Abstract
Control of digit forces for grasping relies on sensorimotor memory gained from prior experience with the same or similar objects and on online sensory feedback. However, little is known about neural mechanisms underlying digit force planning. We addressed this question by quantifying the temporal evolution of corticospinal excitability (CSE) using single-pulse transcranial magnetic stimulation (TMS) during two reach-to-grasp tasks. These tasks differed in terms of the magnitude of force exerted on the same points on the object to isolate digit force planning from reach and grasp planning. We also addressed the role of intracortical circuitry within primary motor cortex (M1) by quantifying the balance between short intracortical inhibition and facilitation using paired-pulse TMS on the same tasks. Eighteen right-handed subjects were visually cued to plan digit placement at predetermined locations on the object and subsequently to exert either negligible force (“low-force” task, LF) or 10% of their maximum pinch force (“high-force” task, HF) on the object. We found that the HF task elicited significantly smaller CSE than the LF task, but only when the TMS pulse coincided with the signal to initiate the reach. This force planning-related CSE modulation was specific to the muscles involved in the performance of both tasks. Interestingly, digit force planning did not result in modulation of M1 intracortical inhibitory and facilitatory circuitry. Our findings suggest that planning of digit forces reflected by CSE modulation starts well before object contact and appears to be driven by inputs from frontoparietal areas other than M1.
Keywords: hand, M1, TMS
successful object grasping and manipulation rely on the application of grip forces on the object that can be flexibly modulated according to object properties (Johansson and Cole 1992; Johansson and Westling 1984; Westling and Johansson 1984). Because of relatively long delays associated with somatosensory feedback, sensorimotor control of digit forces also relies on planning derived from prior experience with the same or similar objects (Johansson and Flanagan 2009). Our recent work suggests that digit force scaling is also a function of where the subject grasps the object (Fu et al. 2010).
Although the above-mentioned studies point to humans' ability to plan digit forces, as well as to correct for erroneously planned forces, the issue of when digit force planning within a reach-to-grasp task takes place and the underlying neural mechanisms remain unexplored. Electrophysiological studies in nonhuman primates have revealed that firing rates of different subsets of primary motor cortex (M1) neurons correlate positively or negatively with grasp force during exertion of active force on the object (Ashe 1997; Cheney and Fetz 1980; Evarts 1968; Hendrix et al. 2009; Hepp-Reymond et al. 1999; Maier et al. 1993; Thach 1978). More recently, Hendrix et al. (2009) have demonstrated that a subset of corticomotor M1 neurons in rhesus monkeys exhibit digit force-related activity before reach onset in preparation for force production on the object using a power grip. Evidence from humans with brain lesions suggests the involvement of the corticospinal tract, parietal and occipital regions of the brain, and cerebellum in planning of digit forces for object grasp and manipulation (Dafotakis et al. 2008a; Nowak et al. 2003; Raghavan et al. 2006; Rost et al. 2005).
Transcranial magnetic stimulation (TMS) studies in neurologically intact individuals demonstrated the role of anterior region of intraparietal sulcus (Davare et al. 2007), corticospinal tract (Loh et al. 2010), and M1 (Chouinard et al. 2005) in planning digit forces to grasp and lift an object based on memory of prior lifts, i.e., sensorimotor memory. However, in addition to sensorimotor memory, successful manipulation depends on appropriate digit force modulation to compensate for trial-to-trial variability in digit positioning on the object (Fu et al. 2010). This phenomenon led to the proposition that subjects plan both digit position and force prior to object contact but adjust forces following contact after sensing a discrepancy, if any, between planned and actual digit positions. This theoretical framework raises the question of whether the design of the above TMS experiments (Chouinard et al. 2005; Loh et al. 2010) might have been suitable to discriminate between planning of digit forces from planning digit forces and positions. Therefore, the extent to which corticospinal excitability (CSE) is modulated with digit force planning for grasping in humans, as well as the time course of CSE modulation, deserves further investigation. Another significant gap is the contribution of intracortical M1 circuitry to digit force planning.
To address these issues, the present study was designed to quantify the extent to which M1 is involved with digit force planning associated with a reach-to-grasp task. We pursued this objective by using single-pulse TMS to quantify the excitability of corticospinal tract, whose fibers predominantly originate from M1 (Lemon 2008), and the role of intracortical circuitry within M1 during digit force planning. For the second objective, we used a paired-pulse TMS approach to assess short intracortical inhibition and facilitation within M1 (Davare et al. 2008; Kujirai et al. 1993; Rothwell et al. 2009). To allow for repeatable trial-to-trial planning of digit position, we studied two reach-to-grasp tasks that differed in terms of whether, following contact on the same points on the object, subjects were asked to exert negligible or significant force on the object without lifting it. This approach was used to isolate the digit force planning component embedded in the reach-to-grasp task, because the only difference between the two tasks was planning of digit force. To quantify the time course of CSE changes associated with digit force planning, we delivered TMS to M1 at different time points within a period starting before reach onset and ending prior to object contact. On the basis of the above-reviewed literature, we hypothesized that force planning-dependent modulation of CSE would occur before reach onset.
METHODS
Subjects
Twenty-two right-handed volunteers aged between 18 and 33 yr (mean ± SD: 22 ± 8 yr; 9 women) with normal or corrected-to-normal vision and no history of musculoskeletal disorders, neurological disease, or upper limb injury were recruited to participate in the study. All subjects were naive to the experimental purpose of the study and gave informed consent to participate in the experiment. Eight subjects participated in experiment 1. Experiment 2a (paired-pulse validation) was performed on 6 subjects, and experiment 2b was performed on 10 subjects (2 of whom participated in experiment 2a). Subjects were screened for potential risk of adverse reactions to TMS according to guidelines by Rossi et al. (2009) using the TMS Adult Safety Screen (Keel et al. 2001). The experimental protocols were approved by the Office of Research Integrity and Assurance at Arizona State University.
Apparatus
Grip device.
