Abstract
Motor adaptation in response to gradual vs. abrupt perturbation schedules may involve different neural mechanisms, potentially leading to different levels of motor memory. However, no study has investigated whether perturbation schedules alter memory of a locomotor adaptation across days. We measured adaptation and retention (memory) of altered interlimb symmetry during walking in two groups of participants over 2 days. On day 1, participants adapted to either a single, large perturbation (abrupt schedule) or a series of small perturbations that increased in size over time (gradual schedule). Retention was examined on day 2. On day 1, initial swing time and foot placement symmetry error sizes differed between groups but overall adaptation magnitudes were similar. On day 2, participants in both groups showed similar retention, readaptation, and aftereffect sizes, although there were some trends for improved memory in the abrupt group. These results conflict with previous data but are consistent with newer studies reporting no behavioral differences following adaptation using abrupt vs. gradual schedules. Although memory levels were very similar between groups, we cannot rule out the possibility that the neural mechanisms underlying this memory storage differ. Overall, it appears that adaptation of locomotor patterns via abrupt and gradual perturbation schedules produces similar expression of locomotor memories across days.
Keywords: motor learning, motor adaptation, locomotion, walking, retention, human
one important feature of locomotion is the ability to adjust the walking pattern in response to altered conditions. The central nervous system (CNS) achieves these adjustments through the use of both feedback (Forssberg et al. 1975) and feedforward (McVea and Pearson 2007; Reisman et al. 2005; Morton and Bastian 2006) control. Motor adaptation is one way in which locomotor patterns are modified in a feedforward manner, specifically in response to some novel or unexpected but predictable perturbation (Shadmehr and Mussa-Ivaldi 1994). During motor adaptation, the CNS experiences a mismatch between the sensory information that is expected and that which actually results from a particular motor command (Kawato 1999; Tseng et al. 2007). This mismatch is thought to produce an error signal (Ito 1989) that drives the updating of feedforward motor commands to better suit the current context. With repetition, the error signal is slowly reduced in magnitude as the adjusted motor commands begin to more closely produce the expected sensory feedback, and the motor behavior becomes more appropriate for the new context.
Recently, there has been considerable focus on how perturbation schedules affect adaptation-based motor learning (Kagerer et al. 1998; Malfait and Ostry 2004; Klassen et al. 2005; Huang and Shadmehr 2009; Criscimagna-Hemminger et al. 2010; Schlerf et al. 2012; Gibo et al. 2013). Typically, this is assessed by comparing adaptation when a perturbation is introduced all at once (abrupt schedule) to adaptation when a perturbation is introduced in small increments over a sustained period of time (gradual schedule). During adaptation with an abrupt schedule, initial error sizes are large. Over the first several trials, the motor commands change substantially in response to the large errors, leading to a rapid plateau in performance. Once this plateau is reached, the newly adapted and successful motor commands are repeated for the remainder of the adaptation period. In contrast, with a gradual schedule, the initial movement errors are relatively small. Because the perturbation is incrementally introduced over the course of the adaptation period, new small errors also appear incrementally. Therefore, during adaptation to gradual schedules, there is very little opportunity for the motor system to repeatedly issue the same motor commands.
It has been suggested that the neural mechanisms for adaptation learning in response to abrupt and gradual schedules may differ (Huang and Shadmehr 2009; Klassen et al. 2005; Schlerf et al. 2012; Malfait and Ostry 2004). For example, given that abrupt schedules induce a prolonged period of repeated motor commands, this schedule may utilize reinforcement-based or repetition-based mechanisms of learning (Huang et al. 2011; Diedrichsen et al. 2010), which may preferentially recruit cortical regions of the motor system. This idea is supported by the recent finding that temporary disruption of the primary motor cortex through transcranial magnetic stimulation reduces learning during the late phase of reach adaptation when the perturbation is applied abruptly but not gradually (de Xivry et al. 2011). Adaptation involving a period of repetition of successful motor commands may also lead to better savings, which is measured as a faster rate of relearning when exposed to a perturbation a second time (Huang et al. 2011).
On the other hand, gradual perturbation schedules do not lead to repetition of the same motor commands, but instead maintain exposure to small errors throughout the adaptation period. Studies have suggested that gradual schedules lead to better transfer of adapted movements to novel contexts compared with an abrupt schedule (Michel et al. 2007; Torres-Oviedo and Bastian 2012). Huang and Shadmehr (2009) argued that when a perturbation is introduced gradually, it is perceived to be more permanent, and as such, the motor memories resulting from this learning should be better retained and decay more slowly. A similar argument has been made in relation to credit assignment (Berniker and Kording 2008), in which the CNS is thought to estimate the source of movement errors. The theory of credit assignment suggests that smaller errors are more likely to be attributed to changes within the body (e.g., limb dynamics) and larger errors are more likely to be assigned to something external, such as a new tool or device. Based on this rationale, motor adaptation in response to small errors would be better retained over long periods of time and better transferred to new contexts than motor adaptation in response to large errors.
Overall, these studies indicate that adapting to gradually and abruptly introduced errors may involve different neural substrates and different mechanisms of learning. Therefore, abrupt and gradual schedules may also result in differing levels of memory, or retention, of newly acquired adaptations over days. However, only a few studies have directly examined long-term retention. After adaptation of the reaching movements to a perturbation of the dynamics of the arm using a gradual schedule, recall of the adapted movement is better than when adaptation occurred using an abrupt schedule (Klassen et al. 2005). Additionally, after reach adaptation using a gradual schedule, deadaptation occurs more slowly after a second exposure (Huang and Shadmehr 2009). Regarding walking performance, it has been shown that split-belt locomotor adaptation transfers to overground walking better when the adapted pattern is acquired with a gradual perturbation schedule (Torres-Oviedo and Bastian 2012). However, retention of the walking pattern was not examined in this study. Each of these studies appears to support the notion that credit assignment is important for increasing retention.
