Abstract
A synergistic effect between silver and UV radiation has been observed that can appreciably enhance the effectiveness of UV radiation for inactivation of viruses. At a fluence of ca. 40 mJ/cm2, the synergistic effect between silver and UV was observed at silver concentrations as low as 10 μg/liter (P < 0.0615). At the same fluence, an MS-2 inactivation of ca. 3.5 logs (99.97%) was achieved at a silver concentration of 0.1 mg/liter, a significant improvement (P < 0.0001) over the ca. 1.8-log (98.42%) inactivation of MS-2 at ca. 40 mJ/cm2 in the absence of silver. Modified Chick-Watson kinetics were used to model the synergistic effect of silver and UV radiation. For an MS-2 inactivation of 4 logs (99.99%), the coefficient of dilution (n) was determined to be 0.31, which suggests that changes in fluence have a greater influence on inactivation than does a proportionate change in silver concentration.
The bactericidal effects of silver have been known since the mid-1800s (11, 31). Given contact times on the order of hours, silver has been shown to be somewhat effective as a disinfectant against coliforms (9, 11) and viruses (41). In water, at concentrations sufficient for bactericidal activity, silver does not impart taste, color, or odor and has no apparent detrimental effects on mammalian cells (41). The only known negative health effect is argyria, an irreversible darkening of the skin and mucous membranes, which has been caused by prolonged silver therapy (34). Accordingly, there is no United States Environmental Protection Agency (USEPA) primary drinking water standard for silver. Two of the principal drawbacks associated with the use of silver as a disinfectant are the need for long contact times (4, 11) and the existence of silver-resistant organisms (5, 15, 33). However, use of silver by some European nations (34) suggests that it is considered feasible and economically viable in some cases. Silver is commonly used to prevent microbial growth in point-of-use filters (4, 32, 39); as a codisinfectant for swimming pool water, which allows for lower chlorine levels in pools (3, 41); and as a codisinfectant in hospital hot water systems (22).
Unlike silver, UV radiation is widely considered a viable process for disinfecting drinking water and wastewater in large-scale treatment systems (40) because it is an effective means of inactivating protozoa such as Cryptosporidium parvum (8, 12, 28) and Giardia lamblia (14, 24) and it does not create significant disinfection by-products (40). As with any disinfection process, an important consideration associated with UV radiation is cost. Power requirements for UV systems are primarily a function of the desired fluence (the product of irradiance and exposure time). In addition to an increase in operating costs, an increase in fluence can also result in a significant increase in capital costs (13). Microbial inactivation goals, which are a function of a target organism, set the UV design fluence. Design fluences for water treatment can vary between 40 and 140 mJ/cm2 (13). Fluences as high as 170 mJ/cm2 have been reported for 4-log (99.99%) inactivation of adenoviruses in tertiary-treated wastewater, which indicates that fluences sufficient for inactivation of coliforms (e.g., ca. 8 mJ/cm2 for Escherichia coli [see reference 40]) may not provide suitable inactivation of human adenoviruses (37). Because viruses are reported to be the pathogens most resistant to UV disinfection, they are likely to influence the fluence requirements of this disinfection process in many cases (7, 23). A reduction in the UV design fluence and subsequent capital and operating costs ought to make UV disinfection more appealing to municipalities that may wish to eliminate disinfection by-products and improve inactivation of protozoa. Methods for improving inactivation of viruses with UV radiation might help in attaining these objectives.
In 1973, Rahn and coworkers (29, 30) reported that silver cations (hereafter referred to as “silver”) can complex with DNA, thereby making it more photoreactive. They hypothesized that this phenomenon might “serve as a useful tool for studying the photobiology of simple systems such as transforming DNA and phage.” It is hypothesized that this phenomenon might also improve the efficacy of UV disinfection in a variety of treatment trains including water and wastewater treatment systems. The objectives of the work reported herein were to (i) determine if a synergistic effect between silver and UV radiation exists for inactivation of coliphage MS-2, a viable surrogate for pathogenic viruses (hereafter referred to as “MS-2”); (ii) quantify inactivation of MS-2 as a function of silver concentration and fluence; and (iii) develop a simple model (a modified Chick-Watson disinfection model) for quantifying the synergistic effect between silver and UV radiation for inactivating MS-2.