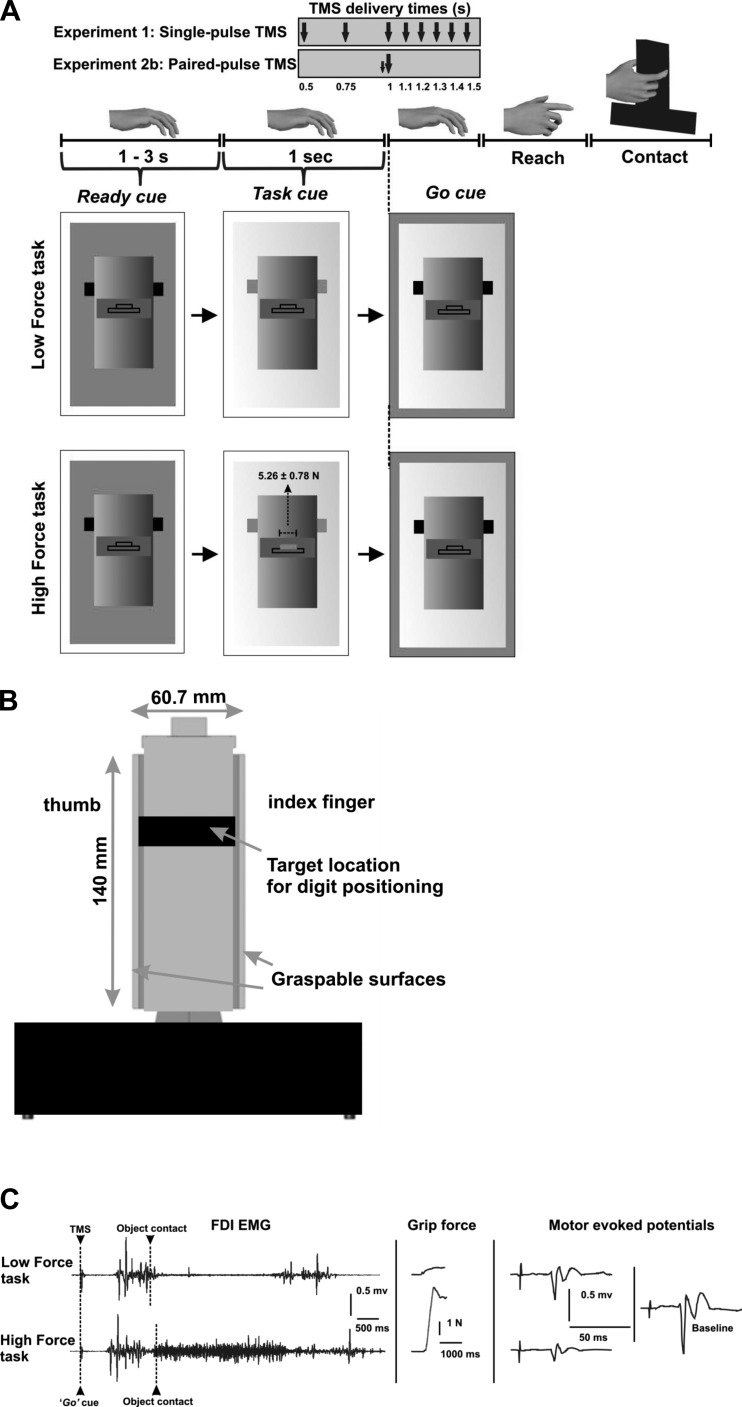
Force exerted by thumb and index finger perpendicular to each gripping surface were measured by two six-dimensional force/torque sensors (Nano-25; ATI Industrial Automation, Garner, NC; Fu et al. 2010; Zhang et al. 2010) mounted on a custom-designed inverted T-shaped grip device (Fig. 1, A and B).
Fig. 1.
Experimental setup, protocol, and representative data. A: experimental protocol. B: grip device. C: electromyographic (EMG) activity from the first dorsal interosseus (FDI) muscle, grip force exerted by thumb and index finger, and motor-evoked potentials (MEP) elicited by transcranial magnetic stimulation (TMS) at the instant of the go cue for the high-force (HF) and low-force (LF) trials (1 representative subject, experiment 1). The baseline MEP was obtained by delivering TMS in between trials while the subject was at rest (see text for more details).
Transcranial magnetic stimulation.
Resting motor threshold (rMT) was estimated using single monophasic TMS pulses delivered by a Magstim model 200 stimulator (Magstim, Whitland, UK). The TMS coil was held tangential to the scalp and perpendicular to the presumed direction of the central sulcus, 45° from the midsagittal line, with the handle pointing backward, inducing current in the posteroanterior direction. Using suprathreshold TMS pulses, we located the region of left M1 that represents the right first dorsal interosseus (FDI) muscle, which in turn corresponds with the hand “knob” area in M1 (Yousry et al. 1997). The position of the coil was adjusted to optimize the motor-evoked potential (MEP) amplitude in all recorded muscles. Following this procedure, the rMT was determined as the TMS intensity that induced 50-μV peak-to-peak MEPs in 5 of 10 trials in FDI muscle (Rossini et al. 1994). The rMT was on average 39 ± 4% (mean ± SE) of the maximal stimulator output. During all experimental procedures following rMT estimation, the TMS coil was stabilized using a coil holder mounted on the TMS chair (Rogue Research, Montreal, QC, Canada). After identification of the optimum coil location in primary motor cortex, the coil was traced on the scalp using a surgical skin marker pen. The location of coil was regularly checked for any displacement that might have occurred during the experimental session.
Experimental Procedures
Tasks.
All experiments were performed in a quiet and well-illuminated room. Subjects were seated in a custom-made chair specifically designed for TMS with the head supported in a head rest (Rogue Research). The grip device was placed on a table in front of the subject chair. The subjects' dominant hand rested pronated comfortably on the table surface at a distance of 30 cm from the grip device. Subjects were instructed to reach and grasp the grip device using the tips of thumb and index finger at a self-selected speed, but without lifting it, using the right hand (Fig. 1A). They were instructed to keep their noninvolved digits, i.e., middle, ring, and little finger, extended during the performance of both tasks.
We studied two task conditions: a low-force (LF) task and a high-force (HF) task. For the LF task, subjects were instructed to reach and grasp the object at predetermined locations by exerting minimal force (<1 N) perpendicular to its gripping surfaces. For the HF task, subjects were instructed to exert 10% of their maximal voluntary force (MVF; Fig. 1). MVF was measured for each subject at the beginning of the session by asking subjects to squeeze as hard as possible with thumb and index finger the same grip device used for the experiment. We selected the largest force of three MVF trials to set the target level for HF task. Subjects were asked to grasp the object at the same locations for both tasks. The location of the instructed digit placement on the grasp surfaces of the grip device was denoted by a colored tape attached on the front panel of the device.
