Abstract
Neurological rehabilitation involving motor training has resulted in clinically meaningful improvements in function but is unable to eliminate many of the impairments associated with neurological injury. Thus there is a growing need for interventions that facilitate motor learning during rehabilitation therapy, to optimize recovery. d-Cycloserine (DCS), a partial N-methyl-d-aspartate (NMDA) receptor agonist that enhances neurotransmission throughout the central nervous system (Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Arch Gen Psychiatry 61: 1136–1144, 2004), has been shown to facilitate declarative and emotional learning. We therefore tested whether combining DCS with motor training facilitates motor learning after stroke in a series of two experiments. Forty-one healthy adults participated in experiment I, and twenty adults with stroke participated in experiment II of this two-session, double-blind study. Session one consisted of baseline assessment, subject randomization, and oral administration of DCS or placebo (250 mg). Subjects then participated in training on a balancing task, a simulated feeding task, and a cognitive task. Subjects returned 1–3 days later for posttest assessment. We found that all subjects had improved performance from pretest to posttest on the balancing task, the simulated feeding task, and the cognitive task. Subjects who were given DCS before motor training, however, did not show enhanced learning on the balancing task, the simulated feeding task, or the associative recognition task compared with subjects given placebo. Moreover, training on the balancing task did not generalize to a similar, untrained balance task. Our findings suggest that DCS does not enhance motor learning or motor skill generalization in neurologically intact adults or in adults with stroke.
Keywords: stroke, d-cycloserine, motor learning, behavioral training
the prevalence of stroke and the extensive costs associated with persistent disability after stroke have generated an urgent need for multidisciplinary neurorehabilitation research. It is now understood that the nervous system has remarkable adaptive capacity. Specifically, the central nervous system retains its ability to reorganize in structure and function in response to behavioral experience following neurological injury. Stroke rehabilitation involves cognitive learning and motor learning that guide the adaptation of the central nervous system. Recent stroke rehabilitation trials with intensive behavioral training have resulted in statistically (Lo et al. 2010) and sometimes clinically meaningful improvements (Duncan et al. 2007; Wolf et al. 2006), but improvements do not restore prestroke function. As it has become increasingly clear that behavioral training alone will not be able to eliminate stroke-related deficits and disability, there is a growing need to find an effective pharmacological agent that will facilitate the neurobiological processes of learning, thus improving human stroke outcomes.
In the fields of psychology and psychiatry, a novel strategy is emerging in which exposure therapy, thought to be a kind of emotional learning, is combined with a pharmacological enhancer of learning and memory. The agent used for many of these recent studies is the learning and memory agonist d-cycloserine (DCS). DCS was originally approved by the U.S. Food and Drug Administration in 1964 for use as an antibiotic to treat tuberculosis. It is now known that DCS has a second action: mediating memory formation. Specifically, DCS acts at the glycine/serine regulatory site of the N-methyl-d-aspartate (NMDA) receptor (NMDAR), boosting long-term potentiation (LTP). When tested as an augmenting agent in exposure therapy in patients with acrophobia, oral DCS given immediately before 30-min exposure sessions enhanced habituation (Ressler et al. 2004). Similar pilot studies in social phobia, post-traumatic stress disorder, and panic disorder have been successful in improving exposure therapy response (Davis 2010; Guastella et al. 2008; Hofmann et al. 2006a,b; Norberg et al. 2008).
So far, tests of DCS in humans have focused primarily on facilitating emotional and extinction-based learning in the context of cognitive behavioral therapy. The NMDARs through which DCS promotes LTP, however, are also utilized in motor learning pathways (Butefisch et al. 2000; Hess et al. 1996).
To our knowledge, there is no literature about the use of DCS in combination with motor training to promote motor learning in humans. It has been found, however, that DCS successfully facilitated recovery of motor and memory function after closed head injury in a mouse model (Yaka et al. 2007). Moreover, studies investigating the impact of DCS administered in combination with brain stimulation have found increased cortical plasticity in humans (Kuo et al. 2008; Nitsche et al. 2004). An important next step is to evaluate whether DCS, given in combination with motor training, facilitates motor learning in humans. Such a combination, if successful, could make rehabilitation training more efficient, more effective, and more long-lasting.
The purposes of this study were to 1) determine if DCS facilitates learning in two types of motor tasks, and 2) determine if the learning that occurs through the practice of the two motor tasks generalizes to another, related motor task. We initially investigated this combination in a neurologically intact population (experiment I) and then in a population of individuals with chronic stroke (experiment II). The design parameters of this study were selected based on the parameters reported in previous clinical studies that used DCS. A single 250-mg dose was used for our assay because studies based on a single dose have found improved outcomes (Hofmann et al. 2006a; Ressler et al. 2004; Wilhelm et al. 2008), while chronic treatment with DCS has not shown efficacy (Fakouhi et al. 1995; Quartermain et al. 1994). The motor training tasks were a lower extremity stability platform task (Taubert et al. 2010) and an upper extremity simulated feeding task (Schaefer and Lang 2012). In addition to these two motor tasks, a declarative learning task that would be expected to be facilitated by NMDAR agonism was added to experiment II to explicitly test the proposed mechanisms of enhancing NMDAR function. The generalizability of motor learning was evaluated by having the participants perform an untrained balance task at pretest and posttest only. Our hypotheses were 1) that both the neurologically intact and stroke participants would demonstrate improved performance with practice, 2) that training on the stability platform task would result in improved performance on the untrained balance task (generalization effect), and 3) that participants given DCS would demonstrate greater improvements in performance than individuals given placebo (time × drug interaction).
