unaltered TS-EPSC failure rate and mean variance of its amplitude, as well as mEPSC frequency (indicators of presynaptic alterations). Such results are consistent with its postsynaptic immunolocalization [present study and Riad et al. (2000)]. While TS-EPSC amplitude was reduced by 5-HT1AR activation, the lack of changes in s/mEPSC amplitude suggests a need to summate smaller 5-HT1AR-mediated events to observe larger end responses, such as those occurring during synchronized TS-EPSCs. The 5-HT1AR-mediated decrease in sEPSC frequency then likely originates from reduced activity of cells that synapse onto the recorded cell. However, while our results are consistent with studies showing a decrease of glutamatergic transmission with 5-HT1AR activation in other central neurons (Ciranna 2006; Costa et al. 2012; McCall and Clement 1994), including a decrease in nTS firing rate (Feldman 1995), they are not consistent with a recent nTS study demonstrating 1 μM 8-OH-DPAT did not alter synaptic transmission in Wistar rat slices (Takenaka et al. 2011). Such disparate results may be due to technical differences, rat strain, drug dosage, or perhaps the influence of GABAergic transmission. Taken together, we demonstrate 5-HT1AR restrains glutamatergic neurotransmission in the nTS to alter cardiorespiratory parameters.
5-HT1AR could attenuate TS-EPSC amplitude via a decrease in postsynaptic AMPA currents or reduction of AMPARs on the membrane. It has been shown that 5-HT1AR activation diminishes AMPA currents via decreased GluR1 subunit phosphorylation and inhibition of Ca2+/calmodulin-dependent kinase II (Cai et al. 2002). Conversely, blockade of 5-HT1ARs increases AMPAR phosphorylation, potentially increasing AMPA currents (Schiapparelli et al. 2005). Although long-term 5-HT1AR modulation may alter AMPAR membrane expression, it is unlikely that postsynaptic AMPAR expression is changed during our brief 5-min exposure of 8-OH-DPAT as mEPSCs were unaltered. Whether such mechanisms occur in nTS neurons requires further investigation.
While glutamate is the primary excitatory neurotransmitter in the nTS, it is counterbalanced by the inhibitory neurotransmitter GABA. We show that 5-HT1AR activation also decreased GBZ-sensitive nTS-evoked IPSCs in the majority of cells, and significantly reduced inhibitory nTS network activity (sIPSCs). Moreover, activation of the 5-HT1AR reduced spontaneous excitatory and inhibitory currents by a similar magnitude (∼30%). However, because the frequency of basal sEPSCs is greater than that of sIPSCs [4.8 Hz (Table 2) vs. 0.9 Hz (Table 3)], the net effect of 5-HT1AR activation to reduce excitatory events may predominate. Of note, while there was some tonic influence of 5-HT1ARs on IPSCs, it was relatively weak, and as a whole this effect did not reach statistical significance. Such results are consistent with reports that 5-HT1AR activation inhibits sIPSCs, but its blockade alone had no effect (Chen et al. 2008, 2012; Lee et al. 2008). Thus the inhibitory influence of 5-HT1ARs may be greater on glutamatergic than GABAergic transmission, altering its influence on activity in the nTS circuit and thus respiration. However, the importance of nTS-derived GABA and the influence of 5-HT1AR has been observed by others. For instance, 5-HT1AR activation decreased nTS-derived IPSCs within the dorsal motor nucleus of the vagus (Browning and Travagli 1999), as well as nTS-derived IPSCs within the nucleus ambiguus (Chen et al. 2012).
8-OH DPAT is a prototypical 5-HT1AR agonist, but may also bind to the 5-HT7R, a relatively new member of the 5-HTR family. However, the observed effects of 8-OH-DPAT in this study are likely due to 5-HT1AR, since the decrease in EPSCs was abolished by application of the specific 5-HT1AR antagonist WAY-100635, and prevailed during blockade of the 5-HT7R using the established antagonist SB-269960. While there may be some pharmacological overlap between both receptor subtypes, their primary pathways have been shown to differ as activation of 5-HT1ARs typically inhibits adenylyl cyclase to decrease cAMP production, whereas 5-HT7R activation causes the opposite effect (Millan et al. 2008). Likewise, 5-HT1ARs inhibit synaptic transmission in hippocampal neurons, whereas 5-HT7Rs produce an increase in transmission (Costa et al. 2012).