To date, no studies have examined whether, for a locomotor adaptation, gradual and abrupt schedules lead to different levels of long-term motor memory, as measured by reduced error magnitudes during a second exposure (Blanchette et al. 2012). Thus the purpose of this study was to compare the magnitude of long-term (24-h) motor memory for a recently learned locomotor adaptation that was initially acquired using an abrupt vs. a gradual perturbation schedule. We theorized that if the duration of repeated successful motor commands was the most critical factor determining memory levels, then a group who initially acquired a locomotor adaptation using an abrupt schedule would show greater memory across days. On the other hand, if credit assignment was more critical for memory, then participants who initially acquired a locomotor adaptation using a gradual schedule would show greater memory across days.
METHODS
Participants.
Thirty-two young, healthy adults with no known orthopedic, neurological, or cardiovascular conditions were recruited for this study. Participants were assigned to one of two groups, either gradual (n = 16, 8 females, age 25.5 ± 3.1 yr, and body wt 71.0 ± 11.3 kg; means ± 1SD) or abrupt (n = 16, 8 females, age 25.4 ± 3.4 yr, and body wt 68.3 ± 10.9 kg). Group assignment was counterbalanced to ensure similar distributions of age, gender, and body weight (unpaired t-tests, P > 0.29 for age and weight). All procedures were in compliance with the Declaration of Helsinki, and the study was approved by the University of Iowa's Institutional Review Board. All participants provided their written informed consent.
Experimental design.
The general experimental paradigm used in this study has been described previously (Savin et al. 2010). Briefly, all participants walked on a treadmill at the same speed (1.34 m/s) while holding onto handrails, on 2 consecutive days. Day 1 consisted of two walking test periods, baseline (5 min) and adaptation (12 min), while day 2 testing consisted of three walking test periods, baseline (5 min), adaptation (12 min), and washout (10 min). All participants had a Velcro strap attached to either the right or left distal lower leg before the initial bout of walking. The choice of which leg to perturb was counterbalanced within groups. Participants had the same leg perturbed on both days of testing. We will refer to the leg to which the Velcro strap was attached as the perturbed leg and the leg without the strap as the unperturbed leg.
On day 1, during the baseline period, participants walked on the treadmill with only the Velcro strap attached to their leg. Between baseline and adaptation periods, a rope was hooked onto a loop attached to the back of the leg strap and fed through a pulley system. At the end of the pulley system, a small container was suspended by the rope. The container was secured at the top by the rope and at the bottom by a resistive band (Theraband; The Hygenic, Akron, OH), which served only to eliminate any slack in the rope. In this way, the rope, pulleys, and container (which would be filled with various sized weights) provided a novel resistance to the forward advancement of one leg during its swing phase.
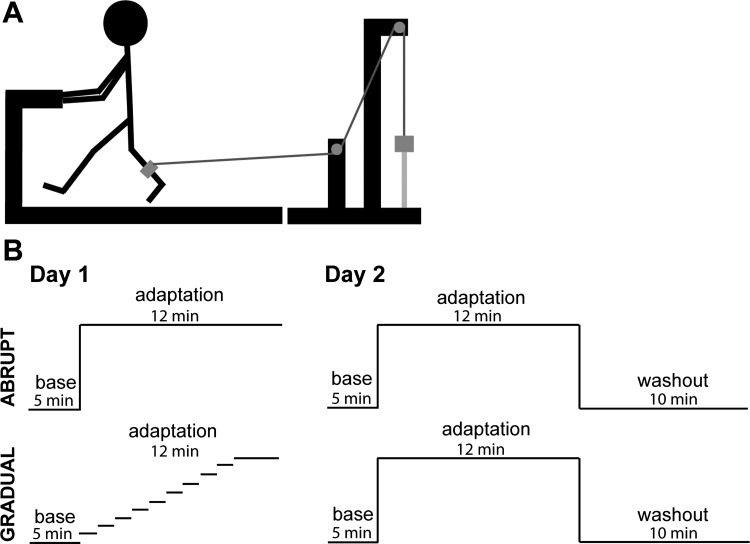
During the adaptation period, small weights were added to the container, either incrementally throughout the course of adaptation (gradual schedule) or all at once just before the beginning of the adaptation period (abrupt schedule). After adaptation, day 1 testing was complete and subjects were instructed to resume their normal daily routines. Participants returned to the laboratory ∼24 h later (range, 21.5–25.5 h) for day 2 testing. On day 2, all participants completed baseline testing in the same manner as day 1. During adaptation, however, the abrupt schedule was used for all participants. That is, the full perturbation amount used on day 1 was introduced all at once just before the beginning of the adaptation period for all subjects in both groups. At the end of adaptation, participants stepped onto the side sleds of the treadmill and the rope attached to the pulley system was unhooked from the leg strap. Participants then stepped back onto the moving treadmill belt and completed the washout period, which consisted of continuous walking with the perturbation removed. See Fig. 1 for an illustration of the experimental procedures.
Fig. 1.
Illustration of the experimental set up. A: participants walked on a treadmill while holding onto handrails. The resistive weight is depicted by the grey square. B: illustration of the experimental timeline for the abrupt and gradual groups on days 1 and 2. Base, baseline period.