METHODS
General Experimental Design
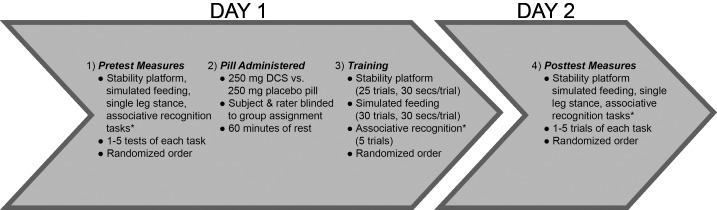
This study used a repeated-measures design with two sessions (Fig. 1). Experiment I examined the effects of DCS combined with motor training on learning in neurologically intact participants while experiment II examined the effects of DCS plus motor training on learning in individuals with chronic stroke. Experiment I was completed before experiment II. This study was approved by the Washington University Human Research Protection Office and was conducted in compliance with the Helsinki Declaration. All participants provided informed consent before beginning the study and were compensated for their time.
Fig. 1.
General experimental design. DCS, d-cycloserine. *Associative recognition task only included in experiment II.
Experiment I: Neurologically Intact Participants
Forty-one neurologically intact adults participated in experiment I. Participants were included if they were 1) 18 years of age or older, and 2) had sufficient cognitive skills to actively participate. People were excluded if they had 1) history of neurological condition or injury; 2) balance impairment or vestibular disorder; 3) history of upper or lower extremity condition, injury, or surgery that could compromise performance and safety on motor training tasks; 3) any cognitive, sensory, or communication problem that would prevent completion of the study; 4) current use of medications for, or diagnosis of, seizure disorder, kidney disease, or liver disease; 5) current substance abuse or dependence; or if they were 6) women who were pregnant, possibly pregnant, or breastfeeding.
Experiment II: Stroke Participants
Twenty adults with stroke participated in experiment II. Additional inclusion criteria for people with stroke were that they 1) had a confirmed ischemic or hemorrhagic stroke, 2) were 6 or more months poststroke, and 3) had unilateral upper extremity and lower extremity weakness. People with stroke were excluded if they had 1) any other neurological diagnosis, or 2) current participation in other stroke treatment, or if they had any of the other exclusion criteria listed for the control participants.
Medication
d-cycloserine is approved by the U.S. Food and Drug Administration for use as an antibiotic to treat tuberculosis. Peak blood levels occur within 2–8 h after dosing, the half-life is estimated to be 10 h, and DCS is central nervous system penetrant. DCS was selected as the pharmacological agent for this study because of its additional role in mediating memory formation through its action as a partial NMDAR agonist (Flood et al. 1992; Hood et al. 1989). The 250-mg dose was selected because previous studies have shown that doses of 25–250 mg of DCS are associated with improved behavioral outcomes (Kuriyama et al. 2011; Otto et al. 2010; Ressler et al. 2004), while a dose of >250 mg does not yield superior outcomes (Ressler et al. 2004) and doses greater than 500 mg DCS can lead to NMDA antagonist effects (Heresco-Levy et al. 2013). In this study, DCS was taken orally 1 h before motor training which is consistent with the timing reported in current literature (Gottlieb et al. 2011; Hofmann et al. 2006a; Otto et al. 2010).
Tasks
We deliberately selected three specific motor tasks that are similar to the types of tasks used in traditional neurorehabilitation and that are relevant to daily function.
Primary trained motor task.
The primary trained motor task was a stability platform balance task (Lafayette Instrument; model 16030L) (Taubert et al. 2010). Successful performance of this task requires that a subject anticipate changes in posture and coordinate muscle activation in response to self-induced perturbations. The stability platform task was selected because of its ecological validity, as decreased dynamic balance is a significant contributor to fall risk (Toraman and Yildirim 2010) and maintaining stability during dynamic activities is required for many daily activities including walking and reaching.
For each 30-s trial, participants were instructed to stand on the movable platform with feet facing forward and to keep the platform level for as much time as possible. The use of a supporting handrail was permitted for initial balance and positioning on the platform, but no upper extremity support was permitted once the trial began. If participants with stroke were unable to maintain the stability platform level for 5 s during a 30-s pretrial, the task was modified by attaching bungees from the midline of the undercarriage of stability platform to each side of the support frame. This adjustment increased the resistance of the platform and reduced the difficulty of the task. Each subject performed 5 pretest trials, 25 training trials, and 5 posttest trials on the stability platform with a 2-min seated rest break after every 5 trials to prevent fatigue. Performance was quantified as the total amount of time (to the tenth of a second) that a subject was able to maintain the platform ± 3° of horizontal during each trial.
Secondary trained motor task.
The secondary trained motor task consisted of spooning beans from one bowl to another (Schaefer and Lang 2012). Successful performance of this task requires coordination of arm and hand muscle activation as well as fine control of the magnitude of muscle force generation across the limb. This task was selected because it simulates feeding, a task that is relevant to daily function and self-care (Blennerhassett et al. 2008; Duncan et al. 2001).
For the simulated feeding task, participants were seated in a chair without armrests at a table. For each 30-s trial, participants were instructed to spoon as many beans as possible, transferring two beans at a time, from one bowl to another bowl. The bowl nearest the participant from which the beans were spooned contained 200 beans at the beginning of each trial and was positioned 21 cm from the target bowl. Neurologically intact participants were required to perform the simulated feeding task with their nondominant hand so that the task was novel and underpracticed. Participants with stroke performed the simulated feeding task with the upper extremity most affected by their stroke. The use of the dominant/unaffected hand was permitted for initial positioning of the spoon in the nondominant/affected hand, but no use of the s hand was permitted once the trial began. If participants with stroke were unable to spoon six or more beans (three successful repetitions) in a 30-s pretrial, the task was modified by sliding a piece of cylindrical foam over the spoon's handle to increase the diameter of the handle to 2.5 mm and/or by repositioning the target bowl to a position 10 cm away from the starting bowl. These modifications reduced the difficulty of the task and allowed participants with limited dexterity and grip strength to participate. Each participant performed 5 pretest trials, 30 training trials, and 5 posttest trials on the simulated feeding task with a 2-min seated rest break after every 10 trials to prevent fatigue. Performance for each trial was quantified as the number of beans transferred from the starting bowl to the target bowl.