In summary, we demonstrate 5-HT1ARs reduce glutamatergic and GABAergic neurotransmission within the nTS. These effects are tonic at rest. However, the tonic activation is greater for sEPSCs than for IPSCs. 5-HT is implicated in modulation of the cardiovascular, respiratory and gastrointestinal systems under a variety of conditions. For instance, direct evidence has shown that 5-HT1ARs in the nTS reduce bronchopulmonary C-fiber-induced apneas (Zhuang et al. 2012), whereas indirectly they have been suggested to induce bradycardia, depressor responses and sympathoinhibition (Feldman and Galiano 1995; Itoh and Bunag 1991). Moreover, 5-HT has been implicated in sudden infant death syndrome (SIDS) and sleep apnea (Hilaire et al. 1993; Kinney 2005). Children who succumb to SIDS have lower 5-HT1ARs in the nTS (Machaalani et al. 2009). In the intermittent hypocapnic hypoxia piglet, a SIDS model, 5-HT1AR-IR is reduced in the nTS (Say et al. 2007). These data demonstrate a powerful physiological role for 5-HT1ARs in the nTS. Moreover, these results advance our understanding of the 5-HT/5-HT1AR-glutamate and GABA systems and their interactions and provide potential mechanisms for the role of 5-HT in vital autonomic and respiratory systems.
GRANTS
This study was supported by National Institutes of Health Grants RO1 HL-085108 (D. D. Kline) and multi-PI RO1 HL-098602 (E M. Hasser and D. D. Kline), and by American Heart Association Grant 12POST11670002 (T. D. Ostrowski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.D.O., D.O., E.M.H., and D.D.K. conception and design of research; T.D.O. and D.O. performed experiments; T.D.O. and D.O. analyzed data; T.D.O., D.O., E.M.H., and D.D.K. interpreted results of experiments; T.D.O. and D.O. prepared figures; T.D.O. and D.O. drafted manuscript; T.D.O., D.O., E.M.H., and D.D.K. edited and revised manuscript; T.D.O., D.O., E.M.H., and D.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Heather Dantzler for technical expertise in immunohistochemistry and immunoblots.
REFERENCES
- Accorsi-Mendonca D, Castania JA, Bonagamba LG, Machado BH, Leao RM. Synaptic profile of nucleus tractus solitarius neurons involved with the peripheral chemoreflex pathways. Neuroscience 197: 107–120, 2011 [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Sanders-Bush E. Serotonin. In: Neuropsychopharmacology, 5th Generation of Progress, edited by Davis KL, Charney D, Coyle JT, Nemeroff C. Philadelphia, PA: Lippincott Williams & Wilkins, 2002, p. 15–34 [Google Scholar]
- Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience 190: 207–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994 [DOI] [PubMed] [Google Scholar]
- Austgen JR, Fong AY, Foley CM, Mueller PJ, Kline DD, Heesch CM, Hasser EM. Expression of Group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience 159: 701–716, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgen JR, Hermann GE, Dantzler HA, Rogers RC, Kline DD. Hydrogen sulfide augments synaptic neurotransmission in the nucleus of the solitary tract. J Neurophysiol 106: 1822–1832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal BG, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol 517: 873–891, 2009 [DOI] [PubMed] [Google Scholar]
- Besnard S, Khemiri H, Masse F, Denise P, Verdaguer M, Gestreau C. Differential respiratory control of the upper airway and diaphragm muscles induced by 5-HT1A receptor ligands. Sleep Breath 16: 135–147, 2012 [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV). Br J Pharmacol 128: 1307–1315, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem 277: 36553–36562, 2002 [DOI] [PubMed] [Google Scholar]
- Carter AG, Soler-Llavina GJ, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J Neurosci 27: 8967–8977, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Bechtold AG, Tabor J, Bonham AC. Exercise reduces GABA synaptic input onto nucleus tractus solitarii baroreceptor second-order neurons via NK1 receptor internalization in spontaneously hypertensive rats. J Neurosci 29: 2754–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol 562: 535–551, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang L, Zhou X, Ge D, Yuan W, Wang J. Agonist of 5-HT1A/7 receptors but not that of 5-HT2 receptors disinhibits tracheobronchial-projecting airway vagal preganglionic neurons of rats. Neuroscience 207: 78–87, 2012 [DOI] [PubMed] [Google Scholar]