For both tasks, a computer monitor placed behind the grip device presented three sequential visual cues: the first, a “ready” cue, signaled the beginning of a trial and was followed by a “task” cue presented at random delays (1–3 s) after the ready cue. The task cue consisted of a schematic “box” representing the grip device (Fig. 1A) and informed the subject about whether the upcoming task was an LF or a HF task. Finally, the “go” cue appeared 1 s after the task cue to instruct subjects to initiate the reach-to-grasp task. Note that both LF and HF task cues showed a force target, the only difference between the targets being the required force magnitude. The experimenter ensured that subject's performance was consistent with the task cue during each trial. Force feedback was provided to the subjects to ensure they would not go beyond a ±1 N window centered at the target force for each task. For the HF task, subjects were instructed to apply force to reach the target shown on the computer monitor during the task cue presentation (Fig. 1A). Subjects were allowed to practice both LF and HF tasks a few times before data collection started. We should emphasize that LF and HF tasks were identical in terms of reach distance, cue sequence, and visual display of force feedback, with the only difference being that for the HF task subjects were asked to exert a much greater normal force than for the LF task.
TMS: corticospinal excitability (experiment 1).
To assess the influence of digit force planning on the CSE, we delivered single-pulse TMS at 120% of rMT to left M1 of 8 subjects using a 50-mm-diameter custom-made figure-of-eight coil at 1 of 8 latencies from the task cue in a random order: 500, 750, 1,000 (coinciding with the go cue), 1,100, 1,200, 1,300, 1,400, and 1,500 ms. This procedure was used for both LF and HF tasks. LF and HF tasks were presented randomly across 6 blocks of 40 trials each. Each combination of task condition and TMS delivery time was repeated 15 times. During each block, each subject received eight single-pulse TMS randomly interspersed between trials to assess CSE at rest (baseline MEP) for the normalization procedure (see below). TMS pulses were spaced at least 5 s apart. Because both tasks required digit positioning on the object at the same locations, any task-related difference in CSE at one or more TMS delivery times would be due to digit force planning.
Validation of paired-pulse TMS protocol (experiment 2a).
It has been shown that when the interval between the first (conditioning) pulse delivered at subthreshold intensity and the second (test) pulse delivered at suprathreshold intensity is between 1 and 5 ms (interstimulus interval, ISI), there is a significant inhibition (short intracortical inhibition, SICI) of the response to the test pulse. However, if the ISI is between 6 and 50 ms, there is a significant facilitation (short intracortical facilitation, SICF) of the response to the test pulse (Kujirai et al. 1993). To validate our paired-pulse TMS protocol, either a single pulse (test alone) or two (paired) pulses with an ISI of 1, 2, 4, 8, 10, 12, and 15 ms randomized across trials were delivered to 6 subjects using a 70-mm-diameter custom-made figure-of-eight coil with subjects at rest. Fifteen trials were delivered per single pulse and each paired-pulse ISI with a total of 120 stimulations. The first (conditioning) pulse was delivered at 80% of rMT, followed by another (test) pulse at 120% of rMT (Davare et al. 2008).
Assessment of intracortical inhibition and facilitation (experiment 2b).
We used a paired-pulse TMS approach on 10 subjects to quantify the balance between intracortical inhibitory and facilitatory circuits within M1 during digit force planning. Using the same above-described tasks (LF and HF), we delivered either a single pulse (“test” stimulus) or paired-pulses with an ISI of 1, 2, 8, 10, and 15 ms randomized across trials at the moment of the go cue using a single TMS coil placed over the M1 hand area. We focused on this time only because the results of experiment 1 revealed that CSE was modulated as a function of task only at the time of the go cue. Subjects performed 15 trials per task per TMS pulse (single or paired). Each subject performed 6 blocks of 30 trials each. The stimulation intensity was the same as for experiment 2a. Similarly to experiment 1, subjects received five single-pulse TMS randomly interspersed between trials to assess CSE at rest during each block.
Data Analysis
We recorded electromyographic (EMG) activity using Ag-AgCl bipolar surface electrodes from three intrinsic muscles of the right hand (first dorsal interosseus, FDI; abductor pollicis brevis, APB; abductor digiti minimi, ADM) and one forearm muscle (flexor carpi radialis, FCR). For experiment 1, ADM and FCR were used as control muscles to determine whether the effects of digit force planning on CSE during movement preparation (if any) were specific to the muscles involved in the execution of the tasks (FDI and APB; task-specific muscles). For experiment 2b, we used only ADM as a control muscle because both controls used in the first experiment showed similar but no task-specific effects. The MEP peak-to-peak amplitude was measured to assess the CSE during preparation for each of the two grasp tasks. To avoid any influence of ongoing EMG activity on MEPs, trials with EMG activity greater than two times the standard deviation of the mean background EMG activity occurring 100 ms before the TMS pulse were excluded (<10% of trials). About 40% of trials for TMS elicited at 1,400 and 1,500 ms after the task cue (400 and 500 ms after the go cue, respectively) were excluded because the reach was initiated, as indicated by an increase in the EMG activity of one or more muscles, before the delivery of the TMS pulse. Therefore, MEPs computed for these two time points were excluded from statistical analysis (experiment 1).
For experiment 1, we computed the ratio between MEPs elicited at each of the above-described time points and “baseline” MEP recorded between trials to normalize MEP data across subjects. The baseline MEPs for FDI, APB, ADM, and FCR muscles were 2.49 ± 0.3, 2.41 ± 0.6, 1.5 ± 0.4, and 0.5 ± 0.1 mV, respectively. Despite not having comparable EMG baselines between task-specific muscles (FDI and APB) and the control muscles (ADM and FCR), we observed CSE suppression during movement preparation in the control muscles for both tasks (LF and HF) but no task-dependent CSE modulation (see results). This suggests that the TMS intensity used to induce MEPs in control muscles should have been sensitive enough to detect task-dependent effects. Repeated-measures analysis of variance (rmANOVA) was used to determine the effects of within-subject factors of task (2 levels: LF, HF), TMS delivery time (6 levels: 500, 750, 1,000, 1,100, 1,200, and 1,300 ms), and muscle (4 levels: FDI, APB, ADM, FCR) on the normalized MEP (nMEP) amplitude. To assess potential task-dependent modulation of EMG activity, the amplitude of EMG activity from each muscle was quantified by computing the root mean square (RMS) of the EMG signal from go to object contact and over a period of 1,500 ms following object contact on each trial and task, and pooled across different TMS delivery times. For each subject, we computed the ratio of EMG activity during HF trials to EMG during LF trials.