The amount of perturbation was individualized and totaled 1.5% body wt. For participants in the gradual group, an initial perturbing mass of 0.15% body weight was placed in the container just before the beginning of the adaptation period. After each minute of adaptation, participants stepped onto the side sleds of the treadmill and another 0.15% body wt was added. Participants then stepped back onto the treadmill with their unperturbed leg first and continued walking. This continued until the 10th minute for a total perturbing mass of 1.5% of body wt. Participants then walked for an additional 2 min during adaptation with the full 1.5% perturbation in place. For participants in the abrupt group, the full perturbation amount of 1.5% of body weight was added to the container just before the beginning of adaptation. To make the adaptation procedures otherwise equivalent between groups, we also had participants in the abrupt group step onto the side-sleds of the treadmill every minute for the first 10 min of adaptation, remain there for 5–10 s, and then step back onto the moving treadmill with the unperturbed leg first to continue walking. For both groups, participants were instructed to walk as normally as possible throughout adaptation.
Data collection.
The Optotrak motion capture system (Certus System; NDI, Waterloo, ON) was used to record position data from both legs during walking. All strides of all testing periods were recorded continuously. Infrared markers were placed bilaterally on the heads of fifth metatarsals, lateral malleoli, and greater trochanters to mark the locations of the fifth metatarsophalangeal joints, ankles, and hips, respectively. Position data were sampled at 100 Hz.
Data analysis.
Position data were analyzed using a set of custom-written Matlab programs (MathWorks, Natick, MA). Data were low-pass filtered at 8 Hz. Heel strike was defined as the time at which the ankle marker was at its most anterior position and toe off was defined as the time at which the metatarsophalangeal marker was at its most posterior position (Noble and Prentice 2006). The primary outcome measures included one temporal measure and one spatial measure: swing time symmetry and foot placement symmetry. Swing time was defined as the time between toe off of one leg until the heel strike of that same leg and reported as a percentage of the gait cycle duration. Foot placement was defined as the anterior-posterior distance between the ankle marker and the hip marker on the leading leg at the time of heel strike. Swing time and foot placement were measured for each leg, and interlimb symmetry measures were calculated by subtracting the performance of the unperturbed leg from the perturbed leg for all strides. Thus perfect symmetry in both measures would be indicated by a value of zero. We then averaged performance over several key epochs during both days. On day 1, we averaged performance over late baseline, early adaptation and late adaptation. On day 2, we averaged performance over early and late baseline, early and late adaptation, and early and late washout. For all, early epochs consisted of the first five strides of that period, and late epochs consisted of the last 40 strides of that period. To minimize effects of any potential initial baseline asymmetries present on day 1 and to reduce intersubject variability, we also normalized the data by subtracting out the late baseline performance from day 1 from all values on both days for each individual.
Statistical analyses.
All statistical analyses were completed using Statistica (StatSoft, Tulsa, OK) software. The dependent variables were swing time interlimb symmetry and foot placement interlimb symmetry. First, to determine whether day 1 initial error sizes were different between groups and whether both groups adapted to similar levels despite differences in perturbation schedule, we compared early and late adaptation epochs across groups using a group × epoch factorial ANOVA, with repeated measures on the factor epoch. To determine whether either group showed significant retention across days (defined as reduced initial error when reexposed to the perturbation on day 2), we compared day 2 early adaptation epochs from both the abrupt and gradual groups to the day 1 early adaptation epoch from the abrupt group using unpaired t-tests. The unpaired t-test was performed to compare both the abrupt and gradual groups on day 2 to only the abrupt group on day 1 because perturbation sizes were necessarily very different between groups on day 1 and both groups performed the abrupt schedule on day 2. Next, separate group × epoch factorial ANOVAs were performed to compare 1) retention and readaptation magnitudes on day 2 (comparison of early vs. late adaptation epochs between groups), and 2) washout effects on day 2 (comparison of late baseline vs. early washout vs. late washout between groups). Finally, to determine if between-day aftereffects were present, a group × epoch factorial ANOVA was performed to compare late baseline on day 1 to early baseline on day 2 for both groups. This would determine whether there were any significant alterations in interlimb symmetry during the first five strides of day 2 baseline testing after a single day of exposure. When an ANOVA revealed a significant effect, Tukey's honestly significant difference test was performed for post hoc comparisons. Statistical significance was set at an alpha of 0.05 for all comparisons.
RESULTS
All participants walked in a grossly symmetrical manner during day 1 baseline. In both groups, when the weight was introduced at the beginning of adaptation, participants showed, for swing time, an increase on the perturbed leg and a decrease on the unperturbed leg, and for foot placement, a decrease on the perturbed leg and an increase on the unperturbed leg. These bilateral changes were consistent with previous findings using this paradigm (Savin et al. 2010, 2013) and generated a positive interlimb swing time asymmetry and a negative interlimb foot placement asymmetry at early adaptation. The swing time and foot placement asymmetries were largely corrected for by late adaptation.
