Abstract
Hydroxycinnamates, aromatic compounds that play diverse roles in plants, are dissimilated by enzymes encoded by the hca genes in the nutritionally versatile, naturally transformable bacterium Acinetobacter sp. strain ADP1. A key step in the hca-encoded pathway is activation of the natural substrates caffeate, p-coumarate, and ferulate by an acyl:coenzyme A (acyl:CoA) ligase encoded by hcaC. As described in this paper, Acinetobacter cells with a knockout of the next enzyme in the pathway, hydroxycinnamoyl-CoA hydratase/lyase (HcaA), are extremely sensitive to the presence of the three natural hydroxycinnamate substrates; Escherichia coli cells carrying a subclone with the hcaC gene are hydroxycinnamate sensitive as well. When the hcaA mutation was combined with a mutation in the repressor HcaR, exposure of the doubly mutated Acinetobacter cells to caffeate, p-coumarate, or ferulate at 10−6 M totally inhibited the growth of cells. The toxicity of p-coumarate and ferulate to a ΔhcaA strain was found to be a bacteriostatic effect. Although not toxic to wild-type cells initially, the diphenolic caffeate was itself converted to a toxin over time in the absence of cells; the converted toxin was bactericidal. In an Acinetobacter strain blocked in hcaA, a secondary mutation in the ligase (HcaC) suppresses the toxic effect. Analysis of suppression due to the mutation of hcaC led to the development of a positive-selection strategy that targets mutations blocking HcaC. An hcaC mutation from one isolate was characterized and was found to result in the substitution of an amino acid that is conserved in a functionally characterized homolog of HcaC.
Aromatic compounds termed hydroxycinnamates are among potential carbon sources released from living and decaying plants. Consisting of variations on a phenolic ring with a propenoate side chain, hydroxycinnamates play versatile roles as components or precursors in plant architecture and defense (9, 48). The ability of bacteria to utilize diverse hydroxycinnamates as sources of carbon and energy is widely distributed among microbial groups (1, 8, 29, 32, 39, 46, 47). As is frequently the case with aromatic compounds (44), hydroxycinnamates tend to be toxic to bacterial cells (38). A bacterial strain that meets the challenge presented by these compounds is Acinetobacter sp. strain ADP1. However, the observation that one class of Acinetobacter mutants blocked in hydroxycinnamate catabolism was extremely sensitive to the presence of hydroxycinnamates led to an investigation of this phenomenon, and this investigation is reported here.
As a derivative of strain BD413 (23), Acinetobacter sp. strain ADP1 has a remarkable proficiency for natural transformation by DNA (30). Another notable feature of the strain is found in the extended regions of DNA where genes encoding catabolic functions are concentrated (20, 35). One cluster contains genes for pathways of dicarboxylate (dca), protocatechuate (pca), quinate or shikimate (qui), and p-hydroxybenzoate (pob) catabolism (35). Characterization of DNA beyond the pob genes in this strain led to the identification of all of the hca genes involved in hydroxycinnamate utilization (37, 46). The pca, qui, pob, and hca genes are functionally related in that quinate, shikimate, p-hydroxybenzoate, and hydroxycinnamates are all degraded via the protocatechuate branch of the β-ketoadipate pathway.
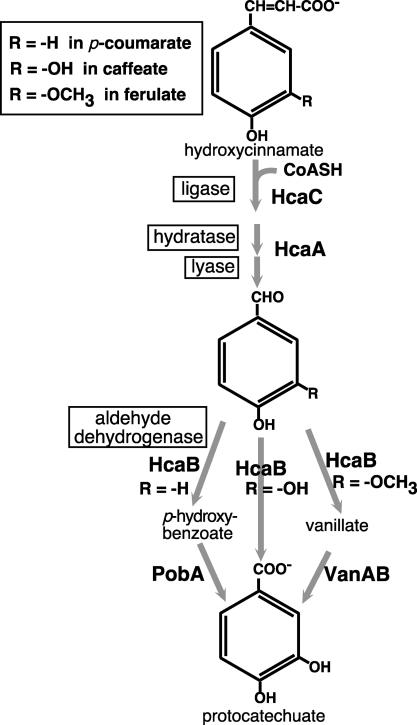
Enzymes encoded by the hca genes exhibit relatively broad substrate specificity, acting on three hydroxycinnamates, caffeate, p-coumarate, and ferulate, or their respective derivatives. In Acinetobacter strains, dissimilation of the latter two compounds occurs via the intermediates p-hydroxybenzoate and vanillate, respectively (Fig. 1) (8, 37, 46). The hca genetic cluster consists of two groups of genes: hcaABCDEFG and the divergently transcribed hcaKR (Fig. 2). Enzymes encoded by hcaC, hcaA, and hcaB carry out three steps in the dissimilation of hydroxycinnamates (Fig. 1). HcaC, hydroxycinnamoyl:CoA ligase, activates hydroxycinnamates to their thioester derivatives. HcaA, a bifunctional hydratase/lyase (13), converts the thioester derivative to an aldehyde intermediate. HcaB, an aldehyde dehydrogenase, transforms the aldehyde to a carboxylated derivative that serves as a substrate for downstream catabolic pathways. A fourth gene, hcaG, encodes an esterase that hydrolyzes chlorogenate to quinate and caffeate (46). An exception to the proximity of related genes is the situation for the van genes for vanillate degradation, which are unlinked to the hca region (43).
FIG. 1.
Pathway for dissimilation of hydroxycinnamates to protocatechuate in Acinetobacter sp. strain ADP1.
FIG. 2.
Physical map of hca genes in Acinetobacter sp. strain ADP1. Arrows indicate directions of transcription but not necessarily the units of transcription; it has been established that at a minimum hcaABCDE forms a unit of transcription (37). The subclones shown below the map were used as transforming DNA to localize second-site suppressor mutations or in tests with E. coli.
Accumulation of the substrate of PcaB, a tricarboxylate intermediate, is toxic to cells (14). Thus, upon their exposure to an appropriate precursor, pcaB-blocked cells give rise to secondary mutants that are protected by their inability to generate the tricarboxylate compound. The application of a positive-selection procedure based on the toxic effect has resulted in the isolation of informative structure-function mutations in upstream enzymes and their regulators (7, 14, 26, 27, 34). However, this approach had met with limited success in the case of the initial steps of the hydroxycinnamate pathway because the numerically predominant secondary mutants were those blocked in steps towards the end of the catabolic sequence.
The gene responsible for controlling the expression of the hca genes, hcaR, encodes a repressor which is homologous to the Escherichia coli repressor, MarR, and the inductive triggers for hca gene expression were found to be the thioester products of the HcaC ligase reaction (37). The latter finding was based in part on results obtained with strain ADP8114, which contains two mutations: a promoterless lacZ fused to hcaE, and ΔhcaA1. In the presence of only 10−6 M p-coumarate, strain ADP8114 expressed LacZ at a level representing a 270-fold increase over the uninduced level, whereas other hcaE::lacZ strains with wild-type or hca mutant backgrounds had the background level of expression in the presence of low concentrations of hydroxycinnamates.