MATERIALS AND METHODS
Reagents.
Silver nitrate (Alfa Aesar, Ward Hill, Mass.) stock solutions (100 or 1,000 mg of Ag/liter) were measured by a colorimetric procedure (Hatch, Loveland, Colo.; detection limit, 0.05 mg of Ag/liter). Subsequent solutions containing silver below the detection limit were made by dilution of the stock solutions. Silver neutralizer solutions contained 11.5 g of 0.93 M sodium thiosulfate (J. T. Baker, Phillipsburg, N.J.) and 0.88 M sodium thioglycolate (Sigma, St. Louis, Mo.) (41). The solution was filter sterilized and stored in acid-washed, sterile glass bottles. Fresh silver and silver neutralizer stock solutions were prepared on the day of each experiment. The phosphate buffer (20 mM, pH 7.2) was autoclaved before use. All glassware used to handle MS-2 and/or silver was soaked in 10% HNO3 overnight and rinsed in deionized water to remove adsorbed silver (10) and other contaminants prior to use.
Preparation of purified MS-2.
A culture of E. coli (ATCC 15597) was grown in tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.) at 37°C and 150 rpm. Freeze-dried MS-2 (ATCC 15597-B1) was mixed with 1.5 ml of a 24-h culture of E. coli and 3.0 ml of melted (45°C) TSB soft agar (0.5% [wt/vol] agar). The mixture was overlaid on TSB agar (1.5% [wt/vol] agar) plates and incubated at 37°C for 24 h. Six milliliters of the 20 mM phosphate buffer was added to the plate and incubated for 1 h. The phosphate buffer was removed and passed through a 0.22-μm-pore-size filter, and the filtrate was used as the MS-2 stock suspension, having an initial density of ca. 109 PFU/liter.
Collimated beam setup.
The collimated beam apparatus used in this study (Suntec Environmental, Concord, Ontario, Canada) was modified to hold a stir plate and to allow for easy and reproducible vertical and horizontal adjustment. The two low-pressure mercury vapor lamps in the instrument were warmed up for at least 30 min before all experiments. Lamp irradiance was quantified with a UV detector (IL1400A; International Light, Newburyport, Mass.) by placing the detector at the same height as the sample surface. It was determined that variations in lamp irradiance across the surface of the samples were negligible by moving the detector in the horizontal plane at distances equivalent to the sample surfaces (see below), which resulted in a Petri factor (PF) of unity. Fluence was determined by placing the detector in the integration mode following the removal of a shutter and recording the required exposure time. Variations in fluence resulting from drift in lamp output were typically less than ca. 0.5%. However, lamp output was verified periodically during the course of an experiment to compensate for slight changes resulting from drift. The average fluence (mW · s/cm2 or mJ/cm2) was determined as follows (6):
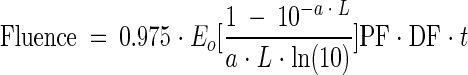 |
(1) |
where Eo is the lamp intensity at the center of the sample dish (mW/cm2), a is the sample absorption coefficient (cm−1), L is the depth of the sample (cm), t is exposure time (s), and DF is divergence factor.
The divergence factor was determined as follows (6):
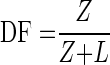 |
(2) |
where Z is distance from lamp to sample surface (cm).
Irradiation of samples.