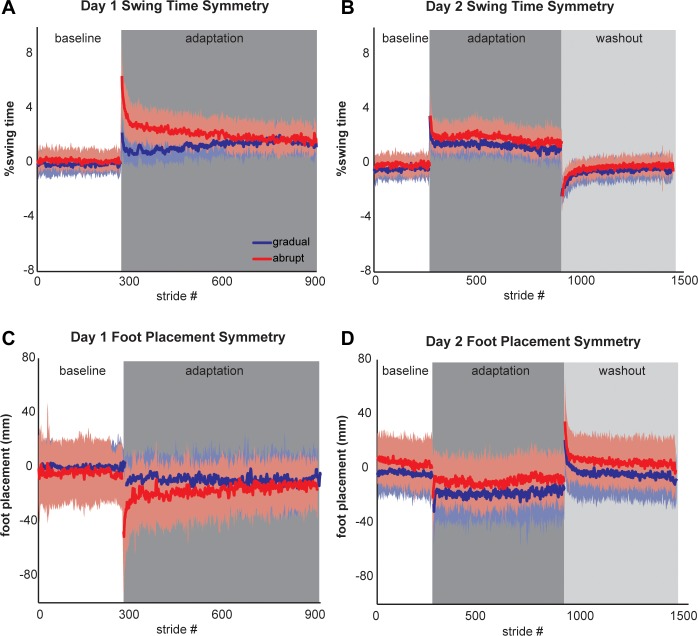
Figure 2 shows group average walking interlimb symmetry for all strides in the abrupt and gradual schedules. On day 1, the initial deviations from baseline symmetry during early adaptation indicate the relative error size in swing time and foot placement for each group. Note that the abrupt group showed larger swing time and foot placement deviations than the gradual group at the beginning of adaptation (i.e., the error sizes were, as expected, different between the 2 groups). Both groups appeared to show at least some degree of retention of swing time and foot placement symmetry across days (comparing day 2 early adaptation in both groups to day 1 early adaptation in the abrupt group). Also, both groups demonstrated robust negative aftereffects for swing time and foot placement symmetry (the reverse swing time and foot placement asymmetry) during the day 2 washout period.
Fig. 2.
Group average swing time (A and B) and foot placement (C and D) symmetry shown for all strides of walking for the abrupt group (red) and gradual group (blue) on days 1 and 2. Shading, ±1SD.
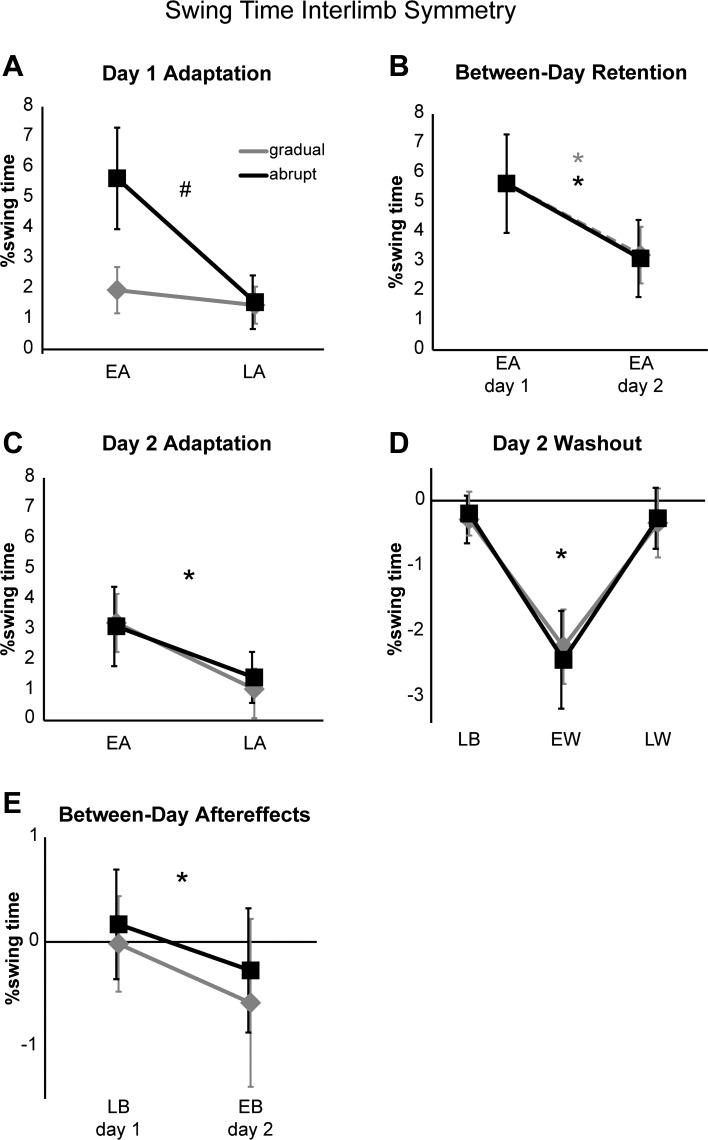
Group results for swing time interlimb symmetry are summarized over all key epochs in Fig. 3. For adaptation performance on day 1 (Fig. 3A), the ANOVA revealed a significant group × epoch interaction (P < 0.001). Post hoc testing indicated that the gradual and abrupt groups performed similarly during late adaptation (P > 0.99), but differently during early adaptation, where the abrupt group showed a larger swing time interlimb asymmetry error than the gradual group (P < 0.001). Additionally, swing time symmetry during early and late adaptation differed in the abrupt group (P < 0.001), whereas it did not differ in the gradual group (P > 0.46). Retention of the adapted swing time symmetry across days was evident in both groups as a reduction in early adaptation asymmetry on day 2 compared with the initial asymmetry in the abrupt group on day 1 (Fig. 3B, P < 0.001 for both). On day 2, the ANOVA comparing early vs. late adaptation across groups showed a significant effect of epoch (P < 0.001) but no group nor group × epoch interaction effects (P > 0.23 for both) indicating that there were no between-group differences in retention levels on day 2 during early adaptation or in overall readaptation magnitudes achieved during day 2 late adaptation (Fig. 3C). Washout effects are shown in Fig. 3D. Here, there was again a significant effect of epoch (P < 0.001), but no significant effect of group nor a group × epoch interaction (P > 0.27 for both). Post hoc testing revealed that early washout swing time asymmetries were significantly greater than both late baseline and late washout asymmetries (P < 0.001 for both) and that participants returned to baseline performance by the end of day 2 testing (late baseline vs. late washout, P > 0.80). Figure 3E shows there were persistent but small aftereffects during the first five strides of day 2 baseline testing after just the single session of exposure to either the abrupt or gradual perturbation 24 h previously. The day 1 late baseline epoch was compared with the day 2 early baseline epoch, and revealed a significant effect of epoch (P < 0.001), but no group effect nor a group × epoch interaction (P > 0.20 for both). Thus both groups showed between-day aftereffects of adapted swing time symmetry. Overall, these results demonstrate that, for swing time symmetry, the abrupt and gradual schedules produce similar levels of memory of a locomotor adaptation over 24 h.