The impetus for the investigation reported in this paper was the observation of extreme hydroxycinnamate sensitivity in hcaA mutant strains like strain ADP8114. Experiments were designed to examine the nature of the poisoning, to define the sensitivity of hcaA-deficient cells to external hydroxycinnamates, to determine whether a knockout of hcaR increased such sensitivity, to discover whether the effect was bacteriostatic or bactericidal, and to explore whether the toxic effect could be developed as a method of positive selection targeted to mutations in hydroxycinnamate:CoA ligase.
MATERIALS AND METHODS
Media components.
Luria-Bertani (LB) medium (45) or minimal medium (38) was used to grow cells. Sigma-Aldrich Co. was the source of hydroxy-trans-cinnamates and other growth substrates. Single substrates were succinate at 10 mM, quinate at 5 mM, and hydroxycinnamate (p-coumarate, ferulate, or caffeate) at 2 mM, with hydroxycinnamates being prepared from a 1 M stock solution in dimethyl sulfoxide (DMSO). Dual-substrate medium contained succinate at 10 mM and a hydroxycinnamate at a concentration that depended on the selection applied, as noted below; succinate used in liquid minimal medium was added prior to autoclaving. Pantothenate-supplemented minimal medium was tested by using the vitamin at 2 μg ml−1. For antibiotic selections, LB medium was supplemented with ampicillin at a concentration of 100 μg ml−1 or with kanamycin at 15 μg ml−1 (for Acinetobacter cells) or 25 μg ml−1 (for E. coli cells). LB medium containing 5% sucrose was used to select against cells that carried a sacB-Kmr cassette.
Plasmid and strain construction.
Table 1 lists strains and plasmids used in this study. Standard methods were used for molecular biology manipulations (3, 42). Cells of Acinetobacter were made naturally competent by published methods (22). Crude lysates were prepared by the resuspension of pelleted cells from a 5-ml culture in 500 μl of lysis buffer (22), followed by incubation at 60°C for 1 h; lysates of cells for PCRs were similarly prepared from 1 ml of a succinate overnight culture.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Genotype | Reference or source |
|---|---|---|---|
| E. coli strain | |||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK−) supE44 λ−thi-1 gyrA96 relA1 | Invitrogen | |
| Acinetobacter sp. strains | |||
| ADP1 | Wild-type strain (also known as BD413) | 23 | |
| ADP853 | pcaB frameshift mutation created by filling in the ends of an NcoI site | pcaB853 | 34 |
| ADP1027 | 100-bp deletion in hcaC; blocked in hydroxycinnamate-CoASH ligase | ΔhcaC1 | 46 |
| ADP8078 | Kmr; Δ(hcaRorf1)1::sacB-Kmr in the ADP1 chromosome | Δ(hcaR-orf1)1::sacB-Kmr | 37 |
| ADP8079 | Kmr; Δ(hcaB′ AK′)1::sacB-Kmr in the ADP1 chromosome | Δ(hcaB′ AK′)1::sacB-Kmr | 37 |
| ADP8083 | 217-bp HincII deletion in hcaR in the ADP8078 chromosome | ΔhcaR1 | 37 |
| ADP8085 | 163-bp HpaI deletion in hcaA in the chromosome of ADP8079 | ΔhcaA1 | 37 |
| ADP8089 | Kmr; Δ(hcaB′ AK′)1::sacB-Kmr from pZR8227 introduced into the ADP8083 chromosome | ΔhcaR1 Δ(hcaB′ AK′)1::sacB-Kmr | This study |
| ADP8090 | Kmr; Δ(hcaB′ AK′)::sacB-Kmr from pZR8227 introduced into the ADP853 chromosome | pcaB853 Δ(hcaB′ AK′)::sacB-Kmr | This study |
| ADP8093 | ΔhcaA1 from pZR8216 introduced into the chromosome of ADP8089 | ΔhcaA1 ΔhcaR1 | This study |
| ADP8095 | ΔhcaA1 from pZR8216 introduced into the chromosome of ADP8090 | pcaB853 ΔhcaA1 | This study |
| ADP8150 | Spontaneous hcaC mutation in ADP8095 mutant background | pcaB853 ΔhcaA1 hcaC2 | This study |
| ADP8209 | Spontaneous hcaC mutation in wild-type background | hcaC2 | This study |
| ADP8292 | ADP8150 with pcaB mutation corrected with pZR3 | ΔhcaA1 hcaC2 | This study |
| Plasmids | |||
| pBKS | Apr; narrow-host-range cloning vector | Stratagene | |
| pBKSΔ1 | Apr; pBKS (ΔSmaI-HincII) | 37 | |
| pRMJ1 | AprKmr; contains sacB-Kmr cassette for genetic replacement by positive selection | 21 | |
| pRK415 | Tcr; broad-host-range vector | 24 | |
| pUC18 | Apr; narrow-host-range cloning vector | 49 | |
| pZR3 | Apr; 2.75-kb HindIII fragment containing pcaB′DK′ from strain ADP1 in pUC18 | 10 | |
| pZR8200 | Apr; 16.5-kb insert which includes the hca genetic cluster; in pUC19 | 37 | |
| pZR8208 | Tcr; 6.5-kb SacI insert of hcaK′ABCD′ from pZR8200 in pRK415 | This study | |
| pZR8210 | Apr; 6.5-kb SacI insert of pZR8200 in pBKSΔ1 | 37 | |
| pZR8216 | Apr; 163-bp HpaI deletion of hcaA in pZR8210 | 37 | |
| pZR8221 | Apr; 1.2-kb KpnI deletion of pZR8210; the insert contains hcaABC′ and hcaK′ | This study | |
| pZR8227 | Apr Kmr; sacB-Kmr cassette of pRMJ1 inserted at the site of the PstI deletion of hcaB′ hcaA hcaK′ in pZR8220 | 37 | |
| pZR8240 | Tcr; 3.3-kb SacI-BglII insert of hcaC plus the 3′ part of hcaB in pRK415 | 37 | |
| pZR8247 | Apr; 2.1-kb KpnI-BglII subclone of pZR8240 in pUC18 | 37 | |
| pZR8251 | Apr; 1.3-kb HindIII deletion of pZR8247 | This study | |
| pZR8266 | Apr; 3.4-kb PstI insert of hcaB′CD′ from pZR8208 in pUC18 | This study | |
| pZR8272 | Apr; 3.4-kb PstI-SphI insert of hcaB′CD′ in pUC18 | This study | |
| pZR8273 | Apr; pZR8272 with a 1.7-kb SmaI-to-ClaI deletion, leaving the 3′ end of hcaC | This study |
Strains with designed deletions were created by using intermediary strains containing a sacB-Kmr cassette from pRMJ1 in the targeted site. Cells having the cassette will not grow at room temperature in medium supplemented with 5% sucrose (21). Thus, the cassette affords positive selection for its replacement by DNA carrying a deletion. For example, strain ADP8095 was constructed from strain ADP853 by transformation of the latter with pZR8227, which contained a PstI deletion covering hcaB through hcaK; a sacB-Kmr cassette was inserted in the deletion site. Following selection and purification on medium supplemented with kanamycin, the resultant isolate ADP8090 was transformed with pZR8216, which contained ΔhcaA1. Colonies that grew in the presence of sucrose were screened for the absence of the cassette's Kmr marker as well as for the absence of vector antibiotic resistance. One of these colonies was isolated as strain ADP8095. The construction of other strains by following similar steps can be traced in Table 1. PCRs were performed to confirm that the desired insertion or deletion mutations were present in the Acinetobacter chromosome.