Samples were prepared by combining 1,000 ± 6 μl of MS-2 viral stock suspensions with 9 ± 0.1 ml (8.9 ± 0.1 ml for samples containing silver) of phosphate buffer in acid-washed, sterile Pyrex glass petri dishes. All samples containing silver were prepared such that 100 ± 0.6 μl of a particular silver stock solution was added to the MS-2 suspensions in the petri dishes to minimize effects of dilution. Samples containing silver were incubated at 25 ± 0.1°C for a predetermined time. The total volume (without stir bar) and depth (with stir bar) of the viral stock suspensions in the petri dishes were 10 ml and 0.6 cm, respectively. The viral stock suspensions were placed under the collimated beam and irradiated for a period sufficient to achieve the predetermined fluence. Samples were stirred slowly to prevent forming a vortex in the water (6). The incubation period for silver and MS-2, prior to and including the time of UV radiation exposure, was held at 10 min unless stated otherwise. Samples containing silver were neutralized (to halt disinfection) following the incubation period by adding approximately 10 μl of the stock neutralizer solution, which was determined to be sufficient for neutralizing the highest silver concentration (10 mg/liter) used in this work. Because photoreduction of silver, due to ambient light, has been reported to have a negligible influence on the bactericidal efficiency of silver (10, 11), exposure to visible light during the short periods when the samples were set up was considered negligible. Each experiment was conducted at least in triplicate. For all samples, a minimum of three dilutions were plated in triplicate by the standard double agar overlay technique as described elsewhere (1, 23) with an E. coli (ATCC 15597) host grown at 37 ± 0.1°C for 3 to 6 h (41). Plates were incubated at 37 ± 0.1°C and enumerated at 24 ± 1 h. The dilution giving the highest number of PFU less than 300 was averaged and used to obtain the MS-2 survival (23). Control assays were conducted in triplicate, and controls were plated at various times during each experiment to ensure that conditions during the course of an experiment did not influence the number of PFU in the stock suspensions. Neutralizer was also added to selected controls to verify that it did not influence MS-2 inactivation.
RESULTS AND DISCUSSION
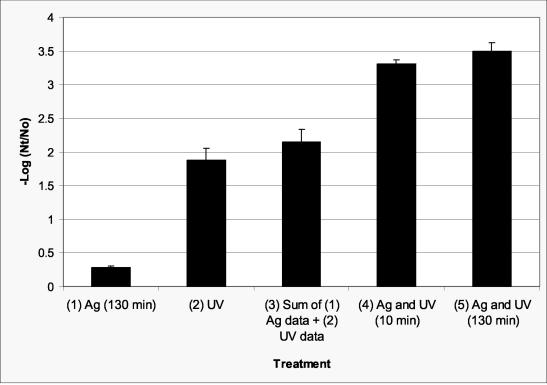
MS-2 was used in this study because it has been proposed as the benchmark for validation of full-scale UV reactors (23, 25) and it has been proposed as a surrogate for pathogenic enteric viruses such as noroviruses (see reference 38). Data for inactivation of MS-2 by silver (0.1 mg/liter), UV radiation (target fluence of 40 mJ/cm2, corrected to 37 mJ/cm2 according to equation 1, where a = 6.4 × 10−2 cm−1, Z = 86 cm, and DF = 1.00), and combinations of silver and UV radiation are presented in Fig. 1. A target fluence of 40 mJ/cm2 was used because it has been proposed as a recommended fluence for water treatment systems (13, 38). Considered individually, the silver, given 130 min of contact time, and UV radiation resulted in approximately 0.28-log (47.52%) and 1.87-log (98.65%) inactivation, respectively. With the use of this silver concentration only, a measurable inactivation was not observed for 10 min of contact time. When silver was followed by UV radiation, resulting in a total silver contact time of 10 min, a synergistic effect resulted in ca. 3.30-log (99.95%) inactivation (column 4). An increase to ca. 3.49-log inactivation (99.97%) occurred when silver was neutralized 120 min after exposure to UV radiation, which resulted in a total silver contact time of 130 min (column 5). The difference between column 3 and column 5 was found to be statistically significant (P < 0.0001). The data clearly show that there is a synergistic effect when silver and UV radiation are combined. The additional inactivation between column 4 and column 5 is comparable to inactivation by 0.1 mg of silver/liter alone (column 1) given the same contact time (130 min).
FIG. 1.