For experiment 2a, CSE modulation for seven different ISIs at rest was analyzed using rmANOVA with TMS pulse (8 levels: 1, 2, 4, 8, 10, 12, 15, test alone) as a within-subject factor. We used post hoc Dunnett's test to compare MEP for each ISI with the test-alone MEP. For experiment 2b, paired-pulse MEP data were normalized to single-pulse MEP data to assess the influence of the conditioning stimulus on the test stimulus. We performed rmANOVA with task (2 levels: LF, HF), ISI (5 levels: 1, 2, 8, 10, 15), and muscle (3 levels: FDI, APB, ADM) as within-subject factors on normalized paired-pulse MEPs.
Finally, we performed rmANOVA with task (2 levels: LF, HF) and muscle (3 levels: FDI, APB, ADM) as within-subject factors on nMEPs pooled across subjects from experiments 1a and 2b (single-pulse TMS data; n = 18). We applied Huynh-Feldt corrections when sphericity assumption was violated. We performed post hoc comparisons using paired t-test with Bonferroni corrections. Significance level was set at 0.05.
RESULTS
All subjects complied with the task instructions by exerting significantly greater grip force on the object during the HF than the LF task (experiments 1 and 2b; n = 18; paired t-test: t17 = −9.1, P < 0.001). For the HF task, subjects exerted grip force of 5.26 ± 0.78 N (equivalent to 10.07 ± 0.36% of MVF, mean ± SE) following accurate digit positioning on the object, whereas for the LF task subjects exerted negligible grip force (1.06 ± 0.27 N, mean ± SE).
Corticospinal Excitability During Planning LF vs. HF Tasks (Experiment 1)
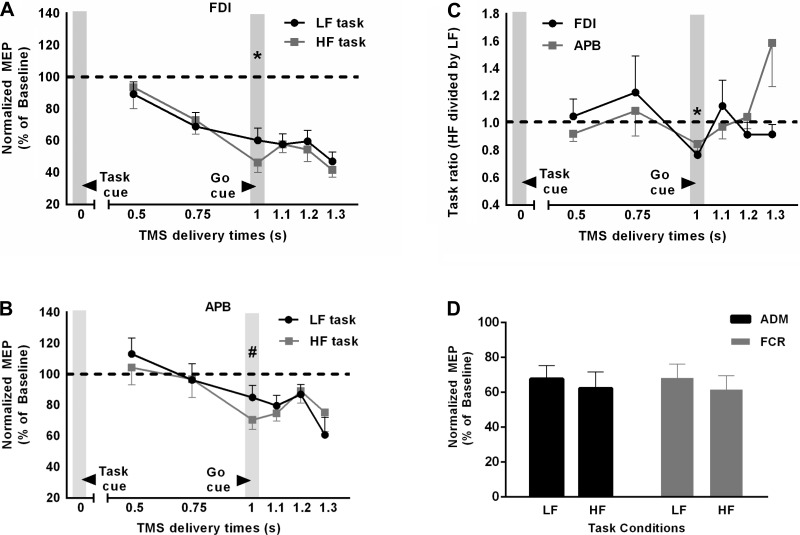
We determined whether digit force planning influences CSE during movement preparation and before object contact using two grasp tasks. MEP peak-to-peak amplitude elicited during two tasks was reduced compared with that during resting state MEP (Fig. 2, A and B), which is consistent with previous literature (Cattaneo et al. 2005; Prabhu et al. 2007). However, this reduction in MEP amplitude was significantly different across single-TMS pulse timings [Fig. 2, A and B; significant task × TMS delivery time interaction; F(5, 35) = 4.534, P = 0.003]. Furthermore, MEP amplitude for the two tasks was different across four muscles [significant task × muscle interaction; F(3, 21) = 4.351, P = 0.016]. For FDI, greater reduction in MEP amplitude was observed for HF task vs. LF task, but only at 1,000 ms after the task cue presentation, i.e., at the time of the go cue (post hoc paired t-test: t7 = 5.457, P = 0.001; adjusted α = 0.025). For APB, this difference was close to, but failed to reach, the adjusted α level of 0.025 (t7 = 2.3, P = 0.053). For ADM and FCR, although we observed CSE suppression at the time of the go cue, the difference between the CSE for two tasks was not significant (P > 0.1; Fig. 2D). Furthermore, the RMS amplitude of EMG during the reach was not significantly different across the two task conditions [no main effect of task: F(1,7) = 3.6, P = 0.1; no task × muscle interaction: F(3,21) = 2.1, P > 0.1]. The lack of task difference in EMG amplitudes of intrinsic and forearm muscles would suggest negligible or no difference in finger kinematics. Thus, because our two tasks differed only in terms of the amount of normal force required following digit positioning at the same locations on the object, our findings suggest that the difference in MEP amplitude observed at the moment of go cue presentation was associated with digit force planning. Furthermore, the task-dependent modulation in CSE was only observed in muscles directly involved in the grasp (Fig. 2, C and D). Additionally, we observed that the increase in FDI EMG activity during performance (a 1,500-ms window following object contact) of HF task vs. LF task (EMG ratio: 7.3 ± 1.95, mean ± SE) was significantly greater than that for other muscles (APB: 1.74 ± 0.26; ADM: 1.97 ± 0.16; FCR: 1.56 ± 0.06; all P <0.05).
Fig. 2.
Corticospinal excitability as a function of time and task. A and B: time course of corticospinal excitability (CSE), denoted by MEP amplitude normalized relative to baseline MEP, for the FDI and abductor pollicis brevis (APB) muscles, respectively, as a function of TMS delivery time and task. *P < 0.01; #0.05 < P < 0.1. C: time course of the ratio between HF-task MEP and LF-task MEP for the FDI and APB muscles. Values >1 denote greater CSE for HF task than for LF task. Data are averages of all subjects (vertical bars denote SE). *P < 0.025, values significantly different from 1. D: normalized MEP amplitude for the APB and flexor carpi radialis (FCR) muscles at the time of the go cue.
Validation of Paired-Pulse TMS Protocol (Experiment 2a).