Untrained motor task.
Experiments I and II also included an untrained balance task to determine if training on the primary balance task generalized to improved balance performance on a similar, but untrained task. Single leg stance on a balance beam was selected as the untrained task because it is a sensitive tool for assessing balance capacity (Curtze et al. 2010) and it has the ability to be graded for different abilities by changing the beam width. Additionally, the task is clinically relevant because single leg balance is a significant predictor of injurious falls in older persons (Vellas et al. 1997) and reflects the single leg support phase of gait required for walking and stair climbing.
For this task, participants were instructed to place their foot on the balance beam (19 cm high, 60 cm long, and 1¾ cm wide), lift their other leg off of the support surface, and try to maintain balance for as long as possible without touching down with any part of their body. Although the data indicate that there is no difference in unilateral postural stability between the dominant and nondominant lower extremities (Hoffman et al. 1998) for consistency, neurologically intact participants performed the single leg stance balance beam task on their nondominant leg. Participants with stroke balanced on the lower extremity most affected by their stroke. The use of upper extremity support was permitted for initial balance and positioning on the balance beam, but no upper extremity support was permitted once the trial began. If a subject with stroke scored less than a 2 on item 14 (single leg stance) of the Berg Balance Test (Berg 1995; Blum and Korner-Bitensky 2008), a modification was made and the subject performed single leg stance on the floor rather than on the narrow balance beam. This modification reduced the level of difficulty of the task by increasing the amount of area that the foot had in contact with the support surface. Each participant performed five pretest trials and five posttest trials of the single leg stance task. Performance for a given trial was quantified as the amount of time (to the tenth of a second) that a subject maintained balance.
Trained cognitive task.
An associative recognition cognitive task was added to the training paradigm for experiment II as an explicit test of NMDAR function. Associative recognition tasks have been shown to activate the hippocampus, striatum, frontal cortex, and anterior cingulate and employ NMDAR-dependent LTP mechanisms (Bunge et al. 2004; Duzel et al. 2003; Yonelinas et al. 2001). Associative recognition requires the ability to form new associations between unrelated items. The specific associative recognition task selected was one in which participants were instructed to associate and remember 60 “target” nonword-image pairs. Before the pretest and each training trial, participants participated in a study session during which they were shown all 60 target pairs in a random order (stimulus presentation = 1.5 s; interstimulus interval = 1.0 s) and were instructed to associate and remember the target pairs. Following a study session, participants completed a test session in which they were shown 60 slides in a random order and had to indicate whether each pair was a target or “foil” by pressing the 1 or 2 key, respectively, on a standard keyboard (stimulus presentation + response time = 1.0 s; interstimulus interval = 1.0 s). Foil pairs consisted of pairs in which the nonword, the image, or both were not target items or in which the nonword and the image were incorrectly paired target items. The number of target pairs included in a test session ranged from 15 to 30 pairs. Each participant performed one pretest, five training, and one posttest trial(s) of the associative recognition task. No study session was given before the posttest; participants had to recall target pairs after a 24- to 48-h period between sessions.
Instructions, stimuli, and feedback were presented electronically on a laptop computer screen using the E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Nonwords (e.g.,“blauds,” “woodcug”) were displayed in Calibri, size 72 font on the left side of the screen while the image of a familiar object (e.g., comb, apple) appeared on the right side of the screen. Nonwords were selected from a database of nonwords provided by the English Lexicon Project (Balota et al. 2007). All nonwords were pronounceable, five to seven letters in length, and had no more than two syllables. Images were selected from a bank of Microsoft Clip Art. All images were single items, considered to be familiar to most American adults.
Performance on the associative recognition task was measured as the percentage of correct responses (0–100%) for the trial. Responses were considered correct if a participant identified a target pair as a target pair or identified a foil pair as a foil pair. Responses were incorrect if a participant indicated that a target pair was a foil pair or responded that a foil pair was a target pair, or if the participant did not enter a response in the allotted timeframe following stimulus presentation. The response reaction time was gauged as the time interval from stimulus presentation to the number one key or the number two key being struck, indicating a response. If a participant did not enter a response in the allotted time, reaction time was entered as 1,000 ms. Participants received immediate feedback about the accuracy of each response as the word(s) “correct,” “incorrect,” or “no response detected” appeared on the screen for 0.5 s after each stimulus.
Feedback.
For all tasks, feedback regarding objective performance was given at the end of each trial (e.g., number of beans transferred, number of seconds in balance and correct or incorrect response). No feedback was provided concerning the specific techniques implemented during task performance. This allowed participants to engage in discovery learning in which they adapted their technique and movement strategies based on trial and error. This form of learning was selected because it is associated with higher levels of engagement in rehabilitation (Castronova 2002).
Order of Experiment
During the first session in experiment I, a trained clinical interviewer provided informed consent, gathered demographic data, and gathered baseline measurements of performance on 2–5 trials of 1) a stability platform balancing task, 2) a simulated feeding task, and 3) a single leg stance task, in a randomized order. Experiment II included stroke-specific descriptive measures in addition to the items listed for experiment I. These consisted of the National Institutes of Health Stroke Scale (Brott et al. 1989), Short Blessed Test (Katzman et al. 1983), Action Research Arm Test (Lang et al. 2006), and the Berg Balance Test (Berg 1995; Blum and Korner-Bitensky 2008), which served to describe stroke severity, cognition, upper extremity function, and balance, respectively.
After assessment of baseline measures and before behavioral training, participants were provided with either 250 mg DCS or placebo, taken orally. Medication assignment was randomized, with concealed allocation. DCS and placebo pills were manufactured to look identical and were individually packaged in identical, opaque pill bottles labeled with an ID number corresponding to the subject ID number. Randomization was done by separate personnel located in another building and pills were distributed to the participants by a blinded experimenter. Following the administration of DCS or placebo, participants rested for 60 min.