Inactivation of MS-2 by silver (0.1 mg/liter for 130 min) (column 1) and UV radiation (ca. 40 mJ/cm2) (column 2); (column 3) sum of columns 1 and 2; (column 4) silver (0.1 mg/liter for 10 min) followed by UV radiation (ca. 40 mJ/cm2) and then neutralized immediately (10-min total silver exposure); (column 5) silver (0.1 mg/liter for 10 min) followed by UV radiation (ca. 40 mJ/cm2) and then neutralized 120 min after exposure to UV (130-min total silver exposure). The difference between column 3 and column 5 was found to be statistically significant (P < 0.0001). Error bars represent 1 standard deviation.
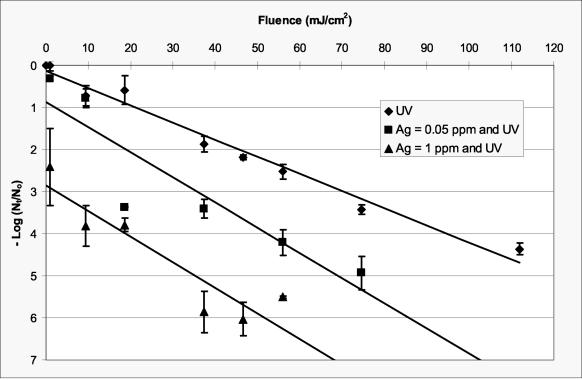
Data for inactivation of MS-2 as a function of UV fluence are presented in Fig. 2. Samples of MS-2 that were first exposed to silver (0.05 or 1 mg of Ag/liter) for 73 min and then exposed to UV radiation are also presented in this figure. An incubation time of 73 min was used here because the influence of incubation time on the synergistic effect between silver and UV was not yet evaluated. It was later determined that silver incubation time does not influence the synergistic effect, but it does provide additional inactivation by silver alone. Inactivation of MS-2 by 0.05 and 1 mg of Ag/liter, given 73 min of contact time without UV irradiation, was less than 0.3 and 1.2 logs, respectively, which is consistent with data published in the literature for inactivation of MS-2 with silver (41). At a silver concentration of 0.05 mg/liter, which is one-half the USEPA secondary drinking water standard of 0.1 mg/liter, the fluence required to achieve a 4-log inactivation of MS-2 was reduced by ca. 45% (from 95 to 52 mJ/cm2). Approximately a 2-log inactivation of MS-2 was achieved at a fluence of 20 mJ/cm2 and a silver concentration of 0.05 mg/liter. Should future regulatory goals for UV disinfection become focused on a 2-log inactivation of MS-2 (25), then a reduction in fluence from ca. 40 to 20 mJ/cm2 would be possible if a silver concentration of 0.05 mg/liter was used in conjunction with UV disinfection.
FIG. 2.
Inactivation of MS-2 as a function of fluence and silver concentration in a phosphate-buffered solution (pH 7.2). Fluence data for UV only (⧫) pass through the origin. The lowest fluence data for the samples containing silver (▪ and ▴) were 1 mJ/cm2. Samples that were first exposed to silver (0.05 or 1 mg of Ag/liter) were incubated for 73 min including the time required to achieve the specified fluence. Error bars represent 1 standard deviation.
In order to compare UV data for MS-2 reported in the literature (38) with data in the present study, the data in Fig. 2 were fit with linear functions (no silver, slope = 4.1 × 10−2 cm2/mJ, y intercept = 0.13, R2 = 0.98; 5 × 10−2 mg of silver/liter, slope = 6.0 × 10−2 cm2/mJ, y intercept = 0.87, R2 = 0.83; and 1.0 mg of silver/liter, slope = 6.1 × 10−2 cm2/mJ, y intercept = 2.85, R2 = 0.84). Thurston-Enriquez et al. (38) reported a slope of 3.1 × 10−2 cm2/mJ for MS-2 inactivation in a phosphate-buffered solution, which compares favorably to the slope of 4.1 × 10−2 cm2/mJ observed in this study for inactivation of MS-2 in the absence of silver. The slopes for the regression lines through the silver-UV data are slightly greater than those for UV alone. Because the y axis corresponds to silver alone, the y-intercept values should be a function of the silver concentration and the incubation time. The y-intercept values, obtained via linear regression, are somewhat higher than the values for silver alone given 73 min of contact time (see above), which indicates that the synergistic effect between silver and UV radiation was apparent at the lowest fluence evaluated (1 mJ/cm2) and that a nonlinear model may be more appropriate for modeling the synergistic effect as a function of fluence and silver concentration, particularly at low fluences.