Acinetobacter growth tests.
Comparative growth tests with succinate-supplemented medium were conducted with cultures grown at 37°C with shaking at 250 rpm. After overnight growth at the expense of succinate, a 100-μl aliquot of a culture was added to 5 ml of fresh minimal medium containing succinate with or without the test compounds; the initial cell concentration was about 107 cells ml−1. The succinate control tubes also contained the amount of DMSO added with the highest level of hydroxycinnamate in a given experimental set. When cells exposed to succinate alone had reached the late exponential to early stationary phase (after about 4.5 h), turbidity was measured for all cultures of that strain. Pantothenate-supplemented medium was tested for strain ADP8085 grown in the presence of 10−5 M p-coumarate.
Absorbance of caffeate in minimal medium at 600 nm can interfere with the measurement of turbidity. This was not a problem with cells that can utilize caffeate readily. For cells with an hcaA or hcaC mutation, which are unable to utilize caffeate, the optical density at 600 nm (OD600) of control tubes with caffeate at 10−3 or 10−4 M (without cells present) was subtracted from the values obtained with cells exposed to the respective concentrations of the compound. Similar results were obtained when cells were centrifuged from medium containing caffeate and resuspended in minimal medium. These results correlated well with apparent cell densities in other tubes containing known concentrations of cells.
E. coli growth tests.
Three independent isolates of E. coli strain DH5α freshly transformed with a ligation mix were cultured in liquid LB medium with ampicillin and yielded three separate preparations of pZR8266, carrying a full-length copy of hcaC. SphI deletion plasmids were prepared from each of the three pZR8266 stocks. The deletion plasmids, termed pZR8272, also had the full-length hcaC insert. A further ClaI-SmaI deletion of one pZR8272 preparation removed the 5′ half of hcaC along with insert DNA between the ClaI site and the vector multiple-cloning site, creating pZR8273.
E. coli cells were transformed with the three separate ligation mixes for the pZR8272 constructions as well as the ligation mix for the pZR8273 construction. Transformants that arose on LB plates containing ampicillin were patched onto the same medium alone and onto the same medium containing 1 mM p-coumarate; four colonies were patched for each independent construction.
Viability tests.
To gauge the effect of hydroxycinnamoyl-coenzyme A (CoA) accumulation on cell viability, overnight cultures of cells grown on succinate were inoculated into medium containing 1 mM hydroxycinnamate and no other carbon source; the initial cell concentration was about 5 × 105 cells ml−1. Control tubes with no carbon source added were amended with DMSO as noted above. Viable counts of cultures were determined after incubation for 48 h at 30°C with shaking at 250 rpm.
Isolation of hcaC secondary mutant strains.
All transformations described in this section were carried out by spreading 150 μl of competent recipient cells on solidified nonselective medium containing succinate. Aliquots of DNA were placed on the cells at marked locations. Alternatively, when numerous mutations were mapped, cells were replica plated onto solidified medium that had been spread with 10 μl of a dilute DNA lysate of E. coli cells carrying a subclone. Following incubation at 37°C for 3 h, cells exposed to DNA were patched or replica plated onto selective medium along with control spots where no DNA or control DNA was added.
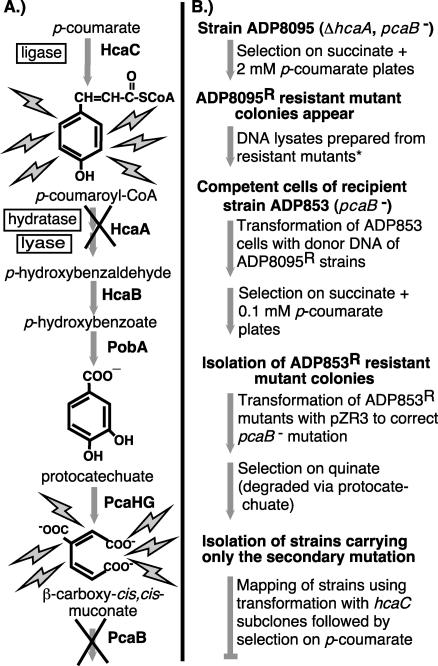
Figure 3 gives an overview of a procedure that was used to isolate hcaC mutants. Individual colonies of strain ADP8095 (pcaB853 ΔhcaA1) were patched onto solidified medium containing succinate plus 2 mM p-coumarate (Fig. 3B). Colonies that appeared out of the background of nongrowing cells were purified on the same selective medium, and lysates were prepared. An aliquot of each crude lysate was treated with the protein precipitation solution of the Wizard genomic DNA purification kit (Promega Corp.) in order to ensure the full digestibility of the DNA. Further steps with the Wizard kit for purification of DNA from gram-negative bacteria, carried out with proportionately reduced volumes, were followed to completion. Digestion of the purified DNA with PstI physically separated hcaC from ΔhcaA (Fig. 2).
FIG. 3.
Procedure for positive selection of strains whose sole impediment to growth with hydroxycinnamates is a mutation in HcaC, the hydroxycinnamate:CoA ligase. (A) Pathway for catabolism of p-coumarate to β-carboxy-cis,cis-muconate. A block at either of two steps, HcaA or PcaB, can result in a toxemia that prevents growth in the presence of precursor substrates. Mutation of an enzyme that acts upstream in generating the nonmetabolizable intermediate can overcome the inhibitory effect. (B) Flowchart showing the steps taken to isolate strains having a single mutation which lies in a gene other than hcaA or pcaB. The manipulations shown in lightface text were applied to the Acinetobacter strain in boldface text that headlines the steps that follow. The asterisk indicates that DNA prepared from the ADP8095 resistant mutants was digested with PstI to separate the hcaA deletion from secondary mutations, such as those in hcaC, which can block the conversion of p-coumarate to growth-inhibiting compounds. The relevant PstI site between hcaA and hcaC is shown in Fig. 2. Although the procedure for strain isolation seems elaborate, it is efficient because of the ability to process multiple strains readily due to the natural transformability of the strains and the ease of doing multiple transformations on a single plate.
A subsequent selection required the use of competent cells of pcaB mutant strain ADP853 as recipients for the PstI-digested DNA of strains containing a putative hcaC mutation. The parental strain ADP8095 was constructed to contain the pcaB853 mutation of strain ADP853 as well as the ΔhcaA1 mutation. The strain was constructed in this way in order to avoid simply correcting the pcaB mutation of strain ADP853 rather than introducing the secondary mutation. The selective medium for plating the ADP853 transformants contained succinate and 0.1 mM p-coumarate. After purification of selected colonies on the same medium, the pcaB mutation in each strain was corrected by an analogous procedure. Transformation with pZR3, which carries pcaB, was followed by selection for growth at the expense of quinate, and strains were purified on the same medium (Fig. 3B).