Fig. 3.
Group average swing time interlimb symmetry during day 1 adaptation (A), between-day retention (B), i.e., early adaptation on day 1 (abrupt group only) vs. day 2 (both groups), day 2 adaptation (C), day 2 washout (D), and between-day aftereffects (E), i.e., late baseline on day 1 vs. early baseline on day 2. Black squares represent the abrupt group; grey diamonds represent the gradual group. Errors bars, ±1SD. LB, late baseline; EA, early adaptation; LA, late adaptation; EW, early washout; LW, late washout; EB, early baseline. #Significant group × epoch interaction; *significant main effect of epoch.
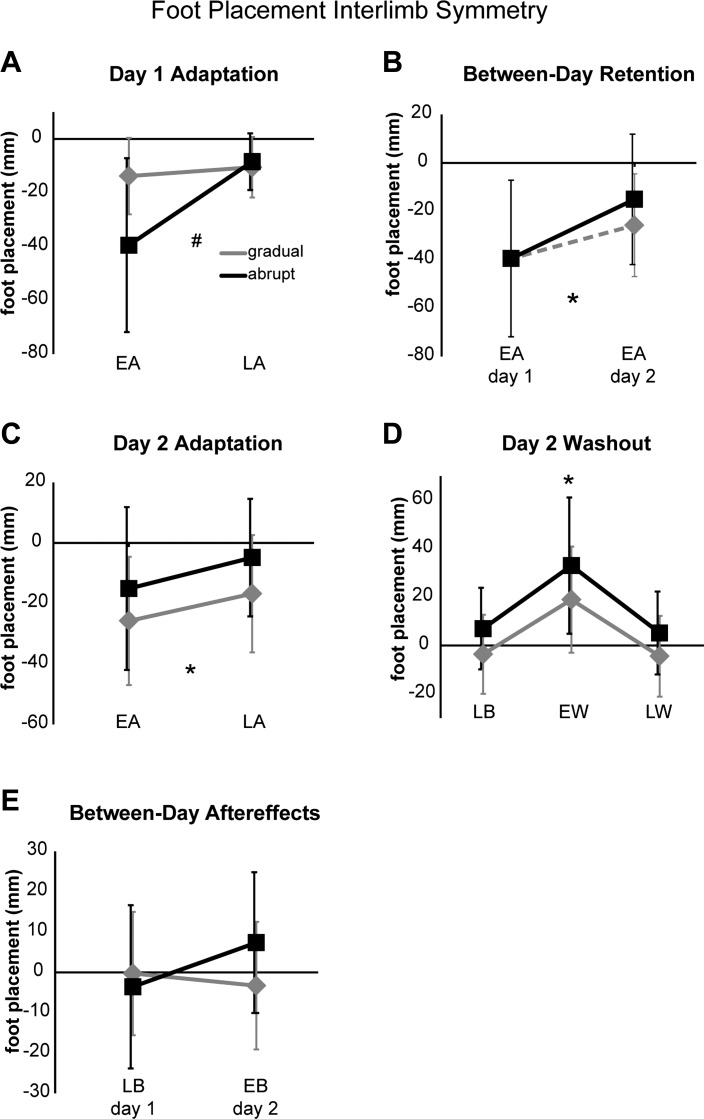
Group results for foot placement interlimb symmetry are summarized over all key epochs in Fig. 4. During day 1 adaptation (Fig. 4A), there was a significant group × epoch interaction (P < 0.05), with post hoc testing indicating that the abrupt group had a significantly greater foot placement error during early adaptation than the gradual group (P < 0.001) but that both groups reached similar levels of symmetry by late adaptation (P > 0.99). Additionally, participants in the abrupt group demonstrated significantly greater asymmetries during early vs. late adaptation (P < 0.001), whereas participants in the gradual group did not (P > 0.93). The abrupt group showed significant retention of adapted foot placement symmetry across days (P < 0.04), but this comparison did not reach significance in the gradual group (Fig. 4B, P = 0.169). On day 2, the comparison of early and late adaptation epochs (Fig. 4C) revealed a significant effect of epoch (P < 0.001), but no significant effect of group nor a significant interaction (P > 0.14 for both), indicating that there were no between-group differences in retention levels (or lack thereof) during day 2 early adaptation or in readaptation magnitudes during day 2 late adaptation. For the comparison of late baseline to early and late washout symmetry on day 2 between groups (Fig. 4D), there was a significant effect of epoch (P < 0.001), but no significant group effect nor a group × epoch interaction (P > 0.09 for both). Post hoc testing showed that early washout asymmetries were greater than both late baseline and late washout asymmetries (P < 0.001 for both), indicating the presence of large negative aftereffects that returned to baseline levels by late washout (difference between late baseline and late washout, P > 0.86). Last, the assessment of between-day aftereffects (comparisons of late baseline on day 1 to early baseline on day 2, Fig. 4E) revealed a significant group × epoch interaction (P < 0.04). However, post hoc testing of the interaction failed to show any significant differences either between groups or epochs (P > 0.10 for all). Overall, these results indicate that for foot placement symmetry significant retention across days was only observed in the abrupt schedule. However, neither schedule produced significant between-day aftereffects, and both schedules produced similar levels of retention during day 2 early adaptation, similar readaptation levels during day 2 late adaptation, and similar aftereffect sizes.