The inactivation data for MS-2 exposed to UV radiation, in the absence of silver (Fig. 2), are congruent with data reported in the literature (23, 38). Appreciable tailing was not observed for this inactivation curve, indicating that MS-2 did not have a tendency to clump (17, 38). Variations between replicate experiments in the presence of silver were more pronounced than those for UV only because silver might have complexed with particulate or dissolved matter, which could have been added with the MS-2 to the phosphate-buffered solutions. Silver has been reported to complex with many of the amino acids and constituents commonly found in growth media (5, 9, 19, 21, 26) that could be comparable to debris in the MS-2 suspensions. Although the suspension evaluated in this work has been referred to as “demand free” relative to UV radiation (38), it is probably not demand free relative to silver.
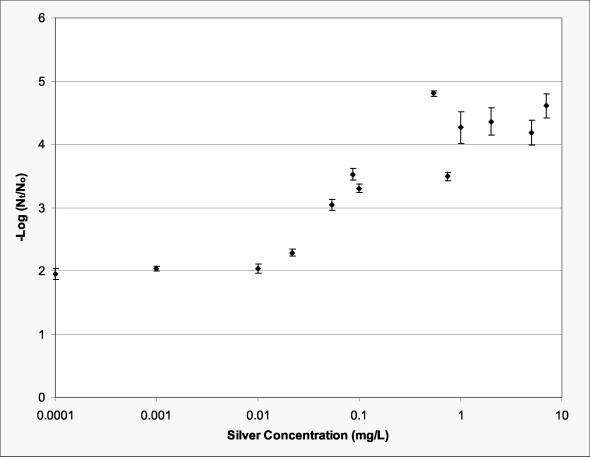
Inactivation of MS-2 as a function of silver concentration for a target UV radiation fluence of 40 mJ/cm2 (corrected fluence of 37 mJ/cm2) is presented in Fig. 3. The synergistic effect between silver and UV was most sensitive to silver concentration between 0.01 and 1 mg/liter. There appeared to be no additional inactivation above a silver concentration of 1 mg/liter under the conditions evaluated. At approximately the anticipated regulatory fluence of 40 mJ/cm2 (13), a synergistic effect between silver and UV was observed at silver concentrations as low as 10 μg/liter (P < 0.0615). At the same fluence, an MS-2 inactivation of ca. 3.7 logs (99.97%) was achieved at a silver concentration of 0.1 mg/liter, a significant (P < 0.0001) improvement over the ca. 1.8-log (98.42%) inactivation of MS-2 in the absence of silver.
FIG. 3.
Inactivation of MS-2 as a function of silver concentration for a fluence of ca. 40 mJ/cm2. Silver incubation time was 10 min. Error bars represent 1 standard deviation.
The well-known Chick-Watson disinfection model has produced an adequate fit to data on microbial inactivation (16, 17). Furthermore, it is the regulatory basis for the USEPA's Surface Water Treatment Rule, which governs drinking water disinfection. The Chick-Watson model was modified, by replacing contact time with fluence, and used here as a first approximation to model the synergistic effect of silver and UV radiation as follows:
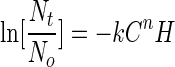 |
(3) |
where C is silver concentration (mg/liter), H is fluence (mJ/cm−2), n is coefficient of dilution, and k is die-off constant [(mg/liter)−n/(mJ/cm2)]. The empirical constants, k and n, were determined as described elsewhere (36) for 4-log inactivation. Under these conditions, n = 0.31 and k = 0.57 · [(mg/liter)−0.31/(mJ/cm2)]. Because the coefficient of dilution was determined to be less than unity, the model predicts that a proportionate change in fluence has a greater impact on inactivation than does a proportionate change in silver concentration (16). This finding is congruent with the data presented in Fig. 2 and 3, which illustrate that silver concentration must change by several orders of magnitude to have the same impact on MS-2 inactivation as that of fluence.