Mapping of hcaC mutant strains.
Resultant p-coumarate-negative strains were screened to verify that they did not carry pZR3 vector antibiotic resistance, that they contained a wild-type hcaA gene, and that the sole defect preventing their growth on hydroxycinnamates lay in hcaC. The last two conditions were established by testing the ability of pZR8240, which contains primarily the wild-type hcaC gene (Fig. 2), to correct the p-coumarate-negative phenotype. Mutations in strains that were found to have defects in hcaC were localized by means of other hcaC subclones (Fig. 2).
Frequency of second-site suppressor mutants.
The frequency of second-site suppressor mutants of strains ADP8085 and ADP853 was determined by plating cells on selective medium containing 10 mM succinate plus p-coumarate at 0.1 and 2 mM, respectively. The fraction of the total viable count that appeared as CFU on the selective medium after 40 h at 37°C was measured as the second-site mutant frequency.
PCRs and DNA sequencing.
Template DNA was prepared as a crude chromosomal lysate. A 2.3-kb amplicon of hcaC containing a spontaneous mutation was amplified by PCR. Conditions for PCR were 94°C for 3 min and 30 cycles of denaturing at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for an appropriate length of time. For each strain, reaction products were purified by a QIAquick PCR purification step (QIAGEN Inc.) prior to sequencing. On the basis of localization of spontaneous mutations, the relevant section of hcaC containing a spontaneous mutation was sequenced. Primer sets were as follows: HCACF1 (5′-TGTTCGCTTGGCAAATGACAGTGAGT-3′) and HCACR1 (5′-AACCCCATACGAAATGAGCTTGAGAA-3′) were used to amplify hcaC, HCACF3 (5′-CATCAGATTGCCAAGTTTTTATTTAC-3′) was used to sequence hcaC2, and DPZR82F6 (5′-GGGTCACACTTAATCGTCCTCACAA-3′) and DPZR82R3 (5′-TCTTCATTCTGATCCCATGTCAGCT-3′) were used to confirm the genotypes of strains ADP8093 and ADP8095.
ABI PRISM terminator cycle sequencing with AmpliTaq DNA polymerase was carried out at the Yale Keck Biotechnology Resource Lab.
RESULTS
Sensitivity of strain ADP8085 (ΔhcaA1) cells to hydroxycinnamates.
A deletion in the hydratase/lyase gene, ΔhcaA1, eliminates the ability of Acinetobacter cells to grow at the expense of the three hydroxycinnamates (caffeate, p-coumarate, and ferulate) that are growth substrates for the wild-type strain (37). The mutation also blocks the cells' ability to grow in the presence of these compounds. The provision of wild-type hcaA by transformation of ΔhcaA1 mutant strain ADP8085 with plasmid pZR8221 restored the hydroxycinnamate-positive phenotype.
To determine relative inhibitory concentrations of p-coumarate, caffeate, and ferulate, strain ADP8085 (ΔhcaA) and wild-type ADP1 cells at 107 cells ml−1 were exposed to decreasing concentrations of the three hydroxycinnamates in the presence of succinate (Fig. 4). Under these conditions, growth inhibition of strain ADP8085 was virtually complete at p-coumarate and caffeate concentrations of 10−5 M and higher and at a ferulate concentration of 10−3 M. Over the 4.5-h time period of the experiments, the growth of strain ADP1027 (ΔhcaC1) was similar to that of the wild-type strain in the presence of a 1 mM concentration of each of the three compounds (data not shown), indicating that the toxic effect observed under these conditions was not simply the result of the hydroxycinnamates being converted to toxins in the absence of metabolism.
FIG. 4.
Effects of hydroxycinnamates on wild-type strain ADP1 and ΔhcaA1 strain ADP8085. Cells were grown at the expense of succinate with decreasing amounts of the three hydroxycinnamates added. For each set of data, OD600 was measured when succinate-grown cells had reached the late exponential to early stationary phase. The initial cell concentration was 107 cells ml−1, which corresponded to an OD600 of 0.03. The averages for three independent trials are shown with their standard deviations (error bars).
The relative sensitivity of pcaB mutant strain ADP853 to p-hydroxybenzoate and to p-coumarate was determined by the same method. In this case, growth inhibition was virtually total in the presence of each of the two compounds at 10−4 M (data not shown). Thus, the toxic effect of p-coumarate on an hcaA mutant strain appears to be somewhat more potent than the effect of this compound on the carboxymuconate-accumulating pcaB mutant strain ADP853.
It should be noted that the toxic effect is dependent on the cell concentration. Thus, a 10-fold-lower initial cell density resulted in the total absence of the growth of strain ADP8085 cells in the presence of 10−6 M p-coumarate by the time the control cells on succinate alone had fully grown (data not shown).
Sensitivity of strain ADP8093 (ΔhcaA1 ΔhcaR1) cells to hydroxycinnamates.
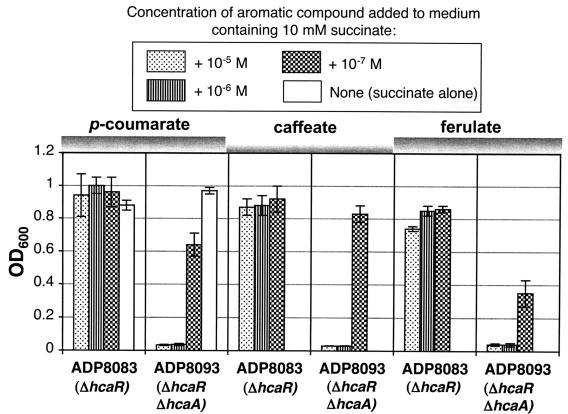
A possible interpretation of the hydroxycinnamate toxic effect observed with ΔhcaA1 mutant strain ADP8085 is that elevated expression of hca genes, triggered by the accumulation of hydroxycinnamoyl-CoA thioester inducers (37), places a deleterious biosynthetic load on cells. This interpretation was tested by deleting the HcaR repressor, thereby ensuring uniform hca gene expression in the derived strain in the presence of different concentrations of hydroxycinnamates and eliminating induction as a factor (37).
Strain ADP8093 (ΔhcaA1 ΔhcaR1) was grown in the presence of different levels of hydroxycinnamates in parallel with its parental strain ADP8083 (ΔhcaR1). As shown in Fig. 5, strain ADP8083 grew well in the presence of hydroxycinnamates, whereas sensitivity to hydroxycinnamates was higher in strain ADP8093 than in strain ADP8085 (ΔhcaA1). When the concentration of p-coumarate, caffeate, or ferulate was lowered to 10−7 M, cultures of strain ADP8093 were able to grow somewhat (Fig. 5).
FIG. 5.