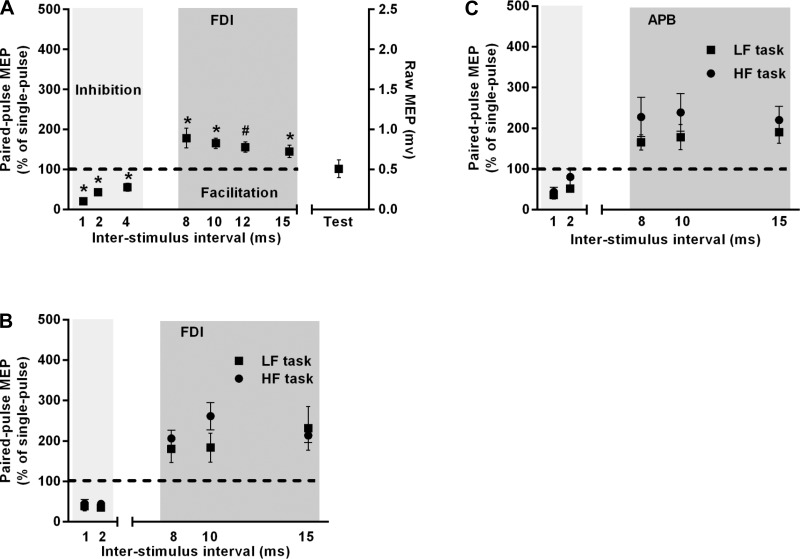
Using paired-pulse TMS over M1 at rest, we confirmed previous findings (Davare et al. 2008; Kujirai et al. 1993) of reduced MEP amplitude with an ISI ranging between 1 and 5 ms and larger MEP amplitude with an ISI between 8 and 15 ms [Fig. 3A; significant main effect of TMS pulse: F(2.65, 13.24) = 19.8, P < 0.0001]. We observed a significant difference between MEP amplitudes elicited using paired-pulse TMS at all ISIs (except 12 ms) and that elicited using single-pulse (test alone) TMS (post hoc Dunnett's test: all adjusted P < 0.05 except 12 ms: P = 0.09).
Fig. 3.
Paired-pulse TMS. A: validation of short intracortical inhibition and facilitation protocol in the FDI muscle at rest as a function of different TMS interstimulus intervals. Test data were obtained by delivering single-pulse TMS. *Adjusted P < 0.05; #0.05 < adjusted P < 0.1. B and C: short intracortical inhibition and facilitation assessed at the time of the go cue, i.e., during preparation but before movement onset of HF and LF tasks in the FDI and APB muscles, respectively. Data are averages of all subjects (vertical bars denote SE).
Intracortical Inhibition and Facilitation (Experiment 2b)
We investigated the modulation of intrinsic circuits within M1 when subjects planned for HF vs. LF tasks at the moment of go cue, i.e., 1,000 ms following the task cue, because this was the only stimulation time at which a task effect was found on MEP. We failed to observe a difference in intracortical inhibition (ISIs: 1 and 2 ms) or facilitation (ISIs: 8, 10, and 15 ms) between the two tasks [Fig. 3, B and C; no main effect of condition: F(1, 9) = 3.83, P = 0.1; no condition × ISI interaction: F(2.03, 18.62) = 1.17, P = 0.33]. Furthermore, the amount of suppression in MEP amplitude with shorter ISIs and facilitation in MEP amplitude with longer ISIs for the two tasks were similar across muscles [no main effect of muscle: F(1.46, 13.12) = 0.52, P = 0.55; no condition × muscle interaction: F(2, 18) = 1.48, P = 0.25].
We confirmed our findings from experiment 1. Specifically, MEP amplitude for the two tasks was different across three muscles [main effect of muscle: F(2, 18) = 5.3, P = 0.016; significant task × muscle interaction: F(2, 18) = 4.8, P = 0.02]. Post hoc tests showed greater reduction in FDI and APB MEP amplitudes for HF task vs. LF task (FDI: t9 = 3.69, P = 0.005; APB: t9 = 3.26, P = 0.01, adjusted α = 0.025). For ADM, the difference between the CSE for two tasks was not significant (t9 = −0.75, P = 0.45).
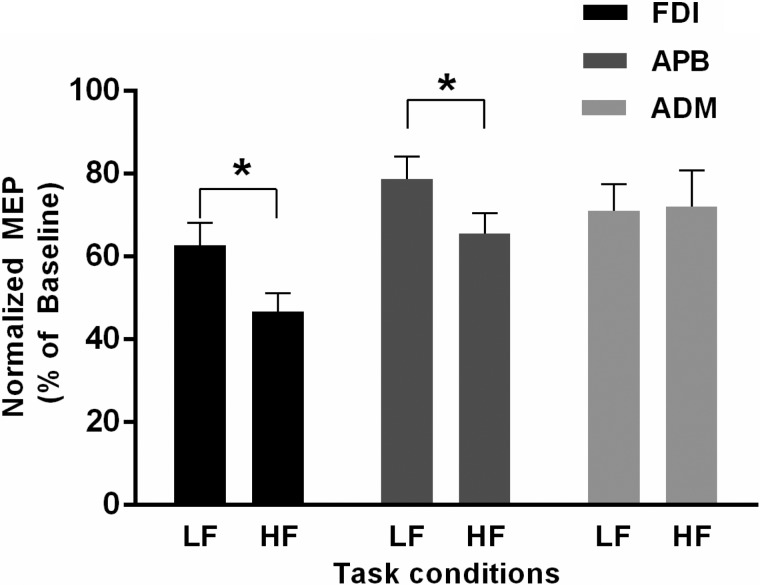
After pooling data from subjects from experiments 1 and 2b (n = 18), we observed that the reduction in MEP amplitude elicited using single-pulse TMS 1,000 ms following the task cue presentation was again significantly greater during preparation for HF vs. LF tasks [main effect of condition: F(1, 17) = 14.5, P = 0.001; significant condition × muscle interaction: F(1.69, 28.8) = 5.1, P = 0.016; Fig. 4]. However, the magnitude of suppression was different across three muscles [main effect of muscle: F(1.565, 26.59) = 8.28; P = 0.003]. The MEP amplitudes for FDI and APB, but not ADM, were significantly reduced for HF vs. LF tasks at the moment of go cue (post hoc paired t-test: FDI: t17 = 5.69, P < 0.001; APB: t17 = 3.88, P = 0.001; ADM: t17 = −0.173, P = 865, adjusted α level = 0.025).
Fig. 4.
MEP data pooled across experiments 1 and 2b. Normalized MEP data obtained through single-pulse TMS pooled across experiments 1 and 2b (n = 18) are shown for the FDI, APB, and ADM muscles at the time of go cue for HF and LF tasks. Data are averages of all subjects (vertical bars denote SE). *P < 0.001, significant differences in MEP between HF and LF tasks.