- Chen YH, Hou LL, Wang JJ. 5-HT1A/7 receptor agonist excites cardiac vagal neurons via inhibition of both GABAergic and glycinergic inputs. Acta Pharmacol Sin 29: 529–538, 2008 [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol Regul Integr Comp Physiol 268: R851–R858, 1995 [DOI] [PubMed] [Google Scholar]
- Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 4: 101–114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J Neurosci 31: 12318–12329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe IA, Brightwell CI, Fletcher A, Forster EA, Mansell HL, Reilly Y, Routledge C, White AC. (S)-N-tert-butyl-3-[4-(2-methoxyphenyl)-piperazin-1-yl]-2-phenylpropanamide [(S)-WAY-100135]: a selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. J Med Chem 36: 1509–1510, 1993 [DOI] [PubMed] [Google Scholar]
- Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus 22: 790–801, 2012 [DOI] [PubMed] [Google Scholar]
- Costa-Silva JH, Zoccal DB, Machado BH. Glutamatergic antagonism in the NTS decreases post-inspiratory drive and changes phrenic and sympathetic coupling during chemoreflex activation. J Neurophysiol 103: 2095–2106, 2010 [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Feldman PD. Effects of serotonin-1 and serotonin-2 receptor agonists on neuronal activity in the nucleus tractus solitarius. J Auton Nerv Syst 56: 119–124, 1995 [DOI] [PubMed] [Google Scholar]
- Feldman PD, Galiano FJ. Cardiovascular effects of serotonin in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 269: R48–R56, 1995 [DOI] [PubMed] [Google Scholar]
- Fong AY, Stornetta RL, Foley CM, Potts JT. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol 493: 274–290, 2005 [DOI] [PubMed] [Google Scholar]
- Fortin G, Champagnat J. Spontaneous synaptic activities in rat nucleus tractus solitarius neurons in vitro: evidence for re-excitatory processing. Brain Res 630: 125–135, 1993 [DOI] [PubMed] [Google Scholar]
- Gillis RA, Hill KJ, Kirby JS, Quest JA, Hamosh P, Norman WP, Kellar KJ. Effect of activation of central nervous system serotonin 1A receptors on cardiorespiratory function. J Pharmacol Exp Ther 248: 851–857, 1989 [PubMed] [Google Scholar]
- Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol 121: 147–162, 2000 [DOI] [PubMed] [Google Scholar]
- Hilaire G, Morin D, Lajard AM, Monteau R. Changes in serotonin metabolism may elicit obstructive apnoea in the newborn rat. J Physiol 466: 367–381, 1993 [PMC free article] [PubMed] [Google Scholar]
- Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes 59: 1890–1898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Bunag RD. Cardiovascular and sympathetic effects of injecting serotonin into the nucleus tractus solitarius in rats. J Pharmacol Exp Ther 256: 1147–1153, 1991 [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol 8: 507–524, 2005 [DOI] [PubMed] [Google Scholar]
- Kline DD, Hendricks G, Hermann G, Rogers RC, Kunze DL. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J Neurophysiol 101: 2270–2278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 88: 2736–2744, 2002 [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol 476: 117–130, 1994 [PMC free article] [PubMed] [Google Scholar]
- Lando L, Zucker RS. Ca2+ cooperativity in neurosecretion measured using photolabile Ca2+ chelators. J Neurophysiol 72: 825–830, 1994 [DOI] [PubMed] [Google Scholar]
- Lee KS, Han TH, Jo JY, Kang G, Lee SY, Ryu PD, Im JH, Jeon BH, Park JB. Serotonin inhibits GABA synaptic transmission in presympathetic paraventricular nucleus neurons. Neurosci Lett 439: 138–142, 2008 [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience 165: 61–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, De LL, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11: 449–458, 1993 [DOI] [PubMed] [Google Scholar]
- Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol (Berl) 117: 257–265, 2009 [DOI] [PubMed] [Google Scholar]
- Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol 301: 535–553, 1990 [DOI] [PubMed] [Google Scholar]
- McCall RB, Clement ME. Role of serotonin1A and serotonin2 receptors in the central regulation of the cardiovascular system. Pharmacol Rev 46: 231–243, 1994 [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la CC. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci 29: 454–464, 2008 [DOI] [PubMed] [Google Scholar]
- Mueller PJ, Foley CM, Vogl HW, Hay M, Hasser EM. Cardiovascular response to a group III mGluR agonist in NTS requires NMDA receptors. Am J Physiol Regul Integr Comp Physiol 289: R198–R208, 2005 [DOI] [PubMed] [Google Scholar]
- Mueller PJ, Hasser EM. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol 290: R383–R392, 2006 [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev 108: 1614–1641, 2008 [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Otero-Garcia M, Sanchez-Perez AM, Nunez A, Ma S, Gundlach AL. Distribution and targets of the relaxin-3 innervation of the septal area in the rat. J Comp Neurol 520: 1903–1939, 2012 [DOI] [PubMed] [Google Scholar]
- Oskutyte D, Jordan D, Ramage AG. Evidence that 5-hydroxytryptamine(7) receptors play a role in the mediation of afferent transmission within the nucleus tractus solitarius in anaesthetized rats. Br J Pharmacol 158: 1387–1394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci 12: 440–453, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, El MS, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417: 181–194, 2000 [PubMed] [Google Scholar]
- Sapru HN. Neurotransmitters in the nucleus tractus solitarius mediating cardiovascular function. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. New York: Springer, 2004, p. 81–98 [Google Scholar]
- Say M, Machaalani R, Waters KA. Changes in serotoninergic receptors 1A and 2A in the piglet brainstem after intermittent hypercapnic hypoxia (IHH) and nicotine. Brain Res 1152: 17–26, 2007 [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Slow GABA(A) mediated synaptic transmission in rat visual cortex. BMC Neurosci 9: 8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiapparelli L, Del RJ, Frechilla D. Serotonin 5-HT receptor blockade enhances Ca(2+)/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation. J Neurochem 94: 884–895, 2005 [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Bechtold AG, Tham RC, Bonham AC. A novel postsynaptic group II metabotropic glutamate receptor role in modulating baroreceptor signal transmission. J Neurosci 29: 11807–11816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporton SC, Shepheard SL, Jordan D, Ramage AG. Microinjections of 5-HT1A agonists into the dorsal motor vagal nucleus produce a bradycardia in the atenolol-pretreated anaesthetized rat. Br J Pharmacol 104: 466–470, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurney CF, Ohuoha DC, Murray AM, Kleinman JE, Hyde TM. The subnuclear distribution of 5-HT1A receptors in the human nucleus of the solitary tract and selected structures of the caudal medulla. Mcgill J Med 3: 80–85, 1997 [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557–618, 1981 [DOI] [PubMed] [Google Scholar]
- Takenaka R, Ohi Y, Haji A. Distinct modulatory effects of 5-HT on excitatory synaptic transmissions in the nucleus tractus solitarius of the rat. Eur J Pharmacol 671: 45–52, 2011 [DOI] [PubMed] [Google Scholar]
- Thor KB, Blitz-Siebert A, Helke CJ. Autoradiographic localization of 5HT1 binding sites in autonomic areas of the rat dorsomedial medulla oblongata. Synapse 10: 217–227, 1992 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. In vivo effects of 5-hydroxytryptamine receptor activation on rat nucleus tractus solitarius neurones excited by vagal C-fibre afferents. Neuropharmacology 36: 489–498, 1997 [DOI] [PubMed] [Google Scholar]
- Zhuang J, Zhang Z, Zhang C, Xu F. 8-OH-DPAT abolishes the pulmonary C-fiber-mediated apneic response to fentanyl largely via acting on 5-HT1A receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 303: R449–R458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol 52: 79–107, 1997 [DOI] [PubMed] [Google Scholar]