Effects of hydroxycinnamates on ΔhcaR1 strain ADP8083 and ΔhcaA1 ΔhcaR1 strain ADP8093. Cells were grown at the expense of succinate with decreasing amounts of the three hydroxycinnamates added, and they were measured as described in the legend to Fig. 4. The initial cell concentration was 107 cells ml−1, which corresponded to an OD600 of 0.03. The averages for three independent trials are shown with their standard deviations (error bars).
Rescue of an hcaA mutant from hydroxycinnamate toxicity by a second-site mutation.
As explained in the section dealing with hcaC mutant isolation below, suppressor mutants were isolated, and the secondary mutations that rescued hcaA mutant cells from hydroxycinnamate poisoning were localized to hcaC. When strain ADP8292 (hcaC2 ΔhcaA1) was exposed to any of the three hydroxycinnamates at a concentration of 1 mM, it grew at the expense of succinate as well as wild-type cells, unlike ΔhcaA1 strain ADP8085 (data not shown). The rescue of ΔhcaA1 cells by an hcaC mutation is consistent with the conclusion that hydroxycinnamoyl-CoA thioester accumulation inhibits the growth of an hcaA mutant strain in the presence of hydroxycinnamates. This effect could be attributed to the depletion of CoA pools by the sequestering of the CoA as the hydroxycinnamoyl-CoA thioester. To determine whether this postulated metabolic imbalance could be overcome by supplying pantothenate, a precursor of CoA, the vitamin was added to medium containing succinate plus 10−5 M p-coumarate. The pantothenate supplement did nothing to enhance the growth of strain ADP8085 (data not shown).
Effect of p-coumarate on E. coli cells carrying hcaC.
Further evidence that supports hydroxycinnamoyl-CoA thioester buildup as the cause of growth inhibition in an hcaA mutant strain comes from results obtained with E. coli cells. The presence or absence of hydroxycinnamate should not influence regulation in this case. E. coli(pZR8272) cells contain a full-length hcaC expressed off the vector lac promoter, and E. coli(pZR8273) cells carry only half of hcaC (Fig. 2). When freshly plated, independent transformants of E. coli(pZR8272) cells were subcultured onto antibiotic medium with and without 1 mM p-coumarate, they grew only in the absence of the hydroxycinnamate, whereas cells of E. coli(pZR8273), like other isolates without an intact copy of hcaC, grew on both types of media.
Survival of ΔhcaA cells following exposure to p-coumarate or ferulate.
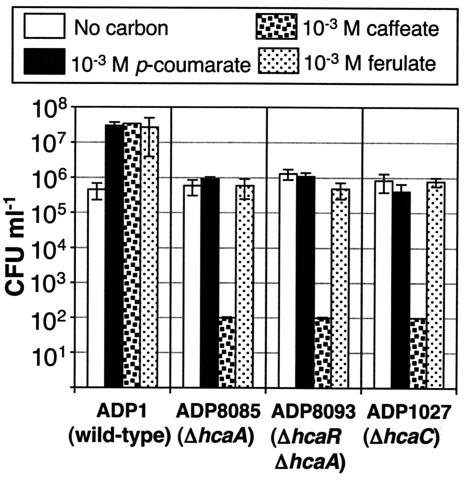
To determine whether the effect of accumulated thioesters is bacteriostatic or bactericidal, succinate-grown cells were inoculated at a low concentration into minimal medium containing only p-coumarate or ferulate at 1 mM. After incubation for 48 h at 30°C, cells were diluted and plated to measure viability. As shown in Fig. 6, cells exposed to the two compounds retained their viability over the 48-h period. An analogous test with pcaB mutant ADP853 exposed to p-hydroxybenzoate generated similar results.
FIG. 6.
Survival of Acinetobacter cells incubated in the presence of a hydroxycinnamate at 1 mM with no other substrate present. Viable counts are shown on a logarithmic scale. Cultures were maintained at 30°C for 48 h. The number of cells in caffeate was at the limit of detection and is actually smaller than the number indicated by the bar. Viable counts are means for three independent experiments, with error bars showing the standard deviations.
Effect of caffeate on cell survival.
One method for measuring substrate toxicity qualitatively is to supply a compound as a localized spot on a plate in or on which cells are distributed uniformly so that the substrate forms a concentration gradient (38). Examined in this way, caffeate appears to be nontoxic to strain ADP1 cells. However, the incubation of strain ADP8085 and other Acinetobacter hca mutant strains with 1 mM caffeate as was done with the other two compounds resulted in a loss of viability (Fig. 6). Because this result occurred with ΔhcaC1 mutant strain ADP1027 as well, it was not possible to draw a conclusion about the mechanism of poisoning caused by the buildup of caffeoyl-CoA thioester.
In further tests of caffeate toxicity, the compound was incubated at a concentration of 1 mM in minimal medium at 37°C for 48 h and, thus prepared, was termed “converted caffeate.” The brown converted caffeate was toxic to wild-type Acinetobacter cells. In medium containing converted caffeate, viable-cell numbers decreased by about 1 log unit after 2 h of incubation and by another log unit at 3 h, and viability was undetectable (less than 5 CFU ml−1) by 5 h, at which time the viable-cell concentration in the control tubes containing only the added DMSO was 5 × 105 CFU ml−1 (standard deviation, 2 × 105 CFU ml−1). The killing effect was reduced upon the lowering of the concentration of converted caffeate or the raising of the cell concentration, each by an order of magnitude.
Isolation of cells with spontaneous mutations in hcaC.
As described in Materials and Methods and outlined in Fig. 3B, resistant mutants derived from strain ADP8095 were selected on solidified medium containing succinate plus 2 mM p-coumarate. p-Coumarate was used as the selective hydroxycinnamate due to the chemical instability of caffeate and the genetic instability of the van region of the ADP1 chromosome, required for the catabolism of ferulate (43). At this point, each resistant mutant of ADP8095 contained three mutations: pcaB853, ΔhcaA, and an unknown secondary mutation, predicted to be in hcaC.
Chromosomal lysates from the resistant mutants were digested with PstI in order to physically unlink ΔhcaA from hcaC (Fig. 2), with the goal being to obtain a strain having a mutation in the latter gene but not the former. Following the transformation of pcaB-negative strain ADP853 with PstI-digested DNA containing the putative hcaC mutation, cells were selected on succinate plus p-coumarate. Because the transforming DNA contained the pcaB853 mutation, protection from toxicity was conferred by the putative hcaC mutation and not by the introduction of a wild-type pcaB gene. In order for the hcaC mutation to confer resistance to p-coumarate in the pcaB-negative background, it was necessary to lower the p-coumarate concentration in solidified medium to 0.1 mM; the rationale for lowering the concentration is discussed in the next section. Correction of the pcaB mutation by an E. coli(pZR3) lysate carrying the wild-type pcaB allele yielded strains that carried only the acquired hcaC mutations.
Frequency and types of suppressor mutants arising in an hcaA or pcaB mutant background at different concentrations of p-coumarate.