DISCUSSION
The neural mechanisms underlying planning and execution of dexterous grasping and manipulation are not well understood. In this study we used two reach-to-grasp tasks that differed in terms of whether significant or negligible normal force was exerted on the object following contact. Using single-pulse TMS, we quantified the influence of digit force planning on the CSE and its time course. We found differential modulation of CSE when subjects planned digit forces, but only when the TMS pulse coincided with the go cue denoting the time to initiate reach. Furthermore, the results from our paired-pulse TMS experiment demonstrated that the modulation of intracortical inhibitory and facilitatory circuits within M1 was similar when subjects planned either task. Overall, our results suggest that digit force planning influences CSE before initiation of reach and that this task-dependent modulation may be mediated by inputs outside of M1.
CSE Suppression During Movement Preparation
Our single-pulse TMS results showed significant reduction of MEP amplitude, a measure of CSE, during movement preparation but before reach onset. It has been suggested that suppression in CSE may represent a “braking” mechanism to suppress the tendency to initiate reach (Prabhu et al. 2007; Prut and Fetz 1999). Consistent with this argument, we observed suppression of CSE in all muscles that we recorded from, i.e., three hand (FDI, APB, and ADM) and one forearm (FCR) muscles. In our study, with the cue to initiate reach (go cue), subjects were required to reach and grasp the object, which also results in concurrent preshaping of the hand to adopt a posture based on the object's shape (Santello 2002). Withholding these movements until the time of the go cue would thus result in suppression of CSE. Suppression of CSE during movement preparation has also been found when subjects prepared for a reaction time task (Duque et al. 2012; Hasbroucq et al. 1997; Touge et al. 1998) and for a motor task to be performed at a self-selected speed (Cattaneo et al. 2005; Prabhu et al. 2007). CSE suppression may originate at the level of the spinal cord as suggested by reduction in H-reflex amplitude (Touge et al. 1998) and inhibition of motor neurons through inhibitory spinal interneurons during movement preparation (Prut and Fetz 1999). However, the authors of the later electrophysiological study in Macaca monkeys argued that because the afferent inputs to the spinal cord do not change during rest and movement preparation, the modulation of spinal interneuron resulted from activity in cortical areas during movement preparation (Prut and Fetz 1999). Recent TMS work in humans identified the role of premotor dorsal area in sending inhibitory inputs to M1, leading to CSE suppression to control the impulse to initiate movement until the cue to initiate movement occurs (Duque et al. 2012).
In contrast to our finding, other studies have reported an increase in MEP amplitude before movement onset (Chen et al. 1998; Chen and Hallett 1999; Starr et al. 1988). Using single-pulse TMS, Chen et al. (1998) reported an increase in MEP amplitude beginning 100 ms before the onset of self-paced thumb abduction movement. However, this discrepancy might be due to differences in the task requirements, since we used an externally cued task consisting of a multi-joint precision grip characterized by contact forces.
Influence of Digit Force Planning on Corticospinal Excitability
We found task-dependent modulation of CSE when the TMS pulse coincided with the go cue while subjects prepared for the HF vs. the LF task. In the HF task, subjects were instructed to apply a significant amount of grip force following accurate digit (thumb and index finger) positioning on the object, whereas the LF task required only accurate digit positioning and minimal grip force. Because the two tasks differed only in the magnitude of force exerted on the object following digit positioning, the difference in CSE resulted from the planning of digit forces during movement preparation but before reach onset. An alternative explanation is that the digit force planning occurs before reach onset and that the presentation of the cue to signal reach onset resulted in the release of the task-specific motor plan. Although we cannot distinguish between these two scenarios, our results suggest that the force-dependent modulation of CSE occurs before reach onset. The muscle specificity of the observed task-dependent modulation further supports the notion that digit force planning was related to the muscles that would have been engaged in generating grip force, i.e., FDI and APB, but not ADM and FCR.
The time course of CSE during digit force planning suggests that force planning-related inputs reach (see below) M1 before initiation of reach. Specifically, task-dependent modulation of CSE was observed when the TMS pulse coincided with the go cue but before reach onset. This suggests an early involvement of M1 in planning digit forces in humans. Virtual lesions elicited by TMS of M1 in humans have been shown to impair the ability to plan digit forces during gripping and lifting based on prior experience with the same object (Berner et al. 2007; Chouinard et al. 2005; Nowak et al. 2005). However, in these studies, disruption of M1 was performed offline and not while subjects prepared for the grip and lift task. Thus it was not clear when grip force planning-related information reached M1. Our results suggest that force planning-related information is available to the corticospinal tract long before the hand contacts the object. More recently, CSE assessed before initiation of reach during a grasp-to-lift task was shown to reflect the memory representation of object weight (Loh et al. 2010). However, these studies (Chouinard et al. 2005; Loh et al. 2010) might not have been suitable to discriminate planning of digit forces from position (see Introduction).
CSE modulation based on task requirements was observed when the TMS pulse coincided with the signal to initiate the grasp, i.e., go cue, at 1,000 ms after task cue presentation. This observation is consistent with Prabhu et al. (2007), who reported significant modulation of CSE when subjects prepared to grasp a handle vs. a disc, but only when a single pulse, coinciding with the cue for initiating a grasp, was delivered 800 ms following object presentation. This task-related modulation in the CSE disappeared when either the TMS pulse and go signal were dissociated or the TMS stimulation signaled go at different time points across trials. Prabhu et al. (2007) inferred that the task-related modulation in CSE is significant when TMS probes the system at a predictable grasp onset time, i.e., “when visuomotor inputs had their greatest anticipatory influence on M1.” In our study, the go cue always appeared at the same time following the task cue during each trial, which would have allowed subjects to predict the onset of reach. Thus the go cue in our study appears to be the time at which the effect of force planning-related neural inputs on CSE is strongest. This is consistent with the finding by Loh et al. (2010) that grasp planning based on sensorimotor memory is revealed by CSE changes at the time of the go cue. However, on the basis of our proposed theoretical framework (Fu et al. 2010), CSE modulation during preparation to grip and lift an object could be due to both digit position and force planning.
It is plausible that presentation of the anticipated go cue to initiate reach might have induced retrieval of memory related to task-specific characteristics or features (Singhal et al. 2013). However, we would not expect to see a change in magnitude of MEP suppression during HF vs. LF tasks if movement initiation would have been internally cued (Thut et al. 2000).