Fig. 4.
Group average foot placement interlimb symmetry during day 1 adaptation (A), between-day retention (B), i.e., early adaptation on day 1 (abrupt group only) vs. day 2 (both groups), day 2 adaptation (C), day 2 washout (D), and between-day aftereffects (E), i.e., late baseline on day 1 vs. early baseline on day 2. Black squares represent the abrupt group; grey diamonds represent the gradual group. Errors bars, ±1SD. #Significant group × epoch interaction; *significant main effect of epoch.
DISCUSSION
In the current study, we compared the extent of long-term (24-h) memory of an error-based locomotor adaptation that was initially acquired in response to either an abrupt or a gradual perturbation schedule. Our main finding was that there was no strong advantage of either the gradual or abrupt perturbation schedule for retention of the locomotor adaptation on day 2. Specifically, there was no difference in retention between abrupt and gradual groups for either swing time or foot placement symmetry (initial errors were not different between groups during day 2 early adaptation; Figs. 3C and 4C). Readaptation magnitudes and aftereffect sizes on day 2 were also similar between groups, as were between-day aftereffects. On the other hand, whereas both groups showed significant retention of swing time symmetry across days, only the abrupt group showed significant retention of foot placement symmetry across days (Fig. 4B) when both were compared with the abrupt group's performance on day 1. That is, although the difference between groups during day 2 early adaptation was not significant (suggesting similar levels of retention between groups), there was, for foot placement symmetry, a significant amount of retention as assessed by the comparison to day 1 early adaptation only in the abrupt group. Likewise, the day 2 adaptation (Fig. 4C) and washout (Fig. 4D) periods, and even the early baseline period (Fig. 4E), showed some apparent trends toward improved retention in the abrupt group for foot placement symmetry, although none were significant. Thus there may be a small benefit of the abrupt schedule on foot placement symmetry retention. Nevertheless, the overall findings suggest that locomotor adaptation using gradual vs. abrupt perturbation schedules does not significantly impact retention of a locomotor adaptation 24 h after initial learning.
At first, this result may appear to conflict with previous studies. Some studies have reported that adapting to a gradual schedule produces better initial learning, recall, and transfer of adapted movement patterns. Particularly, gradual saccade adaptation produced better learning than abrupt saccade adaptation (Wong and Shelhamer 2011), and adaptation of pointing movements to lateral visual displacements using prism goggles occurred more completely when the perturbation was introduced in several small steps (Michel et al. 2007). Torres-Oviedo and Bastian (2012) also reported that locomotor adaptation to a gradual schedule reduced initial learning but improved transfer of the adapted gait pattern to overground walking. One study reported mixed findings regarding recall of adapted reaching movements following adaptation to abrupt vs. gradual schedules (Klassen et al. 2005). In this study, gradual adaptation improved 24-h recall when reaching was adapted in the presence of a force-field perturbation, but 24-h recall after visuomotor adaptation to gradual and abrupt schedules was equivalent (Klassen et al. 2005). Other studies have supported these visuomotor findings by indicating that adaptation to gradual and abrupt schedules produces similar behavioral outcomes. Malfait and Ostry (2004) originally reported that participants who adapted reaching movements to an abrupt schedule showed significant interlimb transfer but participants who adapted to a gradual schedule did not. However, three other studies examining interlimb transfer following adaptation to abrupt and gradual schedules have since disagreed (Taylor et al. 2011; Wang et al. 2011; Joiner et al. 2013). Instead, it was suggested that overall exposure duration (which did not vary in the current paradigm) is the key determinant of the duration of transfer effects and that there may be no difference in the magnitude of transfer effects following adaptation to gradual and abrupt schedules (Joiner et al. 2013). Also, when participants adapt reaching movements to an abrupt or gradual schedule, movement error, movement velocities, and reaction times all reach similar adapted levels regardless of perturbation schedule (de Xivry et al. 2013), suggesting that gradual vs. abrupt schedules do not produce differences in the amount of adaptive learning. Thus the lack of a clear difference in retention levels between abrupt and gradual schedules that we observed here, in retrospect, may not be surprising.