UV radiation causes dimerization of thymine in DNA and uracil in RNA, which can lead to inactivation of an organism (18, 27, 30, 35). It has been known for some time that silver complexes with bases of DNA and RNA (2, 20). Rahn and coworkers (29, 30) reported that the increase in thymine dimerization observed when silver and UV radiation were used together was caused by a heavy atom effect and resulted in an increase in the total inactivation of Haemophilus influenzae for a given fluence. Because silver forms complexes with proteins (5, 9, 26) and the MS-2 capsid contains proteins, the ability of silver to penetrate the capsid and bind with MS-2 RNA is not yet known. However, the mechanism described for inactivation of H. influenzae with silver and UV irradiation (30) might be extensible to MS-2 as well.
This work demonstrates for the first time that a synergistic effect occurs with silver and UV radiation for an RNA virus. Because it has also been reported for a DNA virus (29, 30), it is expected that the synergistic effect between silver and UV radiation might also exist for the inactivation of pathogenic viruses such as poliovirus, noroviruses, and the enteric adenovirus types 40 and 41.
Unlike with silver by itself (9, 11, 41), substantial incubation times were not required to achieve the synergistic effect between silver and UV radiation. In fact, a silver incubation time of 5 min provided almost the same (P < 0.1534) inactivation as did an incubation time of 120 min for a silver concentration of 0.05 mg/liter and a fluence of ca. 40 mJ/cm2. (There was a modest additional inactivation for 120 min resulting from silver contact with MS-2 prior to exposure to UV radiation as described above.) This finding suggests that a large clearwell with a long contact time would not be required to obtain the synergistic effect of silver and UV radiation.
The results reported in this work were obtained in a phosphate-buffered solution, which is typically termed a demand-free system for inactivation of viruses with UV radiation (38). Turbidity, particle counts, and UV absorbance were reported to have a minor influence on UV disinfection of MS-2 (23). Moreover, Butkus et al. (9) reported that hardness, chloride concentration, and turbidity had a minor influence on the inactivation of E. coli by silver. Preliminary data (not shown) indicate that the synergistic effect was not influenced by chloride at concentrations as high as 20 mg of Cl−/liter. The influence of chloride and other common water and wastewater constituents at the point of UV disinfection, e.g., postfiltration, on the synergistic effect between silver and UV radiation for MS-2 and other pathogens warrants further investigation.
Acknowledgments
We gratefully acknowledge the insight on UV disinfection provided by James Malley, Civil Engineering Program, University of New Hampshire, and Elliott Whitby, Suntec Environmental, Concord, Ontario, Canada. We thank Charles P. Gerba, Department of Microbiology and Immunology, The University of Arizona, for his guidance on silver disinfection. The technical assistance of Anand Shetty, Department of Geography and Environmental Engineering, United States Military Academy, is also greatly appreciated.
This project was supported by grants from The Army's PM Soldier Systems Program.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages. Intersciences, New York, N.Y.
- 2.Arakawa, H., J. F. Neault, and H. A. Tajmir-Riahi. 2001. Silver(I) complexes with DNA and RNA studied by Fourier transform infrared spectroscopy and capillary electrophoresis. Biophys. J. 81:1580-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer, C. W., L. E. Guilmartin, T. F. McLoughlin, and T. J. White. 1999. Swimming pool disinfection. J. Environ. Health 61:9-13. [Google Scholar]
- 4.Bell, F. A. 1991. Review of effects of silver-impregnated carbon filters on microbial water quality. J. Am. Water Works Assoc. 83:74-76. [Google Scholar]
- 5.Belly, R. T., and G. C. Kydd. 1982. Silver resistance in microorganisms. Dev. Ind. Microbiol. 23:567-577. [Google Scholar]
- 6.Bolton, J. R., and K. G. Linden. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J. Environ. Eng. 129:209-215. [Google Scholar]
- 7.Brown, N. P., J. G. Jacangelo, C. N. Haas, and C. P. Gerba. 2002. Assessment of existing disinfection practices for inactivation of emerging pathogens. In American Water Works Association Annual Conference Proceedings. American Water Works Association, Denver, Colo.