Following the rest period, behavioral training commenced. This training is described in detail above and consisted of repeated practice of 1) a stability platform task, 2) a simulated feeding task, and 3) an associative recognition task (experiment II only). The order of tasks was randomized. During the second session, which took place 1–3 days after the first session, participants completed the posttest, consisting of five trials of each task. Participants did not receive DCS or placebo during the second session.
Data Analysis
SPSS Statistics 21 (IBM, Armonk, NY) was used for all statistical analyses, and criterion was set at α = 0.05. To test whether DCS augmented learning, we used a 2 × 2 mixed model ANOVA with a within subject factor of time (pretest vs. posttest) and a between subjects factor of drug (DCS vs. placebo). This mixed model analysis was performed for the stability platform task, the simulated feeding task, the single leg stance task, and the associative recognition task. A significant interaction between time and group would support the hypothesis that DCS facilitates learning. The same analyses were performed in experiments I and II.
RESULTS
Participants
Participants were enrolled in experiment I from January 2013 through May 2013 and in experiment II from July 2013 through September 2013. All 41 neurologically intact participants and 20 participants with mild to moderate stroke, who were enrolled, were randomized (Table 1). Half of the participants (19 neurologically intact and 10 with stroke) received the study drug. There were no significant demographic differences between the groups that received DCS vs. those that received the placebo in either experiment (P < 0.05), with the exception of dominant side for experiments I and II and affected side in experiment II (Table 1). Differences in these factors are unlikely to influence the results, because neurologically intact participants all used their nondominant extremity to perform the tasks and participants with stroke all used their affected side to complete motor tasks. There was an average of 1.3 days between sessions for participants in experiment I and an average of 2.2 days between sessions in experiment II. All participants completed both sessions of the experiment, and no adverse effects of participation were reported.
Table 1.
Demographic data
| Neurologically Intact Participants: Experiment I |
Participants with Stroke: Experiment II |
|||||
|---|---|---|---|---|---|---|
| Characteristics | DCS group (n = 19) | Placebo group (n = 22) | Group differences (P value) | DCS group (n = 10) | Placebo group (n = 10) | Group differences (P value) |
| Age (means ± SD) | 44.6 ± 13.7 | 43.7 ± 13.7 | P = 0.950 | 55.3 ± 9.6 | 51.4 ± 11.0 | P = 0.429 |
| Gender | ||||||
| Male | 10 | 15 | P = 0.116 | 7 | 4 | P = 0.398 |
| Female | 9 | 7 | 3 | 6 | ||
| Race | ||||||
| Caucasian | 4 | 4 | P = 0.655 | 7 | 4 | P = 0.398 |
| African American | 15 | 18 | 3 | 6 | ||
| Dominant side | ||||||
| Right | 15 | 20 | P = 0.033 | 6 | 9 | P = 0.003 |
| Left | 4 | 2 | 4 | 1 | ||
| Affected Side | ||||||
| Right | 9 | 3 | P = 0.028 | |||
| Left | 1 | 7 | ||||
| Months since stroke (means ± SD) | 25 ± 11 | 21 ± 13 | P = 0.727 | |||
| Stroke location | ||||||
| Cortical | 6 | 8 | P = 0.520 | |||
| Subcortical | 1 | 1 | ||||
| Posterior circulation | 1 | 0 | ||||
| Unknown | 2 | 1 | ||||
| NIHSS score (median, IQR) | 1,2 | 1,2 | P = 0.582 | |||
| ARAT score (means ± SD) | 34 ± 14 | 41 ± 8 | P = 0.148 | |||
| BBS score (means ± SD) | 46 ± 7 | 47 ± 8 | P = 0.561 | |||
| SB test score (means ± SD) | 2.6 ± 2.6 | 2.8 ± 3.4 | P = 0.228 | |||
Values are counts except where indicated. DCS, d-cycloserine; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale: scores for the motor subset range from 0 to 18, with lower scores indicating less impairment; ARAT, Action Research Arm Test: scores range from 0 to 57, with lower scores indicating more impairment; BBS, Berg Balance Scale: scores range from 0 to 56, with lower scores indicating more impairment; SB, Short Blessed Test: scores range from 0 to 28, with lower scores indicating less impairment.