In the Introduction, we noted that abrupt and gradual perturbation schedules differ in at least two ways that could impact mechanisms of motor learning and memory. First, with abrupt but not gradual schedules, there is a prolonged period of time during which the largely successful motor commands are repeated. Second, with gradual but not abrupt schedules, smaller error sizes may shift credit assignment systems toward attributing sensorimotor errors to the body's own dynamics, rather than towards a specific device or tool (e.g., the treadmill or the resistive strap used in this paradigm). We reasoned that if a long duration of repeated motor commands was the more potent stimulus for retaining memory of the adapted walking pattern across days, then an abrupt schedule would lead to greater retention. Conversely, if adapting to small errors and assigning credit to one's own limb dynamics was the more potent stimulus, a gradual schedule would lead to greater retention. Given the current results of relatively equivalent retention between groups, we conclude that either 1) there are no differences in mechanisms of learning and retention of gradual vs. abrupt perturbation schedules for locomotor adaptation, or 2) there are differing mechanisms at work during adaptation to gradual and abrupt schedules, but the resulting retention induced by experiencing a period of repeating motor commands (abrupt group) is matched by that induced by exposure to small, presumably self-assigned, errors (gradual group). The most parsimonious conclusion would be that gradual and abrupt adaptations rely on the same mechanism.
Indeed, we propose that acquisition and memory of adapted gait patterns, as observed in the current study, likely involves a network of overlapping brain regions that includes (among other areas) the cerebellum and primary motor cortex. Although it was previously reported that individuals with cerebellar damage retain the ability to adapt to gradual perturbations (Criscimagna-Hemminger et al. 2010), more recent evidence has refuted this by carefully controlling for the direction of the force-field perturbation within schedules (Gibo et al. 2013). Importantly, when the direction of force-field perturbation is not allowed to covary with perturbation schedule, individuals with cerebellar damage do show deficits in both abrupt and gradual schedules (Gibo et al. 2013). Other investigators have also confirmed that cerebellar damage impairs adaptation to gradual visuomotor perturbations (Schlerf et al. 2013). Therefore, it seems that both gradual and abrupt forms of adaptation are dependent on the cerebellum (Schlerf et al. 2013; Gibo et al. 2013). It may be that the cerebellum is preferentially involved in acquisition of motor adaptations while the motor cortex may be preferentially involved in memory of the adapted motor commands. For example, when anodal transcranial direct current stimulation is applied over the cerebellum, visuomotor adaptation occurs more quickly. However, when the same stimulation is applied over the primary motor cortex, adaptation is unaffected but deadaptation occurs more slowly (Galea et al. 2011). Additionally, when the motor cortex is disrupted by single-pulse or repetitive transcranial magnetic stimulation during adaptation, memory of the adapted movements is significantly degraded, but initial adaptation is unaffected (Hadipour-Niktarash et al. 2007; Richardson et al. 2006). Taken together, these studies suggest that the primary motor cortex is critically linked to motor memory. In the current paradigm, we suggest that for both perturbation schedules, error-reduction processes were, in part, heavily reliant on the cerebellum, whereas the longer-term memory formation likely recruited motor cortical circuits.
Although the duration of repeating motor commands and the size of the error signal experienced during adaptation were the focus of the current study, as well as other related works (Berniker and Kording 2008; Torres-Oviedo and Bastian 2012; Diedrichsen et al. 2010; Huang et al. 2011; de Xivry et al. 2011), it must be noted that there are a few other differences between groups that are present when using this type of paradigm. For example, the abrupt group was subjected to greater initial perturbing forces applied to the leg and therefore had to generate greater muscular forces and produce more overall effort during early adaptation and throughout the entire adaptation period, compared with the gradual group. The large initial error sizes in the abrupt group may have also led to greater cognitive awareness and/or more attentional resources being devoted to learning in this group. It is possible that one or more of these factors may have influenced learning and retention levels between groups. Further study with greater control of these covariates is warranted to determine the relative roles of these potential differences between gradual and abrupt schedules. Another interesting parallel relates to the work being done in assist-as-needed locomotor robotics training studies (Emken et al. 2007; van Asseldonk et al. 2009). From this work, there is growing evidence that guiding forces from a robot that reduce execution errors may be harmful to adaptation but that enhancing errors may not be useful either (van Asseldonk et al. 2009).
Some caveats to the current study must be mentioned. First, it is important to note that we specifically wanted to assess motor memory 24 h after initial learning. It is possible that within-day motor memories may not be expressed in the same manner as the motor memories observed here. For example, it could be that adaptation in response to a gradual schedule improves short-term motor memory more so than exposure to an abrupt schedule does, in which case we would expect to see greater short-term memory of adapted symmetries in the gradual group. This advantage may then decay over 24 h. However, we think this is unlikely because a recent report of within-day locomotor memory found no evidence for improved memory after adapting to a gradual vs. an abrupt schedule (van Asseldonk et al. 2011). Nevertheless, the time course of the decay of memory for motor adaptations may provide interesting clues into the relative roles of repeating motor commands and error sizes in the maintenance of motor memory. Second, it could be asked whether some of the nonsignificant but trending effects might become significant if there were greater statistical power. However, we have calculated that, given the effect sizes observed here, it would require between 40 and 100 participants per group to achieve a power level of 0.80. This is well beyond the norm for studies of this type, as most recruit between 8 and 17 participants per group (Morton and Bastian 2006; Torres-Oviedo and Bastian 2012; van Asseldonk et al. 2011). This illustrates the point that if perturbation schedule actually does alter locomotor memory, this effect is quite small and certainly much smaller than the effects of perturbation schedule on overground transfer of an adapted walking pattern (Torres-Oviedo and Bastian 2012).