The effect of p-coumarate concentration on the selection of suppressor mutations in hcaA strains versus that in pcaB mutant strains was investigated further. The frequency of suppressor mutants was determined for two strains, ADP8085 (hcaA1) and ADP853 (pcaB853). On succinate plus 2 mM p-coumarate, the suppressor mutant frequency was 1.8 × 10−6 for strain ADP8085 and 7.5 × 10−6 for strain ADP853. On succinate plus 0.1 mM p-coumarate, the second-site mutant frequency was 3.8 ×10−6 for strain ADP8085 and 11 × 10−6 for strain ADP853. It should be noted that the effective cell density was lower under the conditions of these experiments than under the conditions associated with the patch method described above, allowing a sufficient stringency of selection of suppressor mutations from strain ADP8085. The results for suppressor mutant frequencies for strains ADP8085 (hcaA1) and ADP853 (pcaB853) are consistent with the presence of more suppressor targets upstream of the pcaB gene product and with 2 mM p-coumarate providing slightly more stringent selective conditions, but they do not fully explain why a lower p-coumarate concentration is required to obtain hca suppressor mutants from strain ADP853.
p-Hydroxybenzoate is an intermediate in the catabolism of p-coumarate (Fig. 1). Should protection from accumulation of carboxymuconate in a pcaB mutant be afforded by a mutation in pobA, it is conceivable that p-hydroxybenzoate, accumulated by the pcaB pobA cells, could spill out into the medium and affect the growth of pcaB cells that are not similarly protected; an analogous situation would apply to an upstream block in pcaH or pcaG. These effects account for the results described in the next paragraph.
On media containing succinate plus 2 mM p-coumarate, virtually all of the secondary mutants of strain ADP853 (pcaB853) were resistant to 1 mM p-hydroxybenzoate as well, i.e., they were blocked in their metabolism of p-hydroxybenzoate, whereas on medium containing succinate plus 0.1 mM p-coumarate, about 70% of the ADP853 mutants were resistant to p-hydroxybenzoate. Of the ADP853 secondary mutants that were sensitive to 1 mM p-hydroxybenzoate, i.e., putative hca mutants, all were resistant to p-coumarate at a level of 0.1 mM. However, in the presence of 2 mM p-coumarate, the p-hydroxybenzoate-sensitive putative hcaC mutants grew only if p-hydroxybenzoate-resistant cells were excluded from the plate. It is likely that p-hydroxybenzoate-resistant cells cause the accumulation of p-hydroxybenzoate or protocatechuate in the surrounding medium, which prevents the growth of strains that have acquired p-coumarate resistance through an hcaC mutation.
Sequence analysis of a representative HcaC mutation.
Putative hcaC-mutated isolates that now had wild-type hcaA and pcaB alleles were screened to eliminate those that carried a deletion that extended beyond hcaC. Plasmid pZR8240, which carries hcaC (Fig. 2), transformed to wild type those cells for which an hcaC mutation was the sole barrier to growth with hydroxycinnamates. Subsequent mapping of the mutations was performed by testing the ability of the hcaC subclones (Fig. 2) to transform each mutant strain to a p-coumarate-positive phenotype, and sequencing confirmed the mapping results. Ten hcaC spontaneous mutant strains were isolated from the two-step ADP8095-ADP853 procedure. One of the mutations, carried by strain ADP8209, was a thymine-to-cytosine transition positioned 784 bp from the 5′ end of hcaC, altering a tryptophan codon to one for arginine at residue 262 in HcaC. The other hcaC mutations will be presented as part of a separate paper.
DISCUSSION
Sensitivity of cells to a block in hydroxycinnamoyl-CoA thioester catabolism.
A block in the catabolism of hydroxycinnamoyl-CoA thioesters makes Acinetobacter cells sensitive to micromolar concentrations of hydroxycinnamates, a response that is enhanced in a constitutive mutant strain. This susceptibility is complemented by the ability of hcaA mutant cells containing a reporter lacZ gene to detect micromolar levels of hydroxycinnamates and to respond with a high level of induced expression (37). Cells with these characteristics have potential as environmental sleuths to explore the bioavailability of hydroxycinnamates in natural habitats where they may be bound to inert materials and to explore the hypothesis that one source of hydroxycinnamates for Acinetobacter sp. strain ADP1 is the plant matrix suberin, in which they are esterified to dicarboxylic acids (4, 15, 31). This hypothesis has particular resonance because in this strain many dca genes for the catabolism of dicarboxylic acids are just upstream of the pca structural genes (36) and thereby linked to the hca genes.
The elimination of hydroxycinnamate toxicity in an hcaA mutant strain by an hcaC mutation points to acyl-CoA thioester accumulation as a trigger for the toxic effect, as does the sensitivity of hcaC-carrying E. coli cells to p-coumarate. The effect of accumulated p-coumaroyl-CoA or feruloyl-CoA thioesters on cells was bacteriostatic, but that of the caffeoyl-CoA thioester could not be assessed because caffeate itself became bactericidal during the course of the experiment, having undergone oxidative browning (6). Oxidation of caffeic acid to the reactive o-quinone and to condensation products has been observed in simple, abiotic model systems (5, 12, 17, 25).
It remains unclear why accumulated hydroxycinnamoyl-CoA thioesters are growth inhibitory. Sequestering of CoA and interference of the accumulated thioesters with cellular processes are two reasonable hypotheses. In E. coli, reversible growth stasis occurred when the total CoA concentration fell below 5 pmol per 108 cells (19). In an effort to influence the intracellular CoA pool, Acinetobacter ΔhcaA mutant cultures were supplemented with the CoA precursor pantothenate (18), but this step failed to alleviate the growth stasis caused by the presence of p-coumarate. Thus, the cause of growth inhibition by hydroxycinnamoyl-CoA accumulation remains unresolved.
Knockouts of HcaA homologs were created in Pseudomonas sp. strain HR199, Pseudomonas putida WCS358, Pseudomonas fluorescens, and Sphingomonas paucimobilis, but toxic effects resulting from the block were not reported (13, 29, 33, 47). In the case of S. paucimobilis, the HcaA homolog, FerB, had an isofunctional protein that could fill in for its activity (29), but a knockout of enoyl-CoA hydratase/lyase in each of the other species resulted in a ferulate-negative phenotype. It may be that the conditions used for the mutant screens and mutant analysis were not suitable for detecting a ferulate-inhibitory phenotype.
Substrate concentration as a critical parameter in positive-selection schemes targeting genes of aromatic catabolic pathways.
The frequency of second-site suppressor mutations was three- to fourfold higher in strain ADP853 (pcaB mutant) than in strain ADP8085 (hcaA mutant). An explanation for this result is that suppressor mutations of strain ADP8085 largely target hcaC but that those of strain ADP853 may include pobA, pobR, and pcaHG and, in the case of the low concentration of p-coumarate, pcaU and hcaC as well. At the lower p-coumarate concentration, the increase in suppressor mutation frequency is consistent with the emergence of additional colonies with more subtle defects that can protect cells from the selective condition; in addition, as noted above, there are more target sites for resistance in strain ADP853 at the lower concentration.