- 8.Bukhari, Z., T. M. Hargy, J. R. Bolton, B. Dussert, and J. L. Clancy. 1999. Medium pressure UV light for oocyst inactivation. J. Am. Water Works Assoc. 91:86-94. [Google Scholar]
- 9.Butkus, M. A., L. Edling, and M. P. Labare. The efficacy of silver as a bactericidal agent: advantages, limitations, and considerations for future use. J. Water Supply Res. Technol. AQUA 52:407-415.
- 10.Chambers, C. W. 1956. A procedure for evaluating the efficiency of bactericidal agents. J. Milk Food Technol. 19:183-187. [Google Scholar]
- 11.Chambers, C. W., M. Proctor, and P. W. Kabler. 1962. Bactericidal effect of low concentrations of silver. J. Am. Water Works Assoc. 54:208-216. [Google Scholar]
- 12.Clancy, J. L., T. M. Hargy, M. M. Marshall, and J. E. Dyksen. 1998. UV light inactivation of Cryptosporidium oocysts. J. Am. Water Works Assoc. 90:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotton, C. A., D. M. Owen, G. C. Cline, and T. P. Brodeur. 2001. UV disinfection costs for inactivating Cryptosporidium. J. Am. Water Works Assoc. 93:82-94. [Google Scholar]
- 14.Craik, S. A., D. Weldon, G. R. Finch, J. R. Bolton, and M. Belosevic. 2001. Inactivation of Cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 35:1387-1398. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, A., M. Maynes, and S. Silver. 1998. Effects of halides on plasmid-mediated silver resistance in Escherichia coli. Appl. Environ. Microbiol. 64:5042-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas, C. N. 1999. Disinfection, p. 14.1-14.60. In R. W. Letterman (ed.), Water quality and treatment, 5th ed. McGraw-Hill, Inc, New York, N.Y.
- 17.Haas, C. N., and S. B. Kara. 1984. Kinetics of microbial inactivation by chlorine. I. Review of results in a demand-free system. Water Res. 18:1443. [Google Scholar]
- 18.Harris, G. D., V. D. Adams, D. L. Sorensen, and M. S. Curtis. 1987. Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria. Water Res. 21:687-692. [Google Scholar]
- 19.Herrin, R. T., A. W. Anders, and D. E. Armstrong. 2001. Determination of silver speciation in natural waters: binding strength of silver ligands in surface freshwaters. Environ. Sci. Technol. 35:1959-1966. [DOI] [PubMed] [Google Scholar]
- 20.Izatt, R. M., J. J. Christensen, and J. H. Rytting. 1971. Sites and thermodynamic quantities associated with proton and metal ion interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem. Rev. 71:439-481. [DOI] [PubMed] [Google Scholar]
- 21.Liau, S. Y., D. C. Read, W. J. Pugh, F. R. Furr, and A. D. Russell. 1997. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett. Appl. Microbiol. 25:279-283. [DOI] [PubMed] [Google Scholar]
- 22.Lin, Y. E., D. V. Radisav, J. E. Stout, and V. L. Yu. 2002. Negative effect of high pH on biocidal efficacy of copper and silver ions in controlling Legionella pneumophila. Appl. Environ. Microbiol. 68:2711-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden, K. G., L. Batch, and C. Schulz. 2002. UV disinfection of filtered water supplies: water quality impacts on MS2 dose-response curves. In American Water Works Association Annual Conference Proceedings. American Water Works Association, Denver, Colo.