In conclusion, this study examined 24-h motor memory of a locomotor adaptation in response to a perturbation that was applied using either a gradual or an abrupt schedule. We found that adapting using gradual and abrupt perturbation schedules led to similar retention levels, readaptation magnitudes, and aftereffect sizes 1 day after initial acquisition of the adaptation. However, when comparing day 1 abrupt early adaptation to day 2 early adaptation in both groups, the abrupt group showed reduced initial foot placement error when reexposed to the perturbation on day 2 (i.e., significant retention across days), but the gradual group did not. These findings suggest that if memory levels do differ following adaptation to gradual and abrupt schedules, these differences are small. We think these results are consistent with several recent studies suggesting that deadaptation rates, patterns of interlimb transfer, and cerebellar involvement during adaptation using abrupt and gradual schedules are similar (Joiner et al. 2013; Wang et al. 2011; Taylor et al. 2011; Gibo et al. 2013; Schlerf et al. 2013). In summary, we propose that exposure to small errors and a period of repeating motor commands produces similar behavioral expression of locomotor memories across days.
GRANTS
This work was supported in part by the National Institute of Neurological Disorders and Stroke Grant R21-NS-067189 and the Foundation for Physical Therapy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.H. and S.M.M. conception and design of research; S.J.H. performed experiments; S.J.H. and S.M.M. analyzed data; S.J.H. and S.M.M. interpreted results of experiments; S.J.H. prepared figures; S.J.H. drafted manuscript; S.J.H. and S.M.M. edited and revised manuscript; S.J.H. and S.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ryan Chapman, Xin Li, Chu-Ling Yen, Alison Charipar, and Shauna Dummett for assistance with data collection.
REFERENCES
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11: 1454–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette A, Moffet H, Roy JS, Bouyer LJ. Effects of repeated walking in a perturbing environment: a 4-day locomotor learning study. J Neurophysiol 108: 275–284, 2012 [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol 103: 2275–2284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Xivry JJO, Criscimagna-Hemminger SE, Shadmehr R. Contributions of the motor cortex to adaptive control of reaching depend on the perturbation schedule. Cereb Cortex 7: 1475–1484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Xivry JJO, Ahmadi-Pajouh MA, Harran MD, Salimpour Y, Shadmehr R. Changes in corticospinal excitability during reach adaptation in force fields. J Neurophysiol 109: 124–136, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci 30: 5159–5166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emken JL, Benitez R, Reinkensmeyer DJ. Human-robot cooperative movement training: learning a novel sensory motor transformation during walking with robotic assistance-as-needed. J Neuroeng Rehabil 28: 4–8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase-dependent reflex reversal during walking in chronic spinal cats. Brain Res 85: 103–107, 1975 [DOI] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJO, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21: 1761–1770, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibo TL, Criscimagna-Hemminger SE, Okamura AM, Bastian AJ. Cerebellar motor learning: are environment dynamics more important than error size? J Neurophysiol 110: 322–333, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci 27: 13413–13419, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol 102: 931–940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Ann Rev Neurosci 12: 85–102, 1989 [DOI] [PubMed] [Google Scholar]
- Joiner WM, Brayanov JB, Smith MA. The training schedule affects the stability, not the magnitude, of the interlimb transfer of learned dynamics. J Neurophysiol 110: 984–998, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997 [DOI] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Op Neurobiol 9: 718–727, 1999 [DOI] [PubMed] [Google Scholar]
- Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164: 250–259, 2005 [DOI] [PubMed] [Google Scholar]
- Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24: 8084–8089, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea DA, Pearson KG. Long-lasting, context-dependent modification of stepping in the cat after repeated stumbling-corrective responses. J Neurophysiol 97: 659–669, 2007 [DOI] [PubMed] [Google Scholar]
- Michel C, Pisella L, Prablanc C, Rode G, Rossetti Y. Enhancing visuomotor adaptation by reducing error signals: single-step (aware) versus multiple step (unaware) exposure to wedge prisms. J Cogn Neurosci 19: 341–350, 2007 [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt walking. J Neurosci 26: 9107–9116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JW, Prentice SD. Adaptation to unilateral change in lower limb mechanical properties during human walking. Exp Brain Res 169: 482–495, 2006 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005 [DOI] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabre A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci 26: 12466–12470, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Morton SM. Bilateral adaptation during locomotion following a unilaterally applied resistance to swing in nondisabled adults. J Neurophysiol 104: 3600–3611, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Whitall J, Morton SM. Poststroke hemiparesis impairs the rate but not magnitude of adaptation of spatial and temporal locomotor features. Neurorehabil Neural Repair 27: 24–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Bastian AJ, Celnik PA. Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. J Neurosci 32: 11610–11617, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB. Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophysiol 109: 1164–1173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Wojaczynski GJ, Ivry RB. Trial-by-trial analysis of intermanual transfer during visuomotor adaptation. J Neurophysiol 106: 3157–3172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. J Neurophysiol 107: 346–356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- van Asseldonk EH, Koopman B, van der Kooij H. Locomotor adaptation and retention to gradual and sudden dynamic perturbations. IEEE Int Cof Rehabil Robot 2011: 5975379, 2011 [DOI] [PubMed] [Google Scholar]
- van Asseldonk EH, Wessels M, Stienen AH, van der Helm FC, van der Kooij H. Influence of haptic guidance in learning a novel visuomotor task. J Physiol (Paris) 103: 276–285, 2009 [DOI] [PubMed] [Google Scholar]
- Wang J, Joshi M, Lei Y. The extent of interlimb transfer following adaptation to a novel visuomotor condition does not depend on awareness of the condition. J Neurophysiol 106: 259–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Saccade adaptation improves in response to a gradually introduced stimulus perturbation. Neurosci Lett 500: 207–211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]