Experimental Paradigm and Learning
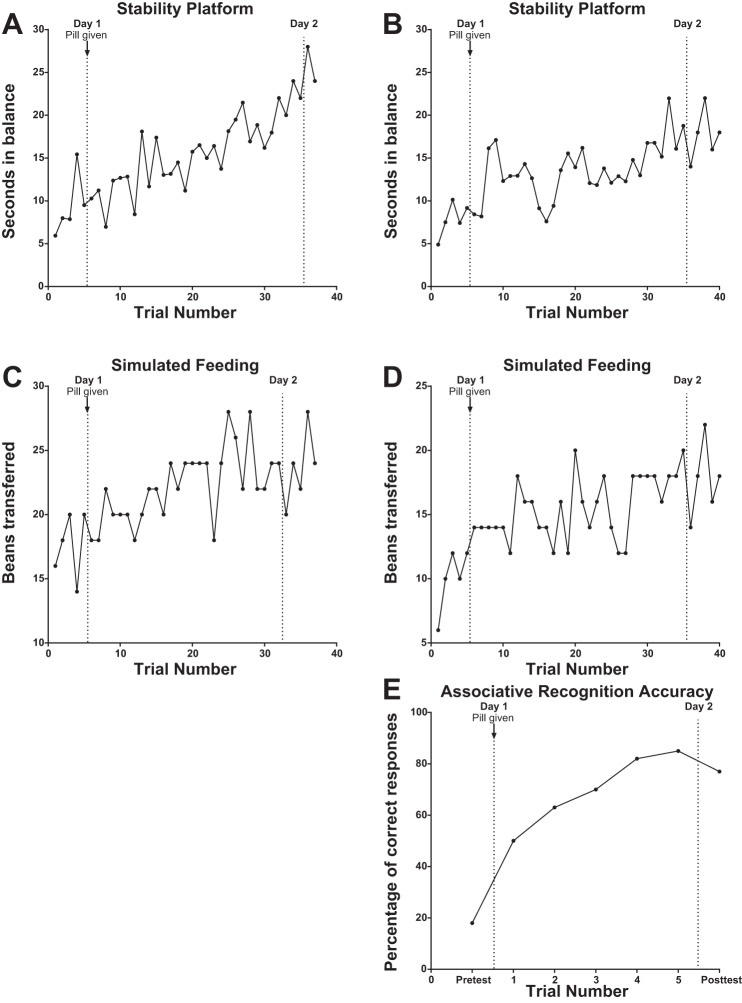
All participants showed improved performance within a session on stability platform, simulated feeding, and associative recognition (experiment II only) tasks. Example data from single participants are provided in Fig. 2. With training, participants were able to maintain the platform within three degrees of horizontal for more seconds per trial on the stability platform (Fig. 2, A and B), to successfully transfer more beans per trial during simulated feeding (Fig. 2, C and D), and to correctly identify more nonword and image pairs as either targets or foils on the associative recognition task (Fig. 2E). Moreover, these performance changes were retained for 1–3 days between sessions. These findings suggest that our assay would have adequate sensitivity to detect facilitation effects.
Fig. 2.
Individual training data. Left: data from experiment I, neurologically intact participants CT18 (A) and CT25 (C). Right: data from experiment II, participants with stroke CTS01 (B), CTS20 (D), and CTS18 (E).
Experiment I
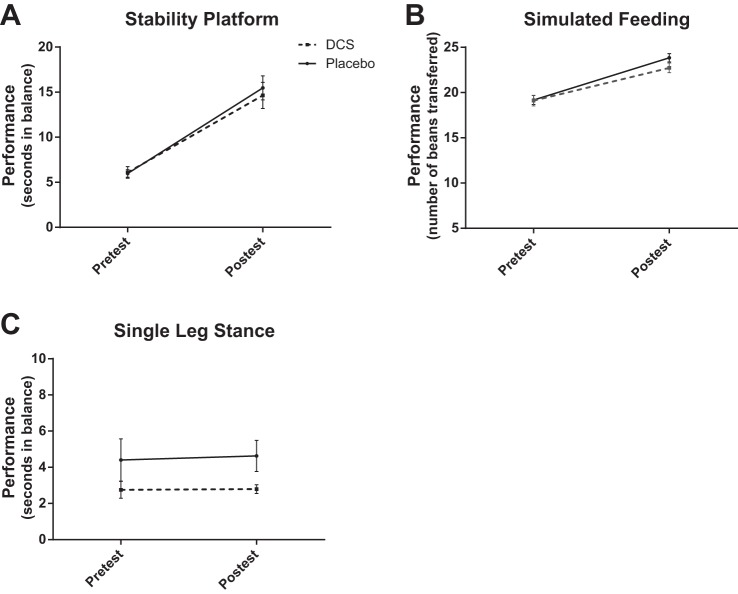
Figure 3 shows group data for the trained stability platform (A), trained simulated feeding (B), and untrained single leg stance (C) tasks. Participants improved on both trained tasks, as indicated by significant main effects of time [stability platform: F = 128.3, degrees of freedom (df) = 1, P < 0.001; simulated feeding: F = 108.6, df = 1, P < 0.001]. Training on the stability platform did not, however, generalize to the untrained single leg stance task, as indicated by a nonsignificant main effect of time (F = 0.06, df = 1, P = 0.802). Moreover, participants who received DCS did not demonstrate enhanced performance on any task compared with participants who were given placebo, as indicated by nonsignificant interaction effects (stability platform: F = 0.36, df = 1, P = 0.555; simulated feeding: F = 1.73, df = 1, P = 0.196; single leg stance: F = 0.03, df = 1, P = 0.864).
Fig. 3.
Mean pretest and posttest performance for each group in experiment I. Error bars represent SE. Subjects improved on both trained tasks [stability platform: P < 0.001 (A); simulated feeding: P < 0.001 (B)] from pretest to posttest but training did not generalize to the untrained single leg stance task [P = 0.802 (C)]. Subjects who received DCS did not demonstrate enhanced performance on any task compared with participants who were given placebo (stability platform: P = 0.555; simulated feeding: P = 0.196; single leg stance: P = 0.864).