It is somewhat counterintuitive to observe greater suppression in MEP amplitude for the HF than the LF task. However, electrophysiological studies in rhesus monkeys found that fewer than 10% of recorded M1 neurons showed significant modulation in their firing rates with respect to baseline activity when the monkeys prepared to reach and grasp objects with different force levels (Hendrix et al. 2009). Thus, if a similar cortical circuitry operates in humans, it could be speculated that such a small subset of M1 neurons might have influenced CSE during digit force planning in our study. Furthermore, a subgroup of M1 neurons has been found to suppress their activity during exertion of large vs. small grip force (Hepp-Reymond et al. 1999; Maier et al. 1993). If a similar suppression of M1 neuron firing rates had occurred at the planning stage of the HF task, it would account for the greater CSE suppression associated with digit force planning. Both speculations, however, require further investigation. It should also be noted that CSE modulation may have resulted from force planning-related inputs from higher-level brain areas (see below). This argument is consistent with the fact that CSE modulation during movement preparation originates from several cortical areas (Prut and Fetz 1999).
Digit Force Planning and Intracortical M1 Circuitry
The findings of experiment 1 motivated a follow-up experiment to determine the source of changes in excitability in the corticospinal tract during digit force planning. To determine the role of the local M1 circuitry within M1 during digit force planning, we used a paired-pulse TMS approach where a subthreshold TMS stimulus preceded delivery of a suprathreshold TMS pulse. Although SICI and SICF are mediated through separate mechanisms, the modulation of MEP size is due to modulation of neural circuits intrinsic to the site of stimulation, i.e., M1 (Chen 2004; Münchau et al. 2002). The modulation of MEP size with different ISI between paired TMS pulses (i.e., SICI and ICF) delivered in synchrony with the go cue but before grasp initiation was similar for HF vs. LF tasks. Our finding of task-dependent modulation of CSE but an absence of modulation in SICI is not consistent with the recent reports from Kouchtir-Devanne et al. (2012). These authors reported an increase in CSE but a decrease in SICI during precision grip using index finger and thumb vs. abduction of the index finger. The authors suggested that the greater reduction in SICI during precision grip allowed larger corticospinal volleys during stimulation, thus recruiting greater proportion of α-motoneurons (Kouchtir-Devanne et al. 2012). However, in our study, task-dependent modulation in CSE did not result from modulation in SICI. The discrepancy between the two studies is that both of our experimental conditions consisted of grasping movements using the index finger and thumb under low and high force conditions, whereas the above-referenced study compared a two-digit grasping task with a single-digit force production task. Overall, our findings suggest that the local circuitry within M1 did not contribute significantly to planning of digit forces at the cue signaling reach onset found through single-pulse TMS (experiment 1).
Cortical Circuitry Involved in Digit Force Planning
In addition to the above described study by Hendrix et al. (2009), parietal, occipital, and subcortical structures (cerebellum and basal ganglia) play a role in grip force planning and control (Berner et al. 2007; Chouinard et al. 2005; Dafotakis et al. 2008b; Davare et al. 2007; Ehrsson et al. 2000, 2001; Murata et al. 1997; Nowak et al. 2005; Prodoehl et al. 2009; Rost et al. 2005). Blood oxygen level-dependent (BOLD)-related cortical activity was significantly greater in right intraparietal cortex when subjects applied a small vs. larger force using a precision grip (Ehrsson et al. 2001). For object manipulation tasks, learning to scale digit forces based on object properties (i.e., weight) also seems to engage a frontoparietal network (Dafotakis et al. 2008a; Nowak et al. 2005; Raghavan et al. 2006). Virtual lesions of M1 (Bäumer et al. 2009; Chouinard et al. 2005) resulted in disruption of grip force scaling based on memory of prior lifts, and virtual lesion of contralateral anterior intraparietal sulcus (aIPS; Davare et al. 2007) led to an overshooting of peak rates of grip and load forces, suggesting a possible interference with the internal representation of object weight. On the basis of these findings, we propose that aIPS and premotor areas may have played an important role in sending force planning-related information to M1. Moreover, this information would reach M1 during movement preparation but before reach onset. This theoretical framework is the subject of ongoing investigation.
Digit Force Control: Planning and Feedback-Driven Corrections
The present findings demonstrate that digit force planning-related processing starts well before object contact. This finding is consistent with the theoretical framework we recently proposed, that planning of digit forces can occur before grasp (Fu et al. 2010). However, it should be noted that early onset of digit force planning as reported here does not preclude corrections to planned digit forces later in the task. According to the sensorimotor control point theory (Johansson and Flanagan 2009), the central nervous system compares planned and actual development of digit forces across critical mechanical events associated with manipulation, e.g., contact, onset of object lift, and onset of static object hold. If a mismatch is detected, subjects make force adjustments according to the task requirements. For example, in a task where subjects can choose digit placement, planned and actual digit positions may not match. In this case, planned digit forces might have to be changed once the mismatch is detected (Fu et al. 2010). Similarly, when object mass is greater than anticipated, the inability to lift the object at the anticipated time triggers feedback-driven force upgrades to scale digit forces and eventually enable object lifting. Besides being used for online digit force corrections, this feedback information is used to update digit force planning for successive lifts (Johansson and Flanagan 2009; Johansson and Westling 1984; Westling and Johansson 1984).
Conclusions
The present study provides two new insights about neural mechanisms underlying force planning for grasping in humans. First, CSE was sensitive to the planned magnitude of digit force for grasping before reach onset. Second, the force-dependent modulation of CSE appears to be driven by inputs from areas other than M1.
GRANTS
M. Davare is funded by a David Phillips Biotechnology and Biological Sciences Research Council Fellowship (UK). P. McGurrin is funded by National Science Foundation Integrative Graduate Education and Research Traineeship no. 1069125.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J.P., M.D., and M.S. conception and design of research; P.J.P. and P.M. performed experiments; P.J.P. analyzed data; P.J.P., M.D., and M.S. interpreted results of experiments; P.J.P. prepared figures; P.J.P. and M.S. drafted manuscript; P.J.P., M.D., P.M., and M.S. edited and revised manuscript; P.J.P., M.D., P.M., and M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jeffrey Kleim for assistance with preliminary data collection and feedback on the experimental protocols.