- 24.Linden, K. G., G. A. Shin, G. Faubert, W. Cairns, and M. D. Sobsey. 2002. UV disinfection of Giardia lamblia cysts in water. Environ. Sci. Technol. 36:2519-2522. [DOI] [PubMed] [Google Scholar]
- 25.Mackey, E. D., T. M. Hargy, H. B. Wright, J. P. Malley, Jr., and R. S. Cushing. 2002. Comparing Cryptosporidium and MS2 bioassays—implications for UV reactor validation. J. Am. Water Works Assoc. 94:62-69. [Google Scholar]
- 26.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzler, D. E. 1977. Biochemistry: the chemical reactions of living cells. Academic Press, New York, N.Y.
- 28.Morita, S., A. Namikoshi, T. Hirata, K. Oguma, H. Katayama, S. Ohgaki, N. Motoyama, and M. Fujiwara. 2002. Efficacy of UV irradiation in inactivating Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 68:5387-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahn, R. O., J. K. Setlow, and L. C. Landry. 1973. Ultraviolet irradiation of nucleic acids complexed with heavy atoms. II. Phosphorescence and photodimerization of DNA complexed with Ag. Photochem. Photobiol. 18:29-38. [DOI] [PubMed] [Google Scholar]
- 30.Rahn, R. O., and L. C. Landry. 1973. Ultraviolet irradiation of nucleic acids complexed with heavy atoms. III. Influence of Ag+ and Hg2+ on the sensitivity of phage and of transforming DNA to ultraviolet radiation. Photochem. Photobiol. 18:39-41. [DOI] [PubMed] [Google Scholar]
- 31.Ravelin, J. 1869. Chemistry of vegetation. Sci. Nat. 11:93-102. [Google Scholar]
- 32.Reasoner, D. J., J. C. Blannon, and E. E. Geldreich. 1987. Microbial characteristics of third-faucet point-of-use devices. J. Am. Water Works Assoc. 79:60-66. [Google Scholar]
- 33.Richards, R. M. E., H. A. Odelola, and B. Anderson. 1984. Effect of silver on whole cells and spheroplasts of a silver resistant Pseudomonas aeruginosa. Microbios 39:151-158. [PubMed] [Google Scholar]
- 34.Russell, A. D., and W. B. Hugo. 1994. Antimicrobial activity and action of silver. Prog. Med. Chem. 31:351-371. [DOI] [PubMed] [Google Scholar]
- 35.Sommer, R., W. Pribil, S. Appelt, P. Gehringer, H. Eschweiler, H. Leth, A. Cabaj, and T. Haider. 2001. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: a comparative approach. Water Res. 35:3109-3116. [DOI] [PubMed] [Google Scholar]
- 36.Tchobanoglous, G., F. L. Burton, and H. D. Stensel. 2003. Wastewater engineering treatment and reuse, 4th ed., p. 1218-1243. Metcalf and Eddy, Inc., Boston, Mass.
- 37.Thompson, S. S., J. L. Jackson, M. Suva-Castillo, W. A. Yanko, Z. E. Jack, J. Kuo, C. L. Chen, F. P. Williams, and D. P. Schnurr. 2003. Detection of infectious human adenoviruses in tertiary-treated and ultraviolet-disinfected wastewater. Water Environ. Res. 75:163-170. [DOI] [PubMed] [Google Scholar]
- 38.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobin, R. S., D. K. Smith, and J. A. Lindsay. 1981. Effects of activated carbon and bacteriostatic filters on microbiological quality of drinking water. Appl. Environ. Microbiol. 41:646-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Environmental Protection Agency. 1996. UV light disinfection technology in drinking water applications—an overview. EPA 811-R-96-002. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 41.Yahya, M. T., T. M. Straub, and C. P. Gerba. 1992. Inactivation of coliphage MS-2 and poliovirus by copper, silver, and chlorine. Can. J. Microbiol. 38:430-435. [DOI] [PubMed] [Google Scholar]