Experiment II
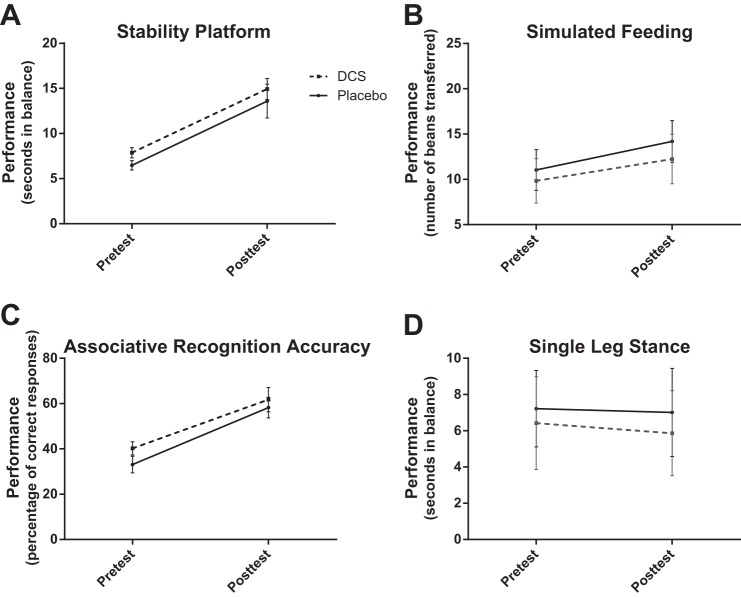
Figure 4 shows group data for the trained stability platform (A), trained simulated feeding (B), trained associative recognition (C), and untrained single leg stance (D) tasks. Subjects improved on all trained tasks, as indicated by significant main effects of time (stability platform: F = 47.45, df = 1, P < 0.001; simulated feeding: F = 22.97, df = 1, P < 0.001; associative recognition: F = 28.30, df = 1, P < 0.001). Training on the stability platform, again, did not generalize to the untrained single leg stance task, as indicated by a nonsignificant main effect of time (F = 0.322, df = 1, P = 0.578). As was the case in experiment I, participants with stroke who received DCS did not exhibit enhanced performance compared with participants who were given placebo, as indicated by nonsignificant interaction effects (stability platform: F = 0.00, df = 1, P = 0.993; simulated feeding: F = 0.429, df = 1, P = 0.521; associative recognition: F = 0.159, df = 1, P = 0.695; single leg stance: F = 0.067, df = 1, P = 0.798).
Fig. 4.
Mean pretest and posttest performance for each group in experiment II. Error bars represent SE. Subjects improved on all trained tasks [stability platform: P < 0.001 (A); simulated feeding: P < 0.001 (B); associative recognition: P < 0.001 (C)] from pretest to posttest but training did not generalize to the untrained single leg stance task [P = 0.578 (D)]. Subjects who received DCS did not demonstrate enhanced performance on any task compared with participants who were given placebo (stability platform: P = 0.993; simulated feeding: P = 0.521; associative recognition: P = 0.695; single leg stance: P = 0.798).
DISCUSSION
We found that administration of a single dose of DCS, 1 h before a motor and cognitive training session, did not enhance the effects of training. This was true for both neurologically intact adults (experiment I) and adults with chronic stroke (experiment II). We also found that DCS did not promote generalization of motor training to an untrained motor task in either participant group. To our knowledge, this study was the first test of DCS in combination with motor training as an enhancer of motor learning in humans. The protocol used to test DCS was quick, easy to administer, and inexpensive. Thus, if it can be shown that performance on these tasks can be enhanced with some agent, the protocol could serve as an economical assay for testing a range of pharmacological agents or other interventions for their ability to facilitate motor learning, before moving to a full-scale, randomized, controlled trial.
There are several possible explanations for why DCS failed to enhance the learning on these motor tasks. One option is that NMDARs were saturated. If NMDARs were saturated and already functioning optimally, the addition of DCS to the system would not enhance performance. We reasoned that this was a highly probable explanation for the negative results found in neurologically intact participants, and we proceeded with experiment II because NMDAR function is chronically reduced following stroke (Dhawan et al. 2010, 2011; Friedman et al. 2000). In spite of the likelihood that NMDAR function was reduced in people with stroke, DCS did not enhance learning.
A second possible explanation may be that the dose of DCS administration or the dose of motor training was inappropriate for enhancing motor learning. We selected a single dose rather than repeated dosing because a single 250-mg dose is well-tolerated (Rodebaugh and Lenze 2013) and avoids receptor desensitization, and changes in NMDAR subunit composition can occur with repeated dosing (Parnas et al. 2005). A number of other studies have found that a wide range of dosing, from 25 to 500 mg, is effective in enhancing cognitive-behavioral outcomes (Javitt 2013; Otto et al. 2010; Ressler et al. 2004). A dose of 250 mg was selected to ensure that the substance would be biologically active in the central nervous system but not so high as to cause NMDAR antagonism that may occur with doses >500 mg (Simeon et al. 1970). Because DCS is a partial NMDA agonist, and learning itself augments NMDAR activity, it remains possible that DCS at the given dosage still resulted in antagonism and receptor saturation. In support of this possibility is the finding that DCS improved LTP-like plasticity at a dosage of 100 mg, but combining the drug with a plasticity induction procedure (transcranial direct current stimulation) reduced motor learning (Kuo et al. 2008; Nitsche et al. 2004). Thus future studies could be designed to test different dosages to rule out the possibility that the missing effect was caused by suboptimal dosing.
With regards to the dose of motor training, this protocol was designed to serve as a simple assay to probe for the effects of combining DCS with motor training. As such, only one dose of DCS and 1 day of training was implemented. Moreover, a single training session showed efficacy in studies that combined DCS with behavioral training in the psychiatric literature (Davis 2010; Guastella et al. 2008) and in a plasticity-induction procedure (Kuo et al. 2008; Nitsche et al. 2004). It is possible that enhancement of motor learning, unlike extinction or emotional learning, requires repeated sessions to occur. Thus a single dose of DCS may have enhanced motor learning if more practice were provided. A study of amphetamine in a monkey model showed that a single dose of the drug resulted in improved motor performance when combined with 2 wk of motor skill training (Barbay et al. 2006).
Another explanation for our results is that the timing of DCS administration might not have been optimal to avoid antagonism. Data from other clinical studies indicate that administration of DCS should take place 1 h before treatment sessions (Guastella et al. 2008; Hofmann et al. 2006b) and that DCS does not improve performance during training but rather that the memory effects of DCS are only detected when tested 24 h or more after training (Kandel 2001). Specifically, it is thought that DCS works to transfer learning from short-term NMDA-independent to longer term NMDA-dependent forms of memory, a phenomenon termed consolidation (Kandel 2001; Norberg et al. 2008). Peak brain levels of DCS are reached 4–8 h after administration, so dosing 1 h before training has the maximal effect after training has ended, as desired (Javitt 2013). Given its role in consolidation, the option of delaying DCS administration until after the training session may be considered in future studies. While DCS would still be circulating during the posttraining “consolidation window,” this would allow researchers to administer DCS only after sessions in which significant improvements are made (Norberg et al. 2008). Other studies, however, indicate that DCS does have a direct effect on neuroplasticity (Nitsche et al. 2004; Teo et al. 2007). If this is the case, timing of DCS administration should occur no later than 2 h before behavioral training to guarantee that peak plasma concentrations occur during motor training. Future studies could be designed to elucidate the optimal timing of DCS administration and clarify whether the effects of the agent are primarily that of direct neuroplasticity or learning consolidation.