Positive selection for mutations in enzymes, such as protocatechuate 3,4-dioxygenase, that act upstream of the enzyme encoded by pcaB has generally used a ΔpcaBDK mutant exposed to substrate concentrations in the millimolar range (7, 14). In the present study, positive selection targeting mutations in HcaC carried out in an hcaA or an hcaA pcaB mutant background required a p-coumarate concentration above 1 mM in order to avoid background growth of cells. This condition was required with strain ADP8095 (pcaB853 ΔhcaA1) cells from a single colony patched onto selective medium, due to the high concentration of cells in a given patch. It may seem counterintuitive that, following transformation with hcaC-mutated DNA, selection for HcaC mutants in a background mutated only in pcaB required that the p-coumarate concentration be dropped to 0.1 mM, particularly since strain ADP853 is less sensitive to the presence of p-coumarate than strain ADP8085, as noted in Results.
The problem appears to have been caused by feeding from non-hca mutants, e.g., pob or pcaHG mutants, in the population under selection. In the case of pob mutants, the level of p-hydroxybenzoate that accumulated in the medium was presumably sufficient to exert a separate selective pressure for second-site suppressor mutations in genes required for the conversion of p-hydroxybenzoate to carboxymuconate.
Positive selection for mutations targeting hydroxycinnamate:CoA ligase.
Ligases that convert hydroxycinnamates to their corresponding CoA thioesters belong to a biotechnologically significant group of enzymes. In the biotransformation of aromatic feedstocks into natural aroma chemicals (16, 40, 41), the microbial ligase is a key catalyst, and 4-coumarate:CoA ligase from plants produces hydroxycinnamoyl-CoA substrates that are central to the biosynthesis of specialized plant phenylpropanoids. The evolutionary lineage of HcaC is similar to that of the homologous Fcs, feruloyl-CoA synthetases, from Pseudomonas sp. strain HR199 (33) and P. putida strain KT2440 (38a); to our knowledge, other close homologs pulled up by a BLAST search (2) are uncharacterized at present. Acinetobacter HcaC and Pseudomonas Fcs proteins are divergent from functionally similar ligases from Amycolatopsis (1) and Sphingomonas (29) as well as from the two major classes of angiosperm 4-coumarate:CoA ligases (11, 28).
In principle, it should be possible to apply positive selection against the accumulation of hydroxycinnamoyl-CoA thioesters to isolate random or site-directed mutations in hydroxycinnamate:CoA ligases of diverse origins or specificities. In this study, one suppressor mutant isolated by the two-step ADP8095-ADP853 selection method contained a missense mutation that translates to the replacement of tryptophan with arginine at residue 262 of HcaC. Trp262 is conserved in the aligned Fcs homologs from Pseudomonas sp. strain HR199 (33) and P. putida strain KT2440 (38a).
It is likely that the accumulation of hydroxycinnamoyl-CoA thioesters is inhibitory to organisms in general, and the fact that their generation from hydroxycinnamates in E. coli cells carrying an hcaC subclone inhibits cell growth presents a potential opportunity for the selection of mutations in other hydroxycinnamate:CoA ligases, be they homologous to or divergent from HcaC. This fact also affords an easy preliminary functional genomics screen of putative ligase genes for which the aligned products are similar to characterized hydroxycinnamate:CoA ligases. Finally, the use of hcaC or a similar gene in a positive-selection cassette analogous to the sacB-Kmr construction (21) is under investigation.
Two methods for isolating hcaC mutants were described in this paper. Both of these methods led to the isolation of diverse HcaC mutations, which will be described in a separate paper. The two-step ADP8095-ADP853 procedure (Fig. 3B) that resulted in the isolation of the mutant strain ADP8209 has the advantage of homing in on hcaC specifically. The alternative, direct selection of strain ADP853 in the presence of a low concentration of p-coumarate, has the advantage of one less step but the disadvantage of yielding a high percentage of mutants that are blocked in steps downstream of the hydroxycinnamate pathway. The two methods for isolating hcaC mutant strains spotlight one application afforded by the discovery of hydroxycinnamoyl-CoA thioester poisoning, and it should now be straightforward to use PCR-mediated mutagenesis coupled with natural transformation (26, 27) to further target hcaC and to enrich for mutations that may be relevant to structure-function analysis of the ligase.
Strategies to deal with a toxic intermediate.
Evidence presented in this paper points to the importance of cell concentration in the evolution of potentially poisonous pathways. Given the toxigenic nature of nonmetabolizable hydroxycinnamoyl-CoA thioesters and the possible toxicity of aldehyde intermediates, patchwork evolution of the pathway of hydroxycinnamate dissimilation found in bacteria like Acinetobacter may have presented special challenges. It is reasonable to hypothesize that the acquisition and expression of hcaC in the absence of hcaA would be selected against except with very low concentrations of hydroxycinnamates or at high cell concentrations, conditions that apply when cells are present in a biofilm, a condition that also facilitates gene transfer. Conversely, the evolution of catabolic pathways that traffic in toxic intermediates likely imparts a special selective value for biofilm formation.
Acknowledgments
Sequence data for hcaC were generated by the Yale Keck Biotechnology Resource Lab.
This research was funded by grant DAAD19-01-0329 from the Army Research Office and grant GM63628 from the National Institutes of Health.
REFERENCES
- 1.Achterholt, S., H. Priefert, and A. Steinbüchel. 2000. Identification of Amycolatopsis sp. strain HR167 genes, involved in bioconversion of ferulic acid to vanillin. Appl. Microbiol. Biotechnol. 54:799-807. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schafer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1993. Current protocols in molecular biology. Wiley, New York, N.Y.