REFERENCES
- Ashe J. Force and the motor cortex. Behav Brain Res 87: 255–269, 1997 [DOI] [PubMed] [Google Scholar]
- Bäumer T, Schippling S, Kroeger J, Zittel S, Koch G, Thomalla G, Rothwell JC, Siebner HR, Orth M, Münchau A. Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest–a bifocal TMS study. Clin Neurophysiol 120: 1724–1731, 2009 [DOI] [PubMed] [Google Scholar]
- Berner J, Schönfeldt-Lecuona C, Nowak DA. Sensorimotor memory for fingertip forces during object lifting: the role of the primary motor cortex. Neuropsychologia 45: 1931–1938, 2007 [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Voss M, Brochier T, Prabhu G, Wolpert DM, Lemon RN. A cortico-cortical mechanism mediating object-driven grasp in humans. Proc Natl Acad Sci USA 102: 898–903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154: 1–10, 2004 [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci 26: 163–169, 1999 [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol 44: 317–325, 1998 [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 44: 773–791, 1980 [DOI] [PubMed] [Google Scholar]
- Chouinard a P, Leonard G, Paus T. Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci 25: 2277–2284, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafotakis M, Grefkes C, Eickhoff SB, Karbe H, Fink GR, Nowak DA. Effects of rTMS on grip force control following subcortical stroke. Exp Neurol 211: 407–412, 2008a [DOI] [PubMed] [Google Scholar]
- Dafotakis M, Sparing R, Eickhoff SB, Fink GR, Nowak DA. On the role of the ventral premotor cortex and anterior intraparietal area for predictive and reactive scaling of grip force. Brain Res 1228: 73–80, 2008b [DOI] [PubMed] [Google Scholar]
- Davare M, Andres M, Clerget E, Thonnard JL, Olivier E. Temporal dissociation between hand shaping and grip force scaling in the anterior intraparietal area. J Neurosci 27: 3974–3980, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol 586: 2735–2742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci 32: 806–816, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 83: 528–536, 2000 [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol 85: 2613–2623, 2001 [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 31: 14–27, 1968 [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhang W, Santello M. Anticipatory planning and control of grasp positions and forces for dexterous two-digit manipulation. J Neurosci 30: 9117–126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res 5: 185–192, 1997 [DOI] [PubMed] [Google Scholar]
- Hendrix CM, Mason CR, Ebner TJ. Signaling of grasp dimension and grasp force in dorsal premotor cortex and primary motor cortex neurons during reach to grasp in the monkey. J Neurophysiol 102: 132–145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp-Reymond M, Kirkpatrick-Tanner M, Gabernet L, Qi HX, Weber B. Context-dependent force coding in motor and premotor cortical areas. Exp Brain Res 128: 123–133, 1999 [DOI] [PubMed] [Google Scholar]
- Johansson RS, Cole KJ. Sensory-motor coordination during grasping and manipulative actions. Curr Opin Neurobiol 2: 815–823, 1992 [DOI] [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10: 345–359, 2009 [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56: 550–564, 1984 [DOI] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112: 720, 2001 [DOI] [PubMed] [Google Scholar]
- Kouchtir-Devanne N, Capaday C, Cassim F, Derambure P, Devanne H. Task-dependent changes of motor cortical network excitability during precision grip compared to isolated finger contraction. J. Neurophysiol. 107: 1522–9, 2012 [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008 [DOI] [PubMed] [Google Scholar]
- Loh MN, Kirsch L, Rothwell JC, Lemon RN, Davare M. Information about the weight of grasped objects from vision and internal models interacts within the primary motor cortex. J Neurosci 30: 6984–6990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Bennett KM, Hepp-Reymond MC, Lemon RN. Contribution of the monkey corticomotoneuronal system to the control of force in precision grip. J Neurophysiol 69: 772–785, 1993 [DOI] [PubMed] [Google Scholar]
- Münchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci 22: 554–561, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol 78: 2226–2230, 1997 [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdörfer J, Topka H. Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol 250: 850–860, 2003 [DOI] [PubMed] [Google Scholar]
- Nowak DA, Voss M, Huang YZ, Wolpert DM, Rothwell JC. High-frequency repetitive transcranial magnetic stimulation over the hand area of the primary motor cortex disturbs predictive grip force scaling. Eur J Neurosci 22: 2392–2396, 2005 [DOI] [PubMed] [Google Scholar]
- Prabhu G, Voss M, Brochier T, Cattaneo L, Haggard P, Lemon R. Excitability of human motor cortex inputs prior to grasp. J Physiol 581: 189–201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Vaillancourt DE. Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev 33: 900–908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature 401: 590–594, 1999 [DOI] [PubMed] [Google Scholar]
- Raghavan P, Krakauer JW, Gordon AM. Impaired anticipatory control of fingertip forces in patients with a pure motor or sensorimotor lacunar syndrome. Brain 129: 1415–1425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994 [DOI] [PubMed] [Google Scholar]
- Rost K, Nowak DA, Timmann D, Hermsdörfer J. Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clin Neurophysiol 116: 1405–1414, 2005 [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Thompson PD, Kujirai T. Short latency intracortical inhibition: one of the most popular tools in human motor neurophysiology. J Physiol 587: 11–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M. Kinematic synergies for the control of hand shape. Arch Ital Biol 140: 221–228, 2002 [PubMed] [Google Scholar]
- Singhal A, Monaco S, Kaufman LD, Culham JC. Human FMRI reveals that delayed action re-recruits visual perception. PLoS One 8: e73629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Caramia M, Zarola F, Rossini PM. Enhancement of motor cortical excitability in humans by non-invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol 70: 26–32, 1988 [DOI] [PubMed] [Google Scholar]
- Thach WT. Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of intended next movement in motor cortex and cerebellum. J Neurophysiol 41: 654–676, 1978 [DOI] [PubMed] [Google Scholar]
- Thut G, Hauert C, Viviani P, Morand S, Spinelli L, Blanke O, Landis T, Michel C. Internally driven vs. externally cued movement selection: a study on the timing of brain activity. Brain Res Cogn Brain Res 9: 261–269, 2000 [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol 109: 489–495, 1998 [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res 53: 277–284, 1984 [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus–a new landmark. Brain 120: 141–157, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang W, Gordon AM, Fu Q, Santello M. Manipulation after object rotation reveals independent sensorimotor memory representations of digit positions and forces. J Neurophysiol 103: 2953–2964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]