It is also possible that the tasks selected for motor training were not challenging enough and that participants experienced a ceiling effect. Even though many participants with stroke had worse performance than healthy subjects, the possibility of a ceiling effect cannot be ruled out because individuals with stroke may have lower maximal performance capacity. The explanation that our results occurred due to a ceiling effect is unlikely, however, because all participants in experiments I and II demonstrated a steady increase in performance during motor training and did not reach performance plateaus. Moreover, other reports using similar tasks have demonstrated continued increases in performance over multiple days of training (McNevin et al. 2003; Schaefer and Lang 2012; Wulf et al. 2003). It should be acknowledged that while we found a null effect of DCS on the two trained motor tasks that were employed in our study, it does not necessarily indicate that DCS would not enhance the learning of other motor tasks. The general nature of our tasks, i.e., selected to mimic rehabilitation tasks, and outcome measures does not allow us to identify which aspects of the tasks may or may not have been learned. Thus additional studies could take a more mechanistic approach with the goal of determining which features of motor learning may or may not be enhanced by DCS.
Finally, while the literature indicates that DCS enhances extinction-based learning in animal models (Davis et al. 2006) and in humans (Hofmann et al. 2006a; Otto et al. 2010; Ressler et al. 2004), it is possible that DCS does not have the same beneficial effect on motor learning and that the motor tasks used in this study (stability platform and simulated feeding) were not NMDAR mediated. If this is the case, enhancing NMDAR function with DCS would not result in improved learning on the motor tasks. The literature, however, indicates that application of NMDAR antagonists block horizontal LTP (Hess et al. 1996) and reduce use-dependent plasticity in the motor cortex. This implies that NMDA-receptor mechanisms operate in use-dependent plasticity in the motor cortex and point to similarities in the mechanisms underlying this form of plasticity and LTP (Butefisch et al. 2000). To address the possibility that our tasks were NMDAR independent and to confirm that DCS operates through hippocampal LTP, we added an associative recognition task to the protocol for experiment II. Associative recognition is known to involve the hippocampus and NMDARs (Bunge et al. 2004; Duzel et al. 2003; Warburton et al. 2013). As we did not see enhanced performance on the associative recognition task with DCS, it is unlikely that this is the explanation for our results.
In conclusion, we found that a single, 250-mg dose of DCS did not enhance the effects of motor or cognitive training on “rehab-like” tasks in neurologically intact adults or in adults with chronic stroke. Moreover, DCS did not promote generalization of motor training to an untrained motor task. One future direction for this line of research is to use this protocol as an assay to test other pharmacological agents which aim to enhance motor learning and neural plasticity. A second research direction could involve designing an intervention that stimulates the production of endogenous neurotropic factors known to enhance motor learning, rather than relying on exogenous DCS which is a relatively “low-efficacy” agonist with a mixed agonist/antagonist profile. Future studies should include a probe at the serum level to confirm that the pharmacological agent administered produced the expected physiological effects.
GRANTS
This work is supported by National Institute of Child Health and Human Development Grants R01-HD-068290 and T32-HD-007434, the Foundation for Physical Therapy, and the Washington University Spencer T. Olin Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.C., E.J.L., and C.E.L. conception and design of research; K.M.C. performed experiments; K.M.C., E.J.L., and C.E.L. analyzed data; K.M.C., E.J.L., and C.E.L. interpreted results of experiments; K.M.C., E.J.L., and C.E.L. prepared figures; K.M.C., E.J.L., and C.E.L. drafted manuscript; K.M.C., E.J.L., and C.E.L. edited and revised manuscript; K.M.C., E.J.L., and C.E.L. approved final version of manuscript.