- 4.Bernards, M. A., and N. G. Lewis. 1998. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry 47:915-933. [DOI] [PubMed] [Google Scholar]
- 5.Cilliers, J. J. L., and V. L. Singleton. 1991. Characterization of the products of nonenzymic autoxidative phenolic reactions in a caffeic acid model system. J. Agric. Food Chem. 39:1298-1303. [Google Scholar]
- 6.Cilliers, J. J. L., and V. L. Singleton. 1989. Nonenzymatic autoxidative phenolic browning reactions in a caffeic acid model system. J. Agric. Food Chem. 37:890-896. [Google Scholar]
- 7.D'Argenio, D. A., M. W. Vetting, D. H. Ohlendorf, and L. N. Ornston. 1999. Substitution, insertion, deletion, suppression, and altered substrate specificity in functional protocatechuate 3,4-dioxygenases. J. Bacteriol. 181:6478-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delneri, D., G. Degrassi, R. Rizzo, and C. V. Bruschi. 1995. Degradation of trans-ferulic and p-coumaric acid by Acinetobacter calcoaceticus DSM 586. Biochim. Biophys. Acta 1244:363-367. [DOI] [PubMed] [Google Scholar]
- 9.Dixon, R. A., and L. Paiva. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doten, R. C., K.-L. Ngai, D. J. Mitchell, and L. N. Ornston. 1987. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J. Bacteriol. 169:3168-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlting, J., D. Büttner, Q. Wang, C. J. Douglas, I. E. Somssich, and E. Kombrink. 1999. Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionary classes in angiosperms. Plant J. 19:9-20. [DOI] [PubMed] [Google Scholar]
- 12.Fulcrand, H., A. Cheminat, R. Brouillard, and V. Cheynier. 1994. Characterization of compounds obtained by chemical oxidation of caffeic acid in acidic conditions. Phytochemistry 35:499-505. [Google Scholar]
- 13.Gasson, M. J., Y. Kitamura, W. R. McLauchlan, A. Narbad, A. J. Parr, E. L. H. Parsons, J. Payne, J. C. Rhodes, and N. J. Walton. 1998. Metabolism of ferulic acid to vanillin. A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J. Biol. Chem. 273:4163-4170. [DOI] [PubMed] [Google Scholar]
- 14.Gerischer, U., and L. N. Ornston. 1995. Spontaneous mutations in pcaH and -G, structural genes for protocatechuate 3,4-dioxygenase in Acinetobacter calcoaceticus. J. Bacteriol. 177:1336-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graca, J., and H. Pereira. 2000. Suberin structure in potato periderm: glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J. Agric. Food Chem. 48:5476-5483. [DOI] [PubMed] [Google Scholar]
- 16.Hagedorn, S., and B. Kaphammer. 1994. Microbial biocatalysis in the generation of flavor and fragrance chemicals. Annu. Rev. Microbiol. 48:773-800. [DOI] [PubMed] [Google Scholar]
- 17.Hapiot, P., A. Neudeck, J. Pinson, H. Fulcrand, P. Neta, and C. Rolando. 1996. Oxidation of caffeic acid and related hydroxycinnamic acids. J. Electroanal. Chem. 405:169-176. [Google Scholar]
- 18.Jackowski, S. 1996. Biosynthesis of pantothenic acid and coenzyme A, p. 687-694. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham and E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 19.Jackowski, S., and C. O. Rock. 1986. Consequences of reduced intracellular coenzyme A content in Escherichia coli. J. Bacteriol. 166:866-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, R. M., V. Pagmantidis, and P. A. Williams. 2000. sal genes determining the catabolism of salicylate esters are part of a supraoperonic cluster of catabolic genes in Acinetobacter sp. strain ADP1. J. Bacteriol. 182:2018-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, R. M., and P. A. Williams. 2003. Mutational analysis of the critical bases involved in activation of the AreR-regulated σ54-dependent promoter in Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 69:5627-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juni, E. 1972. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J. Bacteriol. 112:917-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juni, E., and A. Janick. 1969. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J. Bacteriol. 98:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 25.Kerry, N., and C. Rice-Evans. 1998. Peroxynitrite oxidises catechols to o-quinones. FEBS Lett. 437:167-171. [DOI] [PubMed] [Google Scholar]
- 26.Kok, R. G., D. A. D'Argenio, and L. N. Ornston. 1997. Combining localized PCR mutagenesis and natural transformation in direct genetic analysis of a transcriptional regulator gene, pobR. J. Bacteriol. 179:4270-4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kok, R. G., D. A. D'Argenio, and L. N. Ornston. 1998. Mutation analysis of PobR and PcaU, closely related transcriptional activators in Acinetobacter. J. Bacteriol. 180:5058-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindermayr, C., B. Möllers, J. Fliegmann, A. Uhlmann, F. Lottspeich, H. Meimberg, and J. Ebel. 2002. Divergent members of a soybean (Glycine max L.) 4-coumarate:coenzyme A ligase gene family. Primary structures, catalytic properties, and differential expression. Eur. J. Biochem. 269:1304-1315. [DOI] [PubMed] [Google Scholar]
- 29.Masai, E., Y. Harada, and X. Peng. 2002. Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl. Environ. Microbiol. 68:4416-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melnikov, A., and P. J. Youngman. 1999. Random mutagenesis by recombinational capture of PCR products in Bacillus subtilis and Acinetobacter calcoaceticus. Nucleic Acids Res. 27:1056-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moire, L., A. Schmutz, A. Buchala, B. Yan, R. E. Stark, and U. Ryser. 1999. Glycerol is a suberin monomer. New experimental evidence for an old hypothesis. Plant Physiol. 119:1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narbad, A., and M. J. Gasson. 1998. Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology 144:1397-1405. [DOI] [PubMed] [Google Scholar]
- 33.Overhage, J., H. Priefert, and A. Steinbüchel. 1999. Biochemical and genetic analysis of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl. Environ. Microbiol. 65:4837-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parke, D. 2000. Positive selection for mutations in bioconversion of aromatic compounds in Agrobacterium tumefaciens: analysis of spontaneous mutations in the protocatechuate 3,4-dioxygenase gene. J. Bacteriol. 182:6145-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parke, D., D. A. D'Argenio, and L. N. Ornston. 2000. Bacteria are not what they eat: that is why they are so diverse. J. Bacteriol. 182:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parke, D., M. A. Garcia, and L. N. Ornston. 2001. Cloning and genetic characterization of dca genes required for beta-oxidation of straight-chain dicarboxylic acids in Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 67:4817-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parke, D., and L. N. Ornston. 2003. Hydroxycinnamate (hca) catabolic genes from Acinetobacter sp. strain ADP1 are repressed by HcaR and are induced by hydroxycinnamoyl-CoA thioesters. Appl. Environ. Microbiol. 69:5398-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parke, D., and L. N. Ornston. 1984. Nutritional diversity of Rhizobiaceae revealed by auxanography. J. Gen. Microbiol. 130:1743-1750. [Google Scholar]
- 38a.Plaggenborg, R., J. Overhage, A. Steinbüchel, and H. Priefert. 2003. Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 61:528-535. [DOI] [PubMed] [Google Scholar]
- 39.Priefert, H., J. Rabenhorst, and A. Steinbüchel. 1997. Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. J. Bacteriol. 179:2595-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabenhorst, J. 1996. Production of methoxyphenol-type natural aroma chemicals by biotransformation of eugenol with a new Pseudomonas sp. Appl. Microbiol. Biotechnol. 46:470-474. [Google Scholar]
- 41.Rosazza, J. P. N., Z. Huang, L. Dostal, T. Volm, and B. Rousseau. 1995. Review: biocatalytic transformation of ferulic acid: an abundant natural product. J. Ind. Microbiol. 15:457-471. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Segura, A., P. V. Bünz, D. A. D'Argenio, and L. N. Ornston. 1999. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol. 181:3494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 46.Smith, M. A., V. B. Weaver, D. M. Young, and L. N. Ornston. 2003. Genes for chlorogenate and hydroxycinnamate catabolism (hca) are linked to functionally related genes in the dca-pca-qui-pob-hca chromosomal cluster of Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 69:524-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venturi, V., F. Zennaro, G. Degrassi, B. C. Okeke, and C. V. Bruschi. 1998. Genetics of ferulic acid bioconversion to protocatechuic acid in plant-growth-promoting Pseudomonas putida WCS358. Microbiology 144:965-973. [DOI] [PubMed] [Google Scholar]
- 48.Weisshaar, B., and G. I. Jenkins. 1998. Phenylpropanoid biosynthesis and its regulation. Curr. Opin. Plant Biol. 1:251-257. [DOI] [PubMed] [Google Scholar]
- 49.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]