REFERENCES
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. The English Lexicon Project. Behav Res Methods 39: 445–459, 2007 [DOI] [PubMed] [Google Scholar]
- Barbay S, Zoubina EV, Dancause N, Frost SB, Eisner-Janowicz I, Stowe AM, Plautz EJ, Nudo RJ. A single injection of d-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil Neural Repair 20: 455–458, 2006 [DOI] [PubMed] [Google Scholar]
- Berg K, Wood-Dauphinee S, Williams JI. The balance scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med 27: 27–36, 1995 [PubMed] [Google Scholar]
- Blennerhassett JM, Carey LM, Matyas TA. Clinical measures of handgrip limitation relate to impaired pinch grip force control after stroke. J Hand Ther 21: 245–252; quiz 253, 2008 [DOI] [PubMed] [Google Scholar]
- Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther 88: 559–566, 2008 [DOI] [PubMed] [Google Scholar]
- Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moomaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20: 864–870, 1989 [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn 56: 141–152, 2004 [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97: 3661–3665, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronova J. Discovery learning for the 21st century: what is it and how does it compare to traditional learning in effectiveness in the 21st century? Action Res Exchange 1: 1–12, 2002 [Google Scholar]
- Curtze C, Postema K, Akkermans HW, Otten B, Hof AL. The Narrow Ridge Balance Test: a measure for one-leg lateral balance control. Gait Posture 32: 627–631, 2010 [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 60: 369–375, 2006 [DOI] [PubMed] [Google Scholar]
- Davis M. Facilitation of fear extinction and psychotherapy by d-cycloserine. Zeitschrift Fur Psychologie 218: 149–150, 2010 [Google Scholar]
- Dhawan J, Benveniste H, Nawrocky M, Smith SD, Biegon A. Transient focal ischemia results in persistent and widespread neuroinflammation and loss of glutamate NMDA receptors. Neuroimage 51: 599–605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J, Benveniste H, Luo Z, Nawrocky M, Smith SD, Biegon A. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol 6: 823–834, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Studenski S, Lai SM, Johnson D. Conceptualization of a new stroke-specific outcome measure: the stroke impact scale. Top Stroke Rehabil 8: 19–33, 2001 [DOI] [PubMed] [Google Scholar]
- Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK; LEAPS Investigative Team. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol 7: 39, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci 23: 9439–9444, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakouhi TD, Jhee SS, Sramek JJ, Benes C, Schwartz P, Hantsburger G, Herting R, Swabb EA, Cutler NR. Evaluation of cycloserine in the treatment of Alzheimer's disease. J Geriatr Psychiatry Neurol 8: 226–230, 1995 [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Lanthorn TH. Effect on memory processing by d-cycloserine, an agonist of the NMDA/glycine receptor. Eur J Pharmacol 221: 249–254, 1992 [DOI] [PubMed] [Google Scholar]
- Friedman LK, Belayev L, Alfonso OF, Ginsberg MD. Distribution of glutamate and preproenkephalin messenger RNAs following transient focal cerebral ischemia. Neuroscience 95: 841–857, 2000 [DOI] [PubMed] [Google Scholar]
- Gottlieb JD, Cather C, Shanahan M, Creedon T, Macklin EA, Goff DC. d-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res 131: 69–74, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of d-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry 63: 544–549, 2008 [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, Kremer I. A randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol 16: 501–506, 2013 [DOI] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol 75: 1765–1778, 1996 [DOI] [PubMed] [Google Scholar]
- Hoffman M, Schrader J, Applegate T, Koceja D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects. J Athl Train 33: 319–22, 1998 [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Arch Gen Psychiatry 63: 298–304, 2006a [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with d-cycloserine. CNS Drug Rev 12: 208–217, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. d-Cycloserine: a ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett 98: 91–95, 1989 [DOI] [PubMed] [Google Scholar]
- Javitt DC. Harnessing N-methyl-d-aspartate receptors for new treatment development in psychiatry: positive lessons from negative studies. Am J Psychiatry 170: 699–702, 2013 [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038, 2001 [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry 140: 734–739, 1983 [DOI] [PubMed] [Google Scholar]
- Kuo MF, Unger M, Liebetanz D, Lang N, Tergau F, Paulus W, Nitsche MA. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia 46: 2122–2128, 2008 [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Honma M, Soshi T, Fujii T, Kim Y. Effect of d-cycloserine and valproic acid on the extinction of reinstated fear-conditioned responses and habituation of fear conditioning in healthy humans: a randomized controlled trial. Psychopharmacology (Berl) 218: 589–697, 2011 [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil 87: 1605–1610, 2006 [DOI] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT, Bever CT, Jr, Bravata DM, Duncan PW, Corn BH, Maffucci AD, Nadeau SE, Conroy SS, Powell JM, Huang GD, Peduzzi P. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 362: 1772–1783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNevin NH, Shea CH, Wulf G. Increasing the distance of an external focus of attention enhances learning. Psychol Res 67: 22–29, 2003 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by d-cycloserine. Neuropsychopharmacology 29: 1573–1578, 2004 [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of d-Cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63: 1118–1126, 2008 [DOI] [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, Hofmann SG, Eisenmenger K, Krystal JH, Pollack MH. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry 67: 365–370, 2010 [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to d-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem 83: 224–231, 2005 [DOI] [PubMed] [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with d-cycloserine facilitates learning and retention. Eur J Pharmacol 257: 7–12, 1994 [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61: 1136–1144, 2004 [DOI] [PubMed] [Google Scholar]
- Rodebaugh TL, Lenze EJ. Lessons learned from d-cycloserine: the promise and limits of drug facilitation of exposure therapy. J Clin Psychiatry 74: 415–416, 2013 [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Lang CE. Using dual tasks to test immediate transfer of training between naturalistic movements: a proof-of-principle study. J Mot Behav 44: 313–327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon J, Fink M, Itil TM, Ponce D. d-Cycloserine therapy of psychosis by symptom provocation. Compr Psychiatry 11: 80–88, 1970 [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30, 11670–11677, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Rothwell JC. Further evidence for NMDA-dependence of the after-effects of human theta burst stimulation. Clin Neurophysiol 118: 1649–1651, 2007 [DOI] [PubMed] [Google Scholar]
- Toraman A, Yildirim NU. The falling risk and physical fitness in older people. Arch Gerontol Geriatr 51: 222–226, 2010 [DOI] [PubMed] [Google Scholar]
- Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc 45: 735–738, 1997 [DOI] [PubMed] [Google Scholar]
- Warburton EC, Barker GR, Brown MW. Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74: 41–47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with d-cycloserine for obsessive-compulsive disorder. Am J Psychiatry 165: 335–41; quiz 409, 2008 [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D; EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 296, 2095–2104, 2006 [DOI] [PubMed] [Google Scholar]
- Wulf G, Weigelt M, Poulter D, McNevin N. Attentional focus on suprapostural tasks affects balance learning. Q J Exp Psychol A 56: 1191–211, 2003 [DOI] [PubMed] [Google Scholar]
- Yaka R, Biegon A, Grigoriadis N, Simeonidou C, Grigoriadis S, Alexandrovich AG, Matzner H, Schumann J, Trembovler V, Tsenter J, Shohami E. d-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J 21: 2033–2041, 2007 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NE, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: an fMRI study. Neuroreport 12: 359–363, 2001 [DOI] [PubMed] [Google Scholar]